Effect of (NH4)2SO4 Solution Concentration on Bound Water Content in Ion Adsorption Rare-Earth Raw Ore
Abstract
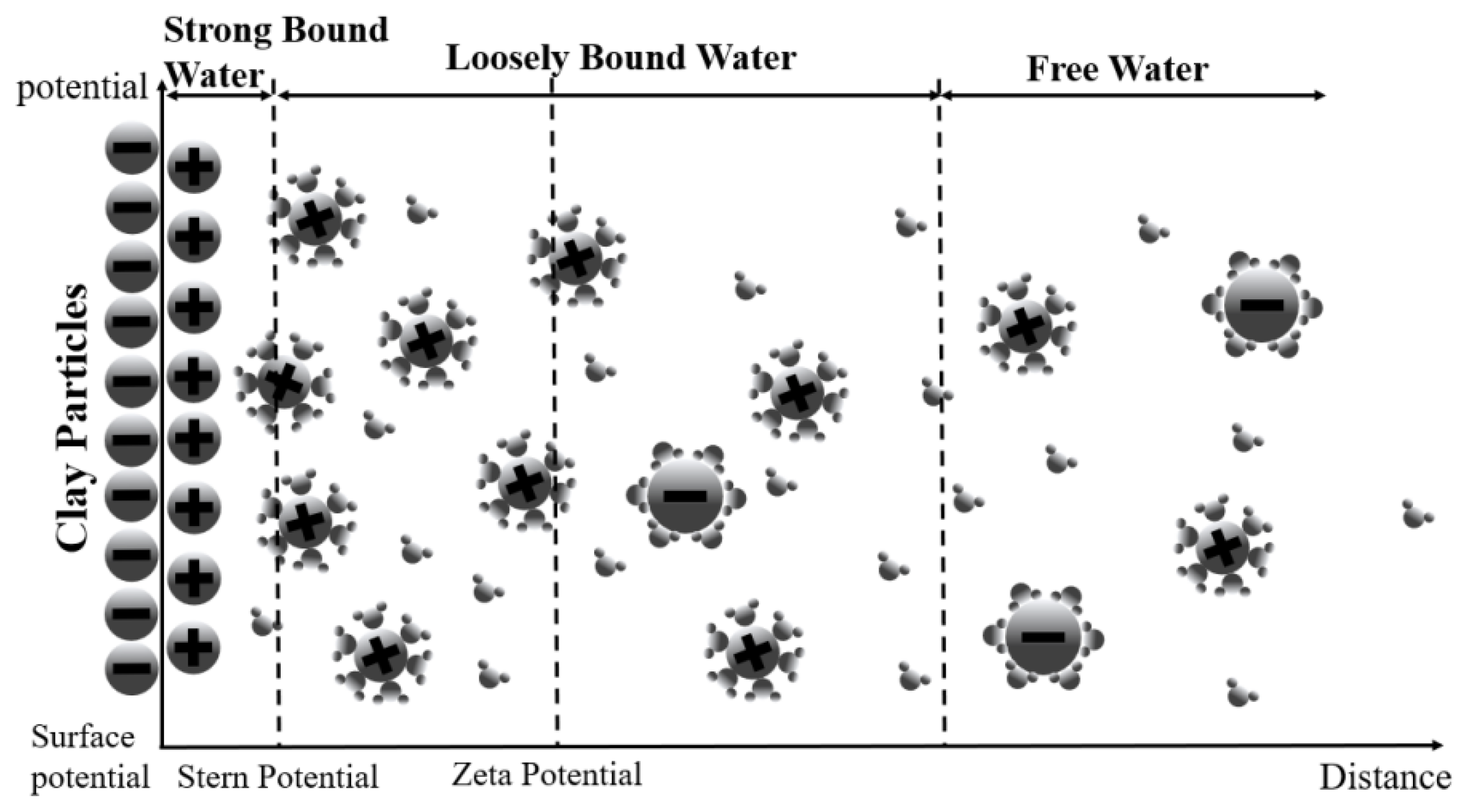
1. Introduction
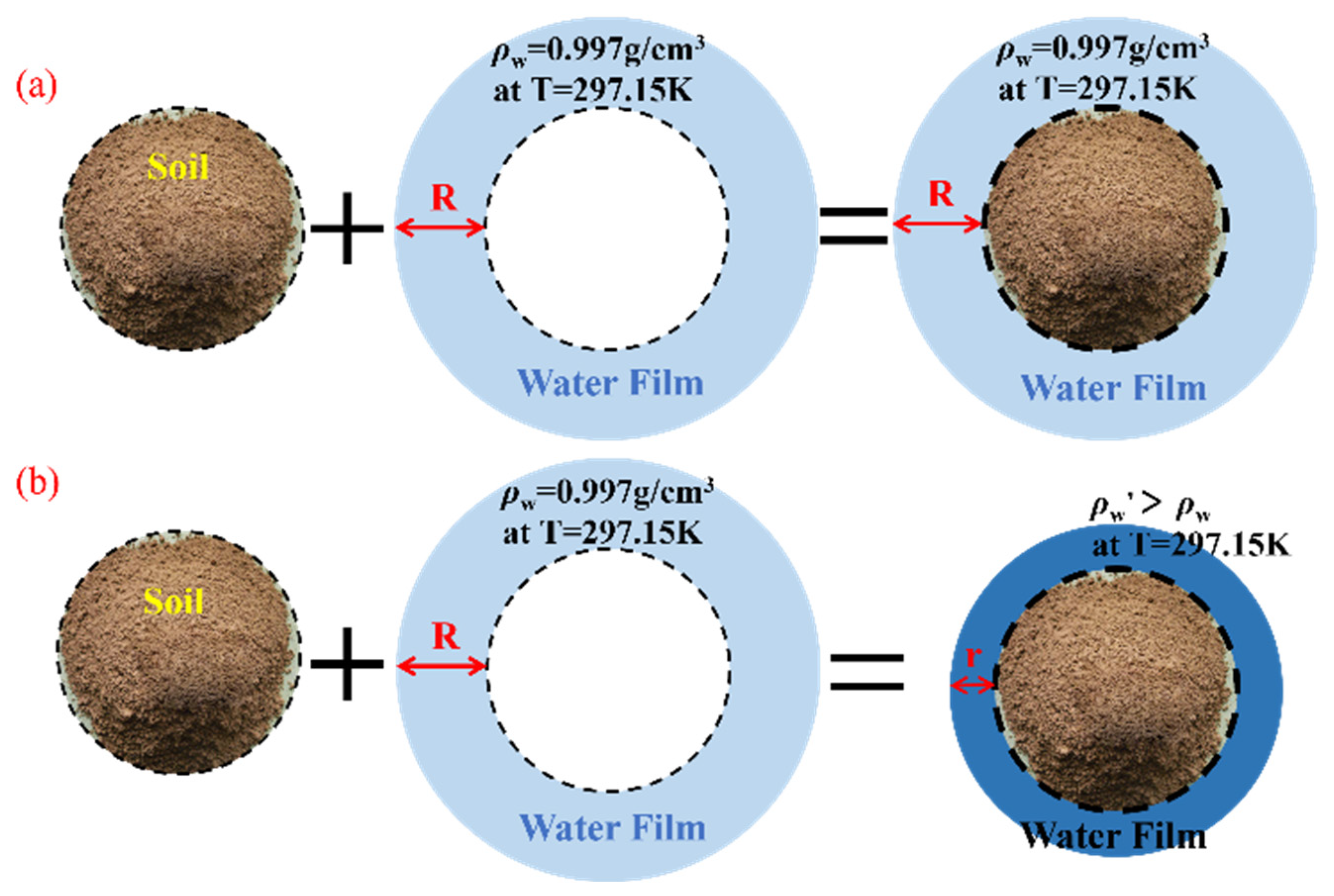
2. Theoretical Derivation
3. Material and Methods
3.1. Experimental Design
