Microstructure of Butt Joint of High-Silicon Steel Made Using CO2 Laser Welding and Inconel 82 Filler
Abstract
1. Introduction
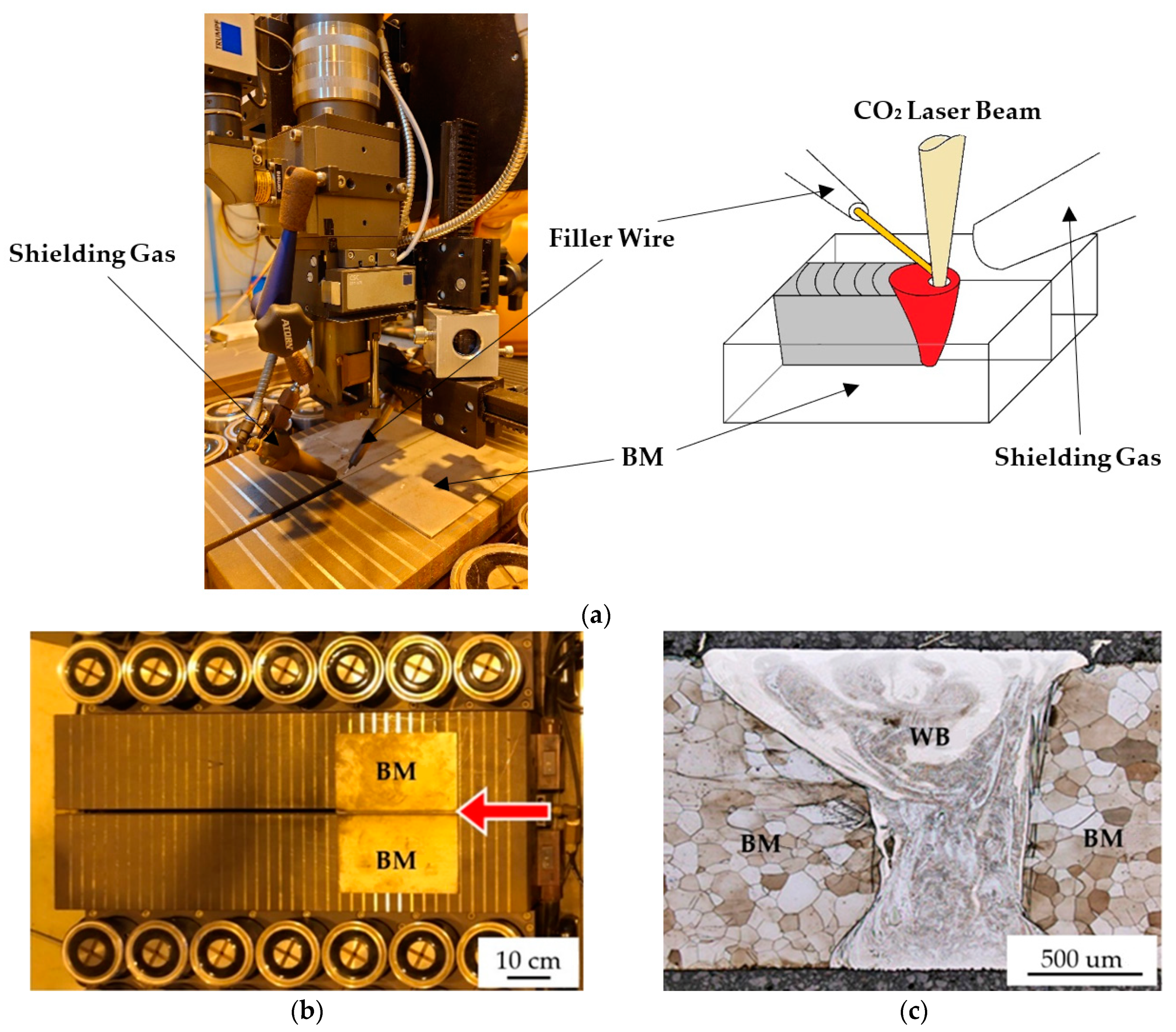
2. Material and Experimental Procedures
3. Results and Discussion
4. Conclusions
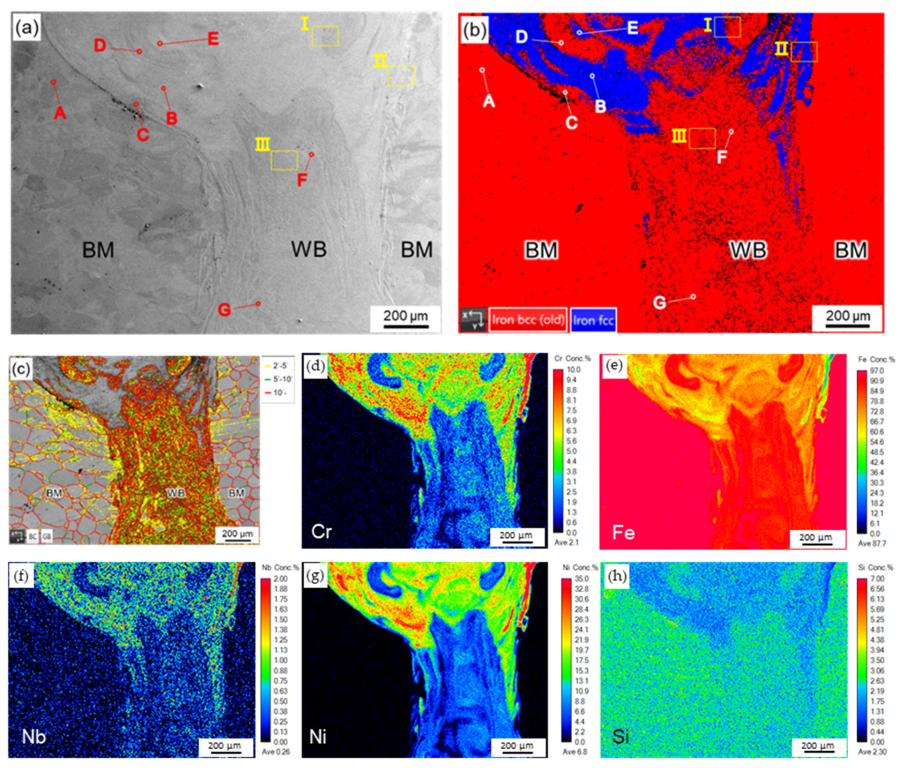
- The heterogeneous WB was mainly composed of austenite in the upper portion and martensite in the middle and bottom portions due to incomplete mixing of the weld pool during laser welding. The trace of convection in the entire laser weld pool was successfully identified from the quantitative Ni mapping in the EPMA analysis.
- The formation of martensite and austenite was determined by the Ni concentration of the WB. The austenite was alloyed with Ni above 20.1 wt%. The austenite was unstable and transformed into martensite if the Ni concentration was decreased below 16.4 wt%. Additionally, there was no retained austenite observed at the lath boundary of martensite.
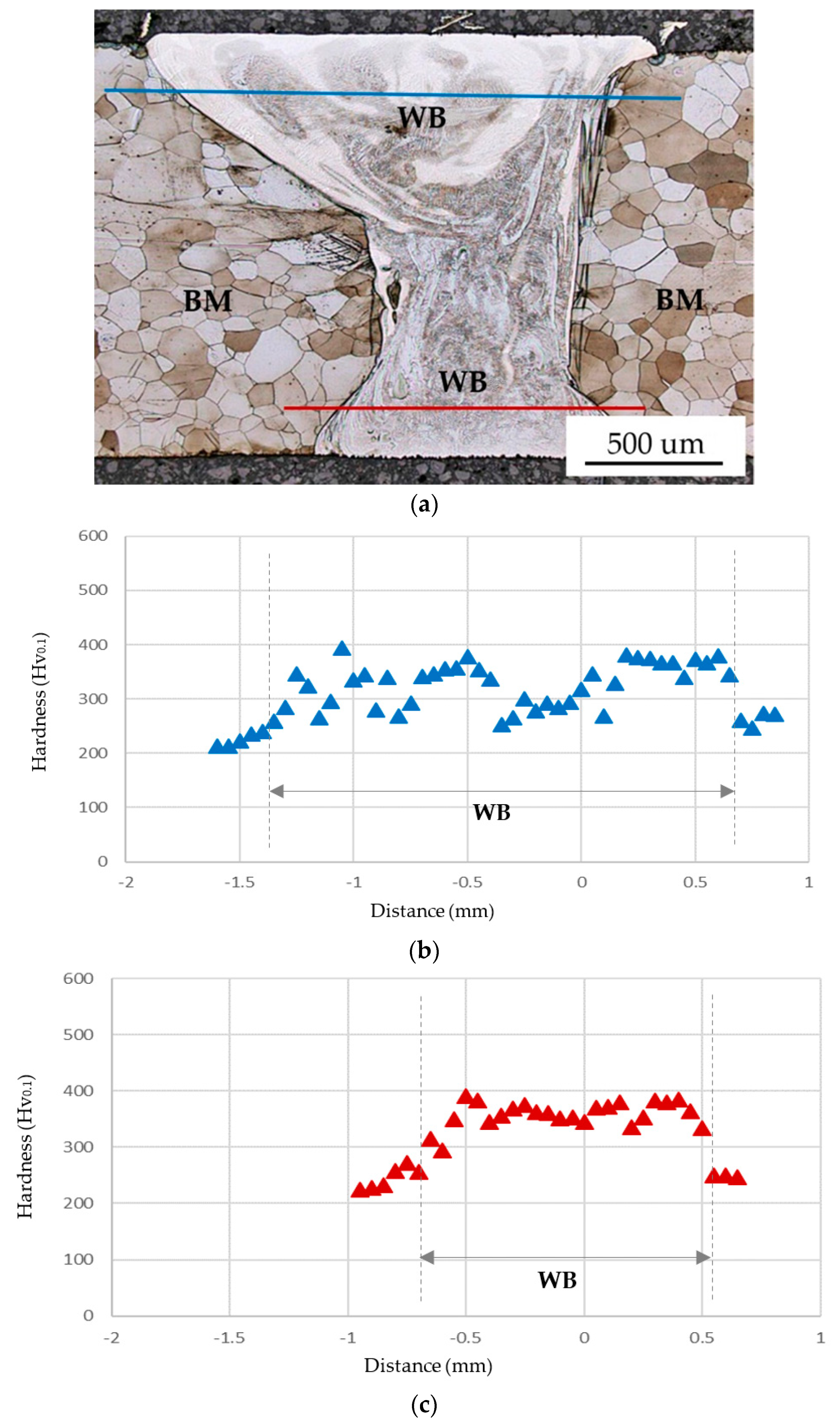
- The austenite in the WB was soft with the hardness range between 250 and 300 Hv0.1. In contrast, the martensite had the highest hardness between 350 and 400 Hv0.1. The high-silicon BM had the lowest hardness of 210 Hv0.1. Therefore, the combination of high-hardness martensite and austenite in the heterogeneous WB was much stronger than the ferrite BM. In