Abstract
High-phosphorus oolitic iron ores (HPOIOs) possess abundant reserves but are incompatible with conventional blast furnace ironmaking, as phosphorus migrates into hot metals during carbothermic reduction, preventing the production of low-phosphorus clean steel. To overcome this limitation, an innovative approach integrating alkaline briquette direct reduction and smelting separation was proposed. Briquettes were prepared from oolitic magnetite concentrate (52.01 wt% Fe, 0.29 wt% P, 0.11 wt% S) with a basicity (R) of 2.0 and 5 wt% MgO added as a desulfurizer. After direct reduction and smelting separation, the resulting metallic iron exhibited a content of 98.56 wt% Fe, with 0.036 wt% P and 0.046 wt% S, achieving an Fe recovery of 87.63%. The dephosphorization and desulfurization efficiencies reached 94.67% and 90.56%, respectively, meeting the clean steel requirements. Phosphorus was effectively stabilized within the gehlenite and merwinite phases as a solid solution of Ca3(PO4)2, inhibiting its transfer to iron. Thermodynamic analyses confirmed that high basicity (R ≥ 2.0) significantly suppressed P2O5 activity, preventing phosphate reduction. The formation of a Ca3(PO4)2–Ca2SiO4 solid solution further obstructed phosphorus migration. This dual mechanism of “chemical fixation and thermodynamic stabilization” enables efficient dephosphorization, offering a sustainable pathway for utilizing HPOIOs.
1. Introduction
China, the world’s leading steel producer, manufactured approximately 1.05 billion tons of crude steel in 2024, reinforcing its pivotal role in global supply chains [1,2]. This production level, however, is accompanied by substantial environmental challenges, with the industry emitting approximately 180 million tons of CO2 annually [3,4]. These emissions account for nearly one-fifth of the country’s industrial greenhouse gas output and contribute an estimated 15% to China’s total carbon footprint, highlighting the critical need for decarbonization strategies within the sector.
The environmental footprint of the steel industry originates predominantly from the blast furnace–basic oxygen furnace (BF–BOF) process, which employs coal and coke as both fuel and reducing agents. This process inherently produces substantial volumes of CO/CO2 emissions due to combustion and reduction reactions [5,6]. In China, carbon emissions associated with BF–BOF steelmaking in 2024 were reported to range from 1.8 to 2.5 metric tons per ton of crude steel, with the blast furnace unit alone contributing approximately 1.5 tons—accounting for 70% to 90% of the total CO2 emissions within integrated steel plants [7,8]. Given its high carbon intensity, this conventional method is increasingly viewed as a critical obstacle to worldwide decarbonization efforts and sustainable industrial development. In light of these challenges, the China Iron and Steel Association (CISA) has introduced stringent energy conservation and coal consumption reduction policies to facilitate a transition toward greener steel production.
Electric smelting furnace (ESF) technology has emerged as a transformative alternative by shifting from coal to direct reduced iron (DRI) or scrap metal as the primary feedstock. Data from the Steel Manufacturers Association (SMA) indicate that the ESF process emits only 0.3 tons of CO2 per ton of steel—a reduction of 75% compared with the 1.6 tons emitted via the conventional BF–BOF route [9]. This position ESF as an environmentally sustainable solution that supports resource circularity and reduced energy consumption is essential for industrial decarbonization. Several demonstration projects, including million-ton-scale DRI–ESF plants launched by the Baowu Group in Zhanjiang and the HBIS Group in Xuanhua, provide valuable references for low-carbon steelmaking in China [10,11,12].
A further significant challenge lies in China’s considerable external dependency on iron ore imports. In 2024, the country’s iron ore imports reached 1.237 billion tons, with more than 80% of this volume supplied by the four leading global mining corporations: Vale, BHP, Fortescue Metals Group (FMG), and Rio Tinto [13,14]. This heavy reliance poses substantial risks to the stability and security of the strategic resource supply chain. Consequently, enhanced utilization of domestic iron ore resources is considered essential for strengthening supply chain resilience and ensuring long-term sustainability. Estimates from the U.S. Geological Survey indicate that China holds more than 80 billion tons of iron ore reserves, comprising over 7 billion tons of high-phosphorus oolitic iron ore (HPOIO), a category of ore with worldwide reserves estimated to exceed 200 billion tons [15]. Despite its abundance, the industrial processing of HPOIO remains challenging owing to its elevated phosphorus content (ranging from 0.1% to 1.5%), complex mineralogical composition, and finely disseminated texture, all of which have thus far limited its large-scale exploitation.
In conventional blast furnace ironmaking, phosphorus-bearing minerals undergo reactions with metallic iron under carbothermic reduction conditions, resulting in the formation of iron phosphides. These compounds impair the crystalline integrity of steel, leading to degraded weldability, inferior thermomechanical properties, and reduced low-temperature toughness while also increasing the propensity for cold brittleness [16,17,18]. In accordance with the Chinese national standard GB/T 699-2015 [19], the phosphorus content in high-quality structural steel is strictly limited to a maximum of 0.035%. This specification underscores the necessity for efficient dephosphorization either prior to or during the smelting process.
Research efforts have been devoted to developing processing strategies for HPOIO. These methods span several technological areas, including conventional mineral beneficiation, hydrometallurgical extraction, and thermal reduction techniques such as magnetization roasting (MR-MS) and direct reduction (DR-MS), both of which are integrated with magnetic separation [20,21,22]. These techniques, however, are subject to considerable limitations: the ultrafine intergrowth of minerals complicates liberation and separation; hydrometallurgical approaches require large quantities of acidic reagents and generate hazardous waste [23,24,25,26]; MR–MS improves processability without substantially reducing phosphorus content; and DR–MS demands temperatures exceeding 1200 °C with high reductant consumption, resulting in elevated energy use and carbon emissions.
Although hot metal pretreatment dephosphorization and basic oxygen furnace (BOF) refining have been extensively investigated, these end-of-pipe methods are incompatible with HPOIO due to stringent requirements for initial phosphorus levels (≤0.2% in hot metals) along with high operational complexity and cost [27,28,29,30,31]. Moreover, conventional dephosphorization depends on simultaneously optimizing three thermodynamically conflicting parameters, namely, low temperature, an oxidizing atmosphere, and high slag basicity, to facilitate the oxidation of [P] to P2O5 and subsequent stabilization in slag as Ca3(PO4)2—conditions unattainable in conventional BF reduction environments.