3.2. Experimental Materials
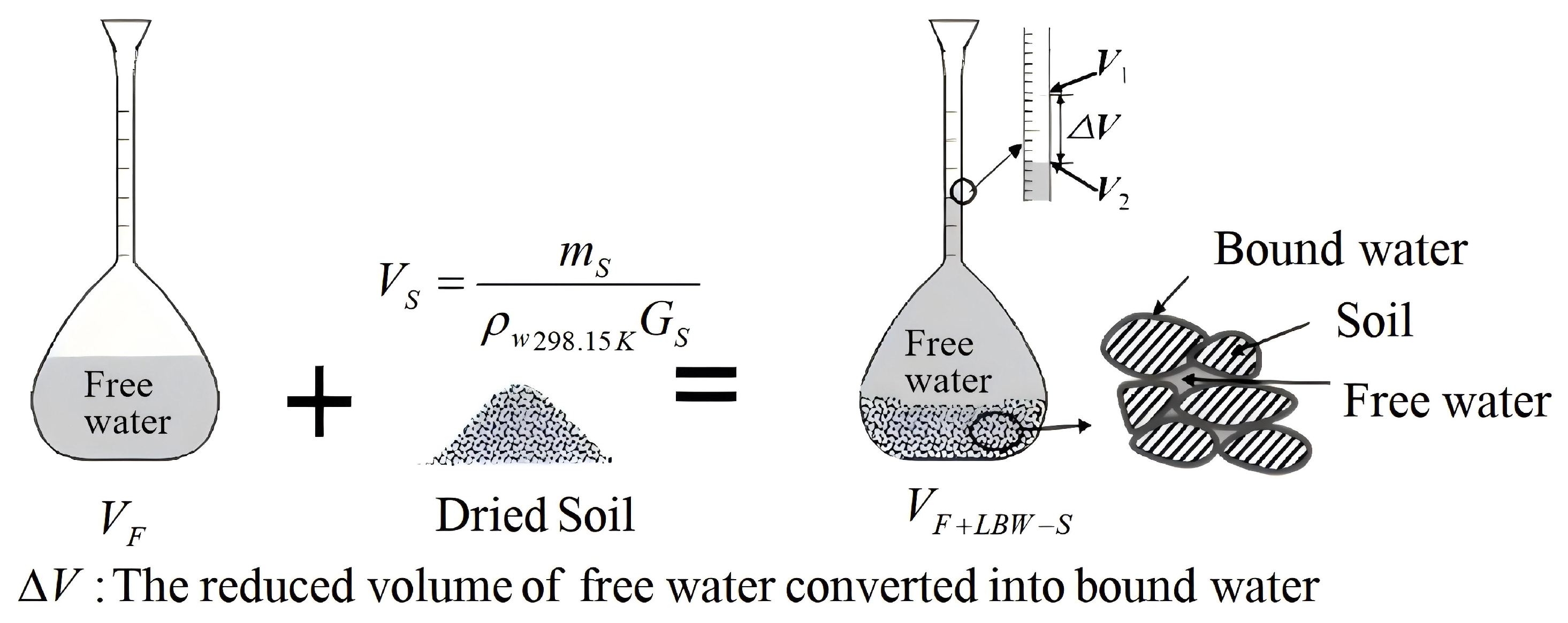
3.3. Experimental Method
4. Results and Discussion
4.1. Analysis of Particle Size Distribution and Property Parameters of the Tested IARE Samples
4.2. Analysis of the Results of the Volumetric Flask Method
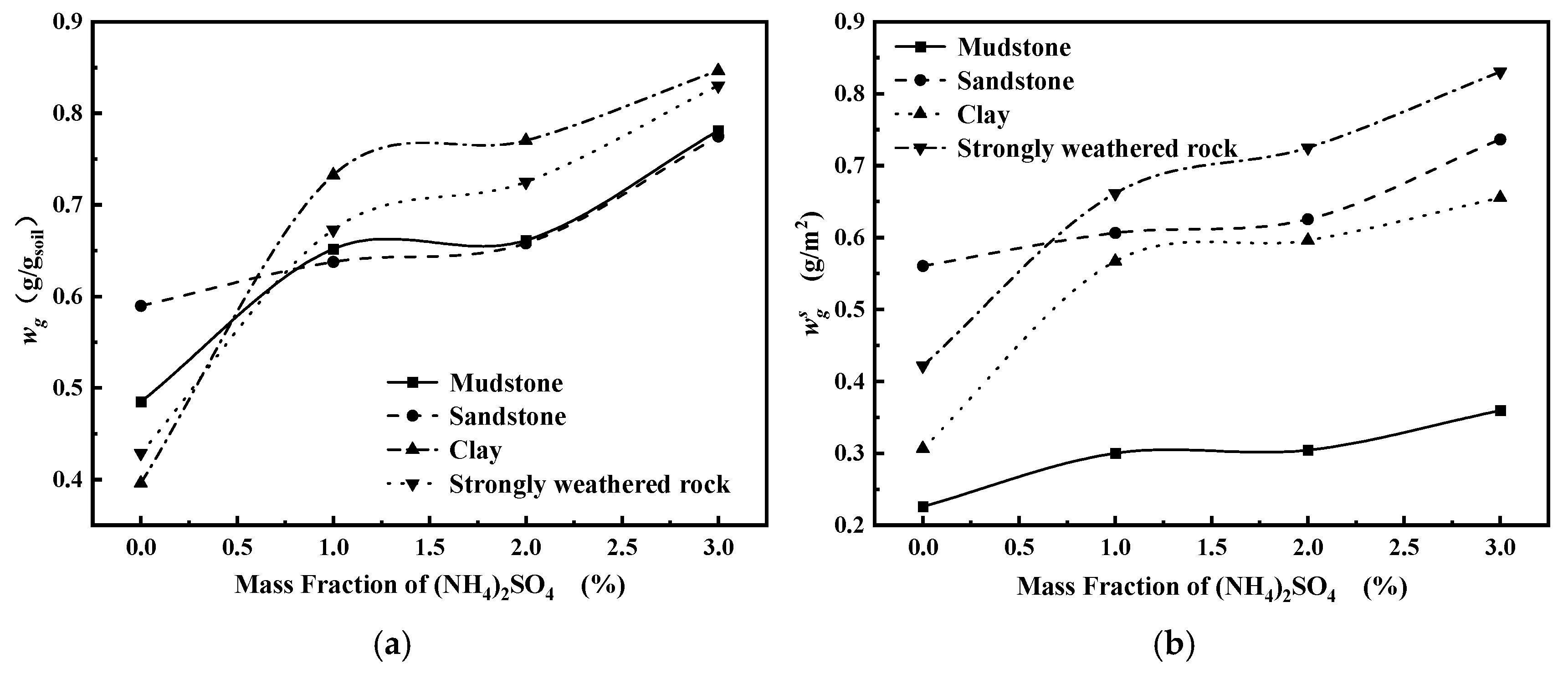