the tensile test, all specimens were fractured in the BM with the tensile strengths between 490 and 500 MPa, and the necking of the BM was observed after the tensile test. The application of the IN82 filler wire in laser welding a high-silicon steel plate can successfully obtain a robust WB.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| BCC | Body-centered cubic |
| BEI | Back-scattered electron image |
| BF | Bright field |
| BM | Base metal |
| EBSD | Electron back-scattered diffraction |
| EDS | Energy-dispersive spectrometer |
| EPMA | Electron probe micro-analyzer |
| FCC | Face-centered cubic |
| FESEM | Field emission scanning electron microscope |
| HAADF | High-angle annular dark-field |
| IN82 | Inconel 82 |
| SADP | Selected area diffraction pattern |
| SEI | Secondary electron imaging |
| STEM | Scanning transmission electron microscope |
| WB | Weld bead |
| WDS | Wavelength dispersive spectrometer |
References
- Greifzu, M.; Tkachov, R.; Stepien, L.; Lopez, E.; Bruckner, F.; Leyens, C. Laser treatment as sintering process for dispenser printed bismuth telluride based paste. Materials 2019, 12, 3453. [Google Scholar] [CrossRef] [PubMed]
- Lee, D. Experimental investigation of laser ablation characteristics on nickel-coated beryllium copper. Metals 2018, 8, 211. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Mian, A. Influence of material property variation on computationally calculated melt pool temperature during laser melting process. Metals 2019, 9, 456. [Google Scholar] [CrossRef]
