Recent Trends in Noble Metal Nanoparticles for Colorimetric Chemical Sensing and Micro-Electronic Packaging Applications
Abstract
1. Introduction
2. Surface Charge Determination on the NPS
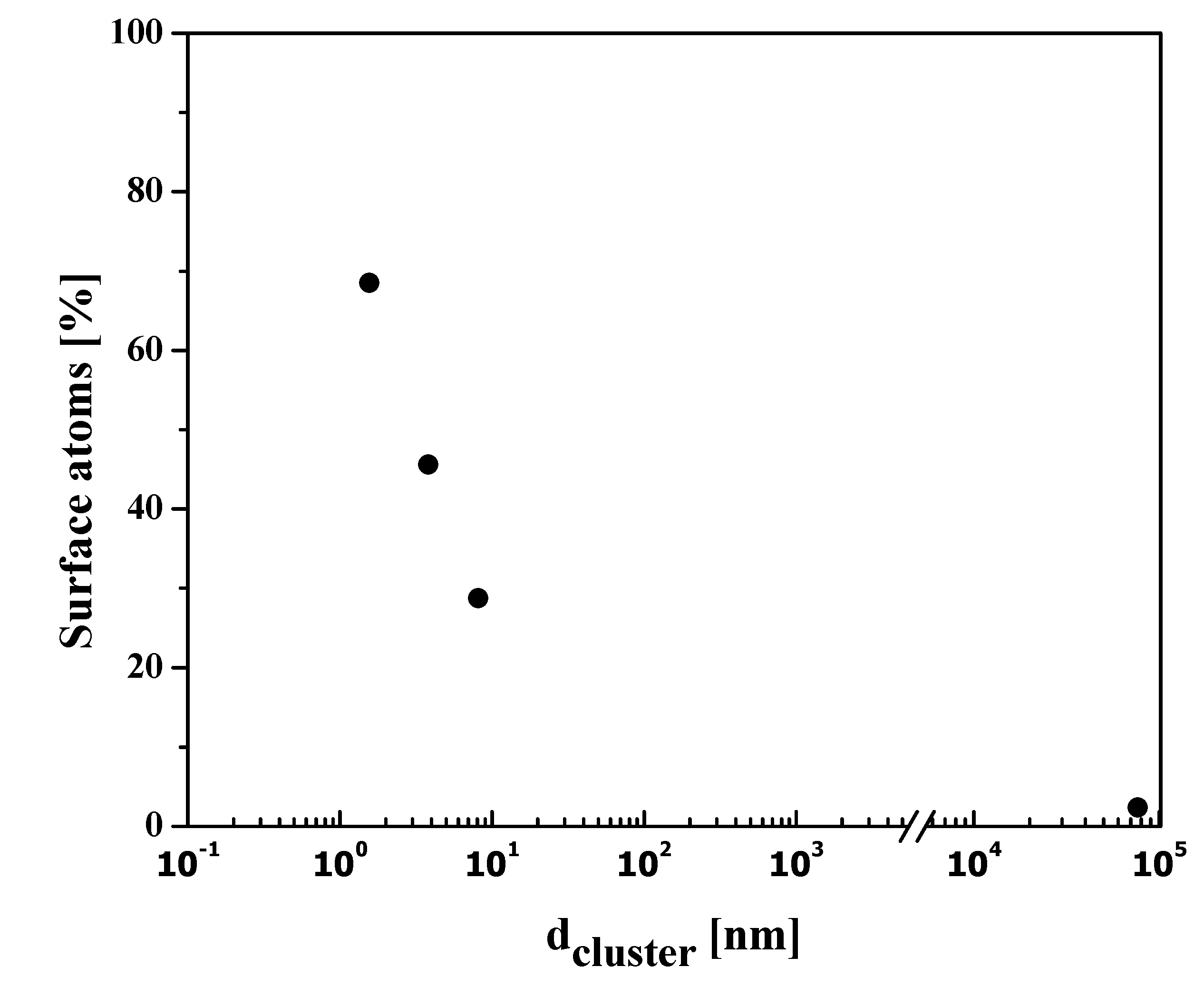
3. Estimation of Surface to Volume Ratio
4. Nucleation and Growth Process
5. Synthetic Methods of Noble Metal NPs
6. Applications of Noble Metal NPs
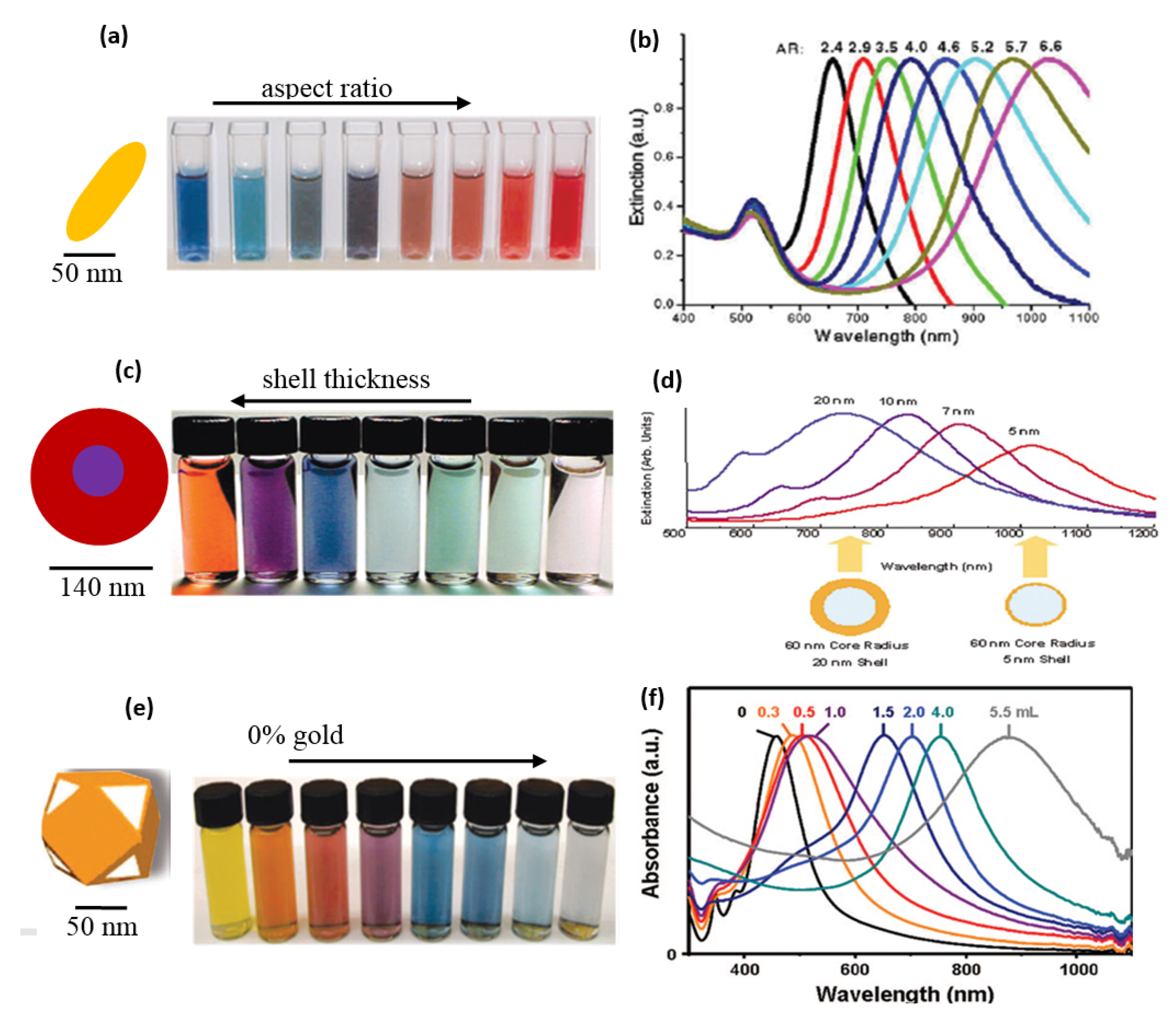
6.1. Colorimetric Sensing
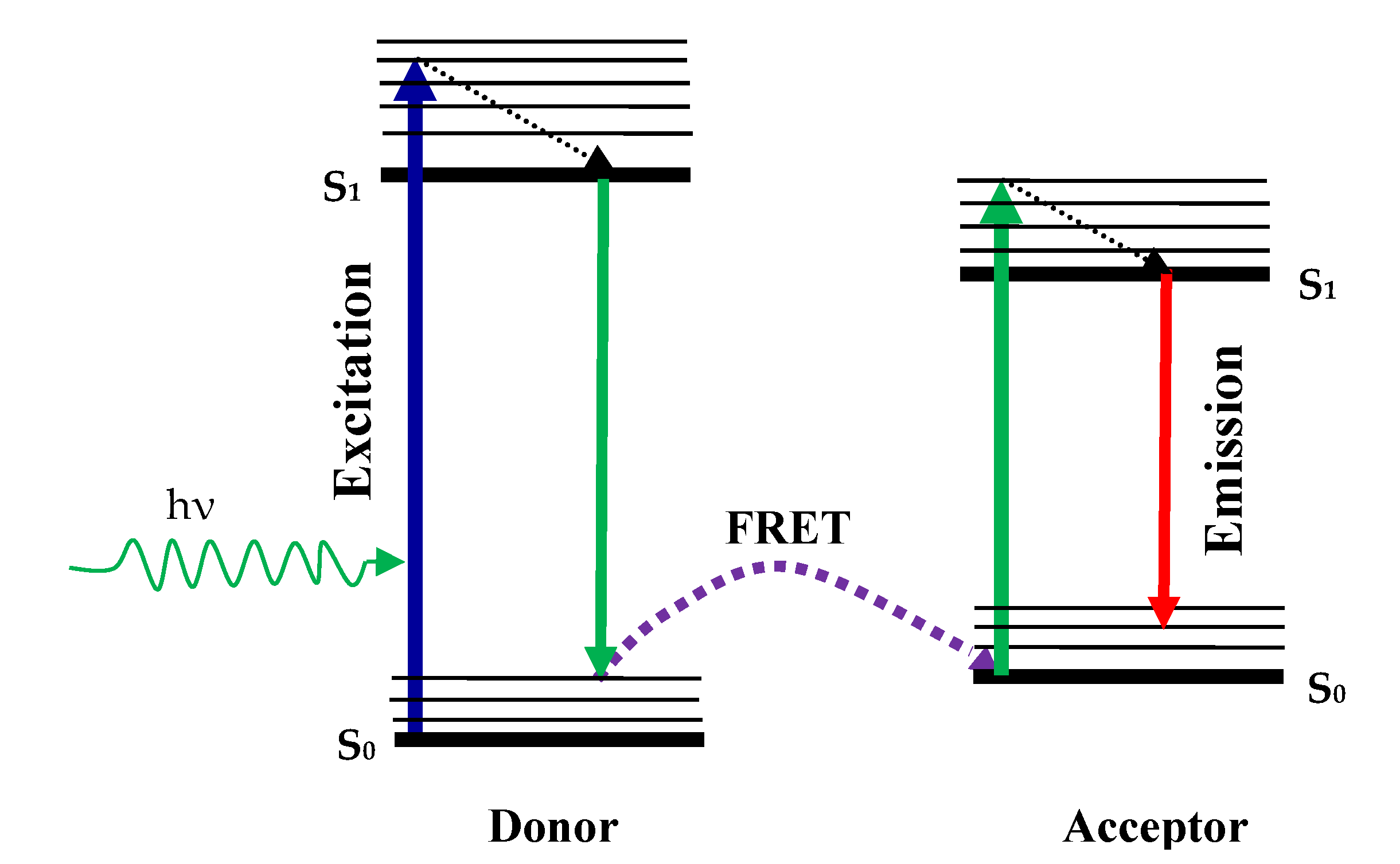
6.2. Fluorimetric Sensing
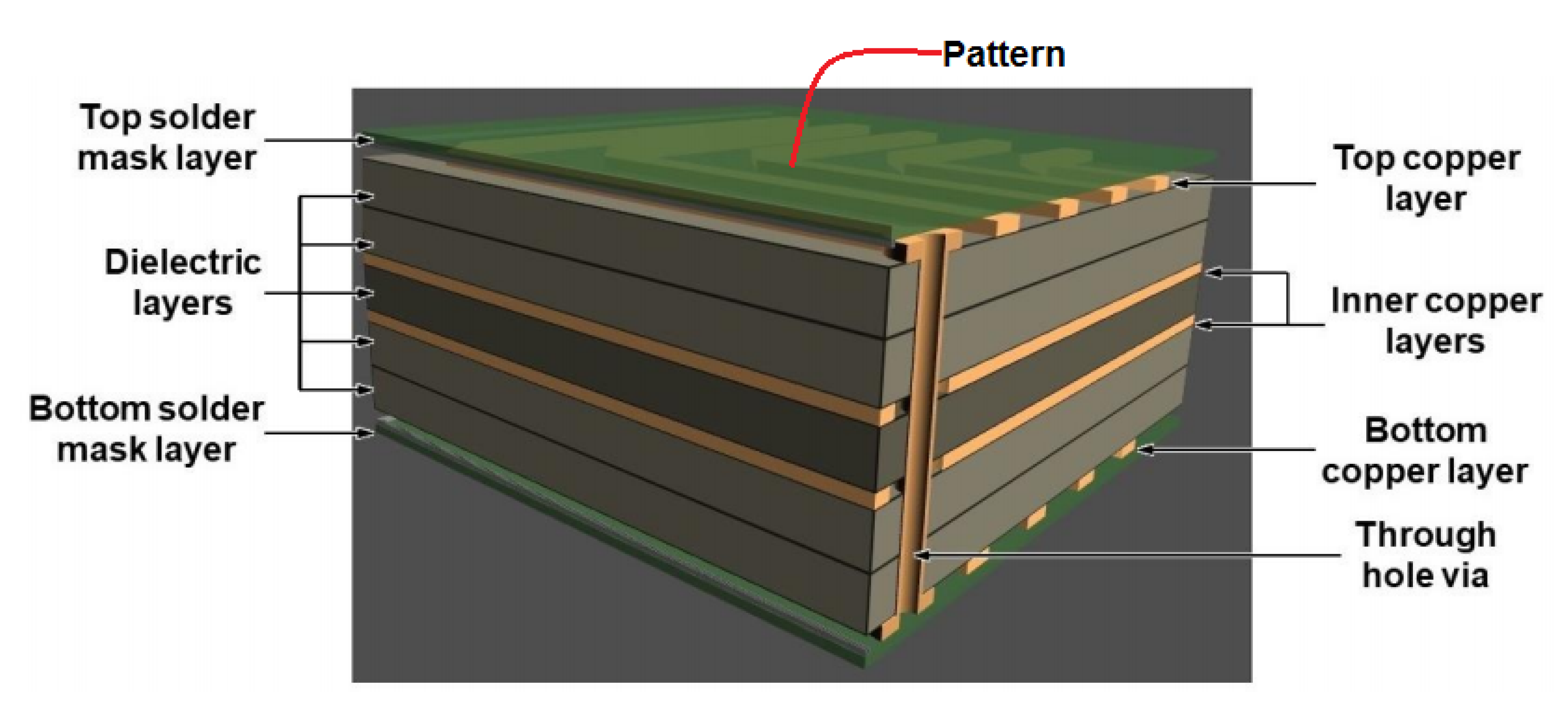
6.3. Metal NPs in Microelectronic Packaging
6.4. Impact of Nanoparticles of Human Health and Enviroments
7. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-Dimensional Metal Nanostructures: From Colloidal Syntheses to Applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef]
- West, J.L.; Halas, N.J. Engineered Nanomaterials for Biophotonics Applications: Improving Sensing, Imaging, and Therapeutics. Annu. Rev. Biomed. Eng. 2003, 5, 285–292. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, Y.; Liu, H.; Wu, X.; Li, Y.; Miao, L.; Shen, Z.; Wu, A. High-Performance Colorimetric Detection of Hg2+ Based on Triangular Silver Nanoprisms. ACS Sens. 2016, 5, 521–527. [Google Scholar] [CrossRef]
- Gautam, A.; Komal, P. Probable ideal size of Ln3+-based upconversion NPs for single and multimodal imaging. Coord. Chem. Rev. 2018, 376, 393–404. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold NPs for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold NPs in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Paull, R.; Wolfe, J.; Hébert, P.; Sinkula, M. Investing in nanotechnology. Nat. Biotechnol. 2003, 21, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Jia, L. Global Governmental Investment in Nanotechnologies. Curr. Nanosci. 2005, 1, 263–266. [Google Scholar] [CrossRef]
- Gharatape, A.; Salehi, R. Recent Progress in Theranostic Applications of Hybrid Gold NPs. Eur. J. Med. Chem. 2017, 138, 221–233. [Google Scholar] [CrossRef]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold Nanocages: From Synthesis to Theranostic Applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, J.; Chen, Q.; Liu, Z. Nanoscale metal-organic frameworks and coordination polymers as theranostic platforms for cancer treatment. Coord. Chem. Rev. 2019, 398, 113009–113023. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Shen, Y. Hydrothermal Synthesis of Gold Nanoplates and their Structure-Dependent LSPR Properties. J. Mater. Res. 2018, 33, 2671–2679. [Google Scholar] [CrossRef]
- Gautam, A.; Ram, S. Preparation and thermomechanical properties of Ag-PVA nanocomposite films. Mater. Chem. Phys. 2010, 119, 266–271. [Google Scholar] [CrossRef]
- Ram, S.; Tripathy, P.; Fecht, H.J. Gold NPs reinforced poly (vinyl alcohol) of self-standing optical films. J. Nanosci. Nanotechnol. 2007, 7, 3200–3206. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Gautam, A.; Fecht, H.J.; Cai, J.; Bansmann, J.; Behm, R.J. A new allotropic structure of silver nanocrystals nucleated and grown over planar polymer molecules. Philos. Mag. Lett. 2007, 87, 361–372. [Google Scholar] [CrossRef]
- Cametti, M.; Džolić, Z. New frontiers in hybrid materials: Noble metal NPs Supramolecular gel systems. Chem. Commun. 2014, 50, 8273–8286. [Google Scholar] [CrossRef]
- Alexander, J. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Gold NPs in Biology and Medicine: Recent Advances and Prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef]
