Abstract
In this study, the Taguchi method was used to investigate the effect of citric acid and malic acid on the leaching of SmCo magnet waste. First, we used a L16(45) orthogonal table to conduct experiments. Second, we conducted a factor effect analysis on the experimental results of the orthogonal table to understand the influence of temperature, acid concentration, solid–to–liquid ratio, time, and hydrogen peroxide on the leaching efficiency and we obtained the priority order of the factors affecting the leaching efficiency. The priorities of citric acid and malic acid were H2O2 > temperature > S/L > time > acid and temperature > time > S/L > H2O2 > acid, respectively. Finally, the optimal leaching parameters were obtained through confirmation experiments. For optimal leaching rates with citric acid, the amount of hydrogen peroxide (H2O2) added was 2.0 vol.%, the temperature was 90 °C, the S/L ratio was 5 g L−1, the time was 135 min and the acid concentration was 1.0 mol L−1, resulting in leaching efficiencies of Sm, Co, Fe, and Cu of 87.62, 93.82, 97.10, and 92.84%, respectively. For optimal leaching rates with malic acid, the temperature was 80 °C, the time was 75 min, the S/L ratio was 7.5 g L−1, the amount of hydrogen peroxide added was 3.5 vol.%, and the acid concentration was 1.5 mol L−1, resulting in leaching efficiencies of Sm, Co, Fe, and Cu of 75.18, 74.58, 82.42, and 1.35%, respectively.
1. Introduction
At present, the demand for rare earth elements is higher than the supply [1] and the supply of rare earth elements often depends on imports from China. We are concerned about China’s monopoly on rare earth elements [2]. Before the emergence of metals that could replace rare earth materials, recycling rare earth elements from waste was the best way to solve this problem [3,4,5,6,7].
Rare earth is often used in computer memory, DVDs, rechargeable batteries, autocatalytic converters, super magnets, mobile phones, LED lighting, superconductors, glass additives, fluorescent materials, phosphate binding agents, solar panels, and magnetic resonance imaging (MRI) agents [8]. The application range of rare earth is very wide and we have to pay attention to its importance.
Among the rare earth elements, Nd and Sm are often made into NdFeB magnets and SmCo magnets [9]. NdFeB magnets are currently the most magnetic [10] and they are often used in hard disks, mobile phones, earphones, etc. [11]. SmCo magnets are often used in the aviation field because their optimum operating temperature (250–350 °C) is higher than that of NdFeB magnets (60,220 °C) [12].
The research on the recovery of NdFeB magnets by hydrometallurgy is very extensive [13,14,15,16,17]. However, less research has been performed on the recycling of SmCo magnets. In the research on the leaching of SmCo magnets, most of the work is based on inorganic acid leaching. Sinha et al. [18] proposed leaching waste SmCo magnets with hydrochloric acid, nitric acid, and sulfuric acid under the conditions of a solid–to–liquid ratio of 100 g L−1, a time of 300 min, a concentration of 4 mol L−1, and a temperature of 95 °C. Hydrochloric acid leached about 100.0% of both Sm and Co, nitric acid leached about 100.0% of Sm and 50.0% of Co, and sulfuric acid leached about 5% of Sm and 55% of Co. Sahoo et al. [19] proposed leaching waste SmCo magnets with hydrochloric acid and sulfuric acid. When the solid–to–liquid ratio was 50 g L−1, the temperature was 80 °C, and the time was 60 min, the efficiencies of hydrochloric acid leaching of Sm and Co were 98.5 and 98.6%, respectively. When the solid–to–liquid ratio was 50 g L−1, the temperature was 80 °C, and the time was 60 min, the efficiencies of sulfuric acid leaching of Sm and Co were 97.5% and 95.6%, respectively. Zhou et al. [20] proposed leaching waste SmCo magnets with sulfuric acid. When the liquid–to–solid ratio was 6 mL g−1, the temperature was 60 °C, and the time was 75 min, the leaching efficiency of Sm and Co by sulfuric acid was 24.1% and 99.9%, respectively. However, the effect of organic acids on leaching SmCo magnets compared with inorganic acids has not been investigated. Moreover, the above studies [18,19,20] only discussed the leaching efficiency of Sm and Co by acid solution and they did not discuss all the metals in the actual waste.
To investigate the effect of organic acids on the leaching of SmCo magnets, we referred to the study of Ji et al. [21]. We found that citric acid and malic acid have the potential to leach waste magnets. Therefore, citric acid and malic acid were chosen for this leaching study. In addition, this study used the Taguchi method to find the optimal leaching parameters. The Taguchi method explores the prioritization of the effects of temperature, the solid-to-liquid ratio, acid concentration, time, and hydrogen peroxide added to the leaching solution. Finally, we successfully obtained an acid solution that can effectively leach SmCo magnets.
2. Materials and Methods
2.1. Materials
The SmCo magnet scrap came from Tai Chuan Metal Co., Ltd. (Taipei, Taiwan) and the scrap was rich in SmCo5 and Sm2Co17. The composition of the SmCo magnet waste is shown in Table 1.

Table 1.
Metal contents in spent SmCo magnets.
Nitric acid (HNO3, 70%) and hydrochloric acid (HCl, 37%) for aqua regia digestion, citric acid monohydrate (99.8%), DL-malic acid (99%), and hydrogen peroxide (H2O2, 30%) for leaching were purchased from Shin-Shin Chemical Co. (Tainan, Taiwan).
2.2. Equipment
In order to control the leaching temperature and stirring rate, the leaching process was carried out in constant-temperature water stirring tank (Shin-Kwang Precision Industry Ltd., New Taipei, Taiwan). After the leaching experiment was completed, the solid–liquid separation was carried out with a filter device (Chemker 300, Rone Scientific Co., Ltd., New Taipei, Taiwan). Afterwards, the concentration of metals in the solution was analyzed using an atomic absorption spectrometer (AAS, PinAAcle 900F AA Spectrometer, PerkinElmer Inc., Waltham, MA, USA).
