Novel Approach to the Surface Degradation Assessment of 42CrMo4 Steel in Marine and Cavitation Erosion Environments
Abstract
1. Introduction
2. Experiment
2.1. Material
2.2. Methods
2.2.1. Corrosion Testing
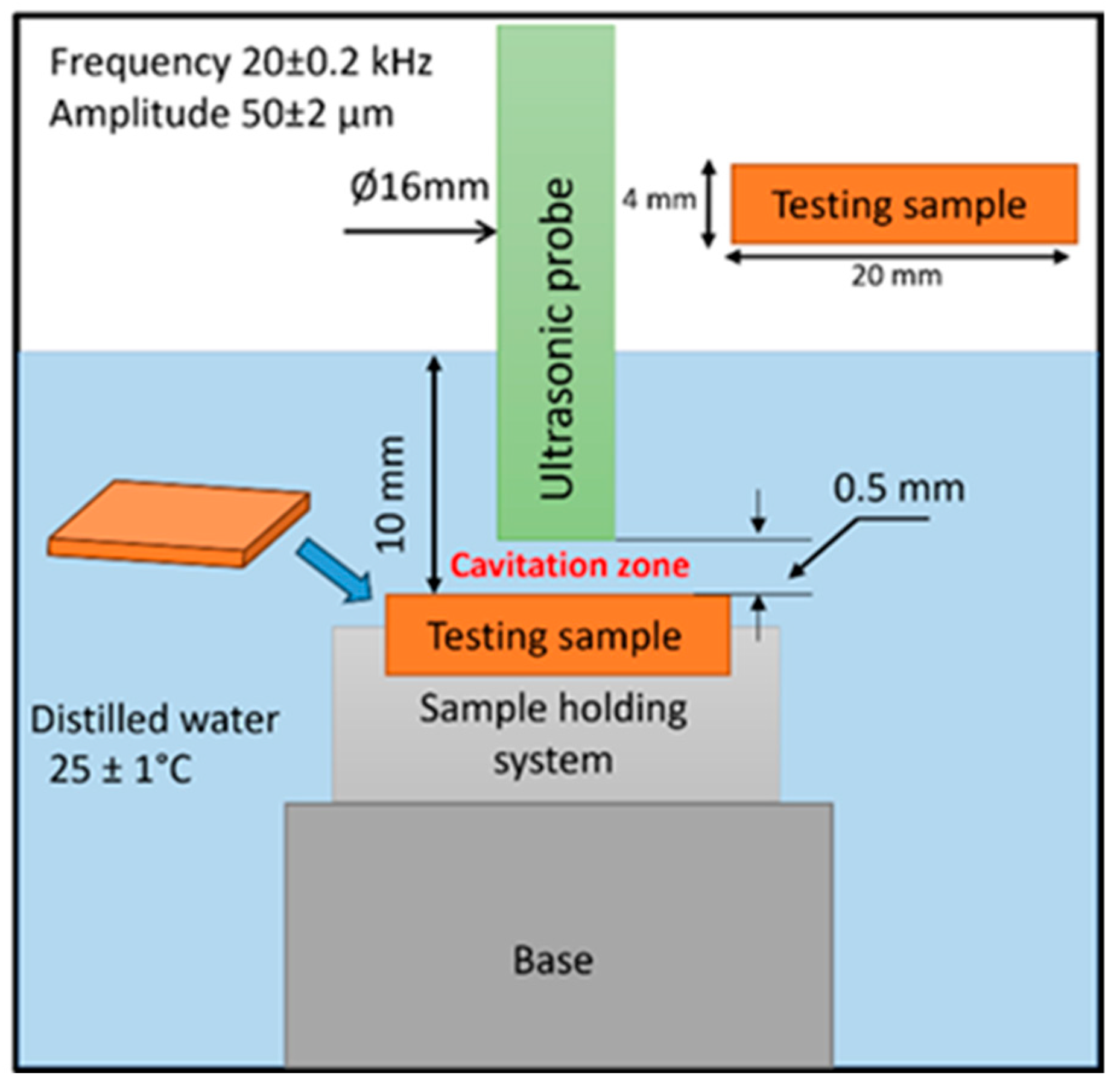
2.2.2. Cavitation Erosion Testing
2.2.3. Methods Applied for Monitoring Cavitation and Corrosion Testing
3. Results and Discussion
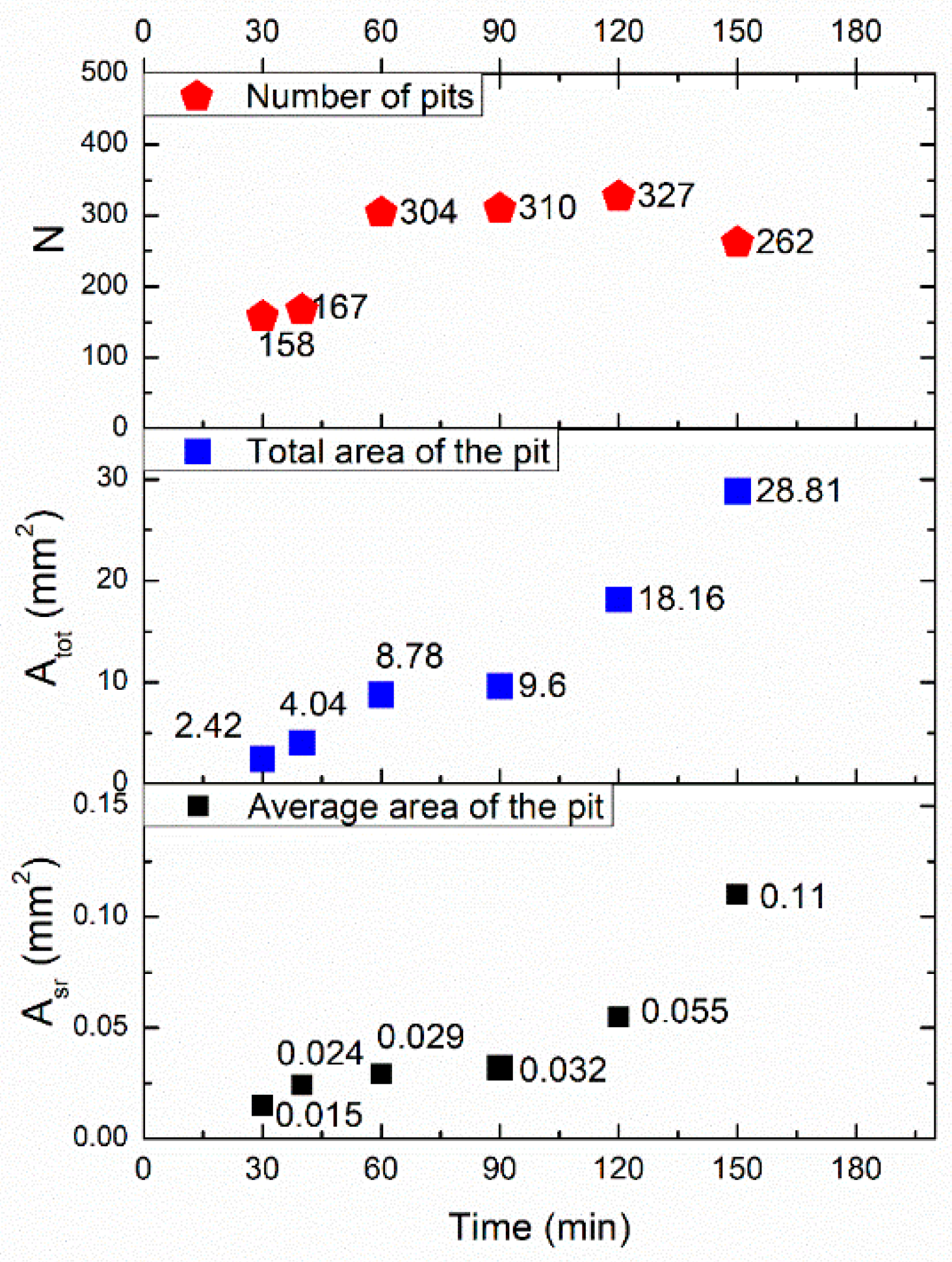
3.1. Corrosion Erosion Experiments
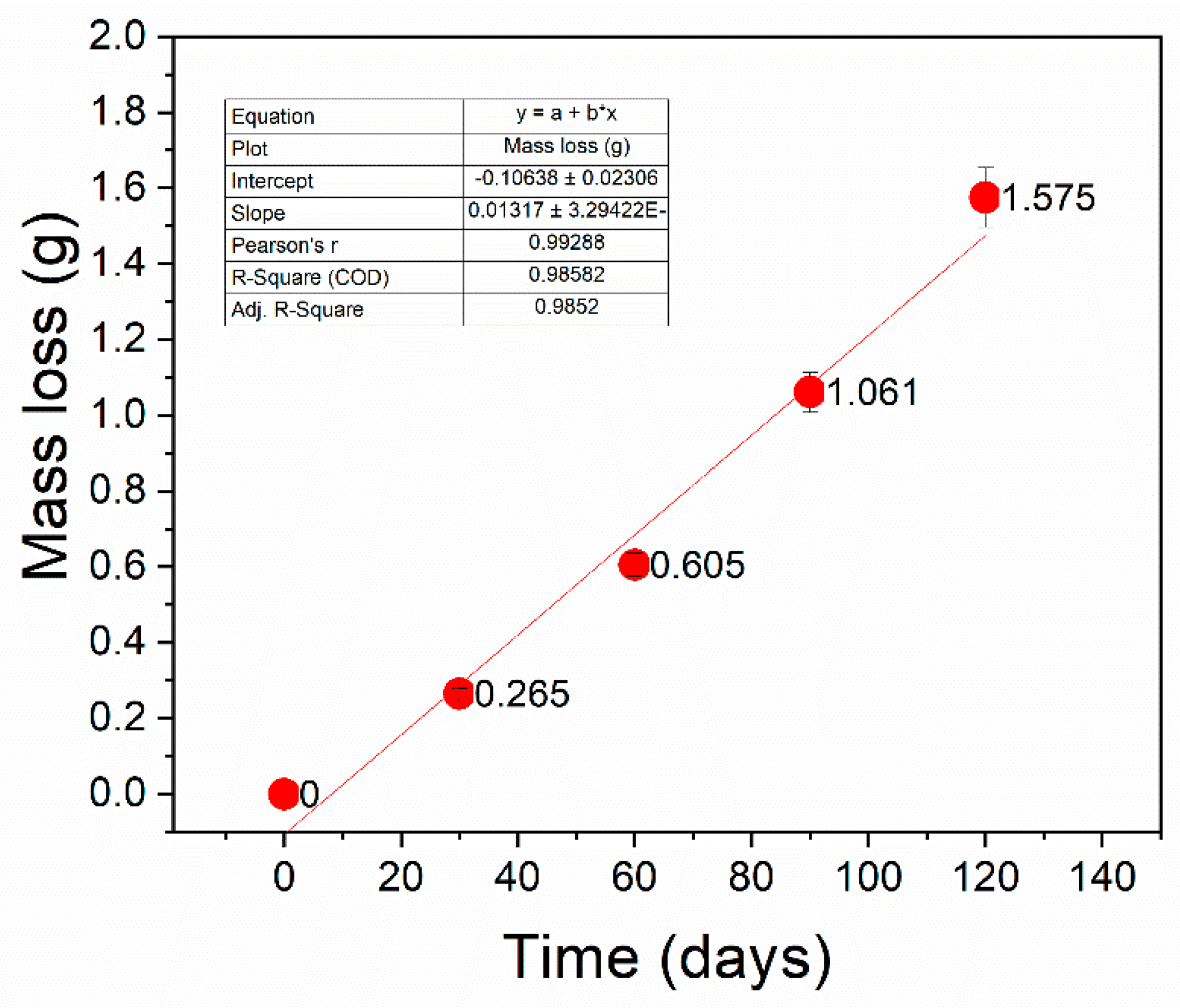
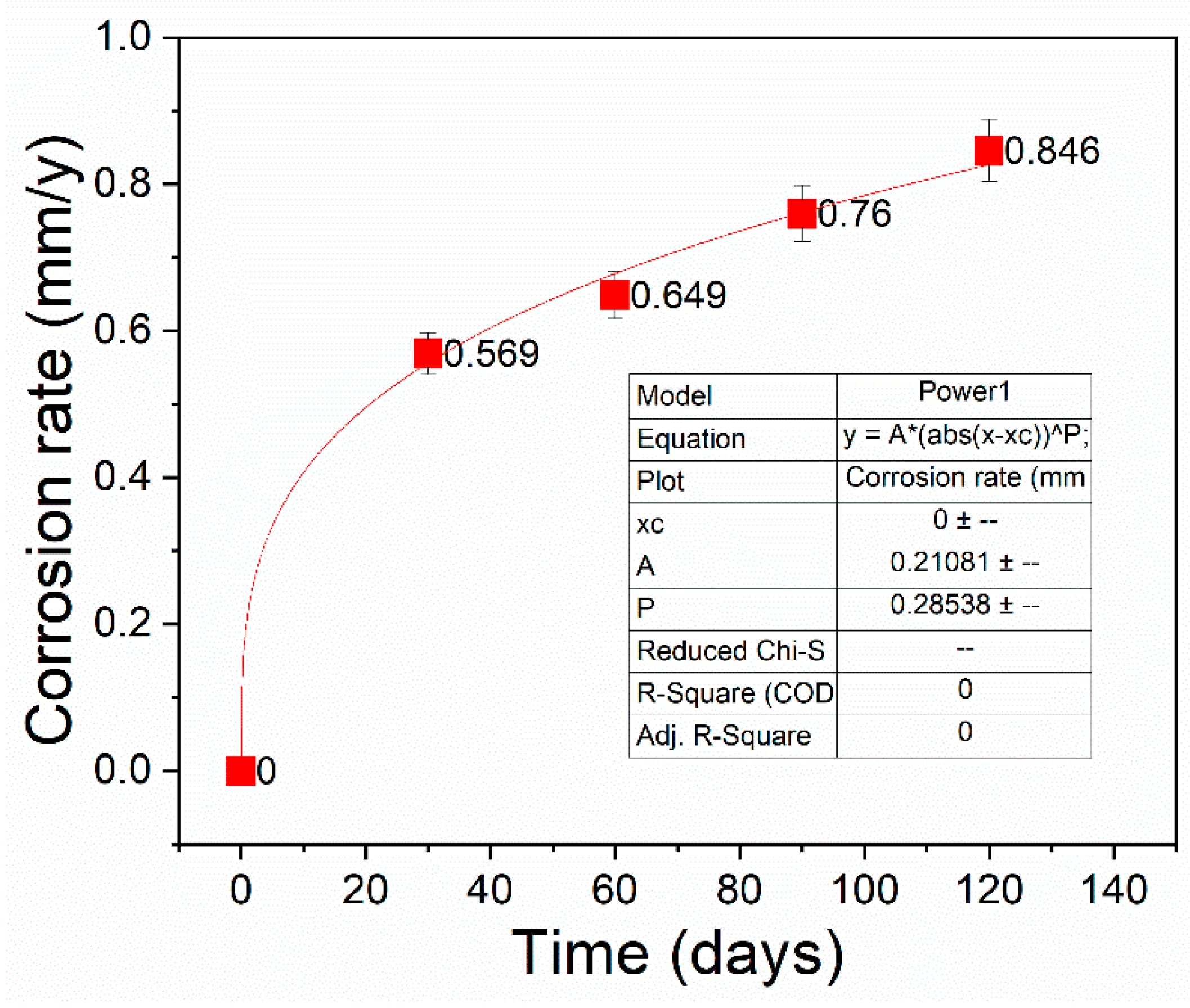
3.1.1. Mass Loss
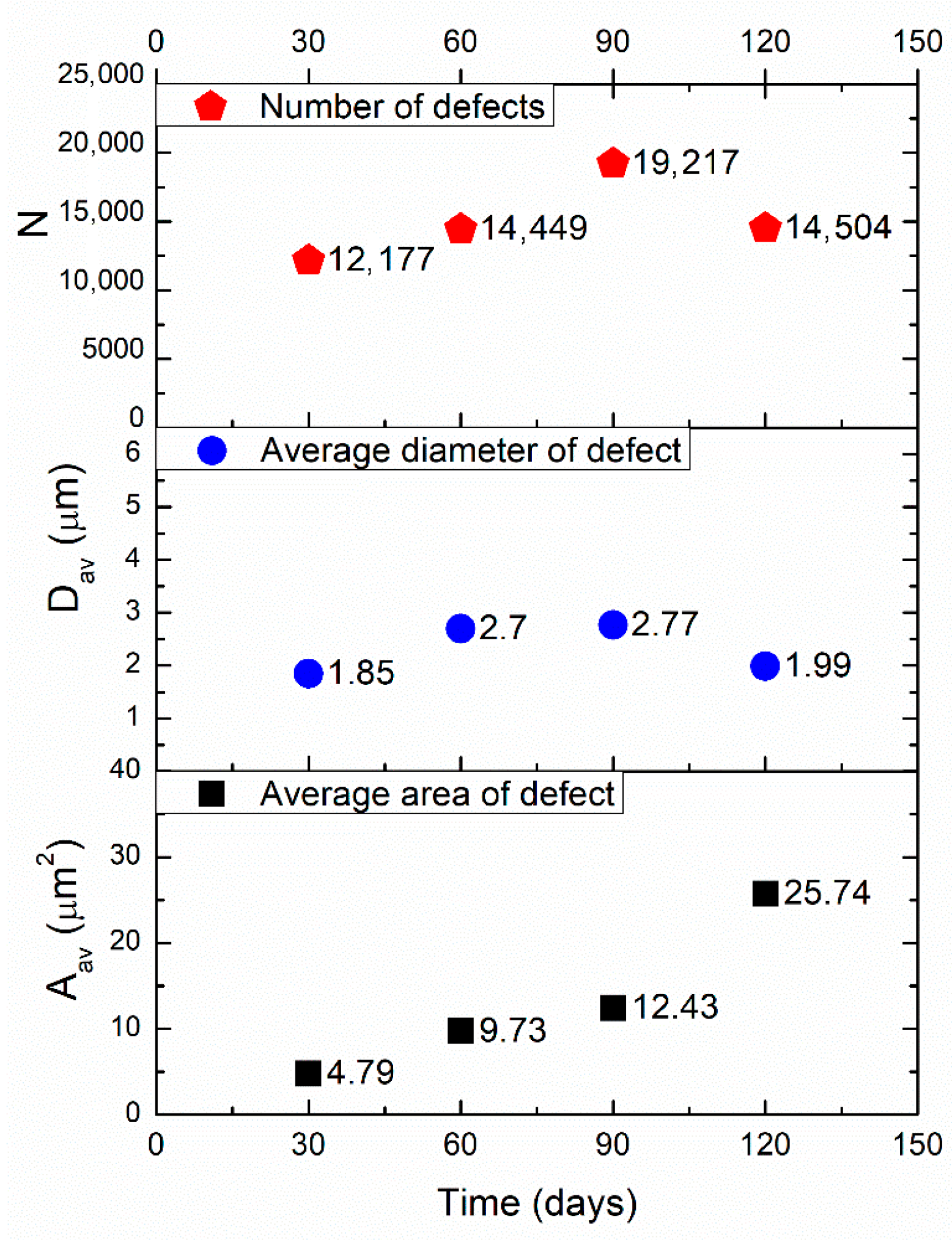
3.1.2. Image Analysis of Defect Morphology
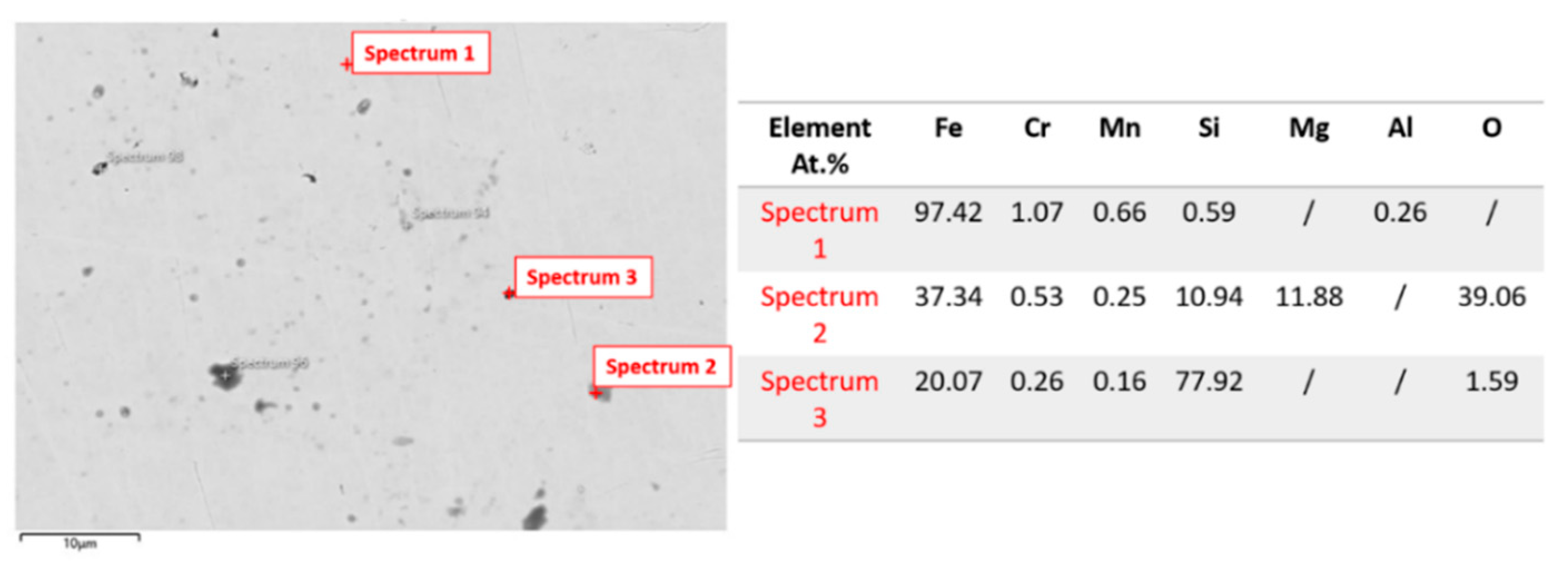
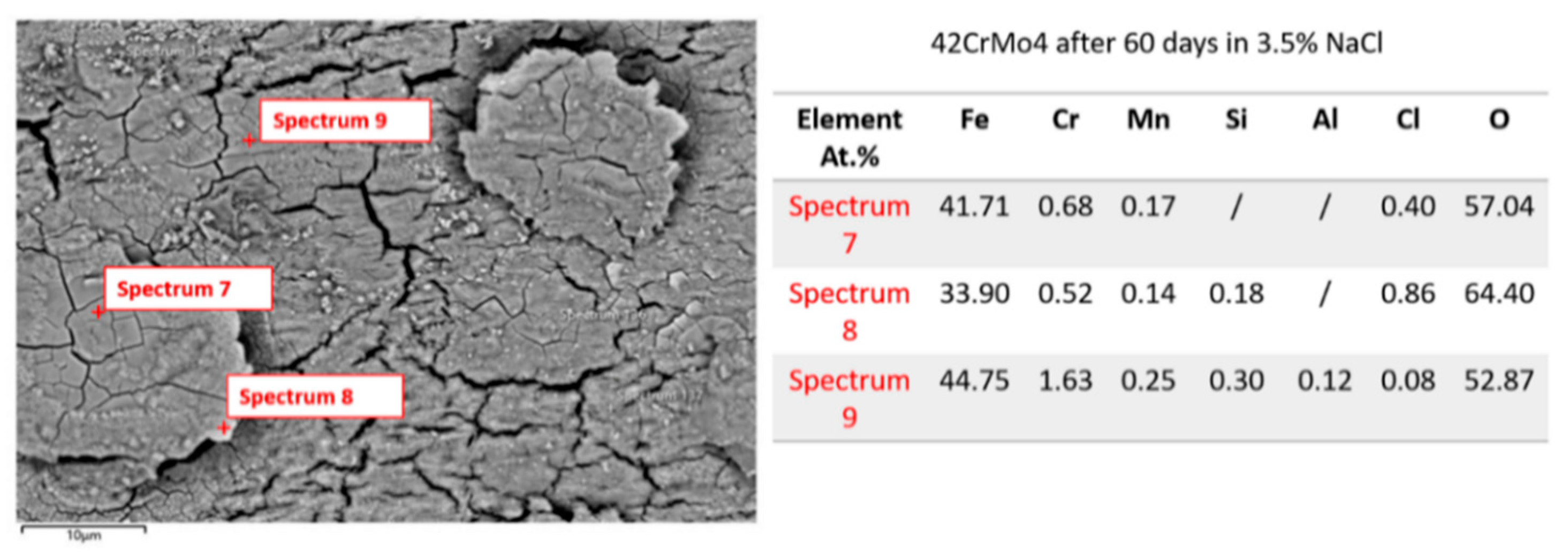
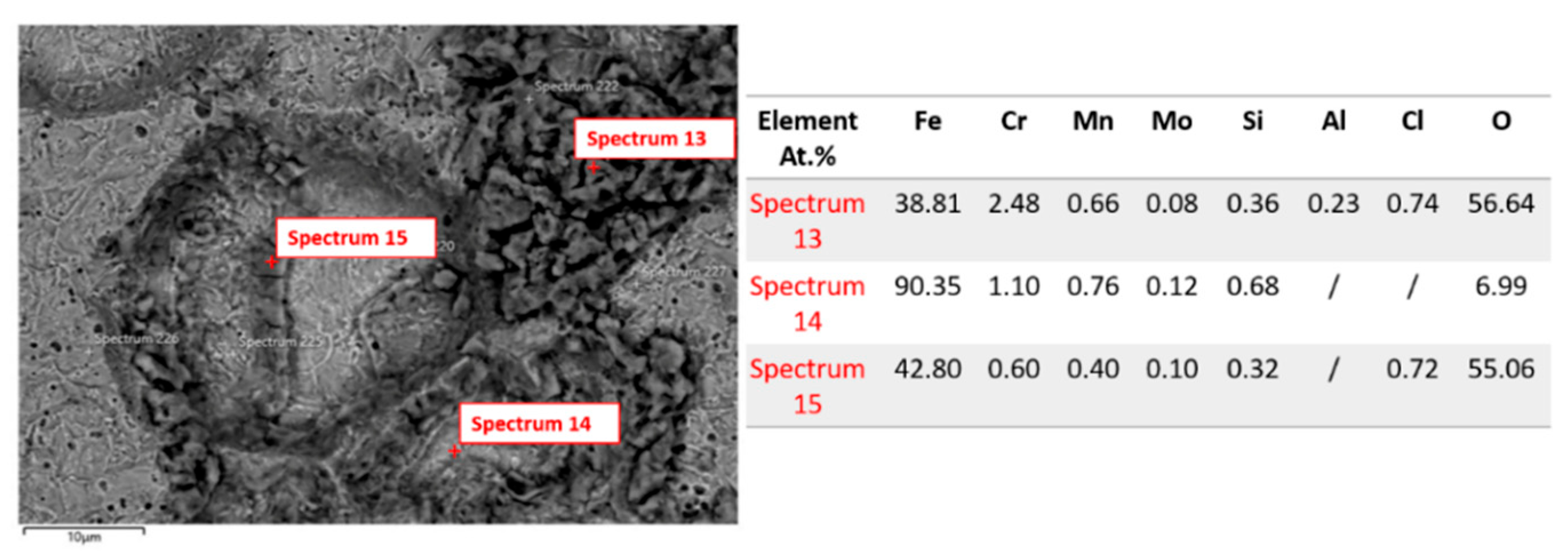
3.1.3. Microstructure Analysis: EDS Analysis
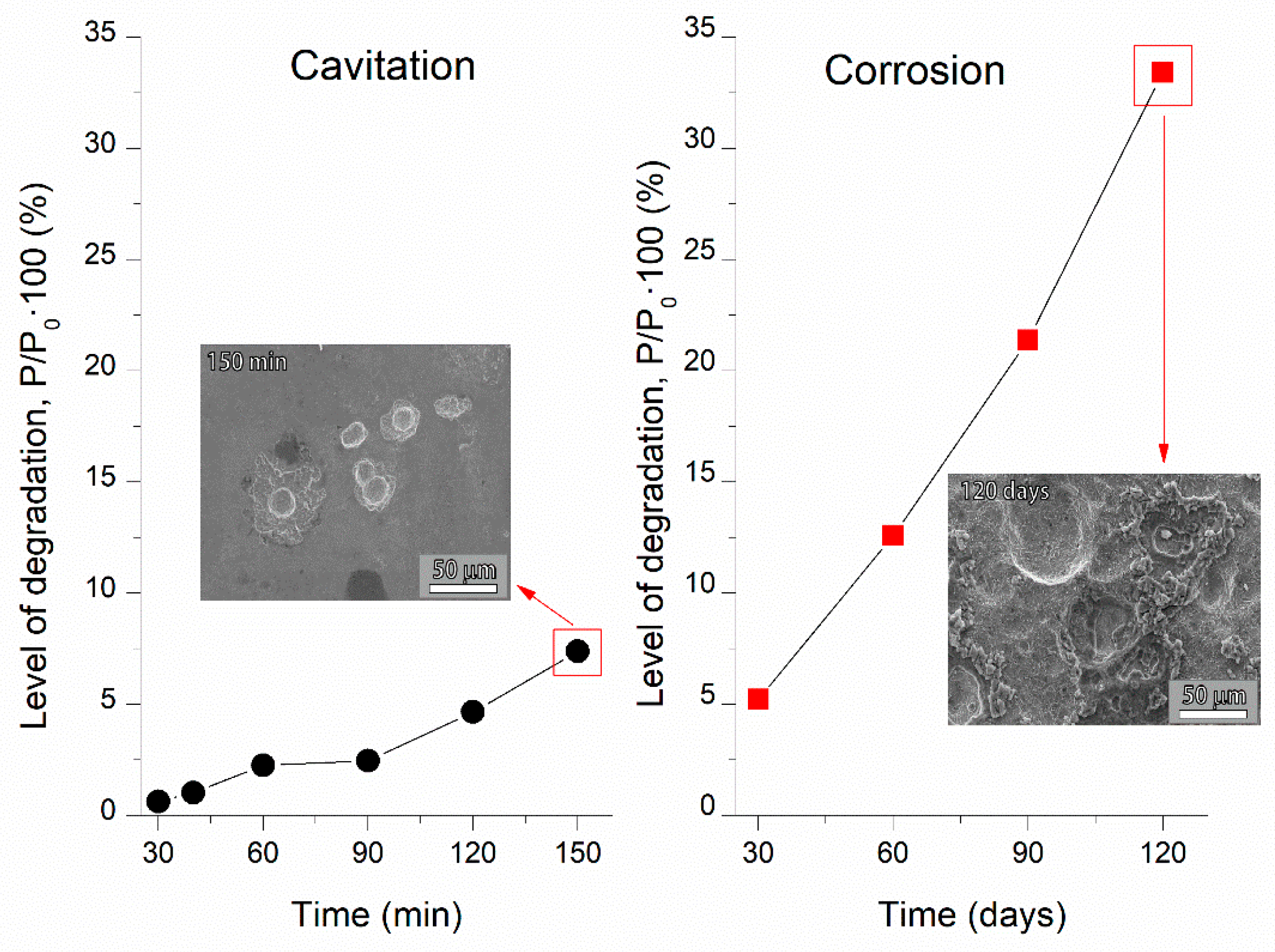
3.2. Cavitation Erosion Experiments
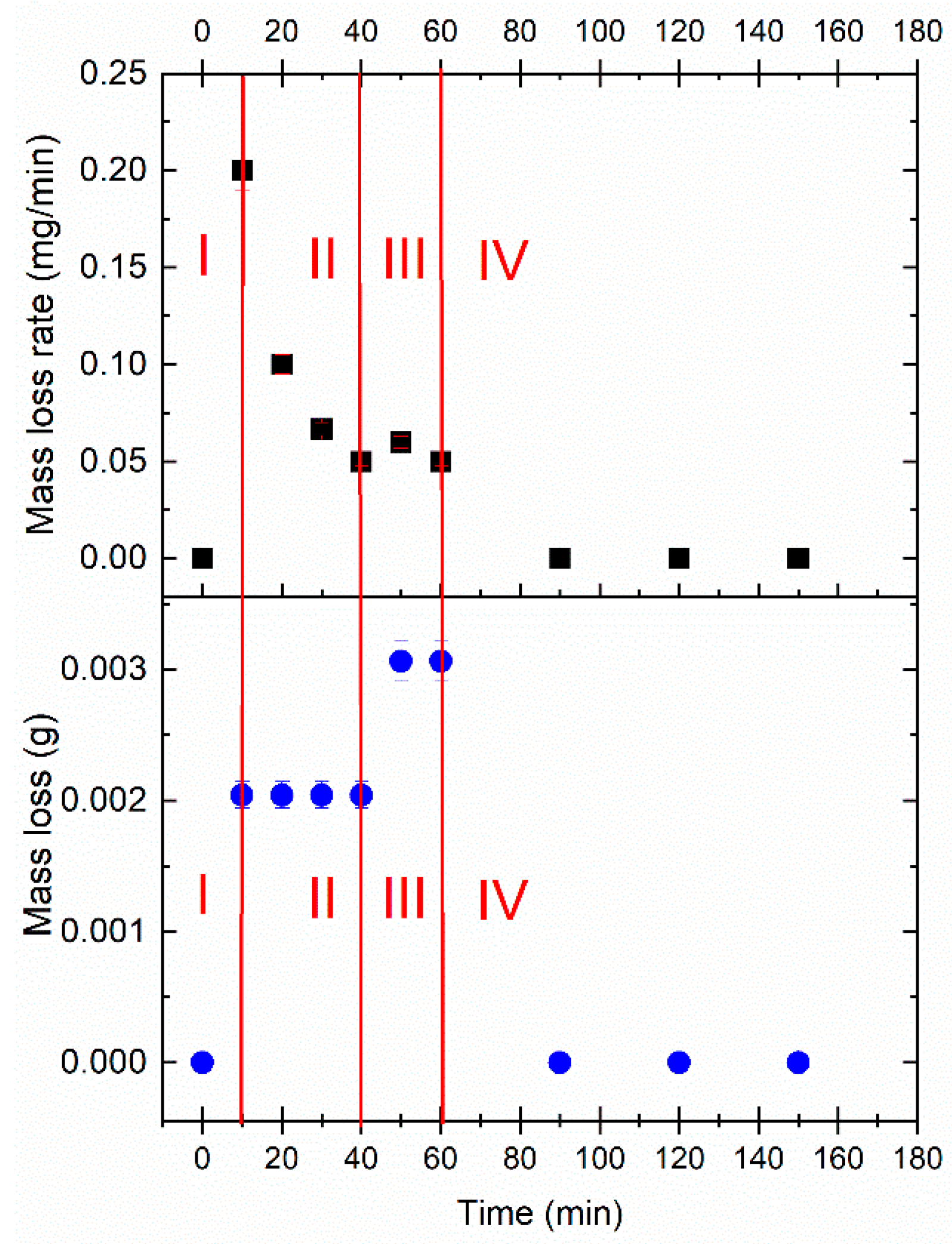
3.2.1. Mass Loss
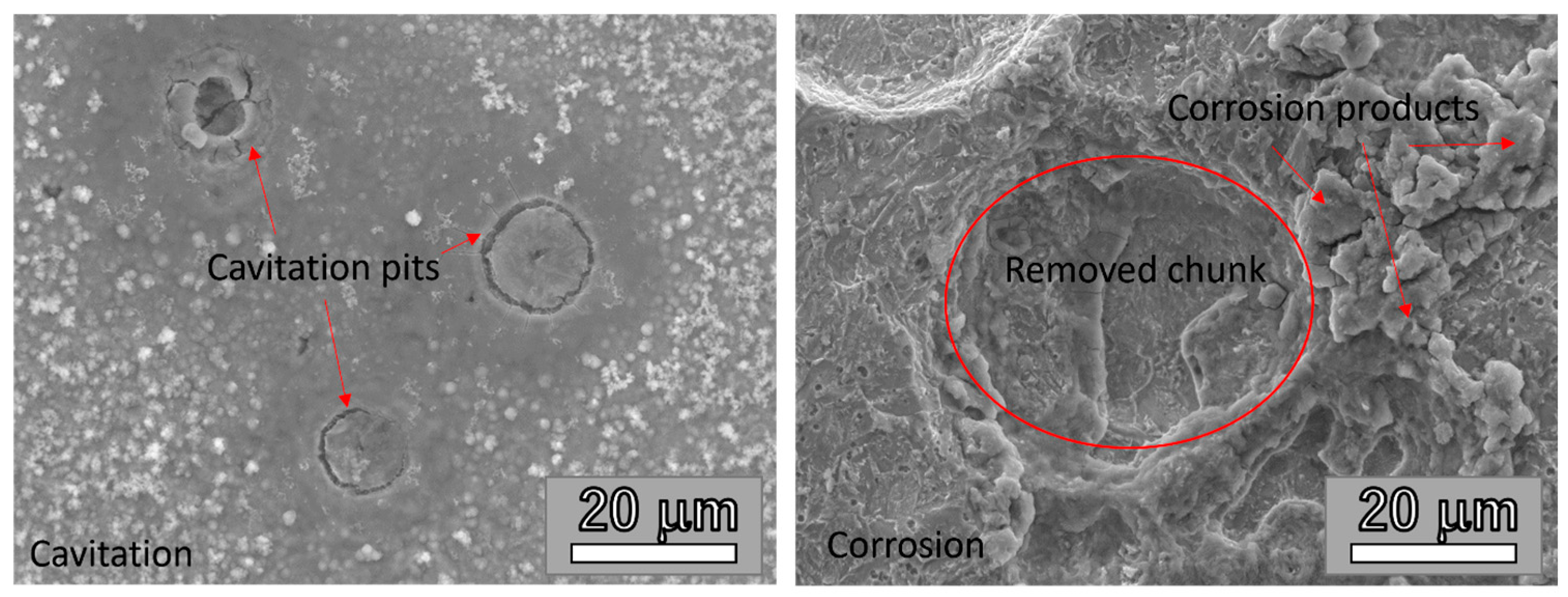
3.2.2. Image and Morphological Characterization of the Samples
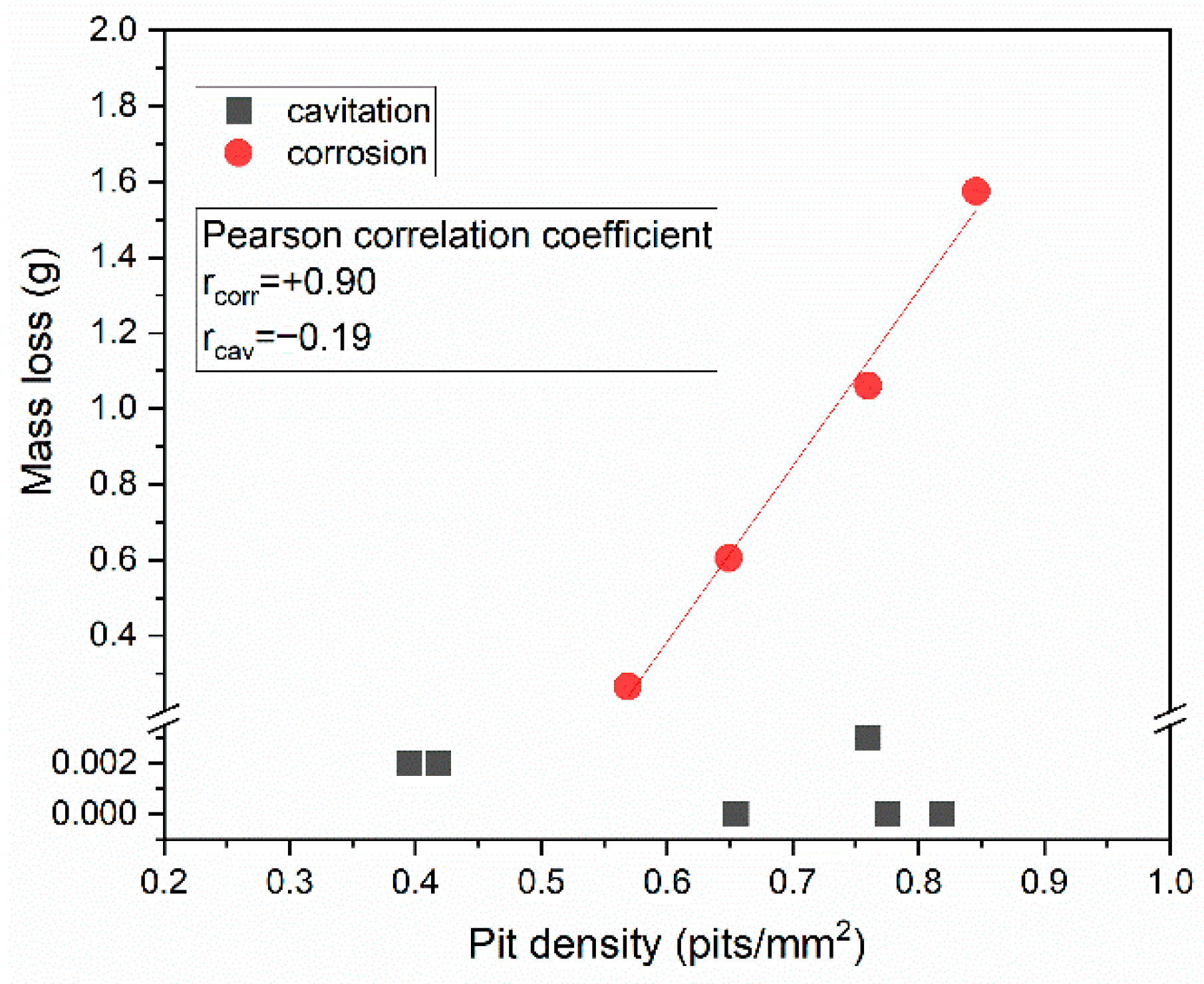