- “Plenty of Room” Revisited. Nat. Nanotechnol. 2009, 4, 781. [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalyst 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Arnal, P.M.; Comotti, M.; Schüth, F. High-Temperature-Stable Catalysts by Hollow Sphere Encapsulation. Angew. Chem. Int. Ed. 2006, 45, 8224–8227. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Zhao, F.; Wang, Y.; Ye, Z.; Hua, C.; Liu, Z.; Bohinc, K.; Guo, X. Spherical Polyelectrolyte Brushes as Templates to Prepare Hollow Silica Spheres Encapsulating Metal Nanoparticles. Nanomaterials 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Bohinc, K.; Bossa, G.V.; May, S. Incorporation of ion and solvent structure into mean-field modeling of the electric double layer. Adv. Colloid Interface Sci. 2017, 249, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Mojarad, N.; Krishnan, M. Measuring the size and charge of single nanoscale objects in solution using an electrostatic fluidic trap. Nat. Nanotechnol. 2012, 7, 448. [Google Scholar] [CrossRef]
- Skaug, M.J.; Schwemmer, C.; Fringes, S.; Rawlings, C.D.; Knoll, A.W. Nanofluidic rocking brownian motors. Science 2018, 359, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Smith, G.N.; Grillo, I.; Rogers, S.E.; Eastoe, J. Surfactants with colloids: Adsorption or absorption? J. Colloid Interface Sci. 2015, 449, 205–214. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Chaichan, M.t.; Kazem, H.A.; Sopian, K. Evaluation and analysis of nanofluid and surfactant impact onphotovoltaic-thermal systems. Case Stud. Therm. Eng. 2019, 13, 100392. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Pulse Electroplating of Ultrafine Grained Tin Coating. In Electroplating of Nanostructures; IntechOpen: Rijeka, Croatia, 2015; pp. 105–129. [Google Scholar]
- Nützenadel, C.; Züttel, A.; Chartouni, D.; Schmid, G.; Schlapbach, L. Critical Size and Surface Effect of the Hydrogen Interaction of Palladium Clusters. Eur. Phys. J. D 2000, 8, 245–250. [Google Scholar] [CrossRef]
- Dubertret, B.; Calame, M.; Libchaber, A.J. Single-Mismatch Detection Using Gold-Quenched Fluorescent Oligonucleotides. Nat. Biotechnol. 2001, 19, 365–370. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Shape and Size Dependence of Radiative, Non-radiative and Photothermal Properties of Gold Nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of NPs in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, Z.; Zhang, Z.; Niu, W.; Li, C.; Yang, N.; Chen, B.; Zhang, H. Two-Dimensional Metal Nanomaterials: Synthesis, Properties, and Applications. Chem. Rev. 2018, 118, 6409–6455. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, X.; Gao, B.; Zou, W.; Dong, L. Facile Ball-Milling Synthesis of CuO/Biochar Nanocomposites for Efficient Removal of Reactive Red 120. ACS Omega 2020, 5, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Korczagin, I.; Golze, S.; Hempenius, M.A.; Vancso, G.J. Surface Micropatterning and Lithography with Poly(Ferrocenylmethylphenylsilane). Chem. Mater. 2003, 15, 3663–3668. [Google Scholar] [CrossRef]
- Kim, S.H.; Liu, B.Y.H.; Zachariah, M.R. Synthesis of Nanoporous Metal Oxide Particles by a New Inorganic Matrix Spray Pyrolysis Method. Chem. Mater. 2002, 14, 2889–2899. [Google Scholar] [CrossRef]
- Susha, A.S.; Ringler, M.; Ohlinger, A.; Paderi, M.; LiPira, N.; Carotenuto, G.; Rogach, A.L.; Feldmann, J. Strongly Luminescent Films Fabricated by Thermolysis of Gold−Thiolate Complexes in a Polymer Matrix. Chem. Mater. 2008, 20, 6169–6175. [Google Scholar] [CrossRef]