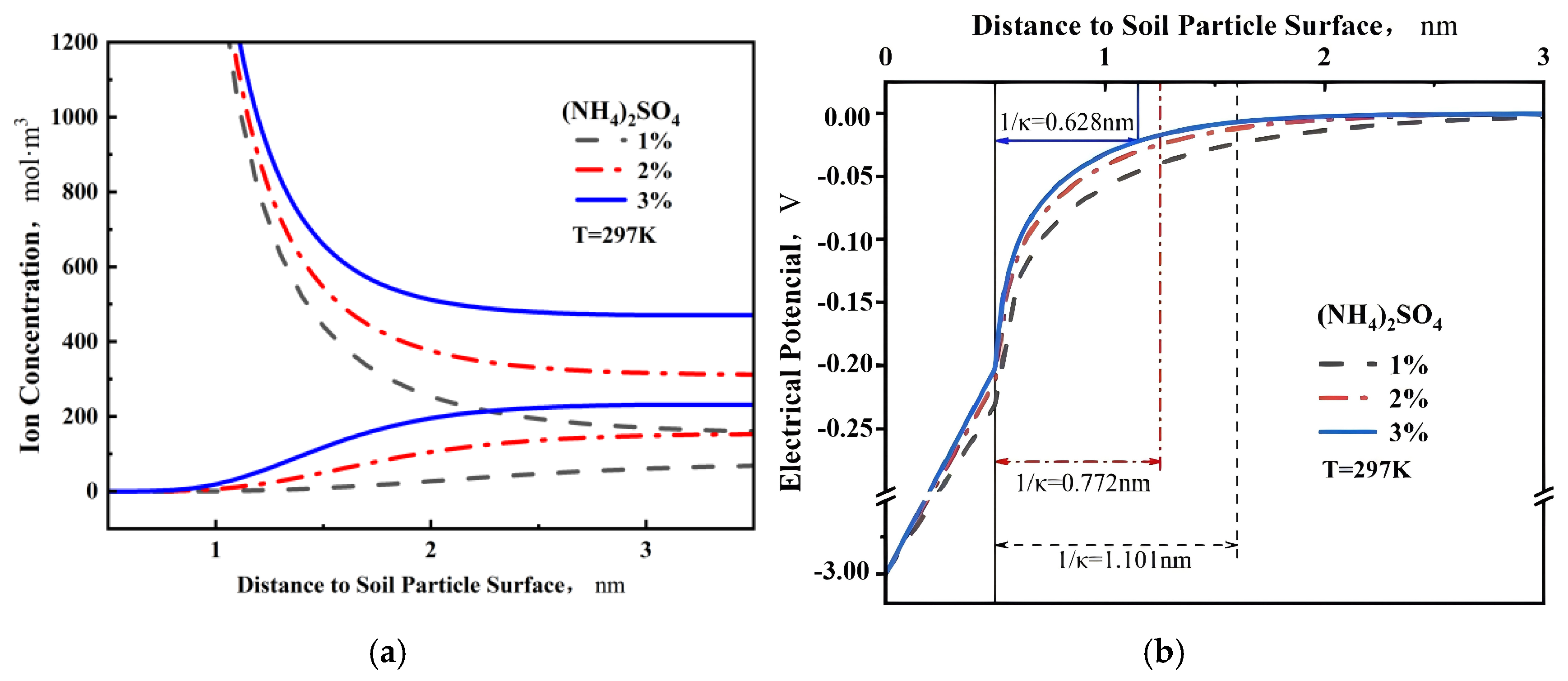
4.3. Analysis of Calculation Results
4.4. Implications for (NH4)2SO4 Leaching (Relevance and Use)
5. Conclusions
- (1)
- The surface charge per unit area of four types of IARE samples, namely mudstone, sandstone, clay, and strongly weathered rock, are 