3.3. Cavitation vs. Corrosion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Q.; Yang, X.; Liu, J.; Jiang, D.; Qiu, Z. Improved Corrosion Resistance of 42CrMo4 Steel by Reconstructing Surface Integrity Using Ultrasonic Surface Rolling Process. Mater. Today Commun. 2023, 35, 105932. [Google Scholar] [CrossRef]
- Bartošák, M.; Horváth, J.; Španiel, M. Isothermal Low-Cycle Fatigue and Fatigue-Creep of a 42CrMo4 Steel. Int. J. Fatigue 2020, 135, 105538. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Y.; Xie, Q.; Li, D.; Gao, N. Mechanical Properties and Dynamic Constitutive Model of 42CrMo Steel. Mater. Des. 2017, 119, 171–179. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Lu, H.; Liu, J.; Cai, G. Surface Integrity and Corrosion Resistance of 42CrMo4 High-Strength Steel Strengthened by Hard Turning. Materials 2021, 14, 6995. [Google Scholar] [CrossRef]
- Maskavizan, A.J.; Quintana, J.P.; Dalibón, E.L.; Márquez, A.B.; Brühl, S.P.; Farina, S.B. Evaluation of Wear and Corrosion Resistance in Acidic and Chloride Solutions of Cathodic Arc PVD Chromium Nitride Coatings on Untreated and Plasma Nitrided AISI 4140 Steel. Surf. Coat. Technol. 2024, 494, 131476. [Google Scholar] [CrossRef]