- Zhu, M.; Baffou, G.; Meyerbröker, N.; Polleux, J. Micropatterning Thermoplasmonic Gold Nanoarrays To Manipulate Cell Adhesion. ACS Nano. 2012, 6, 7227–7233. [Google Scholar] [CrossRef]
- Marques-Hueso, J.; Morton, J.A.S.; Wang, X.; Bertran-Serra, E.; Desmulliez, M.P.Y. Photolithographic Nanoseeding Method for Selective Synthesis of Metal-Catalysed Nanostructures. Nanotechnology 2019, 30, 15302–15309. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for Micro- and Nanoscale Patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- Sreenivasan, S.V. Nanoimprint Lithography Steppers for Volume Fabrication of Leading-Edge Semiconductor Integrated Circuits. Microsyst. Nanoeng. 2017, 3, 17075–17093. [Google Scholar] [CrossRef] [PubMed]
- Garno, J.C.; Yang, Y.; Amro, N.A.; Cruchon-Dupeyrat, S.; Chen, S.; Liu, G.-Y. Precise Positioning of NPs on Surfaces Using Scanning Probe Lithography. Nano Lett. 2003, 3, 389–395. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic NPs. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Gautam, A.; Mukherjee, S.; Ram, S. Controlled Novel Route to Synthesis and Characterization of Silver Nanorods. J. Nanosci. Nanotechnol. 2010, 10, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Singh, G.P.; Ram, S. A Simple Polyol Synthesis of Silver Metal Nanopowder of Uniform Particles. Synth. Met. 2007, 157, 5–10. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Size-Controlled Synthesis of NPs. 1. “Silver-Only” Aqueous Suspensions via Hydrogen Reduction. J. Phys. Chem. B 2004, 108, 13948–13956. [Google Scholar] [CrossRef]
- Tatarchuk, V.V.; Sergievskaya, A.P.; Korda, T.M.; Druzhinina, I.A.; Zaikovsky, V.I. Kinetic Factors in the Synthesis of Silver NPs by Reduction of Ag+ with Hydrazine in Reverse Micelles of Triton N-42. Chem. Mater. 2013, 25, 3570–3579. [Google Scholar] [CrossRef]
- Fountoulaki, S.; Daikopoulou, V.; Gkizis, P.L.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Mechanistic Studies of the Reduction of Nitroarenes by NaBH4 or Hydrosilanes Catalyzed by Supported Gold NPs. ACS Catal. 2014, 4, 3504–3511. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green Nanotechnology: A Review on Green Synthesis of Silver NPs An Ecofriendly Approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Tripathy, P.; Ram, S. Microstructure, topology and X-ray diffraction in Ag-Metal Reinforced Polymer of Polyvinyl Alcohol of Thin Laminates. J. Mater. Sci. 2006, 41, 3007–3016. [Google Scholar] [CrossRef]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of Formation and Structure of Micro Emulsions by Electron Microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Tissandier, C.; Diop, N.; Martini, M.; Roux, S.; Tillement, O.; Hamaide, T. One-Pot Synthesis of Hybrid Multifunctional Silica NPs with Tunable Coating by Click Chemistry in Reverse W/O Microemulsion. Langmuir 2012, 28, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; van Veggel, F.C.J.M. Synthesis of InN@SiO2 Nanostructures and Fabrication of Blue LED Devices. ACS Appl. Mater. Interfaces 2012, 4, 3902–3909. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ganguly, A.; Vaidya, S. Microemulsion-Based Synthesis of Nanocrystalline Materials. Chem. Soc. Rev. 2010, 39, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Alejo, C.J.; D’Alfonso, C.; Pacioni, N.L.; González-Béjar, M.; Grenier, M.; Lanzalunga, O.; Alarcon, E.I.; Scaiano, J.C. Ultraclean Derivatized Monodisperse Gold NPs through Laser Drop Ablation Customization of Polymorph Gold Nanostructures. Langmuir 2012, 28, 8183–8189. [Google Scholar] [CrossRef]