- Biffi, C.A.; Bassani, P.; Carnevale, M.; Lecis, N.; Loconte, A.; Previtali, B.; Tuissi, A. Effect of laser microcutting on thermo-mechanical properties of NiTiCu shape memory alloy. Met. Mater. Int. 2014, 20, 83–92. [Google Scholar] [CrossRef]
- Mohammed, G.R.; Ishak, M.; Ahmad, S.N.A.S.; Abdulhadi, H.A. Fiber laser welding of dissimilar 2205/304 stainless steel plates. Metals 2017, 7, 546. [Google Scholar] [CrossRef]
- Villar, R. Laser cladding. J. Laser Appl. 1999, 11, 64. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Wu, X. A review of laser fabrication of metallic engineering components and of materials. Mater. Sci. Technol. 2007, 23, 631–640. [Google Scholar] [CrossRef]
- Mujumdar, J.D.; Manna, I. Laser material processing. Int. Mater. Rev. 2011, 56, 341–388. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Lippold, J.C. Welding Metallurgy and Weldability; John Wiley & Sons: New York, NY, USA, 2015. [Google Scholar]
- Saunders, F.I.; Wagoner, R.H. Forming of tailor-welded blanks. Metall. Mater. Trans. A 1996, 27, 2605–2616. [Google Scholar] [CrossRef]