- Datta, T.; Pathak, A.D.; Basak, S.; Gollapudi, S.; Sahu, K.K. Fractal Behavior of Surface Oxide Crack Patterns on AISI 4140 High-Strength Low-Alloy Steel Exposed to the Simulated Offshore Environment. Appl. Surf. Sci. Adv. 2021, 5, 100110. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z. Enhancing Surface Integrity and Corrosion Resistance of Laser Cladded Cr–Ni Alloys by Hard Turning and Low Plasticity Burnishing. Appl. Surf. Sci. 2017, 409, 169–178. [Google Scholar] [CrossRef]
- Que, Z.; Saario, T.; Toivonen, A.; Ehrnstén, U. Stress Corrosion Cracking Initiation Susceptibility of Alloy 182 with Different Surface Treatments. Corros. Sci. 2022, 196, 110037. [Google Scholar] [CrossRef]
- Abbas, M.; Rizvi, S.H.M.; Sarfraz, S.; Raza, A.; Khan, A.; Loya, A.; Najib, A. Evaluation of the Influence of Dissolved Nitrates on Corrosion Behaviour of Ship Structural Steel Exposed to Seawater Environment. Ocean Eng. 2024, 298, 117268. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on Corrosion Inhibitors: A Short View. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Montemor, F. Corrosion. In Materials for Construction and Civil Engineering; Springer: Cham, Switzerland, 2014; pp. 679–716. [Google Scholar] [CrossRef]