- Dahal, N.; García, S.; Zhou, J.; Humphrey, S.M. Beneficial Effects of Microwave-Assisted Heating versus Conventional Heating in Noble Metal Nanoparticle Synthesis. ACS Nano. 2012, 6, 9433–9446. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold NPs in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold NPs: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.-H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-Enabled Photonics-Based Imaging and Therapy of Cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Au, L.; Li, X.; Xia, Y. Facile Synthesis of Ag Nanocubes and Au Nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef]
- Polavarapu, L.; Pérez-Juste, J.; Xu, Q.-H.; Liz-Marzán, L.M. Optical Sensing of Biological, Chemical and Ionic Species Through Aggregation of Plasmonic NPs. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold NPs with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, X.; Kumar, A.; Zou, G.; Zhang, X.; Liang, X.-J. Long Genomic DNA Amplicons Adsorption onto Unmodified Gold NPs for Colorimetric Detection of Bacillus Anthracis. Chem. Commun. 2013, 49, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing Colorimetric Approaches Based on Gold and Silver NPs Aggregation: Chemical Creativity Behind the Assay. A Review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef]
- Lee, J.H.; Wang, Z.; Liu, J.; Lu, Y. Highly Sensitive and Selective Colorimetric Sensors for Uranyl (UO22+): Development and Comparison of Labeled and Label-Free DNAzyme-Gold Nanoparticle Systems. J. Am. Chem. Soc. 2008, 130, 14217–14226. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K. Functionalized Gold Nanoparticle Supported Sensory Mechanisms Applied in Detection of Chemical and Biological Threat agents: A review. Anal. Chim. Acta 2012, 715, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Johnson, R.C.; Hupp, J.T. Gold Nanoparticle-Based Sensing of “Spectroscopically Silent” Heavy Metal Ions. Nano Lett. 2001, 1, 165–167. [Google Scholar] [CrossRef]
- Lee, J.-S.; Han, M.S.; Mirkin, C.A. Colorimetric Detection of Mercuric ion (Hg2+) in Aqueous Media Using DNA-Functionalized Gold NPs. Angew. Chem. Int. Ed. Engl. 2007, 46, 4093–4096. [Google Scholar] [CrossRef]
- Xue, X.; Wang, F.; Liu, X. One-Step, Room Temperature, Colorimetric Detection of Mercury (Hg2+) Using DNA/Nanoparticle Conjugates. J. Am. Chem. Soc. 2008, 130, 3244–3245. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly Selective and Ultrasensitive Detection of Hg2+ Based on Fluorescence Quenching of Au Nanoclusters by Hg2+–Au+ Interactions. Chem. Commun. 2010, 46, 961–963. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Z. Colorimetric Detection of Hg2+ by Au NPs Formed by H2O2 Reduction of HAuCl4 Using Au Nanoclusters as the Catalyst. Sens. Actuat. B Chem. 2017, 241, 1063–1068. [Google Scholar] [CrossRef]
- Farhadi, K.; Forough, M.; Molaei, R.; Hajizadeh, S.; Rafipour, A. Highly Selective Hg2+ Colorimetric Sensor Using Green Synthesized and Unmodified Silver NPs. Sens. Actuat. B Chem. 2012, 161, 880–885. [Google Scholar] [CrossRef]
- Sebastian, M.; Aravind, A.; Mathew, B. Green Silver-Nanoparticle-Based Dual Sensor for Toxic Hg(II) Ions. Nanotechnology 2018, 29, 355502. [Google Scholar] [CrossRef] [PubMed]