- Zambon, A.; Ferro, P.; Bonollo, F. Microstructural, compositional and residual stress evaluation of CO2 laser welded superaustenitic AISI 904L stainless steel. Mater. Sci. Eng. A 2006, 424, 117–127. [Google Scholar] [CrossRef]
- Chiang, M.F.; Lo, T.Y.; Chien, P.H.; Chi, C.H.; Chang, K.C.; Yeh, A.C.; Shiue, R.K. The dilution effect in high-power disk laser welding the steel plate using a nickel-based filler wire. Metals 2021, 11, 874. [Google Scholar] [CrossRef]
- Nivas, R.; Das, G.; Das, S.K.; Mahato, B.; Kumar, S.; Sivaprasad, K.; Singh, P.K.; Ghosh, M. Effect of stress relief annealing on microstructure & mechanical properties of welded joints between low alloy carbon steel and stainless steel. Metall. Mater. Trans. A 2017, 48, 230–245. [Google Scholar]
- Chen, Z.R.; Lu, Y.H. TEM observation of martensite layer at the weld interface of an A508III to Inconel 82 dissimilar metal weld joint. Metall. Mater. Trans. A 2015, 46, 5494–5498. [Google Scholar] [CrossRef]
- Sireesha, M.; Albert, S.K.; Sundaresan, S. Influence of high-temperature and mechanical properties of exposure on the microstructure dissimilar metal welds between modified 9Cr-1Mo steel and alloy 800. Metall. Mater. Trans. A 2005, 36, 1495–1506. [Google Scholar] [CrossRef]
- Santosh, R.; Das, G.; Kumar, S.; Singh, P.K.; Ghosh, M. Experimental and computational investigation of structural integrity of dissimilar metal weld between ferritic and austenitic steel. Metall. Mater. Trans. A 2018, 49, 2099–2112. [Google Scholar] [CrossRef]