- Roberge, P. Handbook of Corrosion Engineering; McGraw-Hill Handbooks; Mcgraw-Hill: Columbus, OH, USA, 1999. [Google Scholar]
- Nodoushan, E.J.; Lee, Y.J.; Na, H.-J.; You, B.-H.; Lee, M.-Y.; Kim, N. Effects of NaCl and Temperature on Rheological Characteristics and Structures of CTAB/NaSal Wormlike Micellar Solutions. J. Ind. Eng. Chem. 2021, 98, 458–464. [Google Scholar] [CrossRef]
- Cai, Y.K.; Zhao, Y.; Zhang, Z.K.; Ma, X.B.; Cheng, B. Atmospheric and Marine Corrosion: Influential Environmental Factors and Models. In Proceedings of the International Workshop on Environmental Management, Science and Engineering, Xiamen, China, 16–17 June 2018. [Google Scholar] [CrossRef]
- Hammitt, F.G. Cavitation and Multiphase Flow Phenomena; McGraw-Hill Companies: Columbus, OH, USA, 1980. [Google Scholar]
- Brennen, C.E. Cavitation and Bubble Dynamics; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Sreedhar, B.K.; Albert, S.K.; Pandit, A.B. Cavitation Damage: Theory and Measurements—A Review. Wear 2016, 372–373, 177–196. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Z. Cavitation Erosion Mechanism of 2Cr13 Stainless Steel. Wear 2021, 488–489, 204137. [Google Scholar] [CrossRef]