- Alagan, J.; Dhesingh, R.S. Functionalized Silver Nanoparticle Probe for Visual Colorimetric Sensing of Mercury. Mater. Res. Bull. 2016, 83, 48–55. [Google Scholar]
- Liu, J.; Brown, A.K.; Meng, X.; Cropek, D.M.; Istok, J.D.; Watson, D.B.; Lu, Y. A Catalytic Beacon Sensor for Uranium with Parts-Per-Trillion Sensitivity and Millionfold Selectivity. Proc. Natl. Acad. Sci. USA 2007, 104, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Wang, C.; Wang, T.; Li, L.; Su, Z. Colorimetric Detection of Pb2+ Using Glutathione Functionalized Gold NPs. ACS Appl. Mater. Interfaces 2010, 2, 1466–1470. [Google Scholar] [CrossRef]
- Hung, Y.-L.; Hsiung, T.-M.; Chen, Y.-Y.; Huang, Y.-F.; Huang, C.-C. Colorimetric Detection of Heavy Metal Ions Using Label-Free Gold NPs and Alkanethiols. J. Phys. Chem. C 2010, 114, 16329–16334. [Google Scholar] [CrossRef]
- Patel, K.; Bhamore, J.R.; Park, T.J.; Kailasa, S.K. Selective and Sensitive Colorimetric Recognition of Ba2+ Ion Using Guanine-Functionalized Silver NPs. Chem. Sel. 2018, 3, 10182–10187. [Google Scholar]
- Dau, A.; Komal, P.; Truong, M.; Morris, G.; Evans, G.; Nashmi, R. RIC-3 Differentially Modulates α4β2 and α7 Nicotinic Receptor Assembly, Expression, and Nicotine-Induced Receptor Upregulation. BMC Neurosci. 2013, 14, 14–47. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tian, F.; Lyu, J.; Yang, M. Nanoparticle Based Fluorescence Resonance Energy Transfer (FRET) for Biosensing Applications. J. Mater. Chem. B 2015, 3, 6989–7005. [Google Scholar] [CrossRef] [PubMed]
- Gersten, J.; Nitzan, A. Spectroscopic Properties of Molecules Interacting with Small Dielectric Particles. J. Chem. Phys. 1981, 75, 1139–1152. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-Enhanced Fluorescence and Plasmon Emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef]
- Mendez-Gonzalez, D.; Melle, S.; Calderón, O.G.; Laurenti, M.; Cabrera-Granado, E.; Egatz-Gómez, A.; López-Cabarcos, E.; Rubio-Retama, J.; Díaz, E. Control of Upconversion Luminescence by Gold Nanoparticle Size: From Quenching to Enhancement. Nanoscale 2019, 11, 13832–13844. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Cao, L.; Xu, K.; Zhuo, L.; Ge, J.; Li, Q.; Yu, L. A New Nanobiosensor for Glucose with High Sensitivity and Selectivity in Serum Based on Fluorescence Resonance Energy Transfer (FRET) between CdTe Quantum Dots and Au NPs. Chem. A Eur. J. 2008, 14, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Hong, M.-Y.; Lee, D.; Nam, S.-H.; Yoon, H.C.; Kim, H.-S. Inhibition Assay of Biomolecules Based on Fluorescence Resonance Energy Transfer (FRET) Between Quantum Dots and Gold NPs. J. Am. Chem. Soc. 2005, 127, 3270–3271. [Google Scholar] [CrossRef] [PubMed]
- Angelatos, A.S.; Radt, B.; Caruso, F. Light-Responsive Polyelectrolyte/Gold Nanoparticle Microcapsules. J. Phys. Chem. B 2005, 109, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Zhao, S.; Lu, X.; Tian, J. Gold NPs-based fluorescence resonance energy transfer for competitive immunoassay of biomolecules. Analyst 2012, 137, 5885–5890. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.M.; Kim, G.B.; Kim, Y.-P. Gold Nanoparticle-Based Fluorescence Quenching Via Metal Coordination for Assaying Protease Activity. Gold Bull. 2012, 45, 213–219. [Google Scholar] [CrossRef][Green Version]