- Chien, P.H. The Study of High-Power CO2 Laser Welding High-Silicon Steel Plate. Master’s Thesis, National Taiwan University, Taipei, Taiwan, June 2022. [Google Scholar]
- Okamoto, H.; Massalski, T.B. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Guiraldenq, P.; Duparc, O.H. The genesis of the Schaeffler diagram in the history of stainless steel. Metall. Res. Technol. 2017, 114, 613. [Google Scholar] [CrossRef]
- Kotecki, D.J.; Siewert, T.A. WRC-1992 constitution diagram for stainless-steel weld metals—A modification of the WRC-1988 diagram. Weld. J. 1992, 71, s171–s178. [Google Scholar]
- Ukumoto, S.; Fujiwara, K.; Toji, S.; Yamamoto, A. Small-scale resistance spot welding of austenitic stainless steels. Mater. Sci. Eng. A 2008, 492, 243–249. [Google Scholar] [CrossRef]
- Jaber, H.L.; Pouranvari, M.; Marashi, S.P.H.; Alizadeh-Sh, M.; Salim, R.K.; Hashim, F.A. Dissimilar spot welding of dual phase steel/ferritic stainless steel: Phase transformations. Sci. Technol. Weld. Join. 2014, 19, 565–571. [Google Scholar] [CrossRef]
- Kadoi, K.; Shinozaki, K. Effect of chemical composition on susceptibility to weld solidification cracking in austenitic weld metal. Metall. Mater. Trans. A 2017, 48, 5860–5869. [Google Scholar] [CrossRef]