- Tsybry, I.K.; Vyalikov, I.L. Analysis of Cavitation on the Surface of Steel under the Ultrasonic Cleaning. IOP Conf. Ser. Mater. Sci. Eng. 2017, 177, 012135. [Google Scholar] [CrossRef]
- Volkov-Husović, T.; Ivanić, I.; Kožuh, S.; Stevanović, S.; Vlahović, M.; Martinović, S.; Stopic, S.; Gojić, M. Microstructural and Cavitation Erosion Behavior of the CuAlNi Shape Memory Alloy. Metals 2021, 11, 997. [Google Scholar] [CrossRef]
- Basumatary, J. Study on Cavitation Erosion Resistance of Stellite Alloys by Ultrasonic Vibratory Method. Ph.D. Thesis, University of Southampton, Southampton, UK, 2016. [Google Scholar]
- Dojcinovic, M.; Volkov-Husovic, T. Cavitation Damage of the Medium Carbon Steel: Implementation of Image Analysis. Mater. Lett. 2008, 62, 953–956. [Google Scholar] [CrossRef]
- Knapp, R.T.; Daily, J.W.; Hammitt, F.G. Cavitation; McGraw-Hill: New York, NY, USA, 1970. [Google Scholar]
- Brijkishore; Khare, R.; Prasad, V. Prediction of Cavitation and Its Mitigation Techniques in Hydraulic Turbines—A Review. Ocean Eng. 2021, 221, 108512. [Google Scholar] [CrossRef]