2.3. Experimental Procedures
Firstly, the SmCo magnet wastes were dried in an oven at 70 °C for 7 days to obtain dry waste. Subsequently, the waste was sieved through a 100-mesh sieve to remove the magnets that accidentally fell during the process.
Second, we confirmed the waste composition by digestion with aqua regia. In a 250 mL Erlenmeyer flask, we added 10 mL of nitric acid, 30 mL of hydrochloric acid, and 1 g of SmCo magnet waste. Next, the Erlenmeyer flask was placed in a constant temperature water tank at 90 °C for 1 day to ensure complete dissolution of the metal.
Third, we conducted leaching experiments. We added 100 mL of acid solution (deionized water and the required amount of acid), SmCo magnet powder, and hydrogen peroxide into a 250 mL Erlenmeyer flask and we used a constant temperature water bath to control the experimental temperature.
2.4. Experimental Method
In this study, a L16(45) orthogonal table was used to conduct organic acid leaching studies. The selected control factors and levels were temperature (60–90 °C), solid–to–liquid ratio (5–20 g L−1), time (30–120 min), acid concentration (0.5–2 mol L−1), and hydrogen peroxide addition (0–3 vol.%), as shown in Table 2. The results of the orthogonal table experiments will be used to analyze and understand the influence of each parameter on the leaching efficiency within the selected factors.

Table 2.
Control factors and selection levels.
Then, we used factor effect analysis to understand the prioritization of each control factor on the leaching effect. In order to find the optimal parameters, we performed experiments according to the order of each factor’s influence on the leaching efficiency.
Finally, the optimal leaching parameters were obtained through confirmation experiments. The leaching flow chart is shown in Figure 1.
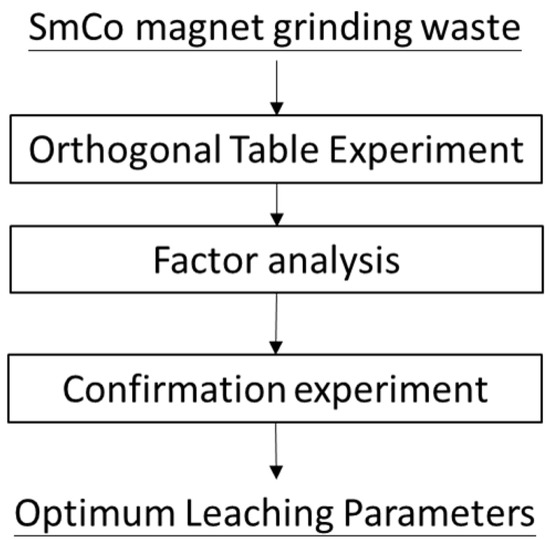
Figure 1.
Leaching flow chart.
3. Results
3.1. Orthogonal Experiment
In this part, a L16(45) orthogonal table was used to conduct leaching experiments to obtain the leaching rates of Sm, Co, Fe, and Cu at different temperatures; the solid–to–liquid ratios; time; acid concentrations; and hydrogen peroxide additions. The citric acid and malic acid leaching efficiencies of Sm, Co, Fe, and Cu under different parameters are shown in Table 3 and Table 4, respectively.

Table 3.
Citric acid leaching rates of Sm, Co, Fe, and Cu under different control factors.

Table 4.
Malic acid leaching rates of Sm, Co, Fe, and Cu under different control factors.
3.2. Factor Analysis
We used the results of orthogonal table experiments to conduct factor effect analysis to understand the priority of each factor on the leaching efficiency. The citric acid leaching efficiency of Sm at temperature K1 in Table 5 represents the average leaching efficiency of Sm at a temperature of 60 °C. The extreme deviation was the difference between the best and worst leaching rates of metals under K1 to K4 conditions. The priority order used extreme deviation to understand the priority of factors that affect metal leaching.

Table 5.
Factor response table for citric acid.
The factor response table of citric acid is shown in Table 5. The order of influence on Sm leaching efficiency was time > H2O2 > temperature > S/L > acid. The order of influence on Co leaching efficiency was H2O2 > temperature > time > acid > S/L. The order of influence on Fe leaching efficiency was H2O2 > temperature > time > acid > S/L. The order of influence on Cu leaching efficiency was S/L > temperature > H2O2 > time > acid.
The factor response table of malic acid is shown in Table 6. The order of influence on Sm leaching efficiency was temperature > time > S/L > acid > H2O2. The order of influence on Co leaching efficiency was temperature > time > S/L > H2O2 > acid. The order of influence on Fe leaching efficiency was time > temperature > S/L > H2O2 > acid. The order of influence on Cu leaching efficiency was time > S/L > temperature > acid > H2O2.

Table 6.
Factor response table for malic acid.
The metals were affected differently by the temperature, solid–to–liquid ratio, time, acid concentration, and hydrogen peroxide addition, so it was impossible to directly know the priority order of each parameter’s influence on the leaching rate. Therefore, we combined the K1, K2, K3, K4, and extreme deviation of the four metals to explore the influence of each parameter on the leaching efficiency.
The citric acid and malic acid results are shown in Table 7 and Table 8, respectively. The order of priority of factors affecting leaching efficiency with citric acid was H2O2 > temperature > S/L > time > acid. The addition of hydrogen peroxide was 3 vol.%, the temperature was 70 °C, the solid–to–liquid ratio was 5 g L−1, the time was 120 min, and the acid concentration was 1 mol L−1, which were the preliminary optimal parameters.