- Mayilo, S.; Kloster, M.A.; Wunderlich, M.; Lutich, A.; Klar, T.A.; Nichtl, A.; Kürzinger, K.; Stefani, F.D.; Feldmann, J. Long-Range Fluorescence Quenching by Gold NPs in a Sandwich Immunoassay for Cardiac Troponin T. Nano Lett. 2009, 9, 4558–4563. [Google Scholar] [CrossRef]
- Amjadi, M.; Abolghasemi-Fakhri, Z.; Hallaj, T. Carbon Dots-Silver NPs Fluorescence Resonance Energy Transfer System as a Novel Turn-on Fluorescent Probe for Selective Determination of Cysteine. J. Photochem. Photobiol. A Chem. 2015, 309, 8–14. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Wang, C.; Li, W.; Qiang, W.; Xu, D. Silver Nanoparticle-Enhanced Fluorescence Resonance Energy Transfer Sensor for Human Platelet-Derived Growth Factor-BB Detection. Anal. Chem. 2013, 85, 4492–4499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, T.; Liu, Y. Silver Nanoparticle Plasmonic Enhanced Förster Resonance Energy Transfer (FRET) Imaging of Protein-Specific Sialylation on the Cell Surface. Nanoscale 2017, 9, 9841–9847. [Google Scholar] [CrossRef]
- Oda, J.M. Metal Nano-Particles. Jpn. Inst. Electron. Packag. 2002, 5, 523–528. [Google Scholar] [CrossRef]
- Wu, B.-H.; Yang, H.-Y.; Huang, H.-Q.; Chen, G.X.; Zheng, N.-F. Solvent Effect on the Synthesis of Monodisperse Amine-Capped Au Nanoparticles. Chin. Chem. Lett. 2013, 24, 457–462. [Google Scholar] [CrossRef]
- Ketelsen, B.; Tjarks, P.P.; Schlicke, H.; Liao, Y.-C.; Vossmeyer, T. Fully Printed Flexible Chemiresistors with Tunable Selectivity Based on Gold NPs. Chemosensors 2020, 8, 116. [Google Scholar] [CrossRef]
- Schmid, G.; Corain, B. Nanoparticulated Gold: Syntheses, Structures, Electronics, and Reactivities. Eur. J. Inorg. Chem. 2003, 3081–3098. [Google Scholar] [CrossRef]
- Castro, T.; Reifenberger, R.; Choi, E.; Andres, R.P. Size-dependent melting temperature of individual nanometer-sized metallic clusters. Phys. Rev. B 1990, 42, 8548–8556. [Google Scholar] [CrossRef]
- Chin, H.; Cheong, K.; Ismail, A. A Review on Die Attach Materials for SiC-Based High-Temperature Power Device. Metall. Mater. Trans. B 2010, 41, 824. [Google Scholar] [CrossRef]
- Wang, G.; Wu, N.; Wang, J.; Shao, J.; Zhu, X.; Lu, X.; Guo, L. Abnormal change of melting points of gold NPs confined between two-layer graphene nanosheets. RSC Adv. 2016, 6, 108343–108346. [Google Scholar] [CrossRef]
- German, R.M. Prediction of Sintered Density for Bimodal Powder Mixtures. Metall. Trans. A 1992, 23, 1455–1465. [Google Scholar] [CrossRef]
- Roh, M.H.; Nishikawa, H.; Jung, J.P. A Review of Ag Paste Bonding for Automotive Power Device Packaging. J. Microelectron. Packag. Soc. 2015, 22, 15–23. [Google Scholar] [CrossRef]
- Jung, D.-H.; Roh, M.H.; Lee, J.H.; Kim, K.H.; Jung, J.P. Transient Liquid Phase (TLP) Bonding of Device for High Temperature Operation. J. Microelectron. Packag. Soc. 2017, 24, 17–25. [Google Scholar] [CrossRef]
- Skandan, G.; Hahn, H.; Kear, B.H.; Roddy, M.; Cannon, W.R. The Effect of Applied Stress on Densification of Nano-structured Zirconia during Sinter-forging. Mater. Lett. 1994, 20, 302–309. [Google Scholar] [CrossRef]
- Matsuba, Y. Direct Patterning Using Metal Nano-Particles. J. Jpn. Inst. Electron. Packag. 2003, 6, 130–135. [Google Scholar] [CrossRef]
- Bishop, P.T.; Ashfield, L.J.; Berzins, A.; Boardman, A.; Buche, V.; Cookson, J.; Gordon, R.J.; Salcianu, C.; Sutton, P.A. Printed Gold for Electronic Applications. Gold Bull. 2010, 43, 181–185. [Google Scholar] [CrossRef]
- Nakamoto, M.; Yamamoto, M.; Kashiwagi, Y.; Kakiuchi, H.; Tsujimoto, T.; Yoshida, Y. In Proceedings of Microelectronics Symposium, 2005; pp. 241–244.