0.7072 × 10−2 mmol/m2, 1.9620 × 10−2 mmol/m2, 1.5418 × 10−2 mmol/m2, and 2.1003 × 10−2 mmol/m2, respectively, with strongly weathered rock having the highest and mudstone having the lowest.
- (2)
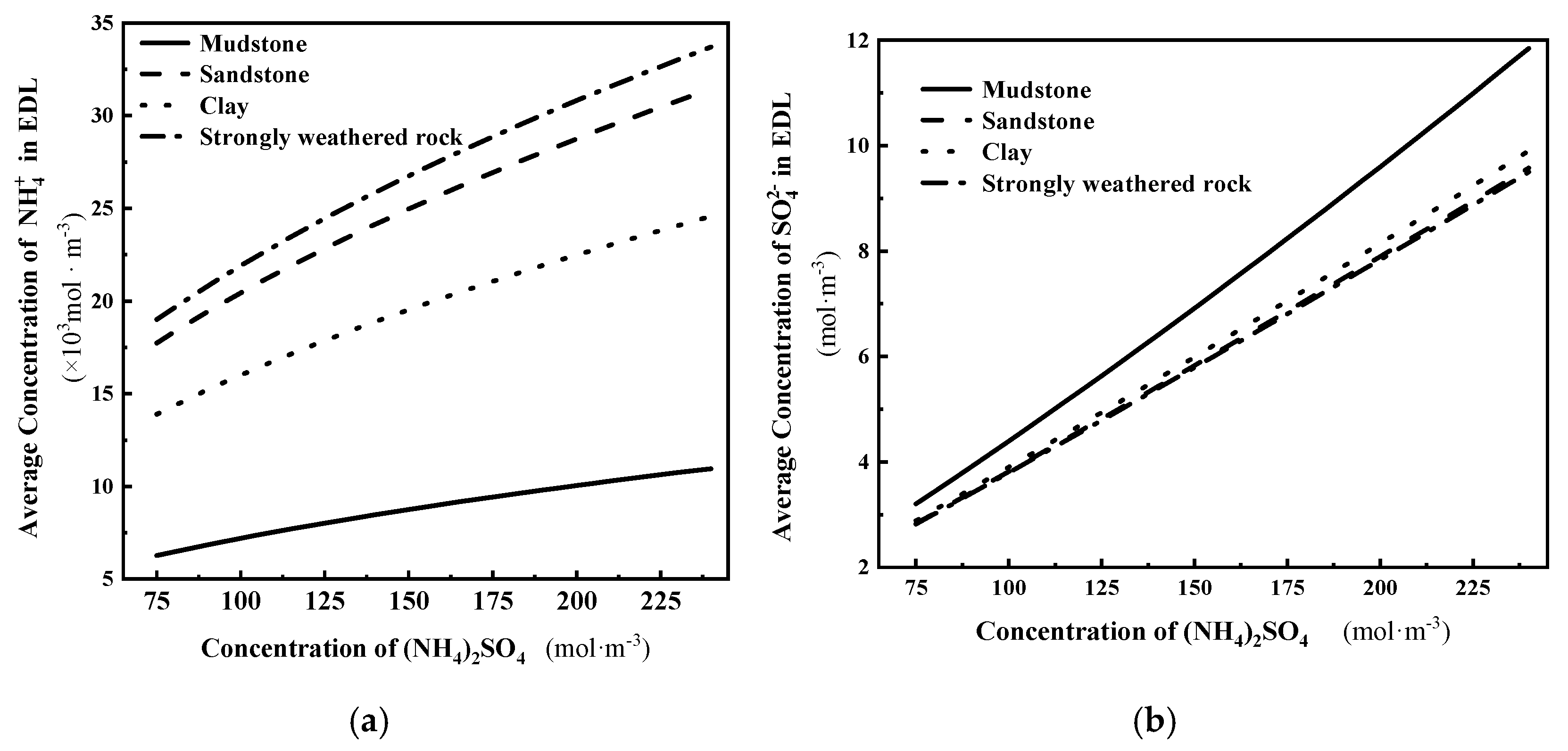
- As the concentration of the solution increases, the thickness of the diffusion double layer on the surface of the IARE sample is compressed, the total amount of adsorbed anions and cations on the surface increases, and the density of the surface water film also increases, leading to a corresponding increase in the quality of adsorbed bound water.