Table 7.
Factor response table for four metals in citric acid.

Table 8.
Factor response table for four metals in malic acid.
The order of priority of factors affecting leaching efficiency with malic acid was temperature > time > S/L > H2O2 > acid. The temperature was 90 °C, the time was 120 min, the solid–to–liquid ratio was 5 g L−1, the addition of hydrogen peroxide was 3 vol.%, and the acid concentration was 1.5 mol L−1, which were the preliminary optimal parameters.
3.3. Confirmation Experiments
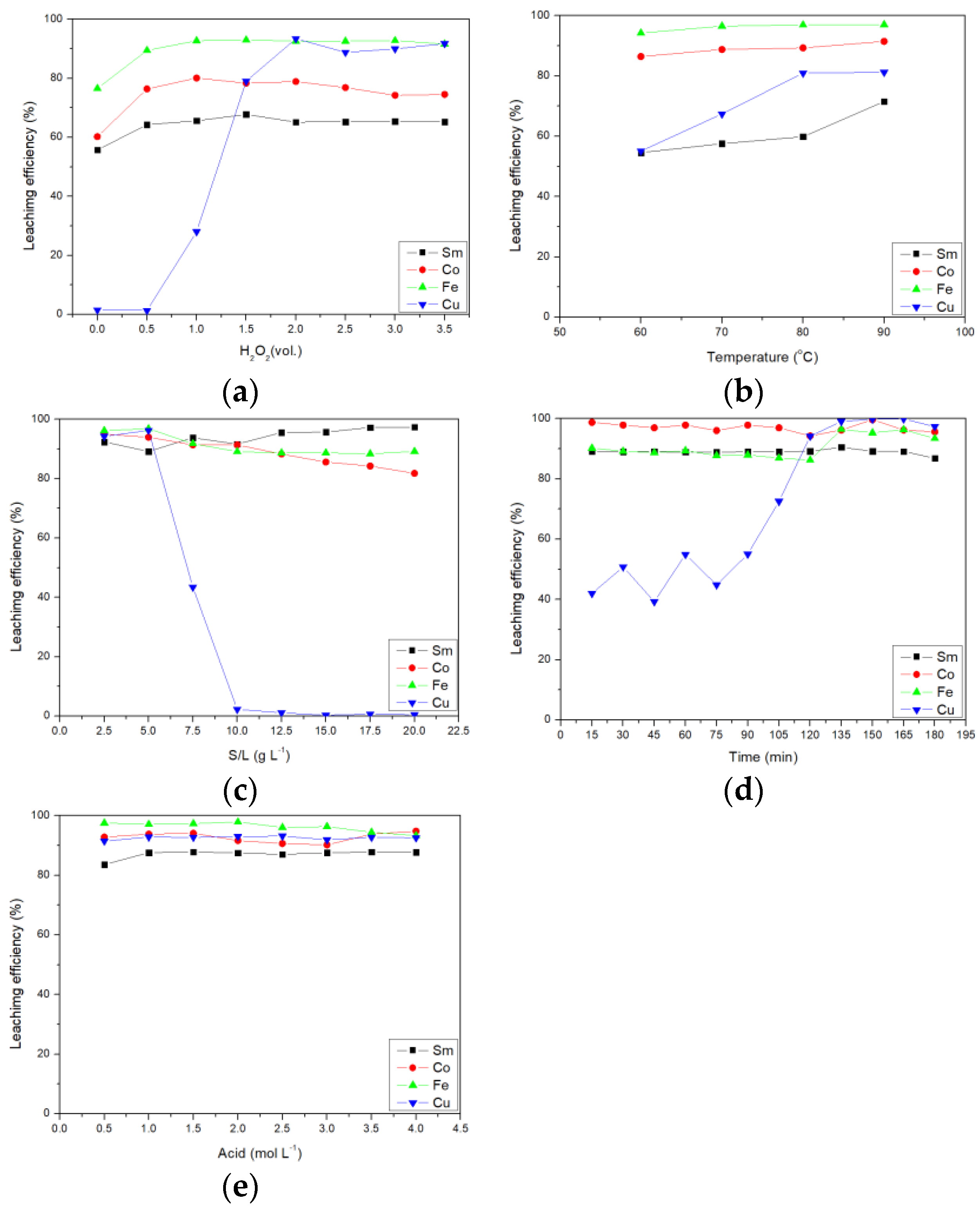
After the preliminary optimal parameters were found, the true optimal parameters were through confirmation experiments. The first part was the effect of the amount of hydrogen peroxide on the leaching efficiency of citric acid and the results are shown in Figure 2a. When no hydrogen peroxide was added, the leaching efficiency of the four metals was less than 80%. When increasing the amount of hydrogen peroxide added to 2.0 vol.%, the leaching efficiencies of Sm, Co, Fe, and Cu increased to 65.18, 78.92, 92.62, and 93.43%, respectively. This result was consistent with the above factor analysis results and hydrogen peroxide was the most important parameter affecting leaching. Hydrogen peroxide can oxidize the metal in the SmCo magnet, so it can improve the leaching efficiency of the metals.
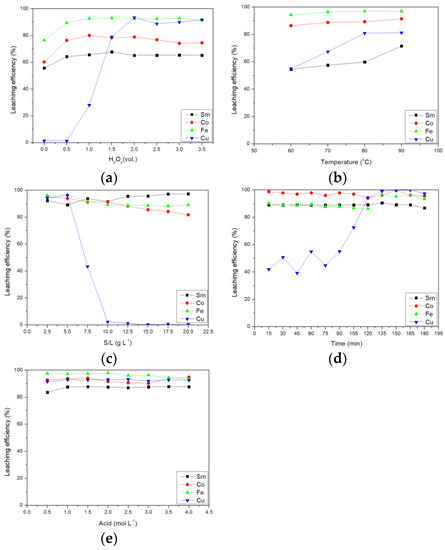
Figure 2.