- Candel, I.; Bunea, F.; Dunca, G.; Bucur, D.M.; Ioana, C.; Reeb, B.; Ciocan, G.D. Detection of Cavitation Vortex in Hydraulic Turbines Using Acoustic Techniques. IOP Conf. Ser. Earth Environ. 2014, 22, 052007. [Google Scholar] [CrossRef]
- Hofmann, J.; Thiébaut, C.; Riondet, M.; Lhuissier, P.; Gaudion, S.; Fivel, M. Comparison of Acoustic and Hydrodynamic Cavitation: Material Point of View. Phys. Fluids 2022, 35, 017112. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Z.; Cai, C.; Zhang, J.; Nie, B. Cavitation Damage Prediction of Stainless Steels Using an Artificial Neural Network Approach. Metals 2019, 9, 506. [Google Scholar] [CrossRef]
- Raami, L.; Varis, T.; Valtonen, K.; Wendler, M.; Volkova, O.; Peura, P. Enhancing the Cavitation Erosion Resistance of AISI 420-Type Stainless Steel with Quenching and Partitioning. Wear 2023, 526–527, 204897. [Google Scholar] [CrossRef]
- Basumatary, J.; Nie, M.; Wood, R.J.K. The Synergistic Effects of Cavitation Erosion–Corrosion in Ship Propeller Materials. J. Bio-Tribo-Corros. 2015, 1, 12. [Google Scholar] [CrossRef]
- Bărbulescu, A.; Dumitriu, C.Ș. Fractal Characterization of Brass Corrosion in Cavitation Field in Seawater. Sustainability 2023, 15, 3816. [Google Scholar] [CrossRef]