- Shamkhalichenar, H.; Bueche, C.J.; Choi, J.-W. Printed Circuit Board (PCB) Technology for Electrochemical Sensors and Sensing Platforms. Biosensors 2020, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Ohsako, Y. In: Abstract of Chemical Engineering, 34th Meeting, K122, 2001.
- Sun, J.; Jiang, J.; Bao, B.; Wang, S.; He, M.; Zhang, X.; Song, Y. Fabrication of Bendable Circuits on a Polydimethylsiloxane (PDMS) Surface by Inkjet Printing Semi-Wrapped Structures. Materials 2016, 9, 253. [Google Scholar] [CrossRef]
- Yin, Z.P.; Huang, Y.A.; Bu, N.B.; Wang, X.M.; Xiong, Y.L. Inkjet Printing for Flexible Electronics: Materials, Processes and Equipments. Chin. Sci. Bull. 2010, 55, 3383–3407. [Google Scholar] [CrossRef]
- Jang, J. Displays Develop A New Flexibility. Mater. Today 2006, 9, 46–52. [Google Scholar] [CrossRef]
- Sharma, A.; Lim, D.U.; Jung, J.P. Microstructure and brazeability of SiC nanoparticles reinforced Al–9Si–20Cu produced by induction melting. Mater. Sci. Technol. 2016, 32, 773–779. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Pulse Electrodeposition of Lead-Free Tin-Based Composites for Microelectronic Packaging. In Electrodeposition of Composite Materials; InTech: Rijeka, Croatia, 2016; pp. 253–274. [Google Scholar]
- Hulander, M.; Hong, J.; Andersson, M.; Gervén, F.; Ohrlander, M.; Tengvall, P.; Elwing, H. Blood interactions with noble metals: Coagulation and immune complement activation. ACS Appl. Mater. Interfaces 2009, 1, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 2001806. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cao, Y.; Yao, Q.; Chai, O.J.H.; Xie, J. Engineering Noble Metal Nanomaterials for Pollutant Decomposition. Ind. Eng. Chem. Res. 2020, 59, 20561–20581. [Google Scholar] [CrossRef]




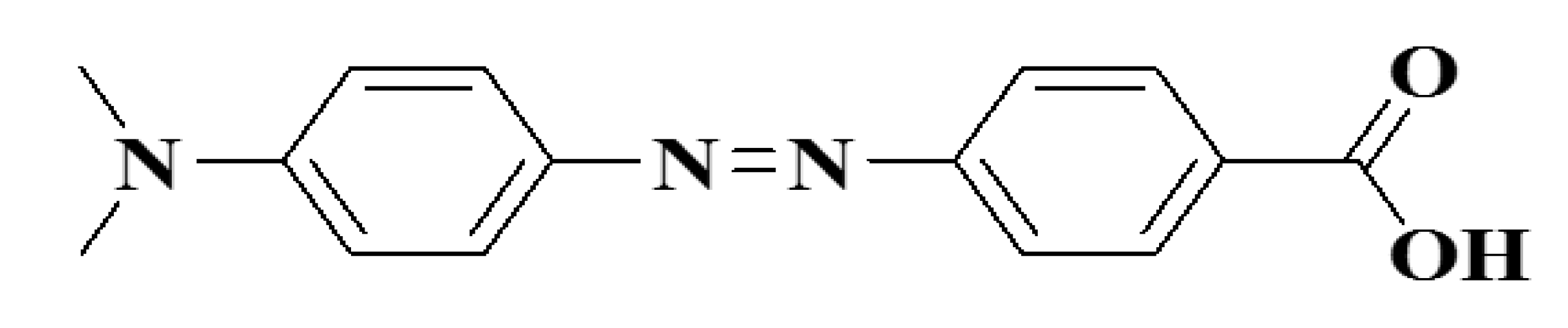
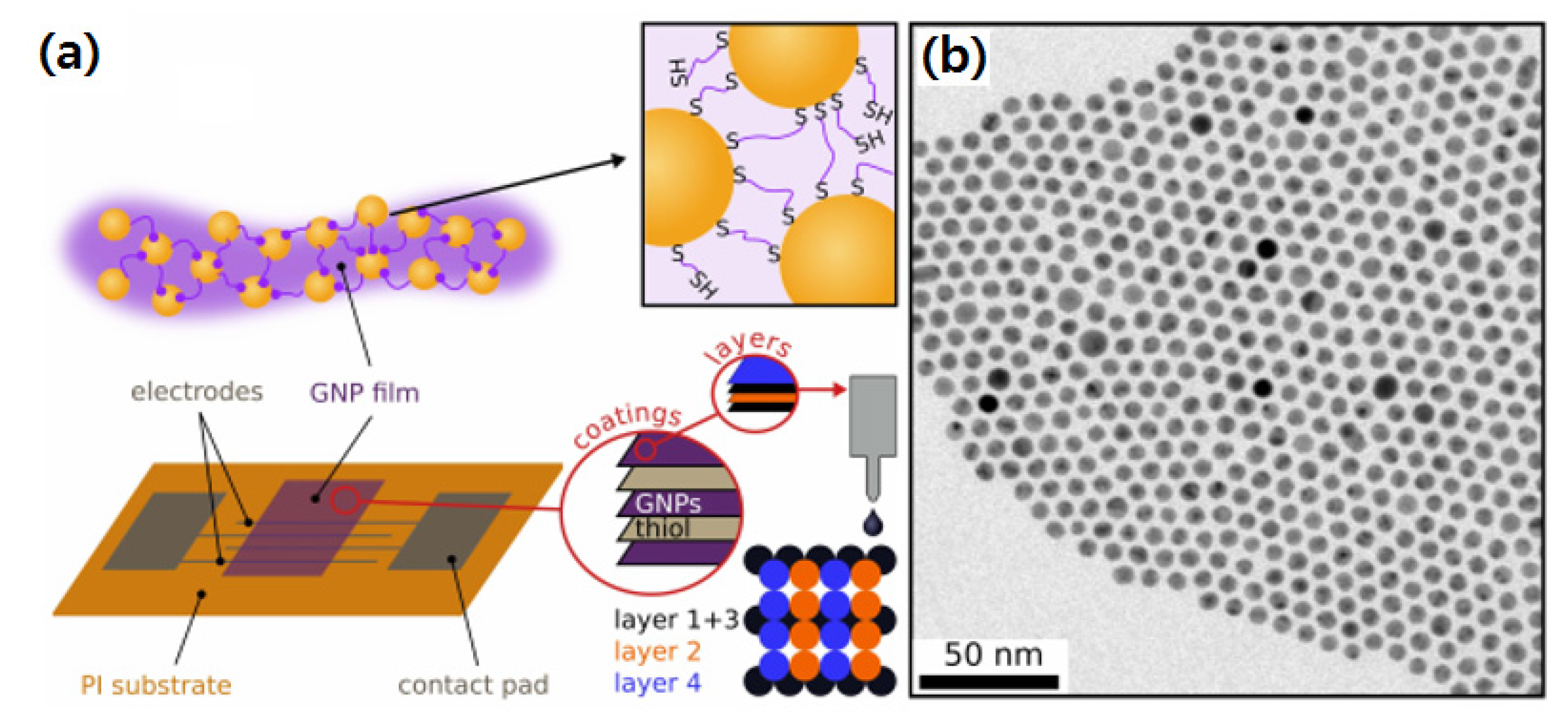



| Analyte | Size of Nps | LOD | NPs | Detection Method | Ref. |
|---|---|---|---|---|---|
| Pb2+,Cd2+, Hg2+ | 13 nm | 400 µM | MUA-functionalized AuNPs | Naked eyes/UVS | [71] |
| Hg2+ | 15 nm | 100 nM | DNA functionalized AuNPs | Naked eyes/UVS | [72] |