Effect of (a) amount of H2O2, (b) temperature, (c) S/L ratio, (d) time, and (e) acid concentration on the leaching efficiency of citric acid.
The second part was the effect of temperature on the leaching efficiency of citric acid. From Figure 2b, it can be seen that the leaching efficiency of Fe and Co did not increase significantly with the increase in temperature. For Sm and Cu, when the temperature was increased from 60 °C to 90 °C, the leaching efficiency was increased by more than 15%. Finally, the leaching efficiencies of Sm, Co, Fe, and Cu were 71.54, 91.49, 97.02, and 81.23%, respectively.
The third part was the effect of the solid–to–liquid ratio on the leaching efficiency of citric acid. It can be seen from Figure 2c that the leaching efficiency of Sm, Co, and Fe was not affected by the solid–to–liquid ratio, but the solid–to–liquid ratio seriously affected the leaching efficiency of Cu. When the solid–to–liquid ratio increased to 10 g L−1, Cu was hardly leached. This result was consistent with the result of factor analysis and the most important factor affecting Cu leaching was the solid–toliquid ratio. In addition, if you conduct separation research, you could consider increasing the solid–to–liquid ratio to keep the Cu in the solid phase.
The fourth part was the influence of time on the leaching efficiency of citric acid and the results are shown in Figure 2d. The Sm, Co, and Fe reacted faster than Cu and the leaching was complete in 15 min. The Cu required 135 min to complete leaching.
The fifth part was the effect of acid concentration on the leaching efficiency of citric acid and the results are shown in Figure 2e. The increase in acid concentration did not affect the leaching efficiency of Sm, Co, Fe, and Cu, so acid concentration had little effect on leaching efficiency.
Finally, when the addition of hydrogen peroxide was 2.0 vol.%, the temperature was 90 °C, the S/L ratio was 5 g L−1, the time was 135 min, and the acid concentration was 1.0 mol L−1, the citric acid Sm, Co, Fe, and Cu leaching efficiencies were 87.62, 93.82, 97.10, and 92.84%, respectively.
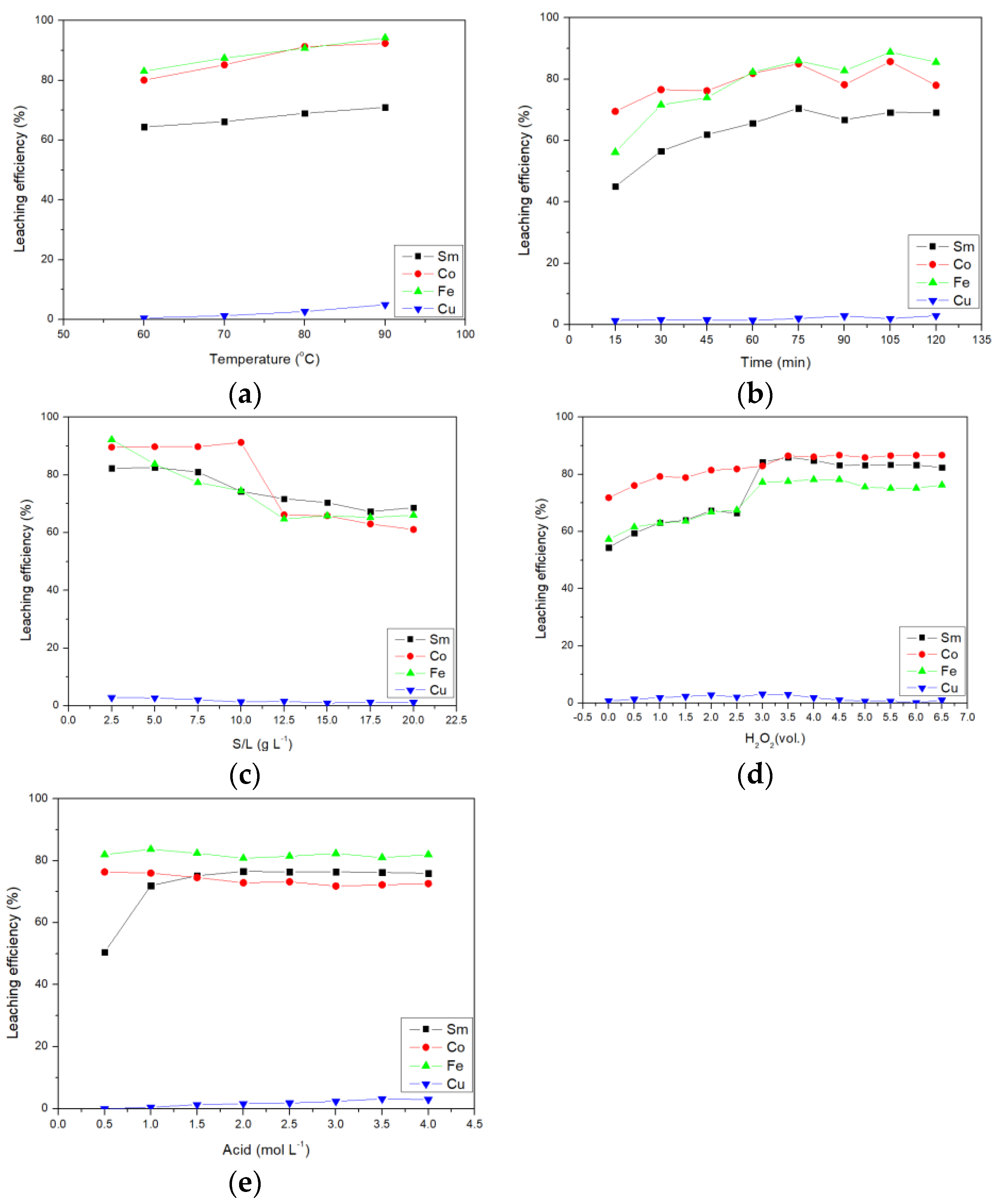
This section shows the results of the confirmation experiment of malic acid. The first effect studied was the effect of temperature on the leaching efficiency of malic acid. From Figure 3a, it can be seen that although the temperature was the most influential factor in leaching, the Cu leaching rate was below 5%. Therefore, Cu cannot be leached by malic acid. Subsequent experiments only considered the influence of other metals on the leaching efficiency.