- Brandi, S.D.; Schon, C.G. A Thermodynamic study of a constitutional diagram for duplex stainless steels. J. Phase Equilibria Diffus. 2017, 38, 268–275. [Google Scholar] [CrossRef]
- Vashishtha, H.; Taiwade, R.V.; Khatirkar, R.K.; Ingle, A.V.; Dayal, R.K. Welding behaviour of low nickel chrome manganese stainless steel. ISIJ Int. 2014, 54, 1361–1367. [Google Scholar] [CrossRef]
- Missori, S.; Koerber, C. Laser beam welding austenitic-ferritic transition joints. Weld. J. 1997, 76, s125–s134. [Google Scholar]
- Landowski, M.; Swierczynska, A.; Rogalski, G.; Fydrych, D. Autogenous fiber laser welding of 316L austenitic and 2304 lean duplex stainless steels. Materials 2020, 13, 2930. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.L.; Wu, S.K.; Liao, H.B.; Wang, X.Y. Microstructure and mechanical properties of CLF-1/316 L steel dissimilar joints welded with fiber laser welding. J. Manuf. Process. 2020, 54, 318–327. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, H.; Jin, X.; Liu, Z. Comparative study on the behavior of keyhole in analogy welding and real deep penetration laser welding. Materials 2022, 15, 9001. [Google Scholar] [CrossRef] [PubMed]
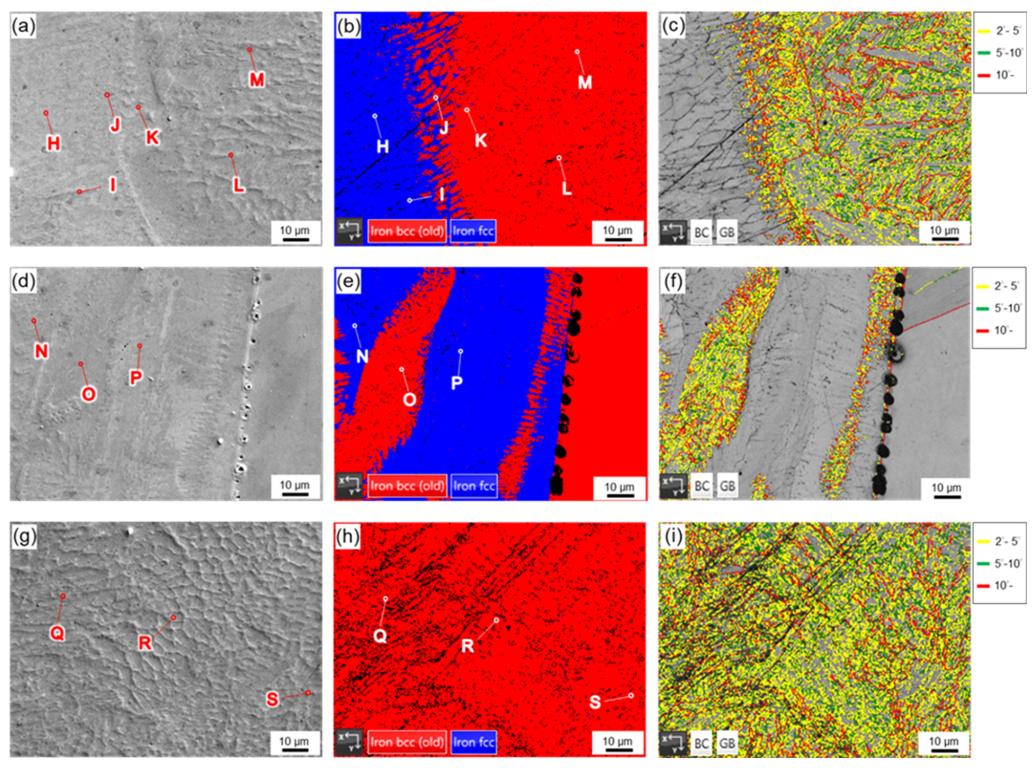
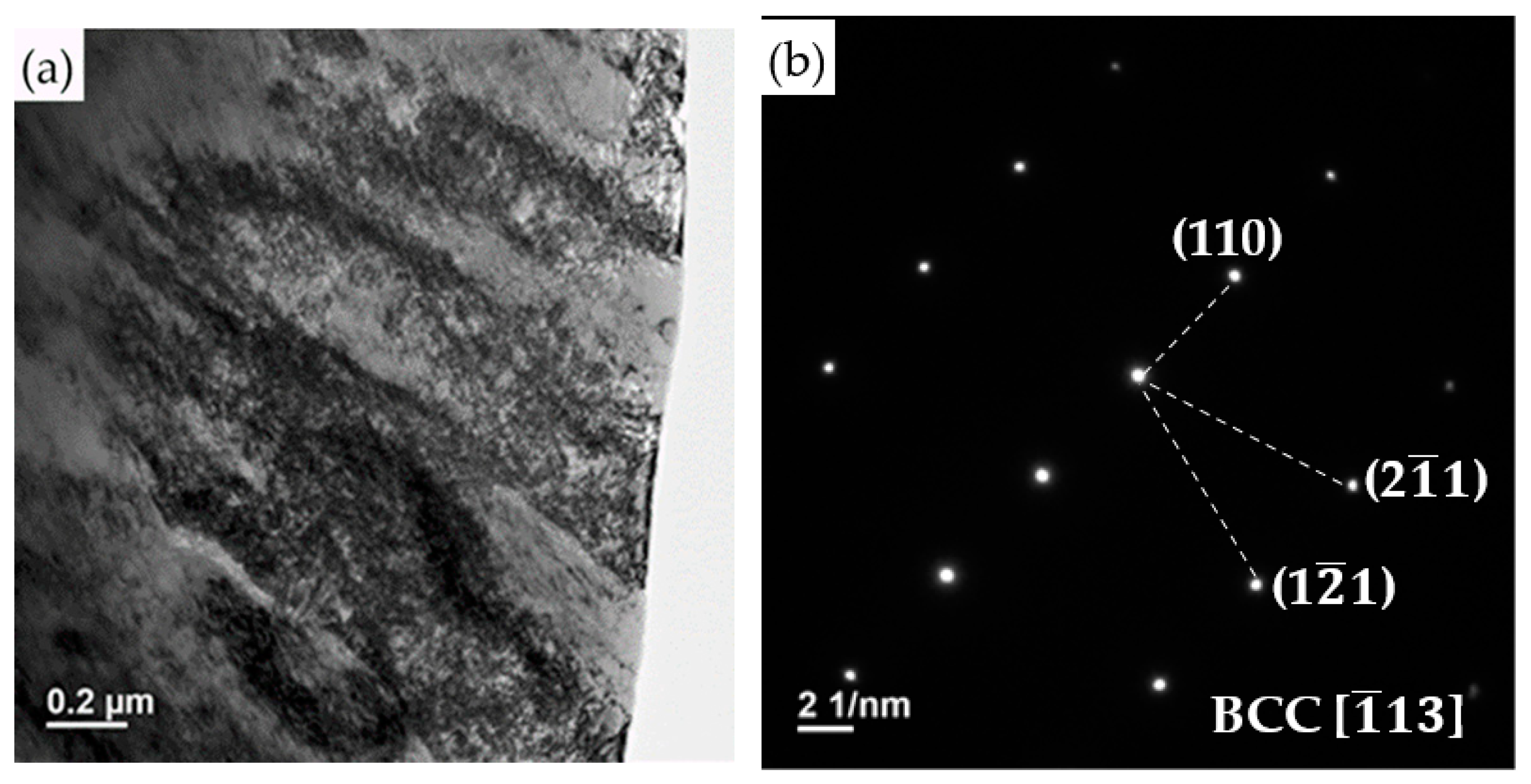
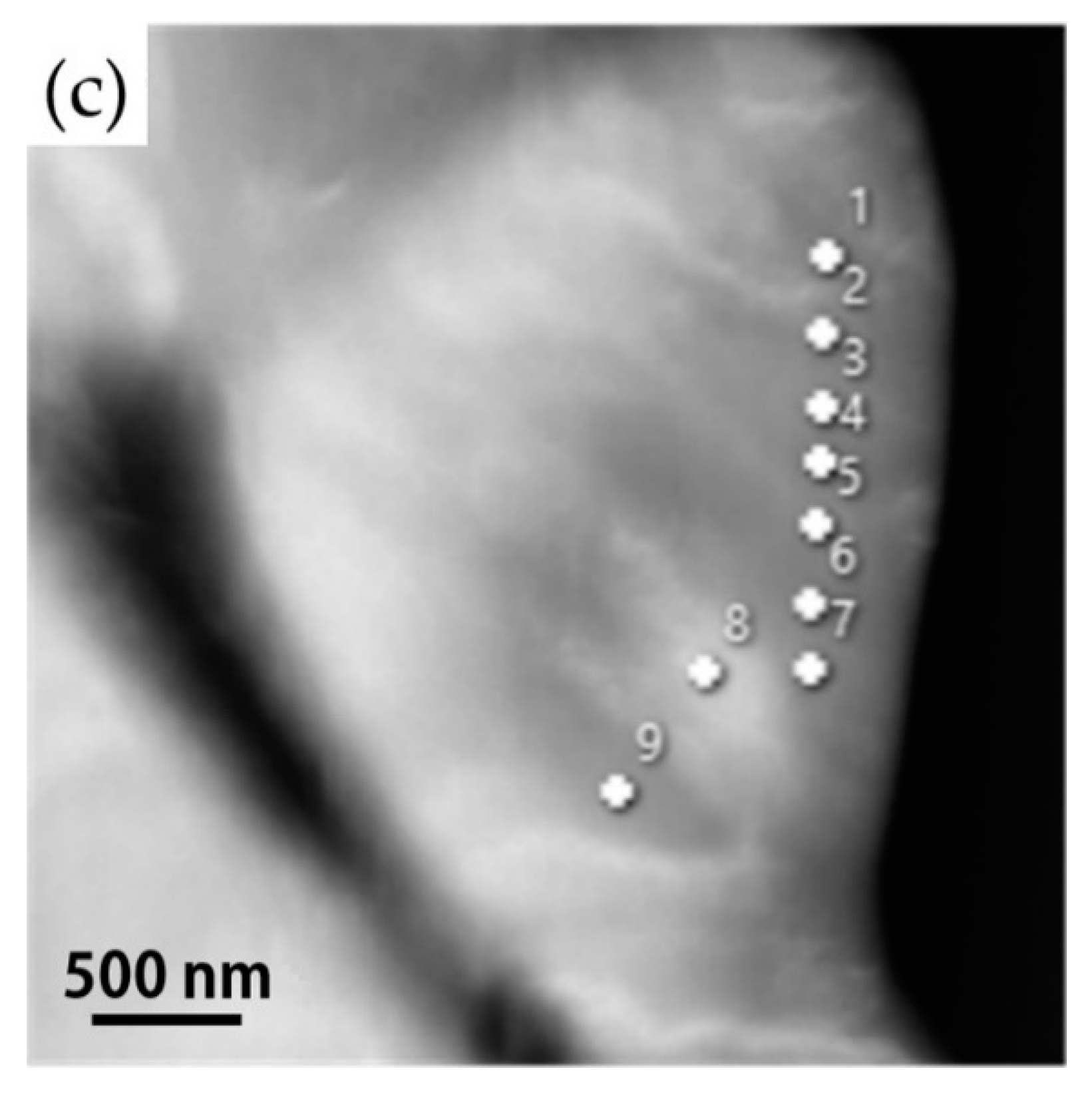

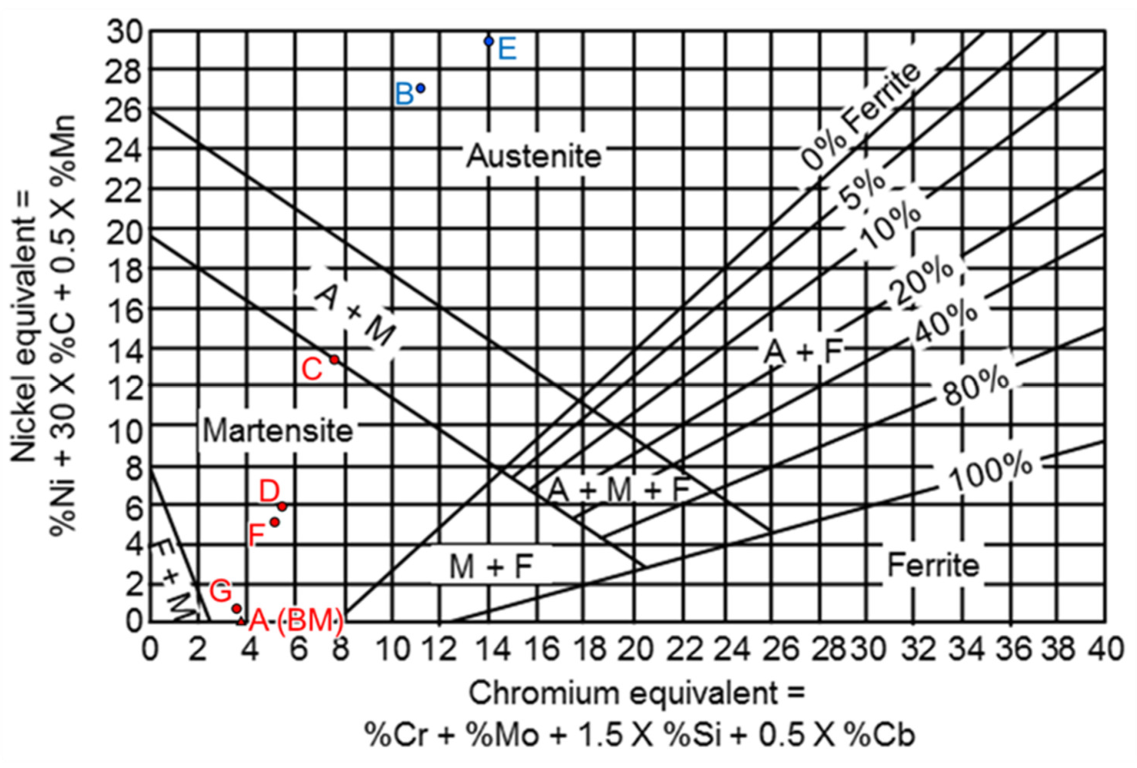
| Element/wt% | Al | Cr | Fe | Mn | Nb | Ni | Si | Phase/Structure |
|---|---|---|---|---|---|---|---|---|