Despite considerable experimental studies on this ore have demonstrated satisfactory performance in iron recovery and dephosphorization, critical challenges remain in existing approaches [32,33]. For instance, hydrometallurgical leaching processes suffer from high acid consumption and difficult disposal of waste acid, while pyrometallurgical routes require large quantities of dephosphorization agents and often result in incomplete phosphorus removal. These limitations severely compromise the quality of the final product. Therefore, it is imperative to develop more efficient and sustainable processing strategies for treating refractory high-phosphorus oolitic iron ore. An innovative process integrating direct reduction of alkaline briquettes with electric furnace smelting was developed for treating oolitic magnetite concentrate (OMC), a preupgraded concentrate derived from HPOIO [27]. The process involves cold-bonded briquetting of OMC with alkaline fluxes, eliminating the need for energy-intensive sintering or pelletization. Subsequent solid-state direct reduction at moderate temperatures facilitates the selective reduction of iron oxides, whereas alkaline additives suppress phosphorus migration into metallic iron. Smelting separation of the reduced briquettes produces a metal phase meeting standard clean steel specifications (P ≤ 0.04%) and a phosphorus-enriched slag phase (P recovery > 94%). This method combines phosphorus fixation during reduction with efficient iron–slag separation, circumventing the complexities and costs associated with downstream dephosphorization.
A comprehensive investigation has been conducted into the reduction behavior, slag–metal partitioning efficiency, and underlying thermodynamics of dephosphorization. This work establishes a short, integrated metallurgical route from refractory high-phosphorus iron ore to clean steel, providing a sustainable and strategically viable pathway for the utilization of challenging domestic iron ore resources.
2. Experimental Section
2.1. Material Characteristics
The two ores utilized in the present research were chemically characterized, and their detailed compositions are quantified in Table 1. Chemical analysis revealed that HPOIO has a total iron (TFe) grade of 35.05%, which is typically inadequate for direct application in conventional iron and steel production. Accordingly, in prior research, HPOIO underwent a preconcentration process consisting of magnetizing roasting and subsequent magnetic separation. This treatment yielded a concentrated product, designated OMC, with a significantly elevated TFe content of 52.06% [27,28]. Despite this, the OMC was found to contain elevated levels of deleterious impurities, specifically Al2O3, SiO2, and P. In particular, the phosphorus content was 0.30%. In traditional blast furnace ironmaking, phosphorus is predominantly reduced and dissolved into the molten iron, with no efficient route for its removal, thereby rendering the production of low-phosphorus steel from such high-phosphorus feed materials unfeasible. Thus, it is essential to develop alternative ironmaking technologies that can effectively process this category of refractory iron ore.

Table 1.
Chemical analysis of ores (wt%).
As evidenced by the mineralogical characterization presented in Figure 1, and Table 2 presents the spectral data obtained from the EDS point scans shown in Figure 1. The OMC is primarily composed of magnetite, hematite, amorphous aluminosilicates, quartz, and fluorapatite. Crucially, the microstructure exhibits a complex concentric ring pattern in which ultrafine-grained magnetite is intimately intergrown with aluminosilicate phases, quartz, and fluorapatite [28]. This interlocking texture encapsulates iron minerals within a gangue matrix, significantly hindering the liberation of magnetite through physical beneficiation methods. Furthermore, extensive substitution of Al3+ into the crystal lattice of iron oxides presents an additional barrier to efficient iron–aluminum separation via conventional physiochemical processing routes. These inherent mineralogical constraints—namely, ultrafine intergrowth and isomorphous substitution—impose substantial challenges for the utilization of this refractory iron ore resource.
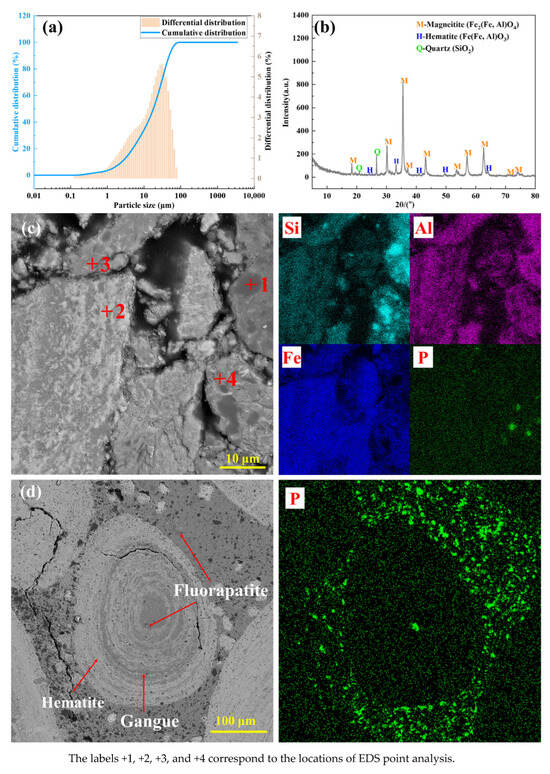
Figure 1.
Ore characteristics. (a–c) OMC; (d) HPOIO.

Table 2.
SEM–EDS analysis.
In this study, pulverized coal served as the reductant. The industrial analysis results (Table 3) indicate that the coal sample consisted of 50.32% fixed carbon (FCad), 10.13% ash (Aad), and 39.55% volatile matter (Vad).

Table 3.
Industrial analysis of coal (wt%).
The alkaline additives employed in this study, including CaCO3 and MgO, were analytical-grade (AR) reagents sourced from the Chemical Platform of Central South University, with chemical purities meeting experimental specifications.