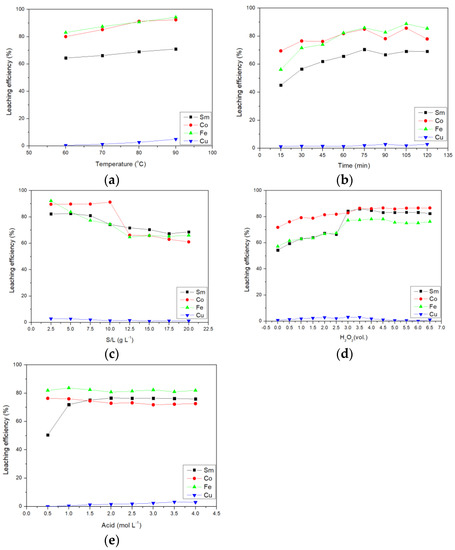
Figure 3.
Effect of (a) temperature, (b) time, (c) S/L ratio, (d) amount of H2O2, and (e) acid concentration on leaching efficiency of malic acid.
The second was the effect of time on malic acid leaching. It can be seen from Figure 3b that the leaching efficiency of malic acid was relatively slow. It took 75 min to leach 70.53, 85.02, 85.96, and 2.02% of Sm, Co, Fe, and Cu, respectively.
The third was the effect of the solid–to–liquid ratio on malic acid leaching. The result is shown in Figure 3c. Until the solid–to–liquid ratio was 7.5 g L−1, the leaching efficiency of valuable Sm and Co did not decrease significantly and the leaching efficiency was still more than 80%. Therefore, a solid–to–liquid ratio of 7.5 g L−1 was selected as the optimal parameter.
The fourth was the effect of the amount of hydrogen peroxide added on the malic acid leaching. The result is shown in Figure 3d. When the amount of hydrogen peroxide added was 3.0 vol.%, the leaching efficiency of Sm and Fe was significantly improved. If hydrogen peroxide was added again, the leaching rate did not increase significantly. Therefore, the amount of hydrogen peroxide added of 3.0 vol.% was selected as the best parameter.