| A | 0.5 | 0.0 | 96.7 | 0.1 | 0.0 | 0.0 | 2.6 | ferrite (BM) |
| B | 0.4 | 7.1 | 60.9 | 1.3 | 1.0 | 26.4 | 2.5 | austenite |
| C | 0.4 | 3.6 | 79.3 | 0.4 | 0.4 | 13.2 | 2.5 | BCC |
| D | 0.4 | 1.7 | 88.9 | 0.2 | 0.2 | 5.8 | 2.4 | BCC |
| E | 0.5 | 6.7 | 55.0 | 1.6 | 3.0 | 28.7 | 3.8 | austenite |
| F | 0.4 | 1.6 | 89.9 | 0.2 | 0.2 | 5.1 | 2.4 | BCC |
| G | 0.4 | 0.1 | 96.0 | 0.1 | 0.0 | 0.7 | 2.5 | ferrite (WB) |
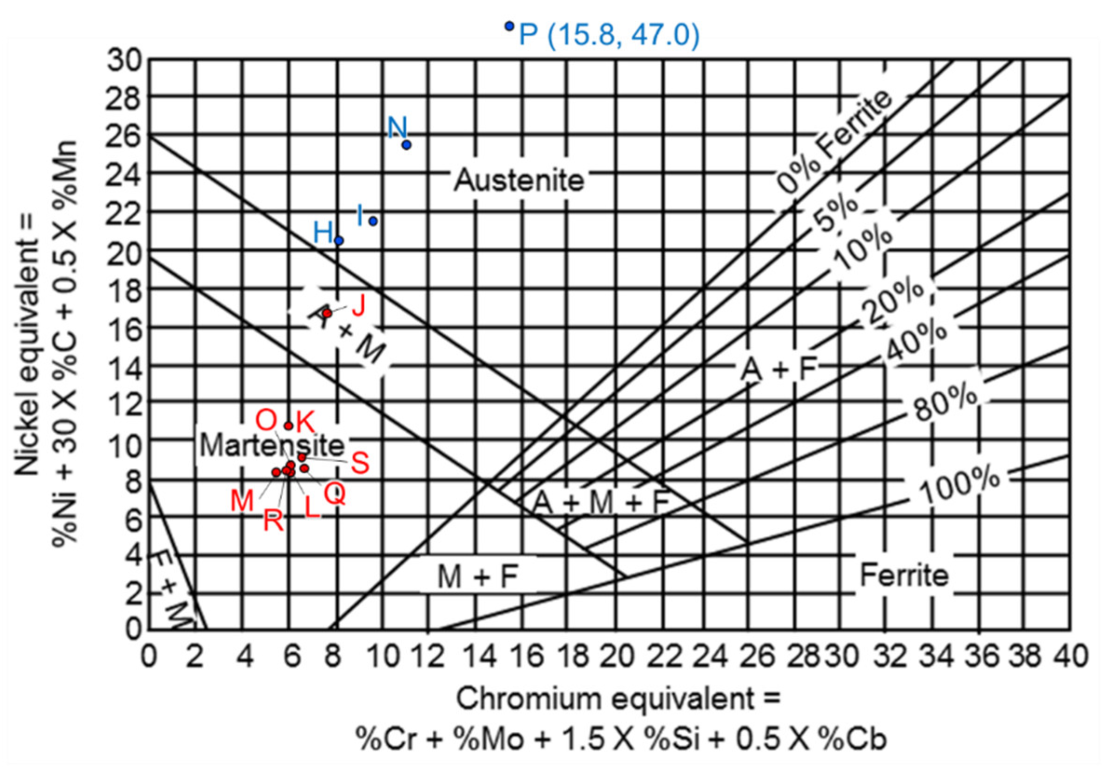
| Element/wt% | Al | Cr | Fe | Mn | Nb | Ni | Si | Phase/Structure |
|---|---|---|---|---|---|---|---|---|
| H | 0.3 | 5.9 | 71.3 | 0.4 | 0.2 | 20.1 | 1.3 | austenite |
| I | 0.3 | 6.3 | 68.7 | 0.6 | 0.4 | 21.2 | 2.1 | austenite |
| J | 0.3 | 5.0 | 75.6 | 0.5 | 0.2 | 16.4 | 1.7 | BCC |
| K | 0.3 | 3.3 | 83.3 | 0.3 | 0.1 | 10.7 | 1.8 | BCC |
| L | 0.4 | 2.4 | 85.8 | 0.2 | 0.3 | 8.2 | 2.4 | BCC |
| M | 0.4 | 2.0 | 88.5 | 0.2 | 0.2 | 6.2 | 2.3 | BCC |
| N | 0.4 | 7.0 | 62.1 | 1.3 | 1.5 | 24.9 | 2.2 | austenite |
| O | 0.4 | 2.6 | 85.6 | 0.4 | 0.3 | 8.4 | 2.2 | BCC |
| P | 0.2 | 13.1 | 34.8 | 2.4 | 1.5 | 45.8 | 1.3 | --- |
| Q | 0.4 | 2.6 | 85.2 | 0.3 | 0.3 | 8.3 | 2.5 | BCC |
| R | 0.4 | 2.7 | 85.9 | 0.2 | 0.2 | 8.3 | 2.0 | BCC |
| S | 0.4 | 2.7 | 84.6 | 0.3 | 0.2 | 9.0 | 2.4 | BCC |
| Element/wt% | Al | Cr | Fe | Mn | Nb | Ni | Si |
|---|---|---|---|---|---|---|---|