- (3)
- As the concentration of the solution increases, the total volume reduction in free water ∆V in the system increases, and the mass of adsorbed bound water per unit mass of IARE sample also increases. This effectively increases particle hydrodynamic size, reduces pore throat apertures and effective porosity, and lowers permeability, which together impede lixiviant penetration and mass transfer. The net effect is reduced contact between the reagent and ore particles and, therefore, a decline in rare-earth extraction efficiency.
- (4)
- At the same solution concentration, the four tested IARE ores exhibit a per-area bound water mass ranking of strongly weathered rock > sandstone > clay > mudstone, consistent with the ordering of surface charge per unit area.
- (5)
- During the leaching of IARE ores, higher lixiviant concentration does not necessarily yield higher leaching efficiency. Efficient IARE leaching results from multiple interacting factors and typically exhibits an ore-specific optimal concentration window. Depending on the raw ore characteristics, staged concentration and soak–drain cycling can be employed to enhance leaching efficiency.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chilingarian, G.V.; Vorabutr, P. Drilling and Drilling Fluids. Eng. Geol. 1982, 19, 74–75. [Google Scholar] [CrossRef]
- Orchiston, H. Adsorption of water vapor. Soil Sci. 1959, 87, 350–352. [Google Scholar] [CrossRef]
- Barshad, I.; Halevy, E.; Gold, H.A.; Hagin, J. Clay Minerals in Some Limestone Soils From Israel. Soil Sci. 1956, 81, 423–438. [Google Scholar] [CrossRef]
- Barshad, I. Absorptive and Swelling Properties of Clay-Water System. Clays Clay Miner. 1952, 1, 70–77. [Google Scholar] [CrossRef]
- Grim, R.E. Applied Clay Mineralogy; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- Zhang, R.; Wu, M.; Kumar, P.; Gao, Q.-F. Influence of loosely bound water on compressibility of compacted fine-grained soils. Adv. Civ. Eng. 2020, 2020, 1496241. [Google Scholar] [CrossRef]
- Kuhn, J.; Castillo-Sanchez, J.M.; Gascon, J.; Calero, S.; Dubbeldam, D.; Vlugt, T.J.H.; Kapteijn, F.; Gross, J. Adsorption and diffusion of water, methanol, and ethanol in all-silica DD3R: Experiments and simulation. J. Phys. Chem. C 2009, 113, 14290–14301. [Google Scholar] [CrossRef]
- Osipov, V.I. Nanofilms of adsorbed water in clay: Mechanism of formation and properties. Water Resour. 2012, 39, 709–721. [Google Scholar] [CrossRef]
- Asay, D.B.; Kim, S.H. Evolution of the adsorbed water layer structure on silicon oxide at room temperature. J. Phys. Chem. B 2005, 109, 16760–16763. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, D. Water adsorption and desorption pretreatment of surfaces increases the maximum amount of adsorbed water molecules by a multiple. Adsorption 2013, 19, 1127–1135. [Google Scholar] [CrossRef][Green Version]
- Chapman, D.L. LI. A contribution to the theory of electrocapillarity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1913, 25, 475–481. [Google Scholar] [CrossRef]
- Gouy, M. Sur la constitution de la charge électrique à la surface d’un electrolyte. Anniue Phys. 1910, 9, 457–468. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, H. Rate of capillary rise in quartz nanochannels considering the dynamic contact angle by using molecular dynamics. Powder Technol. 2020, 372, 477–485. [Google Scholar] [CrossRef]