2.2. Experimental Procedure
A schematic flowchart of the integrated process is presented in Figure 2. The procedure combines direct reduction of basic briquettes with subsequent smelting separation in an electric furnace and consists of three main stages: preparation of basic briquettes, coal-based direct reduction, and smelting separation. In the briquette preparation stage, raw ore powder and CaCO3 were thoroughly homogenized. The powder mixture was then compacted into cylindrical briquettes (11 mm in diameter × 16 mm in length) using a JYP-15-3 hydraulic press (Jiaxinhai, Tianjin, China) under 3-ton pressure with a dwell time of 30 s. In the reduction stage, the prepared briquettes were uniformly mixed with pulverized coal and charged into sealed corundum crucibles. Isothermal reduction was conducted in a JC2-12-10A muffle furnace (Jingcheng Instruments, Qingdao, China) at 1050 °C for 120 min with a C/Fe mass ratio of 2 [28,30]. After reduction, the crucibles were immediately extracted and covered with additional coal to minimize reoxidation during natural cooling to ambient temperature. During the smelting separation stage, the reduced briquettes were placed in a magnesia crucible, which was then positioned in a VSF resistance furnace (Boyuntong Instruments, Nanjing, China) and maintained at 1550 °C for 20 min. The entire melting process was performed under a continuous argon flow of 2 L/min to prevent oxidation. Upon completion, the crucibles were withdrawn from the furnace and cooled to room temperature under the same argon atmosphere. The solidified products were subsequently manually crushed to separate the metallic and slag phases. Moreover, to guarantee the reliability and reproducibility of the findings, all experiments involving reduction and smelting separation were performed a minimum of three times, with the results presented as averages.
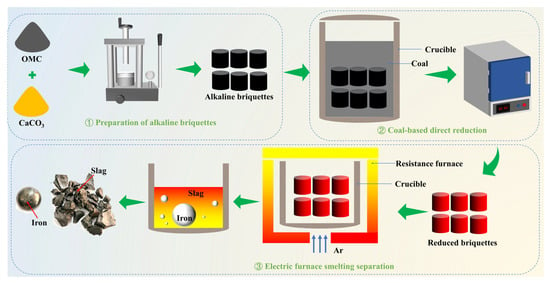
Figure 2.
Flowsheet for the treatment of alkaline briquettes via direct reduction coupled with electric furnace smelting separation.
The metallization degree and basicity of the reduced samples were evaluated via Equation (1) and Equation (2), respectively. Furthermore, the iron recovery efficiency in the metallic phase and the dephosphorization rate were assessed via Equations (3) and (4), respectively.
In Equation (1), the symbol μ signifies the degree of metallization. The terms KFe and NFe designate the contents of metallic iron and total iron (in mass percent) in the reduced briquettes, respectively.
where and denote the mass fractions of CaO and SiO2 in the sample, respectively.
In these formulations, φ represents the iron recovery efficiency (%), where n designates the mass of metallic iron recovered after smelting (g), and w corresponds to the iron content in the metallic product (%). The symbol N denotes the initial mass of the reduced briquettes charged (g), whereas F indicates the total iron grade of the feed material (%). Moreover, ω refers to the dephosphorization rate (%), with q representing the phosphorus content in the metallic phase (%), and S designates the P content in the feed prior to smelting (%).
In parallel, thermodynamic simulations were conducted using the FactSage8.1 software package, employing both the pure substance and oxide databases to calculate the equilibrium phase relationships within the slag system. These calculations provide a theoretical representation of the material equilibrium states under idealized conditions.
3. Results and Discussion
3.1. Effects of Basicity on the Direct Reduction and Melting Separation Processes
In accordance with previous experimental studies [26,27], the optimal conditions for the coal-based direct reduction of cold-bonded briquettes under natural basicity were a roasting temperature of 1050 °C for 2 h and a C/Fe mass ratio of 2. These conditions were subsequently maintained to methodically examine the effect of basicity. The influence of basicity on the extent of metallization achieved during the coal-based reduction process is presented in Figure 3. As the basicity increased from its natural level (R = 0.035) to 1.0, the metallization rate notably increased from 76.75% to 84.41%. Beyond this point, a further increase in basicity up to R = 2.5 resulted in a stabilization of the metallization degree, indicating a plateau in the enhancement effect. These findings suggest that moderately increasing the basicity effectively promotes the reduction efficiency of the briquettes, whereas excessive basicity does not yield additional benefits under the given conditions.
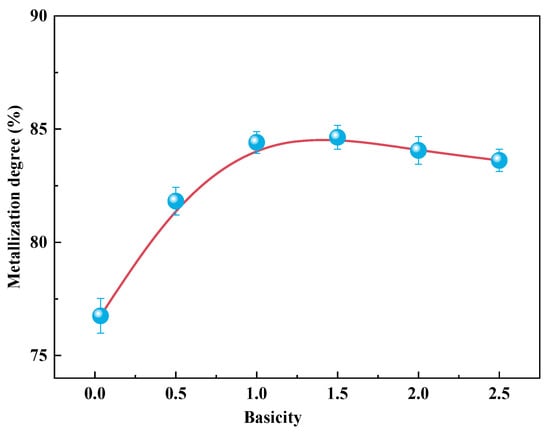
Figure 3.
Effect of basicity on the metallization degree of reduced samples.
Figure 4 shows the macroscopic morphology of slag–iron separation after electric furnace smelting under different basicity conditions. The results show that effective separation between slag and iron was attained as the basicity increased from 0.035 to 2.0. However, at a basicity of 2.5, metallic inclusions were observed within the slag phase, suggesting a decrease in separation efficiency under higher alkaline conditions. Mechanistic analysis indicates that excessive basicity increases the slag viscosity and increases the melting temperature, significantly deteriorating the melt fluidity and thereby impeding the kinetic process of slag–metal interface separation [34,35].
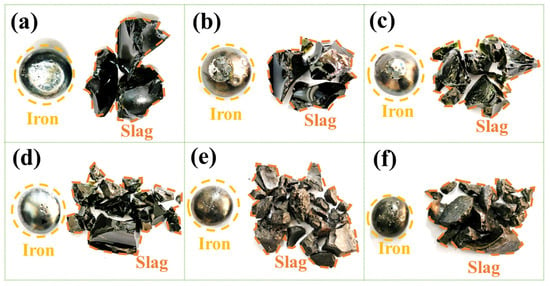
Figure 4.
Macroscopic morphology of slag and iron separation after smelting under different basicity conditions. (a) R = 0.035; (b) R = 0.5; (c) R = 1.0; (d) R = 1.5; (e) R = 2.0; (f) R = 2.5.
The influence of R on the partitioning behavior of iron and phosphorus within the molten iron phase is illustrated in Figure 5. Over the basicity range of R = 0.035–2.5, the purity of the iron remained consistently high, stabilizing between 98% and 99%. In contrast, the iron recovery decreased modestly from 91% to 88% with increasing basicity (Figure 5a).