| 1 | 0.4 | 4.3 | 83.4 | 0.5 | 0.1 | 9.5 | 2.0 |
| 2 | 0.4 | 4.4 | 83.5 | 0.5 | 0.0 | 9.3 | 1.9 |
| 3 | 0.4 | 4.2 | 83.7 | 0.8 | 0.4 | 8.8 | 1.8 |
| 4 | 0.4 | 4.5 | 84.1 | 0.6 | 0.1 | 8.5 | 1.9 |
| 5 | 0.5 | 4.1 | 83.6 | 0.6 | 0.4 | 9.0 | 1.9 |
| 6 | 0.4 | 4.1 | 84.2 | 0.6 | 0.4 | 8.5 | 1.7 |
| 7 | 0.3 | 4.1 | 84.3 | 0.5 | 0.3 | 8.8 | 1.8 |
| 8 | 0.4 | 4.2 | 84.0 | 0.5 | 0.3 | 8.6 | 1.9 |
| 9 | 0.4 | 4.2 | 84.2 | 0.6 | 0.4 | 8.5 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, M.-F.; Chien, P.-H.; Lo, T.-Y.; Shiue, R.-K. Microstructure of Butt Joint of High-Silicon Steel Made Using CO2 Laser Welding and Inconel 82 Filler. Metals 2023, 13, 234. https://doi.org/10.3390/met13020234
Chiang M-F, Chien P-H, Lo T-Y, Shiue R-K. Microstructure of Butt Joint of High-Silicon Steel Made Using CO2 Laser Welding and Inconel 82 Filler. Metals. 2023; 13(2):234. https://doi.org/10.3390/met13020234
Chicago/Turabian StyleChiang, Ming-Feng, Ping-Hui Chien, Tzu-Yuan Lo, and Ren-Kae Shiue. 2023. "Microstructure of Butt Joint of High-Silicon Steel Made Using CO2 Laser Welding and Inconel 82 Filler" Metals 13, no. 2: 234. https://doi.org/10.3390/met13020234
APA StyleChiang, M.-F., Chien, P.-H., Lo, T.-Y., & Shiue, R.-K. (2023). Microstructure of Butt Joint of High-Silicon Steel Made Using CO2 Laser Welding and Inconel 82 Filler. Metals, 13(2), 234. https://doi.org/10.3390/met13020234