Influence of Addition of Al and Ti Solutes and Variable Processing Conditions on Mechanical and Electrical Properties of Cu-Cr Alloys
Abstract
1. Introduction
2. Materials and Methods
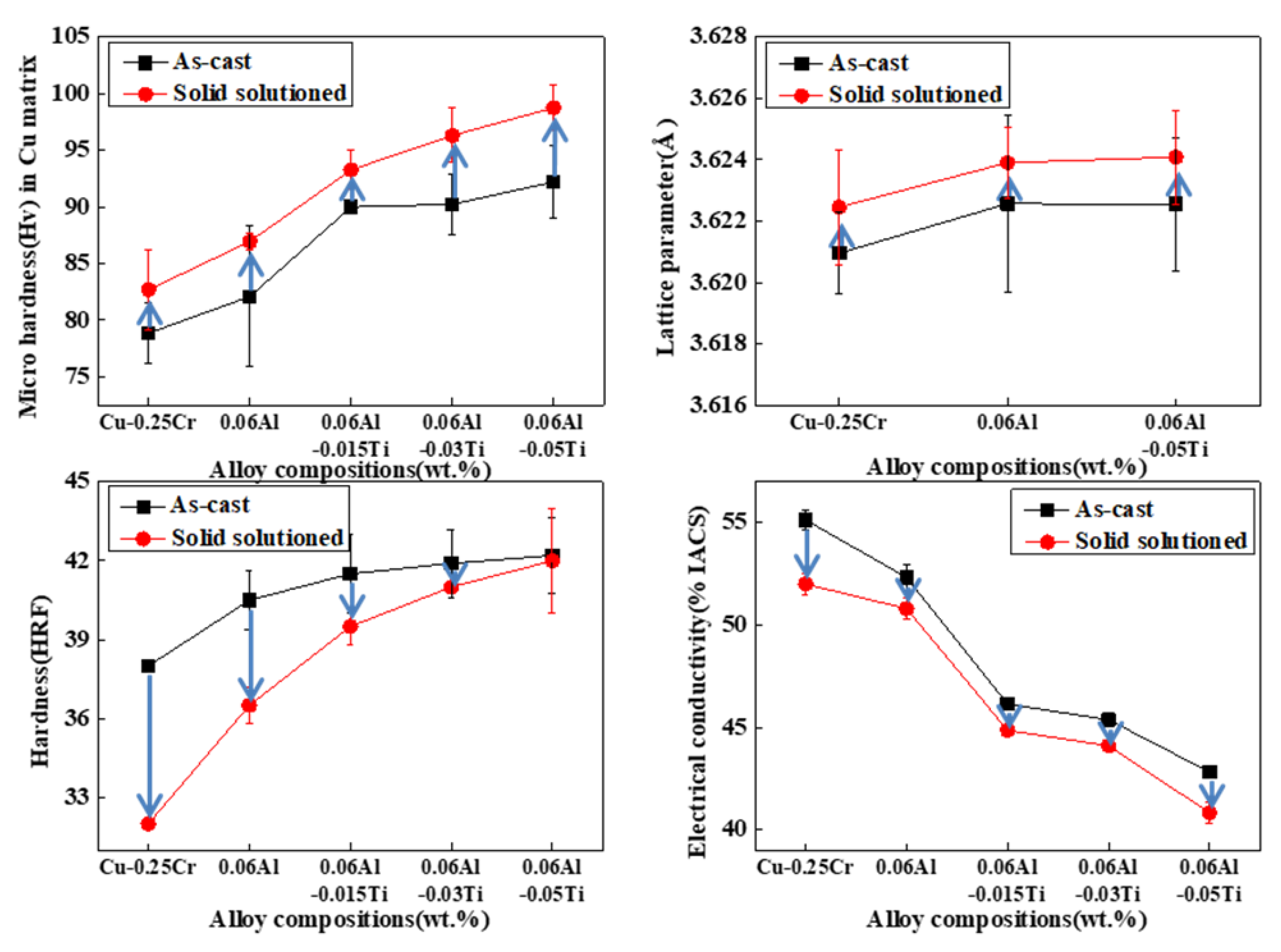
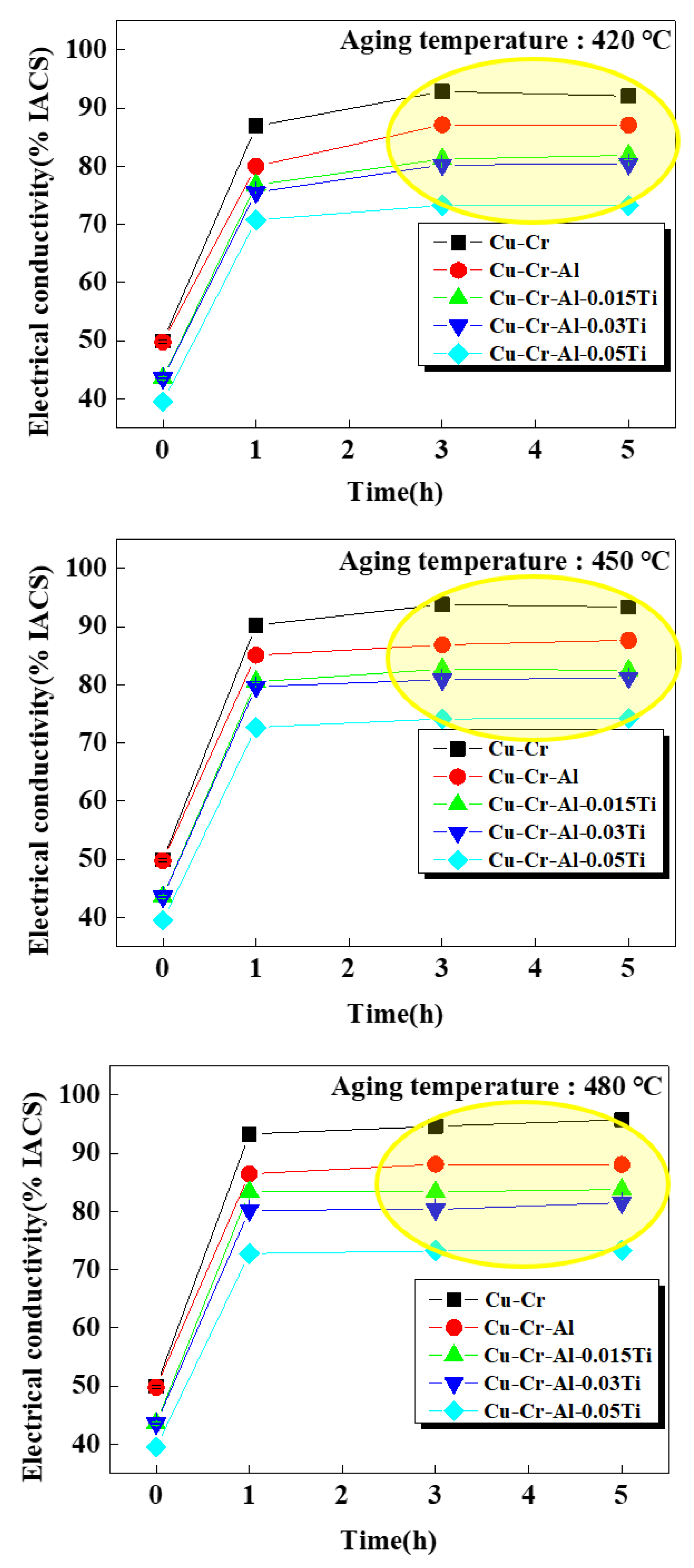
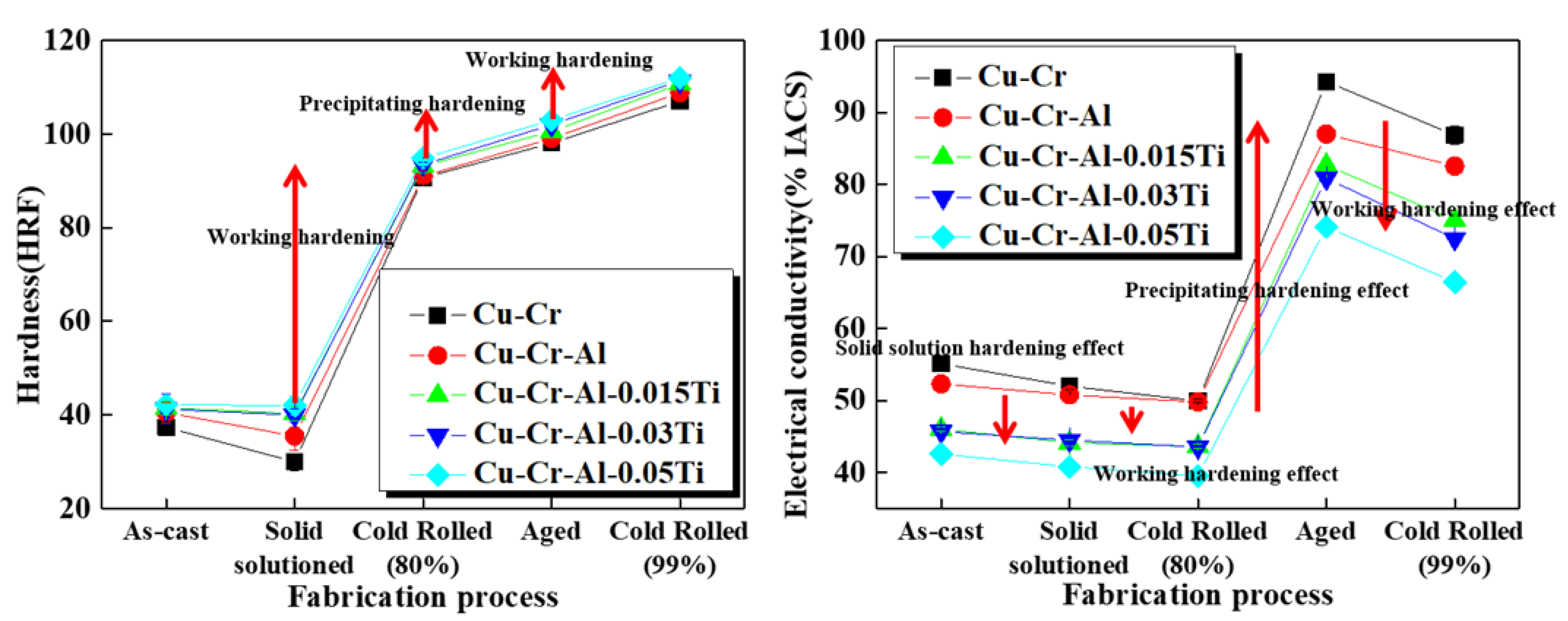