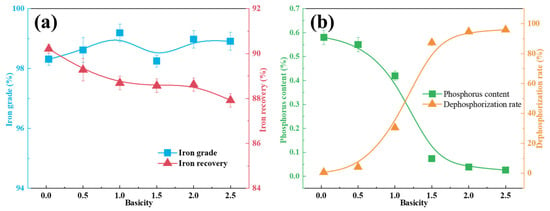
Figure 5.
Influence of basicity on the key constituents of the iron phase after smelting. (a) Iron content index; (b) phosphorus content index.
In contrast, phosphorus partitioning exhibited a strong correlation with basicity (Figure 5b). As R increased from 0.035 to 2.0, the P content in the metallic phase significantly reduced from 0.58% to 0.039%, accompanied by a marked improvement in the dephosphorization efficiency from 0.63% to 94.56%. A further increase in basicity to R = 2.5 resulted in an additional reduction in the phosphorus content to 0.026%, with the dephosphorization rate reaching 95.85%. These results indicate that maintaining the basicity at R = 2 offers an optimal compromise between efficient phosphorus removal and process economics.
3.2. Effect of MgO Application on Direct Reduction and Subsequent Smelting Separation
In the OMC system, sulfur (S) with an initial content of 0.11% requires co-removal alongside phosphorus because of its detrimental induction of hot-working embrittlement, which significantly compromises steel performance. The experimental results demonstrate that at R = 2, the S content in molten iron decreases to 0.09% but still exceeds the conventional steel sulfur threshold of 0.055%. This reveals unresolved optimization potential in the thermodynamic equilibrium control of sulfur within the current process, necessitating enhancements in the sulfur partition coefficients or improvements in the kinetic conditions to achieve deep desulfurization.
Industrial hot metal desulfurization technologies primarily employ two reaction systems: calcium-based agents (CaO, CaC2) and magnesium-based agents (MgO and its derivatives). Compared with that of Ca2+, the ionic radius of Mg2+ is smaller than that of Ca2+, suggesting that MgO has superior sulfur affinity and diffusion kinetics because of its higher charge density, which endows magnesium-based systems with advantages in terms of both the sulfur capacity and desulfurization rate [33]. Building on this foundation, our study innovatively introduces MgO additives to construct a CaO–MgO alkaline slag system, systematically investigating its synergistic regulatory mechanism on the slag–metal interface behavior and migration patterns of impurity elements (P/S) in the iron phase during melting separation.
Figure 6 shows the macroscopic morphology of slag–iron separation after electric furnace smelting under different MgO dosage. Effective slag–iron separation was achieved in both phases as the MgO dosage increased from 0% to 10%. However, at 15% MgO, incomplete separation occurred, with iron particles failing to coalesce and metallic iron entrapped in the slag phase. Mechanistic studies revealed that elevated MgO levels facilitate the formation of refractory silicate phases—including monticellite (CaMgSiO4) and merwinite (Ca3MgSi2O8)—which markedly increase slag viscosity, compromise melt fluidity, and ultimately retard the kinetics of slag–metal separation.

Figure 6.
Macroscopic morphology of slag and iron separation after smelting under different MgO dosage. (a) 0%; (b) 5%; (c) 10%; (d) 15%.
As illustrated in Figure 7, the addition of MgO selectively regulates the separation behavior of Fe, P, and S within the metallic iron phase during smelting. With increasing MgO dosage, the iron grade in the metallic phase gradually decreased from 98.98% to 97.85%, accompanied by a decrease in iron recovery from 88.62% to 85.87% (Figure 7a). These results suggest that excessive MgO addition impairs the enrichment efficiency of iron.
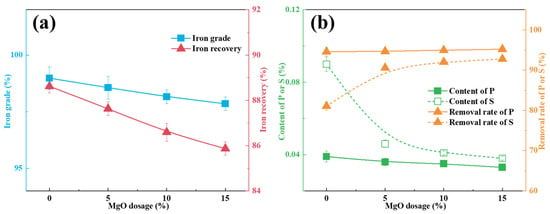
Figure 7.
Effect of the MgO dosage on the composition of the metallic phase following the smelting process. (a) Iron content index; (b) phosphorus content index.
Divergent removal behaviors were observed for P and S, as depicted in Figure 7b. As the MgO dosage increased from 0% to 5%, the sulfur content markedly decreased from 0.09% to 0.046%. In contrast, the phosphorus content only marginally decreased from 0.039% to 0.036%. A further increase in MgO addition to 15% led to a gradual decline in the contents of both elements, reaching 0.038% S and 0.033% P. These results demonstrate the specific removal effect of MgO on sulfur compared with its weaker influence on phosphorus migration. A comprehensive evaluation indicates that the addition of 5% MgO achieves an optimal balance between the slag–metal separation efficiency and impurity removal effectiveness.
As shown in Table 4, the optimized process consistently controls phosphorus and sulfur contents in the metallic phase at 0.036% and 0.046%, respectively, meeting the composition standards for common steel grades (P ≤ 0.045%, S ≤ 0.055%). Concurrently, the slag phase shows significant phosphorus enrichment to 0.528%, confirming effective phosphorus partitioning into the slag. This short-process metallurgical route successfully enables the targeted conversion of HPOIO into low-phosphorus clean steel through coordinated pre-reduction and smelting separation.

Table 4.
Key chemical constituents of the metallic iron phase following smelting (wt%).
4. Characterization of Melting Slags
This study employed XRD phase analysis, XPS chemical state characterization, and EPMA microregional composition mapping to systematically investigate the mineral evolution and interface enrichment mechanisms of phosphorus in smelting slags with various basicity levels (R = 0.035–2.5).
4.1. XRD
As shown in Figure 8, the XRD patterns of slags with varying basicities reveal distinct phase evolution characteristics. At natural basicity (R = 0.035), a broad hump in the 20–40° range (indicative of the amorphous glass phase) coexists with metallic iron diffraction peaks, suggesting the presence of minor incompletely separated iron particles in the slag phase. When R increases to 0.5–1.0, only amorphous scattering patterns are observed, confirming the complete vitrification of the slag phase. Notably, crystallization occurs when R ≥ 1.5: Gehlenite (Ca2Al2SiO7), ferro-magnesian spinel ((Fe,Mg)Al2O4), and merwinite (Ca3MgSi2O8) form at R = 1.5, whereas wüstite (FeO) precipitates at R = 2.0–2.5. This phase evolution demonstrates that higher basicity (R ≥ 1.5) significantly increases the slag liquidus temperature, promoting directional crystallization of thermally stable minerals.