- Kurichetsky, A.H. The Combination of Soil Water Translation; Geological Publishing House Press: Beijing, China, 1982. [Google Scholar]
- Zhang, N. Research Methods for Clay Minerals; Science Press: Beijing, China, 1990. [Google Scholar]
- Low, P.F. Nature and Properties of Water in Montmorillonite-Water Systems. Soil Sci. Soc. Am. J. 1979, 43, 651–658. [Google Scholar] [CrossRef]
- Sposito, G.; Prost, R. Structure of water adsorbed on smectites. Chem. Rev. 1982, 82, 553–573. [Google Scholar] [CrossRef]
- Li, S.; Bo, Z.; Hu, C.; Yang, H.; Wang, A.; Liu, Y.; Jin, H.; Zhu, J. Collection of Translated Works on the Combination of Soil and Water; Geological Publishing House: Beijing, China, 1982. [Google Scholar]
- Li, Z.; Li, J.; Xu, R.; Hong, Z.; Liu, Z. Streaming potential method for characterizing the overlapping of diffuse layers of the electrical double layers between oppositely charged particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 478, 22–29. [Google Scholar] [CrossRef]
- Du, W.; Li, R.; Liu, X.; Tian, R.; Ding, W.; Li, H. Estimating Hofmeister energy in ion-clay mineral interactions from the Gouy-Chapman theory. Appl. Clay Sci. 2017, 146, 122–130. [Google Scholar] [CrossRef]
- Snyder, V.A. Gibbs–Duhem Equation for Ion-Exchanging Mixtures in Donnan Equilibrium with Electrolyte Solutions. Soil Sci. Soc. Am. J. 2011, 75, 2169–2177. [Google Scholar] [CrossRef]
- Falsone, G.; Stanchi, S.; Bonifacio, E. Simulating the effects of wet and dry on aggregate dynamics in argillic fragipan horizon. Geoderma 2017, 305, 407–416. [Google Scholar] [CrossRef]
- Calvet, R.; Prost, R. Cation Migration into Empty Octahedral Sites and Surface Properties of Clays. Clays Clay Miner. 1971, 19, 175. [Google Scholar] [CrossRef]
- Wang, P. Quantitative Analysis of Bound Water on Clay Surface and Study on Hydration Mechanism. Ph.D. Thesis, Southwest Petroleum University, Nanchong, China, 2001. [Google Scholar]
- Saboorian-Jooybari, H.; Chen, Z. Calculation of re-defined electrical double layer thickness in symmetrical electrolyte solutions. Results Phys. 2019, 15, 102501. [Google Scholar] [CrossRef]
- Mi, J.; Gregorich, E.G.; Xu, S.; McLaughlin, N.B.; Ma, B.; Liu, J. Effect of bentonite amendment on soil hydraulic parameters and millet crop performance in a semi-arid region. Field Crops Res. 2017, 212, 107–114. [Google Scholar] [CrossRef]
- Rahimi, L.; Amanipoor, H.; Battaleb-Looie, S. Effect of salinity of irrigation water on soil properties (abadan plain, SW Iran). Geocarto Int. 2021, 36, 1884–1903. [Google Scholar] [CrossRef]
- Aza-Gnandji, C.D.R.; Xu, Y.; Raitt, L.; Levy, J. Salinity of irrigation water in the Philippi farming area of the Cape Flats, Cape Town, South Africa. Water SA 2013, 39, 199–210. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Lu, C.; Deng, L.; Liu, G. Impacts of simulated acid solution on the disintegration and cation release of purple rock (Mudstone) in Southwest China. Geomorphology 2018, 316, 35–43. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Papangelakis, V.G. Recovery of rare earth elements adsorbed on Clay minerals: II. Leaching with ammonium sulfate. Hydrometallurgy 2013, 131–132, 158–166. [Google Scholar] [CrossRef]
- Sposito, G. The Surface Chemistry of Soils; Spenter Inc.: Burbank, CA, USA, 1984. [Google Scholar]
- Lin, F.M.; Wang, G.S.; Qin, L.; Peng, C.L. Analysis of the location of physical adsorption state of ammonium in diffusion double layer on the surface of clay minerals. Chem. Ind. Eng. Prog. 2025, 44, 6093–6101. [Google Scholar] [CrossRef]