The fifth was the effect of acid concentration on malic acid leaching. It was shown in Figure 3e that the acid concentration had little effect on the leaching efficiency. Only the leaching efficiency of Sm was affected.
Finally, when the temperature was 80 °C, the time was 75 min, the S/L ratio was 7.5 g L−1, the addition of hydrogen peroxide was 3.5 vol.%, and the acid concentration was 1.5 mol L−1, the leaching efficiencies of Sm, Co, Fe, and Cu were 75.18, 74.58, 82.42, and 1.35%, respectively.
4. Discussion
4.1. Comparison of Organic Acid Leaching
In a previous study [18,19,20], we found that inorganic acid was effective in leaching waste magnets. Notably, strong acid leachates released toxic gases such as Cl2, SO3, and NOx and the waste acid solution was harmful to the environment [22]. In order to avoid the negative impact of magnet recycling on the environment, we developed a more benign processes.
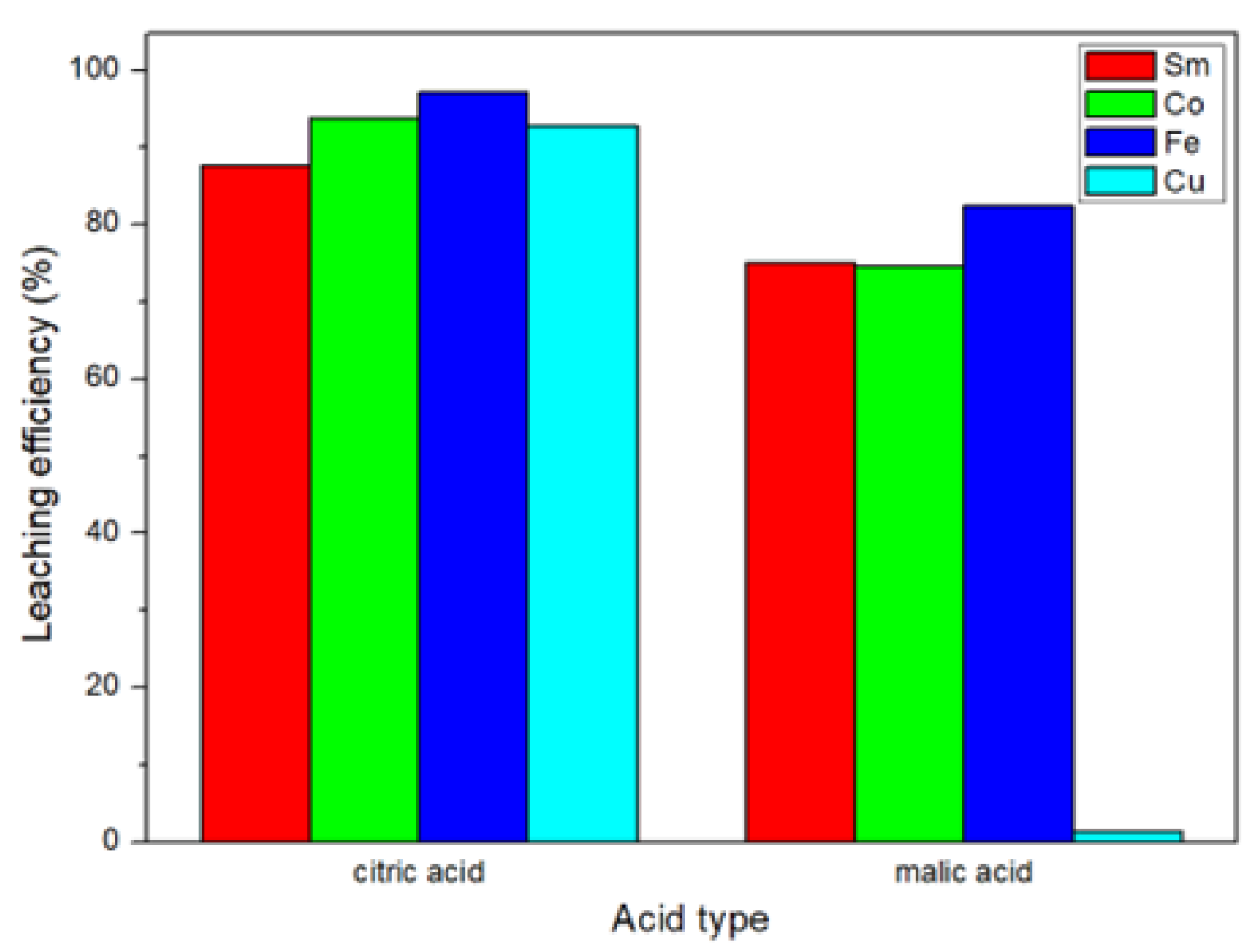
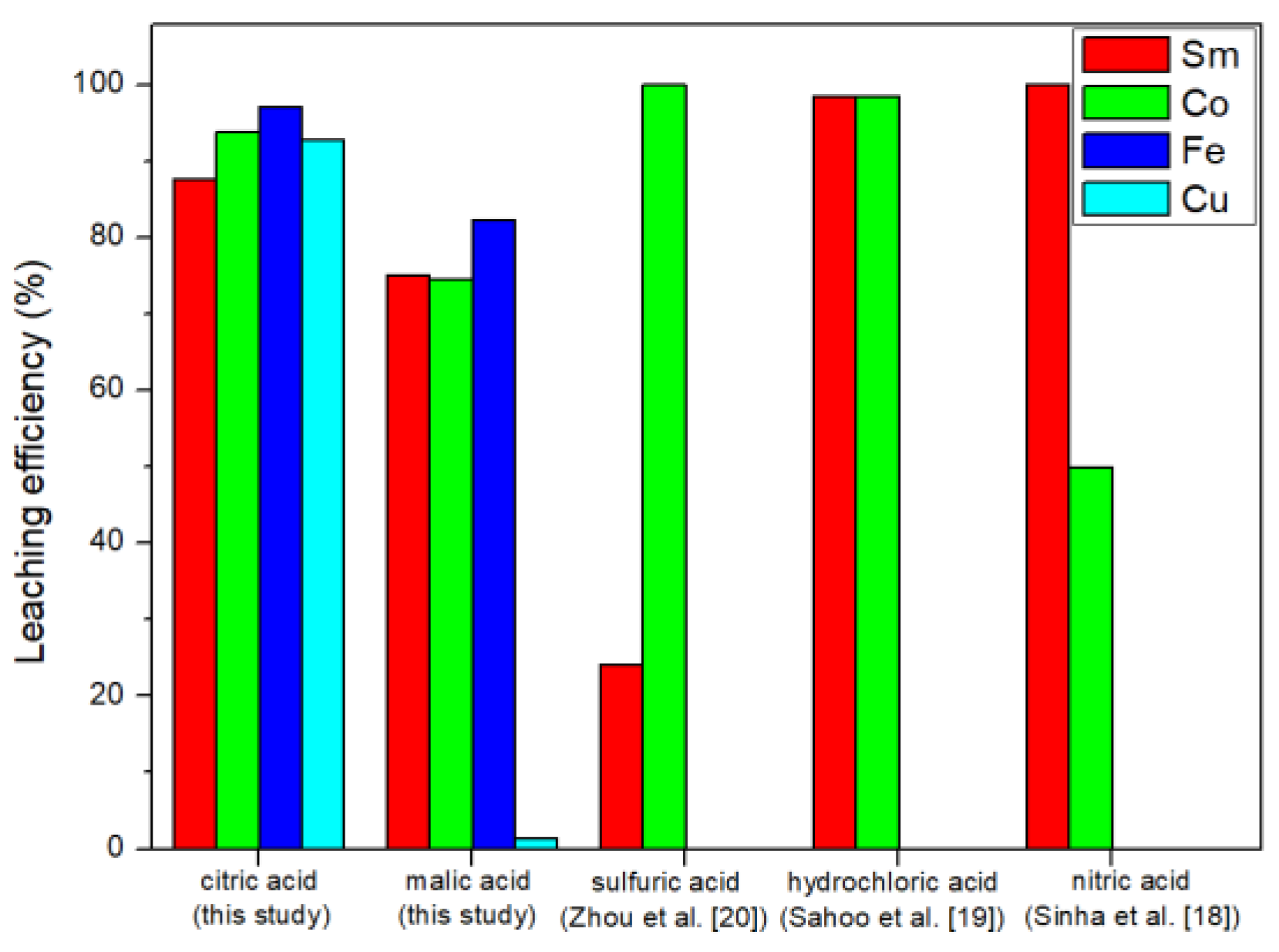
The results of this study are shown in Figure 4. We found that the leaching efficiency of citric acid was better than the leaching efficiency of malic acid. This result was the same as the study of Ji et al. [21]. The Ka (acid dissociation constant) value of citric acid was higher than that of malic acid [23,24]; therefore, it is more capable of leaching metals.