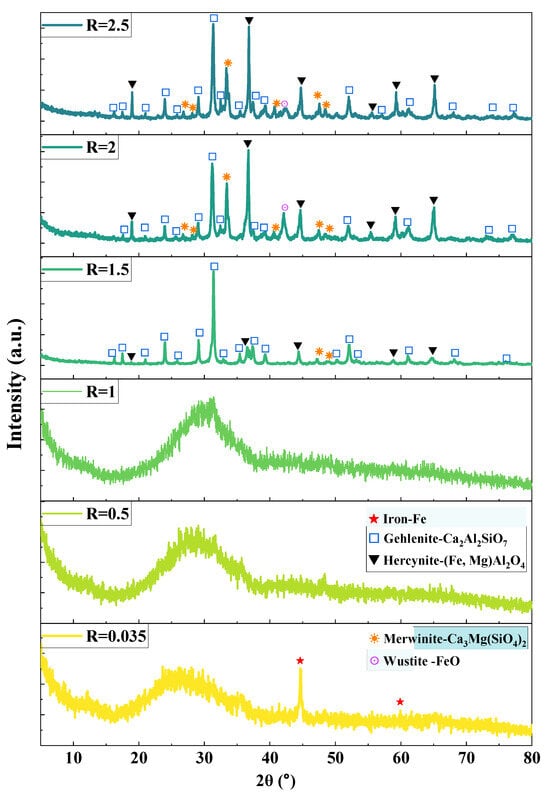
Figure 8.
XRD patterns of the slag samples.
4.2. XPS
As shown in Figure 9, XPS analysis was employed to systematically investigate the speciation transformation of phosphorus within the slag phase. The survey spectrum (Figure 9a) along with the high-resolution P 2p spectra (Figure 9b) revealed that phosphorus enrichment in the slag notably intensified as the basicity increased from 0.035 to 2.5. The characteristic P 2p peak exhibited a positive binding energy shift from 133.12 eV to 133.43 eV (Δ = 0.31 eV), indicating enhanced stability of the chemical environment in the Ca3(PO4)2 crystal structure under high basicity conditions, which facilitates effective phosphorus immobilization in calcium phosphate phases. Quantitative photoelectron intensity analysis (Figure 9c) revealed exponential growth of the phosphorus signal from 26.83 cps·eV to 6335.18 cps·eV as R increased from 0.035 to 2.0, followed by a decrease in the growth rate (6464.28 cps·eV at R = 2.5), corresponding to an increase in the phosphorus accommodation capacity. These series of characterization data collectively demonstrate the positive correlation between basicity and phosphorus enrichment behavior in the slag system.
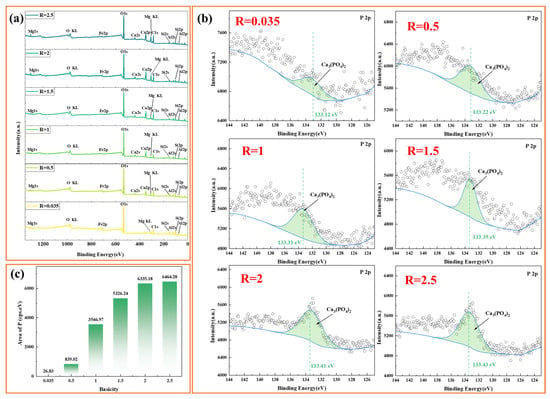
Figure 9.
XPS patterns of the slag samples. (a) XPS survey; (b) 2p fine spectrum of P; (c) area of P.
4.3. EPMA
As shown in Figure 10, EPMA microregional analysis of slag melts with varying basicity levels revealed the phase distribution patterns of phosphorus. Under natural basic conditions (R = 0.035), the slag matrix primarily consisted of an amorphous glass phase (phosphorus was undetectable), whereas the residual metallic iron phase presented a phosphorus content of 0.715% (Figure 10a), demonstrating the inability of low-basicity systems to achieve phosphorus fixation in the slag phase. When R increased to 0.5 and 1.0, phosphorus displayed a homogeneous distribution within the glass phase, with progressively increasing concentration gradients correlated with increasing basicity (Figure 10b,c), confirming the onset of phosphorus migration toward the slag phase.
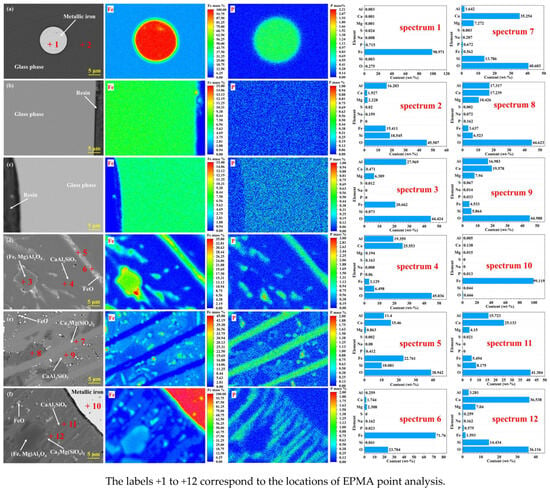
Figure 10.
EPMA of slag samples with different basicities. (a) R = 0.035; (b) R = 0.5; (c) R = 1.0; (d) R = 1.5; (e) R = 2; (f) R = 2.5.