- Lin, F.M.; An, H.R.; Wang, G.S.; Peng, C.L. Adsorption law of ammonium during exchange process of La3+/NH+4 in electric double layer. Chin. J. Nonferrous Met. 2024, 34, 2762–2772. [Google Scholar]
- HJ 889-2017; Soil Quality—Determination of Cation Exchange Capacity (CEC)—Hexamminecobalt Trichloride Solution-Spectrophotometric Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- Editorial Board of Engineering Geology Handbook. Engineering Geology Handbook, 4th ed.; China Construction Industry Press: Beijing, China, 2007. [Google Scholar]
- Xiang, G.; Xu, M.; Cui, J. Study on Particle Composition and Fractal Characteristics of Rock and Soil in Xigeda Formation. J. Chongqing Jiaotong Univ. (Nat. Sci. Ed.) 2018, 37, 60–63+75. [Google Scholar]
- Peng, S. Study on the Engineering Geological Characteristics of Xigeda Formation Clay. Hydrogeol. Eng. Geol. 1986, 57, 16–18. [Google Scholar]
- Wen, L.; Zhu, X.; Bai, Z.; Zou, K.; You, H. Characteristics of Rock and Soil of Xigeda Strata in Xinjiu District of Xi-Pan Expressway. Highway 2005, 7, 145–148. [Google Scholar] [CrossRef]
- You, H. Stability Analysis and Mechanism Study of Xigeda Formation Slope. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2004. [Google Scholar] [CrossRef]
- Wu, F. Some characteristics of adsorption bound water measurement and seepage in cohesive soil. Chin. J. Geotech. Eng. 1984, 6, 84–93. [Google Scholar]
- Liu, X.; Li, H.; Li, R.; Xie, D.; Ni, J.; Wu, L. Strong non-classical induction forces in ion-surface interactions: General origin of Hofmeister effects. Sci. Rep. 2014, 4, 5047. [Google Scholar] [CrossRef] [PubMed]
- Du, W. The Influence of Polarization Effect on Ion Exchange Adsorption in Clay Minerals. Ph.D. Thesis, Southwest University, Chongqing, China, 2017. [Google Scholar]


| IARE Samples | d60 /μm | d30 /μm | d10 /μm | Cu | Cc |
|---|---|---|---|---|---|
| Mudstone | 4.41 | 2.23 | 1.17 | 2.59 | 0.66 |
| Sandstone | 17.65 | 5.43 | 2.15 | 8.21 | 0.79 |
| Clay | 13.78 | 4.25 | 1.73 | 7.97 | 0.75 |
| Strongly weathered rock | 15.05 | 6.28 | 2.40 | 6.27 | 1.09 |
| IARE Samples | Specific Surface Area S/(m2/g) | Cation Exchange Capacity CEC/(cmol/kg) | Specific Gravity Gs |
|---|---|---|---|
| Mudstone | 2.172 | 1.536 | 2.754 |
| Sandstone | 1.052 | 2.064 | 2.735 |
| Clay | 1.292 | 1.992 | 2.638 |
| Strongly weathered rock | 1.017 | 2.136 | 2.650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Wang, J.; Fei, Z.; Peng, C.; An, H.; Fan, Z. Effect of (NH4)2SO4 Solution Concentration on Bound Water Content in Ion Adsorption Rare-Earth Raw Ore. Metals 2025, 15, 1254. https://doi.org/10.3390/met15111254
Liang Y, Wang J, Fei Z, Peng C, An H, Fan Z. Effect of (NH4)2SO4 Solution Concentration on Bound Water Content in Ion Adsorption Rare-Earth Raw Ore. Metals. 2025; 15(11):1254. https://doi.org/10.3390/met15111254
Chicago/Turabian StyleLiang, Yuehua, Jie Wang, Zhikui Fei, Chenliang Peng, Hourui An, and Zhanfeng Fan. 2025. "Effect of (NH4)2SO4 Solution Concentration on Bound Water Content in Ion Adsorption Rare-Earth Raw Ore" Metals 15, no. 11: 1254. https://doi.org/10.3390/met15111254
APA StyleLiang, Y., Wang, J., Fei, Z., Peng, C., An, H., & Fan, Z. (2025). Effect of (NH4)2SO4 Solution Concentration on Bound Water Content in Ion Adsorption Rare-Earth Raw Ore. Metals, 15(11), 1254. https://doi.org/10.3390/met15111254