- Dojcinovic, M.; Markovic, S. The Morphology of Cavitation Damage of Heat-Treated Medium Carbon Steel. J. Serbian Chem. Soc. 2006, 71, 977–984. [Google Scholar] [CrossRef]
- Bordeasu, I.; Popoviciu, M.O.; Ghera, C.; Micu, L.M.; Pirvulescu, L.D.; Bena, T. The Use of Rz Roughness Parameter for Evaluation of Materials Behavior to Cavitation Erosion. IOP Conf. Ser. Mater. Sci. Eng. 2018, 294, 012020. [Google Scholar] [CrossRef]
- Dojcinovic, M. Roughness Measurement as an Alternative Method in Evaluation of Cavitation Resistance of Steels. Hem. Ind. 2012, 67, 323–330. [Google Scholar] [CrossRef]
- Han, S.; Lin, J.H.; Kuo, J.J.; He, J.L.; Shih, H.C. The Cavitation-Erosion Phenomenon of Chromium Nitride Coatings Deposited Using Cathodic Arc Plasma Deposition on Steel. Surf. Coat. Technol. 2002, 161, 20–25. [Google Scholar] [CrossRef]
- SRPS C.A1.011:2004; Methods of Chemical Analyses—Optical Emission Spectrometric Method with Spark Excitation for Quantitative Chemical Analyses of Pig Iron, Cast Iron, Low-Alloy Steels, High-Alloy Steels, Aluminium Alloys and Copper Alloys. Institute for Standardization of Serbia: Belgrade, Serbia, 2004.