Crystalline-dominated regions (R ≥ 1.5) exhibited distinct phase-controlled phosphorus enrichment behaviors. At R = 1.5, phosphorus preferentially resided in elongated Ca2Al2SiO7-FeO-Ca3(PO4)2 composite solid solutions (0.412%, Figure 10d). Increasing the basicity to R = 2.0 induced phosphorus migration into multiphase Ca3Mg(SiO4)2-FeO-Ca3(PO4)2 solid solutions (0.672%, Figure 10e). At R = 2.5, phosphorus further accumulated in elongated Ca3Mg(SiO4)2-Ca2Al2SiO7-FeO-Ca3(PO4)2 solid solutions (0.575%, Figure 10f), with residual iron-phase phosphorus sharply reduced to 0.013%. This evolution pattern confirms that the alkaline briquette prereduction-smelting process involves calcium silicate-mediated lattice entrapment mechanisms (via Ca2Al2SiO7 and Ca3Mg(SiO4)2), effectively inhibiting both Ca3(PO4)2 thermal decomposition and phosphorus diffusion toward metallic phases, thereby providing microstructural evidence for basicity-driven phosphorus removal strategies.
5. Mechanism of Dephosphorization
5.1. Thermal Stability of Ca3(PO4)2
Extensive studies have demonstrated that the reduction and migration of phosphorus minerals are governed by the activity of P2O5 components within the system [26,27]. The fundamental approach to suppressing these processes lies in reducing P2O5 activity. Thermodynamic calculations comparing the P2O5 activity of pure Ca3(PO4)2 and HPOIO under varying basicity conditions (Figure 11) provide critical insights into the feasibility of the phosphorus reaction. Notably, in comparison to the pure Ca3(PO4)2 system, the HPOIO sample demonstrates a sharp exponential enhancement in P2O5 activity under isothermal conditions. This significant enhancement can be largely attributed to the existence of SiO2 and Al2O3 in the ore matrix, which considerably lower the activation energy necessary for the generation of volatile P2O5.
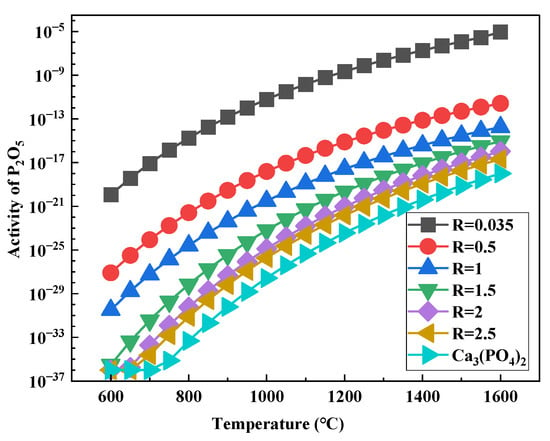
Figure 11.
Activity of P2O5 components in different systems.
Importantly, the increase in basicity from 0.035 to 2.5 induces a substantial reduction in P2O5 activity within the iron ore system, demonstrating the enhanced thermodynamic stability of Ca3(PO4)2 under high-basicity conditions, which effectively suppresses the liberation of reactive P2O5. Furthermore, regardless of the system configuration, P2O5 activity increases exponentially with increasing temperature, confirming that low-temperature operation serves as another critical factor for controlling phosphorus mineral decomposition. The elucidated thermodynamic stabilization mechanism of P2O5 provides guiding significance for industrial electric furnace smelting. To effectively remove phosphorus, the process must be conducted within an operational window of lower temperature and higher slag basicity, thereby promoting the stable fixation of phosphorus within the slag phase.
5.2. Slag Phase Mechanism
Figure 12 presents the high-temperature characteristics of the slag phase. As shown in Figure 12a, with increasing basicity from R = 0.035 to 1.0, the primary crystalline phase transitioned from mullite (3Al2O3·2SiO2) to anorthite (CaAl2Si2O8), accompanied by a reduction in the liquidus temperature from above 1600 °C to approximately 1350 °C. At R = 1.5, the phase assemblage transitioned to gehlenite (Ca2Al2SiO7), accompanied by a rebound in the liquidus temperature to approximately 1500 °C. With a further increase in basicity to R = 2.0–2.5, the system migrated into regions dominated by dicalcium silicate (Ca2SiO4), resulting in liquidus temperatures exceeding 1600 °C. Notably, the Ca3(PO4)2 solid solution identified within dicalcium silicate phases under high-basicity conditions (R ≥ 2.0) presented characteristics consistent with the phosphorus speciation profiles obtained via EPMA. This correlation confirms that phosphorus attains thermal stability via the formation of a Ca3(PO4)2–Ca2SiO4 solid solution, thereby effectively inhibiting its transfer into the metallic iron phase. Analysis of Figure 12b indicates that within the basicity range of 0.5 to 2.5, the slag viscosity increases with rising basicity. Nevertheless, all measured values remain lower than the viscosity at the basicity of 0.035. This implies that operation under high-basicity conditions can satisfactorily meet industrial production requirements.
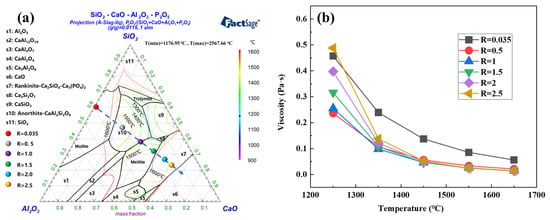
Figure 12.
The high-temperature properties of the slag phase. (a) Liquid phase projection diagram; (b) viscosity of the slag phase.
As illustrated in Figure 13, the dephosphorization mechanism of the alkaline briquette direct reduction–smelting separation process involves three critical stages. Initially, the lattice activation of native Ca3(PO4)2 occurs through interaction with the acidic SiO2-Al2O3 eutectic system, releasing highly reactive P2O5 components. Subsequently, thermally decomposed CaCO3 generates active CaO that combines with P2O5 via interfacial ion-exchange reactions to reconstitute thermodynamically stable Ca3(PO4)2 crystals. Ultimately, the regenerated Ca3(PO4)2 forms a Ca2Al2SiO7-Ca3Mg(SiO4)2-Ca3(PO4)2 solid solution system, establishing a chemical barrier through dual inhibition effects (suppressing the decomposition of both native/regenerated calcium phosphate and phosphorus reduction), thereby achieving targeted phosphorus enrichment in the slag phase.
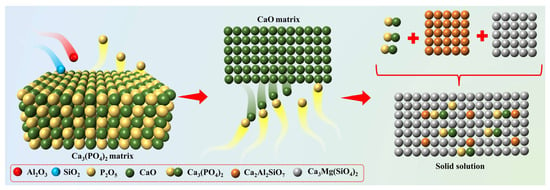
Figure 13.