| Hg2+ | 25 Au-atoms | 50 µM | Au cluster stabilized by Au+ | Naked eyes/UVS | [74] |
| Hg2+ | 15 nm | 10 nM | BSA Stabilized AuNPs | Naked eyes/UVS | [75] |
| Pb2+ | 5–8 nm | 100 nM | GSH- functionalized AuNPs | Naked eyes/UVS | [80] |
| Pb2+ | 14 nm | 45 nM | 2-Mercaptoethanol/AuNPs | Naked eyes/UVS | [81] |
| UO22+ | 45 pM | DNAzyme- AuNp | DNAzyme- AuNPs | UVS | [79] |
| UO22+ | - | 1 nM | Bare | UVS | [69] |
| Ba2+ | 12 | 3 nM | Guanine-AgNPs | Naked eyes | [82] |
| Hg2+ | - | 2.2 µM | Soap plant AgNP | Naked eyes | [76] |
| Hg2+ | 14 nm | 2.1 µM | AgaricusbisporusAgNP | Naked eyes | [77] |
| Biological Target | Donor/Acceptor | Limit of Detection | Detection Range | Ref. |
|---|---|---|---|---|
| Glucose | Quantum dot/AuNp | 50 nM | 0.10–50 µM | [88] |
| Avidin | Quantum dot/AuNp | 10 nM | 10 nm–2 µM | [89] |
| IgG | Fluorophore/AuNp | 1 nM | 1–50 nM | [90] |
| IgM | Fluorophore/AuNp | 42 pM | 0.35 to 5 nM | [91] |
| MMP-7 | Fluorophore/AuNp | 10 ng mL−1 | 10 to 1000 ng mL−1 | [92] |
| cTnT | Fluorophore/AuNp | 0.02 nM | 0.02 to 0.15 nM | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, A.; Komal, P.; Gautam, P.; Sharma, A.; Kumar, N.; Jung, J.P. Recent Trends in Noble Metal Nanoparticles for Colorimetric Chemical Sensing and Micro-Electronic Packaging Applications. Metals 2021, 11, 329. https://doi.org/10.3390/met11020329
Gautam A, Komal P, Gautam P, Sharma A, Kumar N, Jung JP. Recent Trends in Noble Metal Nanoparticles for Colorimetric Chemical Sensing and Micro-Electronic Packaging Applications. Metals. 2021; 11(2):329. https://doi.org/10.3390/met11020329
Chicago/Turabian StyleGautam, Anurag, Pragya Komal, Prabhat Gautam, Ashutosh Sharma, Neeraj Kumar, and Jae Pil Jung. 2021. "Recent Trends in Noble Metal Nanoparticles for Colorimetric Chemical Sensing and Micro-Electronic Packaging Applications" Metals 11, no. 2: 329. https://doi.org/10.3390/met11020329
APA StyleGautam, A., Komal, P., Gautam, P., Sharma, A., Kumar, N., & Jung, J. P. (2021). Recent Trends in Noble Metal Nanoparticles for Colorimetric Chemical Sensing and Micro-Electronic Packaging Applications. Metals, 11(2), 329. https://doi.org/10.3390/met11020329