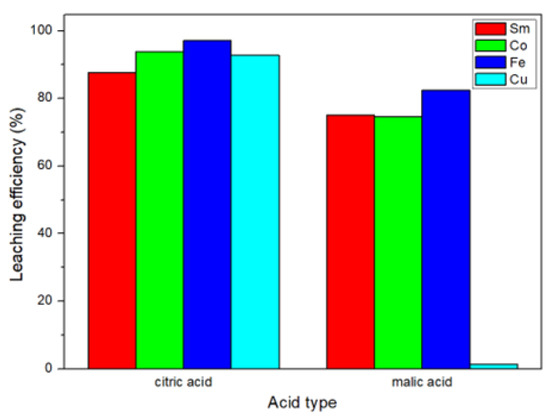
Figure 4.
Comparison of leaching efficiencies of organic acids.
According to the research of [18,19,20], it is known that acid concentration, solid–to–liquid ratio, time, and temperature have an impact on the leaching efficiency. However, these works did not explore the effect of oxidant addition (H2O2) on the leaching effect. In this study, we can see from Figure 2a and Figure 3d that the leaching rate of each metal increased significantly when the oxidant was added. Hydrogen peroxide was beneficial to the oxidation of metals and promoted the leaching process [25].
In the study of Cu leaching with malic acid, the Cu leaching rate was zero when the redox potential (Eh) of copper was low [26]. Therefore, we needed to add more oxidizers for effective leaching. If we added more oxidizers, it would definitely increase the cost. We do not recommend malic acid for leaching. Ultimately, we found that citric acid was able to leach SmCo magnets.
4.2. Leaching Efficiency of Comparison
The leaching results of inorganic acid and organic acid are shown in Figure 5. Sulfuric acid was not recommended for leaching experiments because it was easy to form samarium sulfate precipitates with Sm [20]. Hydrochloric acid and nitric acid are more commonly used in leaching research. Hydrochloric acid can leach about 98.5% of Sm and 98.6% of Co [19], and nitric acid can leach about 99.99% of Sm and 50.00% of Co [18]. Compared with the leaching efficiency of inorganic acid [18,19,20], citric acid Sm, Co, Fe, and Cu leaching efficiencies were 87.62, 93.82, 97.10, and 92.84%, respectively. Therefore, the citric acid leaching of SmCo magnets was competitive. In addition, this study also explored the leaching efficiency of citric acid on Fe and Cu and we obtained good results.
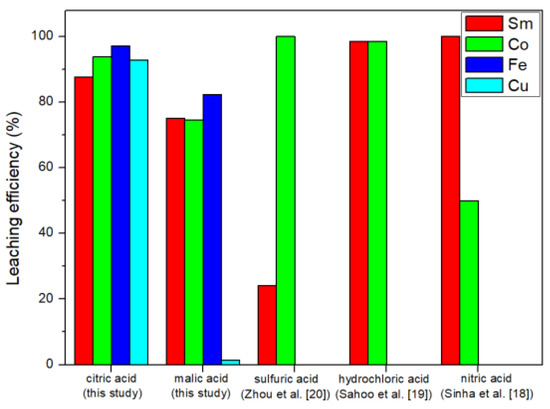
Figure 5.
Comparison of leaching efficiencies.
5. Conclusions
In this study, the Taguchi method was used to investigate the effect of organic acids on the leaching of SmCo magnet waste. Under optimal conditions, citric acid leached 87.62, 93.82, 97.10, and 92.84% of Sm, Co, Fe, and Cu, respectively. Malic acid leached 75.18, 74.58, 82.42, and 1.35 % of Sm, Co, Fe, and Cu, respectively. The leaching efficiency of malic acid was poor. However, the leaching efficiency of citric acid was comparable to the leaching efficiency of inorganic acids and therefore, citric acid can replace inorganic acids for leaching.
This study investigated the effect of various parameters on the leaching of organic acids. We obtained optimal conditions for citric acid and malic acid leaching. Furthermore, we proposed an organic acid solution for efficient leaching.