- EN 10083-3:2006; Steels for Quenching and Tempering—Part 3: Technical Delivery Conditions for Alloy Steels. European Committee for Standardization (CEN): Brussels, Belgium, 2006.
- ASTM G44; Standard Practice for Exposure of Metals and Alloys by Alternate Immersion in Neutral 3.5% Sodium Chloride Solution. ASTM International: West Conshohocken, PA, USA, 2021.
- ISO 11130:2017; Corrosion of Metals and Alloys—Alternate Immersion Test in Salt Solution. ISO: Geneva, Switzerland, 2017.
- ASTM G1-03(2017)e1; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM G32-16; Standard Test Method for Cavitation Erosion Using Vibratory Apparatus. ASTM International: West Conshohocken, PA, USA, 2016.
- Murillo-Marrodán, A.; Bulzak, T.; García, E.; Derazkola, H.A.; Majerski, K.; Tomczak, J.; Pater, Z. Effect of Warm Forming Process Parameters on 42CrMo4 Skew Rolled Bar Mechanical Properties and Microstructure. Arch. Civ. Mech. Eng. 2024, 24, 20. [Google Scholar] [CrossRef]
- Kuc, D.; Bednarczyk, I.; Krawczyk, A.; Paterek, W.; Zygmunt, K.; Woźniak, D.; Hadasik, E. Structure and mechanical properties of rolled bars from steel 42CrMo4. In Proceedings of the 28th International Conference on Metallurgy and Materials, Hotel Voronez I, Brno, Czech Republic, 22–24 May 2019; pp. 391–396. [Google Scholar] [CrossRef]
- Pop, M.; Sas-Boca, I.-M.; Frunză, D.; Popa, F.; Neag, A. The Influence of Hot Deformation on the Mechanical and Structural Properties of 42CrMo4 Steel. Metals 2024, 14, 647. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y. Introduction of Non-metallic Inclusions in Steels. In Handbook of Non-Metallic Inclusions in Steels; Springer: Singapore, 2025. [Google Scholar] [CrossRef]
- Sidorova, E.; Karasev, A.; Kuznetsov, D.; Jönsson, P.G. Investigation of the Initial Corrosion Destruction of a Metal Matrix around Different Non-Metallic Inclusions on Surfaces of Pipeline Steels. Materials 2022, 15, 2530. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, E.; Wang, Q.; Lou, X.; Liu, H.; Zheng, Y.; Wang, B.; Zhu, L. Effect of Mg-Ce Treatment on Inclusion Characteristics and Pitting Corrosion Behavior in EH420 Marine Steel. Metals 2023, 13, 1244. [Google Scholar] [CrossRef]
- Alcántara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine Atmospheric Corrosion of Carbon Steel: A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef]
- Kaczmarczyk, R.; Gurgul, S. Thermodynamic Analysis of Chloride Corrosion in Steel for Energy System Applications in Fe-O-Cl-Na Environments. Energies 2024, 17, 3223. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion Inhibitors—Principles, Mechanisms and Applications. In Developments in Corrosion Protection; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Shin, S.-B.; Song, S.-J.; Shin, Y.-W.; Kim, J.-G.; Park, B.-J.; Suh, Y.-C. Effect of Molybdenum on the Corrosion of Low Alloy Steels in Synthetic Seawater. Mater. Trans. 2016, 57, 2116–2121. [Google Scholar] [CrossRef]
- Yu, X.; Al-Saadi, S.; Zhao, X.-L.; Raman, R.K.S. Electrochemical Investigations of Steels in Seawater Sea Sand Concrete Environments. Materials 2021, 14, 5713. [Google Scholar] [CrossRef]




| 42CrMo4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element (wt. %) | C | Cr | Mo | Mn | Si | Ni | Cu | Al | S | P | Fe |
| 0.40 | 0.93 | 0.20 | 0.65 | 0.29 | 0.03 | 0.04 | 0.044 | 0.003 | 0.009 | Bal. | |
| Morphological Descriptor | Definition | Image |
|---|---|---|
| Area | Area of object. Does not include a hole area | 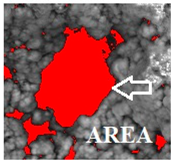 |
| The total area of the pits | The sum of the area of single objects | 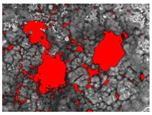 |
| Diameter (mean) | The average length of diameters measured at 2-degree intervals and passing through the object’s centroid. | 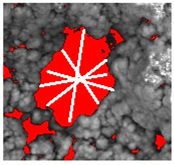 |
| Number of pits/defects | Number of selected objects |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedović, S.; Alil, A.; Martinović, S.; Dikić, S.; Glišić, D.; Volkov-Husović, T. Novel Approach to the Surface Degradation Assessment of 42CrMo4 Steel in Marine and Cavitation Erosion Environments. Metals 2025, 15, 1154. https://doi.org/10.3390/met15101154
Nedović S, Alil A, Martinović S, Dikić S, Glišić D, Volkov-Husović T. Novel Approach to the Surface Degradation Assessment of 42CrMo4 Steel in Marine and Cavitation Erosion Environments. Metals. 2025; 15(10):1154. https://doi.org/10.3390/met15101154
Chicago/Turabian StyleNedović, Stanica, Ana Alil, Sanja Martinović, Stefan Dikić, Dragomir Glišić, and Tatjana Volkov-Husović. 2025. "Novel Approach to the Surface Degradation Assessment of 42CrMo4 Steel in Marine and Cavitation Erosion Environments" Metals 15, no. 10: 1154. https://doi.org/10.3390/met15101154
APA StyleNedović, S., Alil, A., Martinović, S., Dikić, S., Glišić, D., & Volkov-Husović, T. (2025). Novel Approach to the Surface Degradation Assessment of 42CrMo4 Steel in Marine and Cavitation Erosion Environments. Metals, 15(10), 1154. https://doi.org/10.3390/met15101154