Schematic diagram of phosphorus removal by the direct reduction and melting separation processes of alkaline briquettes.
6. Conclusions
Through alkaline briquette direct reduction and smelting separation processes, low-phosphorus steel was successfully produced with supplementary MgO addition for enhanced desulfurization, ultimately yielding clean steel with ultralow phosphorus and sulfur levels. The main conclusions are as follows:
- (1)
- Process optimization demonstrated that coordinated regulation of the basicity (R = 2) and MgO dosage (5%) resulted in 98.56% iron content in the smelted metal phase, with phosphorus and sulfur reducing to 0.036% and 0.046%, respectively. The iron recovery reached 87.63%, accompanied by phosphorus and sulfur removal efficiencies of 94.67% and 90.56%, respectively, meeting standard steel composition requirements.
- (2)
- Slag characterization revealed that higher basicity facilitated phosphorus fixation as Ca3(PO4)2 within Ca2Al2SiO7 and Ca3Mg(SiO4)2 matrices through solid solution formation. This spatial steric hindrance effect effectively suppressed phosphorus migration to the metallic phase, ensuring slag-phase phosphorus enrichment.
- (3)
- Thermodynamic analysis confirmed that under high basicity (R ≥ 2.0), P2O5 activity markedly decreased via Ca3(PO4)2-Ca2SiO4 solid solution formation. This dual mechanism—inhibiting both calcium phosphate decomposition and phosphorus reduction—substantially enhanced system stability. The synergistic mechanism of “chemical immobilization coupled with thermodynamic stabilization” effectively addresses the fundamental challenges associated with achieving deep dephosphorization in high-phosphorus iron ores.
Author Contributions
M.H.: conceptualization, investigation, writing—original draft, and software. D.Z.: project administration, funding acquisition, writing—review & editing. J.P.: methodology, formal analysis, supervision. S.L.: project administration, data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (Nos. 2023YFC3903900, 2023YFC3903904), the National Natural Science Foundation Youth Foundation of China (No. 52404356).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. (The data are not publicly available due to privacy restrictions.)
Acknowledgments
The authors gratefully acknowledge Deqing Zhu for his invaluable guidance in the experimental design. Special thanks are extended to Mengjie Hu for his extensive contributions to the experimental work. The authors also appreciate the analytical support provided by the Changsha Research Institute of Mining and Metallurgy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tang, X.; Liu, S.; Wang, Y.; Wan, Y.; Nubea, M.D. Carbon emission reduction in China’s iron and steel industry through technological innovation: A quadrilateral evolutionary game analysis under government subsidies. Front. Environ. Sci. 2025, 12, 1491608. [Google Scholar] [CrossRef]
- Jiang, W.; Jung, T.; Dai, H.; Xiang, P.; Chen, S. Transition Pathways for Low-Carbon Steel Manufacture in East Asia: The Role of Renewable Energy and Technological Collaboration. Sustainability 2025, 17, 4280. [Google Scholar] [CrossRef]
- Arras, M.; Jeandey, T.-F.; He, Y.; Gupta, P.; Li, Z.; Ma, L. Decarbonizing China’s iron and steel industry: Hydrogen-based mitigation pathway and techno-economic implications. Int. J. Hydrogen Energy 2025, 154, 149946. [Google Scholar] [CrossRef]
- He, Y.; Du, E.; Liu, P.; Li, Z. Decarbonization pathways and layout evolution in China’s steel sector. Renew. Sustain. Energy Rev. 2025, 215, 115588. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.; Wang, F.; Zhang, Y.; Wang, H.; Xu, Q.; Xu, M.; Bai, L. Low-Carbon Production in China’s Iron and Steel Industry: Technology Choices, Economic Assessment, and Policy. Atmosphere 2025, 16, 252. [Google Scholar] [CrossRef]
- Jiang, H.-D.; Liu, Y.-X.; Wang, H.; Li, H.; Jiang, Y. An economy-wide and environmental assessment of an imported supply shortage for iron ore: The case of China. Econ. Anal. Policy 2024, 83, 606–617. [Google Scholar] [CrossRef]
- Xu, Y.; Li, E.; Zhang, Y.; Hong, L.; Yao, X. Research status of new technology for magnetization roasting and reduction of refractory iron ore in China. Miner. Eng. 2024, 218, 109041. [Google Scholar] [CrossRef]
- Liu, Q.W.; Sono, H. The Post-Internationalization Evolution of the Price Discovery Pattern in China’s Iron Ore Markets. Chin. Econ. 2025, 58, 203–216. [Google Scholar] [CrossRef]
- Seaman, J.; Materials, C.R. Economic Statecraft and Europe’s Dependence on China. Int. Spect. 2025, 60, 20–37. [Google Scholar] [CrossRef]
- Liu, Z.J.; Lu, S.F.; Wang, Y.Z.; Zhang, J.L.; Cheng, Q.; Ma, Y.F. Study on optimization of reduction temperature of hydrogen-based Shaft FurnacedNumerical simulation and multi-criteria evaluation. Int. J. Hydrogen Energy 2023, 48, 16132–16142. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Feng, J.E.; Zhao, Z.C.; Tian, H.Y. Preparation of Oxidized Pellets of Vanadium Titanium Magnetite and Direct Reduction Behavior in Hydrogen-Based Shaft Furnace. Steel Res. Int. 2025, 96, 2400480. [Google Scholar] [CrossRef]
- Ali, M.L.; Fradet, Q.; Riedel, U. Particle-resolved computational modeling of hydrogen-based direct reduction of iron ore pellets in a fixed bed. Part I: Methodology and validation. Int. J. Hydrogen Energy 2024, 87, 332–343. [Google Scholar] [CrossRef]
- Kazmi, B.; Taqvi, S.A.A.; Juchelkov, D. State-of-the-art review on the steel decarbonization technologies based on process system engineering perspective. Fuel 2023, 347, 128459. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.S.; She, X.F.; Xue, Q.G.; Wang, G.; Zuo, H.B. Feasibility and comprehensive evaluation of the application of different low-carbon technologies in the iron and steel industry. Fuel 2025, 381, 133434. [Google Scholar] [CrossRef]
- Singh, A.; Singh, V.; Patra, S.; Dixit, P.; Mukherjee, A.K. Review on High Phosphorous in Iron Ore: Problem and Way Out. Min. Metall. Explor. 2024, 41, 1497–1507. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, Z.; Pan, J.; Zhang, F. Synchronous Upgrading Iron and Phosphorus Removal from High Phosphorus Oolitic Hematite Ore by High Temperature Flash Reduction. Metals 2016, 6, 123. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Rao, M.; Zhang, Y.; Jiang, T. Effects of sodium salts on reduction roasting and Fe-P separation of high-phosphorus oolitic hematite ore. Int. J. Miner. Process. 2013, 124, 26–34. [Google Scholar] [CrossRef]
- Yao, G.; Guo, Q.; Li, Y.; Song, J.; Liu, Y.; He, M.; Qi, T. Growth mechanism of metal iron particles with sulfur additives in direct reduction: First-principles calculations and experiments. Powder Technol. 2023, 419, 118287. [Google Scholar] [CrossRef]
- GB/T 699-2015; Quality Carbon Structure Steels. The State Quality Supervision, Inspection and Quarantine Administration of the People’s Republic of China: Beijing, China, 2015.