Author Contributions
Conceptualization, Y.-C.T. and J.-Z.W.; methodology, Y.-C.T. and J.-Z.W.; validation, Y.-C.T. and J.-Z.W.; investigation, Y.-C.T. and J.-Z.W.; resources, Y.-C.T. and J.-Z.W.; data curation, Y.-C.T. and J.-Z.W.; writing—original draft preparation, Y.-C.T. and J.-Z.W.; writing—review and editing, Y.-C.T. and J.-Z.W.; supervision, Y.-H.S.; project administration, Y.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to acknowledge the support of National Cheng Kung University’s Department of Resources Engineering and the Ministry of Science and Technology, R.O.C.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Makarova, I.; Soboleva, E.; Osipenko, M.; Kurilo, I.; Laatikainen, M.; Repo, E. Electrochemical leaching of rare-earth elements from spent NdFeB magnets. Hydrometallurgy 2020, 192, 105264. [Google Scholar] [CrossRef]
- Orefice, M.; Binnemans, K. Solvometallurgical process for the recovery of rare-earth elements from Nd–Fe–B magnets. Sep. Purif. Technol. 2021, 258, 117800. [Google Scholar] [CrossRef]
- Chen, Q.; Ni, S.; Ai, G.; Zhang, T.; Sun, X. A recovery strategy of Sm, Co for waste SmCo magnets by fatty acid based ionic liquids. Miner. Eng. 2020, 158, 106581. [Google Scholar] [CrossRef]
- Mishra, B.B.; Devi, N. D2EHPA is a potential extractant for extraction of europium and samarium from chloride medium. Mater. Today: Proc. 2020, 30, 254–257. [Google Scholar] [CrossRef]
- Onoda, H.; Kurioka, Y. Recovery of samarium from cobalt-samarium solution using phosphoric acid. J. Environ. Chem. Eng. 2015, 3, 2825–2828. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S.; Acharya, M.R. Hydrometallurgical route for recovery and separation of samarium (III) and cobalt (II) from simulated waste solution using tri-n-octyl phosphine oxide—A novel pathway for synthesis of samarium and cobalt oxides nanoparticles. J. Alloy. Compd. 2020, 815, 152423. [Google Scholar] [CrossRef]
- Su, X.; Wang, Y.; Guo, X.; Dong, Y.; Gao, Y.; Sun, X. Recovery of Sm(III), Co(II) and Cu(II) from waste SmCo magnet by ionic liquid-based selective precipitation process. Waste Manag. 2018, 78, 992–1000. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Patil, A.S.; Patil, A.V.; Dighavkar, C.G.; Adole, V.A.; Tupe, U.J. Synthesis techniques and applications of rare earth metal oxides semiconductors: A review. Chem. Phys. Lett. 2022, 796, 139555. [Google Scholar] [CrossRef]
- Severson, M.H.; Nguyen, R.; Ormerod, J.; Palasyuk, A.; Cui, J. A preliminary feasibility study of potential market applications for non-commercial technology magnets. Heliyon 2022, 8, 11773. [Google Scholar] [CrossRef]
- Nababan, D.C.; Mukhlis, R.; Durandet, Y.; Pownceby, M.I.; Prentice, L.; Rhamdhani, M.A. Mechanism and microstructure evolution of high temperature oxidation of end-of-life NdFeB rare earth permanent magnets. Corros. Sci. 2021, 182, 109290. [Google Scholar] [CrossRef]
- Coey, J.M.D. Perspective and prospects for rare earth permanent magnets. Engineering 2020, 6, 119–131. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, M.K.; Pramanik, S.; Sahu, S.K. Recovery of rare earths from spent NdFeB magnets of wind turbine: Leaching and kinetic aspects. Waste Manag. 2018, 75, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chen, Y.J.; Liao, C.H.; Popuri, S.R.; Tsai, S.L.; Hung, C.E. Selective leaching process for neodymium recovery from scrap Nd-Fe-B magnet. Metall. Mater. Trans. A 2013, 44, 5825–5833. [Google Scholar] [CrossRef]
- Niam, A.C.; Wang, Y.F.; Chen, S.W.; You, S.J. Recovery of rare earth elements from waste permanent magnet (WPMs) via selective leaching using the Taguchi method. J. Taiwan Inst. Chem. Eng. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- Parhi, P.K.; Sethy, T.R.; Rout, P.C.; Sarangi, K. Separation and recovery of neodymium and praseodymium from permanent magnet scrap through the hydrometallurgical route. Sep. Sci. Technol. 2016, 51, 2232–2241. [Google Scholar] [CrossRef]
- Yoon, H.S.; Kim, C.J.; Chung, K.W.; Jeon, S.; Park, I.; Yoo, K.; Jha, M.K. The effect of grinding and roasting conditions on the selective leaching of Nd and Dy from NdFeB magnet scraps. Metals 2015, 5, 1306–1314. [Google Scholar] [CrossRef]
- Sinha, M.K.; Pramanik, S.; Kumar, A.; Sahu, S.K.; Prasad, L.B.; Jha, M.K.; Yoo, K.; Pandey, B.D. Recovery of value added products of Sm and Co from waste SmCo magnet by hydrometallurgical route. Sep. Purif. Technol. 2017, 179, 1–12. [Google Scholar] [CrossRef]
- Sahoo, K.; Nayak, A.K.; Ghosh, M.K.; Sarangi, K. Preparation of Sm2O3 and Co3O4 from SmCo magnet swarf by hydrometallurgical processing in chloride media. J. Rare Earths 2018, 36, 725–732. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, A.; Zhang, D.; Zhang, X.; Yang, T. Sulfuric acid leaching of SmCo alloy waste and separation of samarium from cobalt. Hydrometallurgy 2017, 174, 66–70. [Google Scholar] [CrossRef]
- Ji, B.; Li, Q.; Zhang, W. Leaching recovery of rare earth elements from the calcination product of a coal coarse refuse using organic acids. J. Rare Earths 2022, 40, 318–327. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Schuster, J.; Ebin, B. Investigation of indium and other valuable metals leaching from unground waste LCD screens by organic and inorganic acid leaching. Sep. Purif. Technol. 2021, 279, 119659. [Google Scholar] [CrossRef]
- Hussaini, S.; Kursunoglu, S.; Top, S.; Ichlas, Z.T.; Kaya, M. Testing of 17-different leaching agents for the recovery of zinc from a carbonate-type Pb-Zn ore flotation tailing. Miner. Eng. 2021, 168, 106935. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, K.; Cao, Z.; Wang, H.; Liu, S.; Waters, K.E.; Ma, H. Pressure leaching behaviors of copper-cobalt sulfide concentrate from Congo. Sep. Purif. Technol. 2023, 309, 123010. [Google Scholar] [CrossRef]
- Feng, Q.; Tang, W.; Wen, S.; Wang, H.; Zhao, W.; Han, G. Flotation of copper oxide minerals: A review. Int. J. Min. Sci. Technol. 2022, 32, 1351–1364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).