- Liu, F.; Zhang, Y.-C.; Zeng, W.; Ni, J.; Si, Y.-P.; Zhou, H.; Zhang, T.-X.; Wu, S.-L.; Kou, M.-Y. Iron recovery and dephosphorization behaviors from high-phosphorus oolitic hematite by gas-based direct reduction and magnetic separation. J. Iron Steel Res. Int. 2024, 32, 550–563. [Google Scholar] [CrossRef]
- Tang, H.-Q.; Qin, Y.-Q.; Qi, T.-F.; Dong, Z.-L.; Xue, Q.-G. Application of Wood Char in Processing Oolitic High-phosphorus Hematite for Phosphorus Removal. J. Iron Steel Res. Int. 2016, 23, 109–115. [Google Scholar] [CrossRef]
- Huang, D.-B.; Zong, Y.-B.; Wei, R.-F.; Gao, W.; Liu, X.-M. Direct Reduction of High-phosphorus Oolitic Hematite Ore Based on Biomass Pyrolysis. J. Iron Steel Res. Int. 2016, 23, 874–883. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Yan, W.; Xie, J.X.; Diao, C.M. Study on oxygen blowing optimisation of reducing (FeO) content in slag in EAF steelmaking process with high ratio of hot metal to scrap. Ironmak. Steelmak. 2022, 49, 398–404. [Google Scholar] [CrossRef]
- Xi, X.J.; Li, S.Y.; Li, C.M.; Pan, H.T.; Wang, J.; Zhu, R. Research on technical parameters of electrical arc furnace steelmaking based on direct reduced iron as raw material. Ironmak. Steelmak. 2024, 51, 947–960. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, H.; Li, Y.; Liu, T.; Wang, A.; Zhang, H. Resource-saving production of Fe-based amorphous alloys from carbothermal reduction of high-phosphorus oolitic iron ore. J. Non-Cryst. Solids 2022, 579, 121365. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, H.; Li, Y.; Liu, T.; Wang, A.; Zhang, H. Fe-based amorphous alloys with superior soft-magnetic properties prepared via smelting reduction of high-phosphorus oolitic iron ore. Intermetallics 2022, 141, 107441. [Google Scholar] [CrossRef]
- Hu, M.J.; Zhu, D.Q.; Pan, J.; Guo, Z.Q.; Yang, C.C.; Li, S.W.; Cao, W. Efficient Removal of Impurities from Refractory Oolitic Magnetite Concentrate via High-Pressure Alkaline Leaching and Ultrasonic Acid Leaching Process. Minerals 2025, 15, 220. [Google Scholar] [CrossRef]
- Mengjie, H.; Deqing, Z.; Jian, P.; Zhengqi, G.; Congcong, Y.; Siwei, L.; Wen, C. Unique Mineralogical Characteristics of Clay-Rich High-Phosphorus Oolitic Hematite Ore and Combined Process of Iron Enrichment and Phosphorus Removal. Asia-Pac. J. Chem. Eng. 2025, e70064. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, D.; Pan, J.; Guo, Z.; Yang, C.; Li, S.; Cao, W. Efficient Dephosphorization and Enhanced Iron Grain Growth in the Direct Reduction of Refractory High-P Oolitic Iron Ore: Mechanisms Promoted by CaCO3–Na2SO4 Additives. J. Sustain. Metall. 2025, 11, 3043–3059. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, D.; Pan, J.; Guo, Z.; Yang, C.; Li, S.; Cao, W. High-Efficiency Removal of Silica and Alumina from Rough Concentrate of Refractory Oolitic Hematite Ore via High-Pressure Alkaline and Acid-Leaching Process. JOM 2025, 77, 6467–6479. [Google Scholar] [CrossRef]
- Hu, M.J.; Zhu, D.Q.; Pan, J.; Guo, Z.Q.; Yang, C.C.; Li, S.W.; Cao, W. Fe-P Alloy Production from High-Phosphorus Oolitic Iron Ore via Efficient Pre-Reduction and Smelting Separation. Minerals 2025, 15, 778. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, D.; Pan, J.; Li, S. Near-zero carbon iron powder from high-phosphorus oolitic pellets via hydrogen reduction-electromagnetic iron phase reconstruction—Magnetic separation. Powder Technol. 2026, 469, 121719. [Google Scholar] [CrossRef]
- Yang, X.; Sun, F.M.; Yang, J.L.; Liu, F.; Cheng, K.S.; Wang, J.H. Optimization of Low Phosphorus Steel Production With Double Slag Process in BOF. J. Iron Steel Res. Int. 2013, 20, 41–47. [Google Scholar] [CrossRef]
- Ye, M.L.; Xi, X.J.; Yang, S.F.; Li, J.S.; Wang, F. Dephosphorization of hot metal using rare earth oxide-containing slags. High Temp. Mater. Process. 2020, 39, 520–526. [Google Scholar] [CrossRef]
- Zhou, C.G.; Chen, Q.G.; Ji, Y.; Ai, L.Q.; Wang, S.H.; Chen, Q.Y.; Yuan, T.X. Effect of highly oxidizing converter dephosphorization slag on dephosphorization behaviour of molten steel. Ironmak. Steelmak. 2023, 50, 958–968. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).