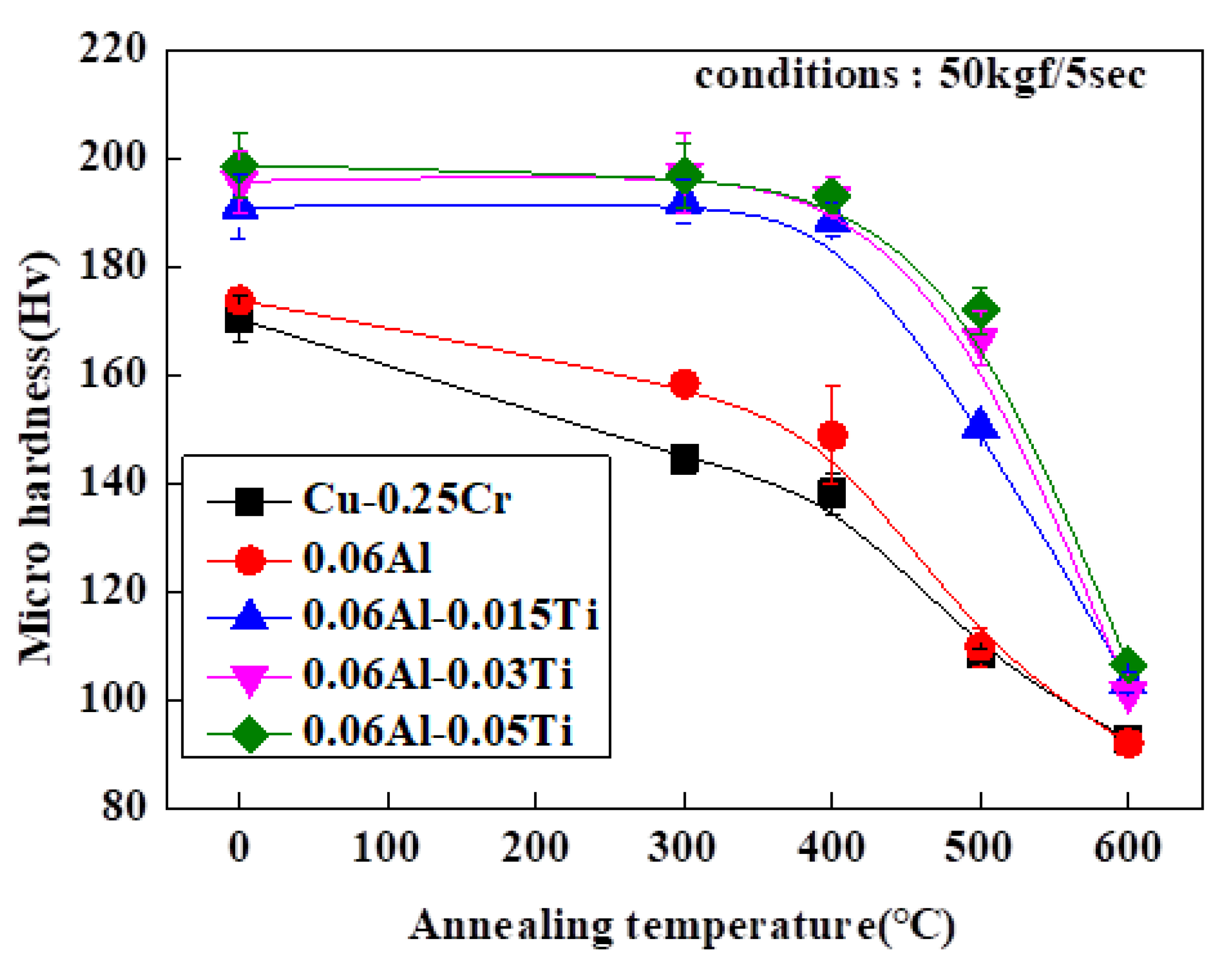
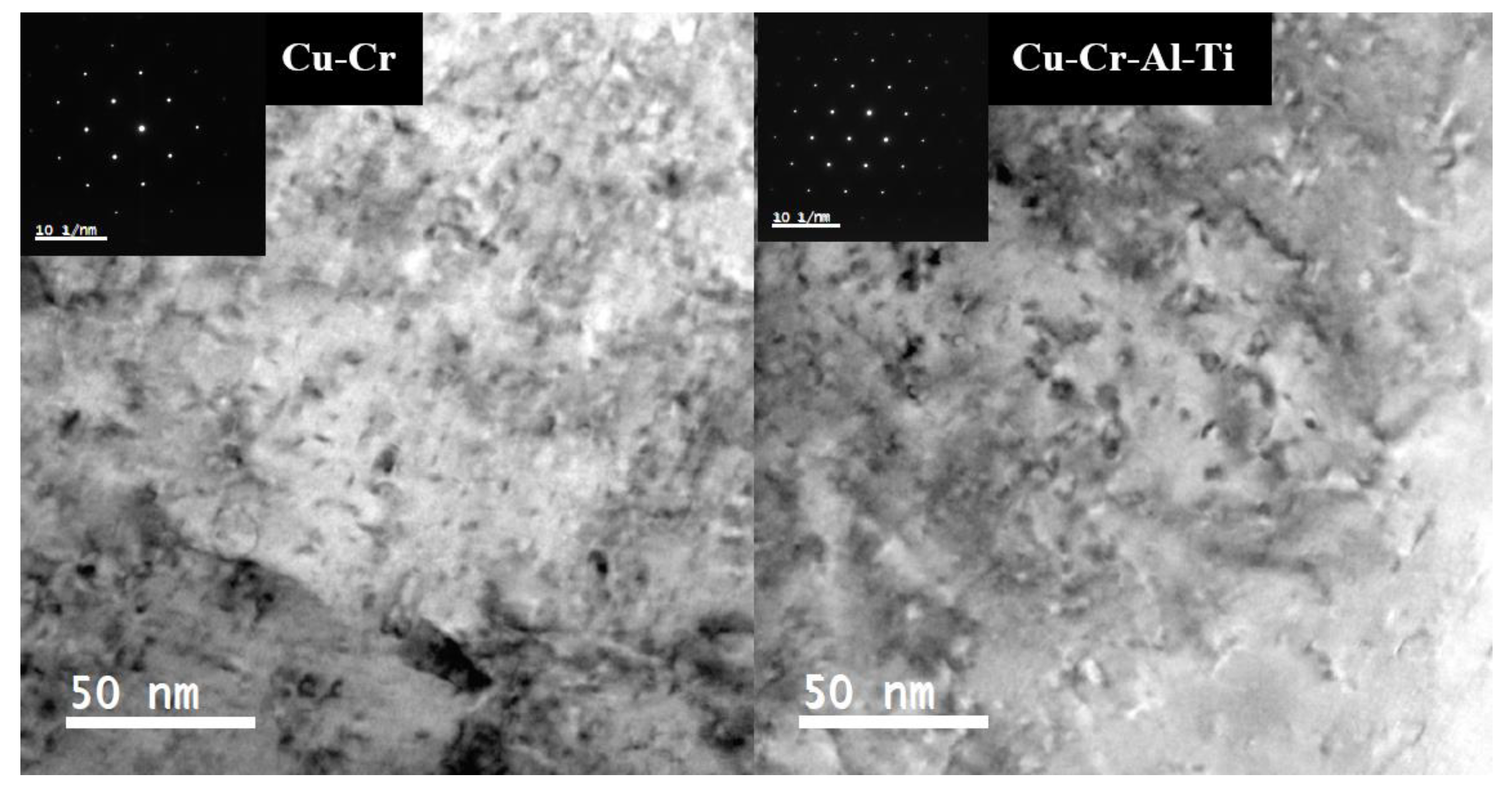
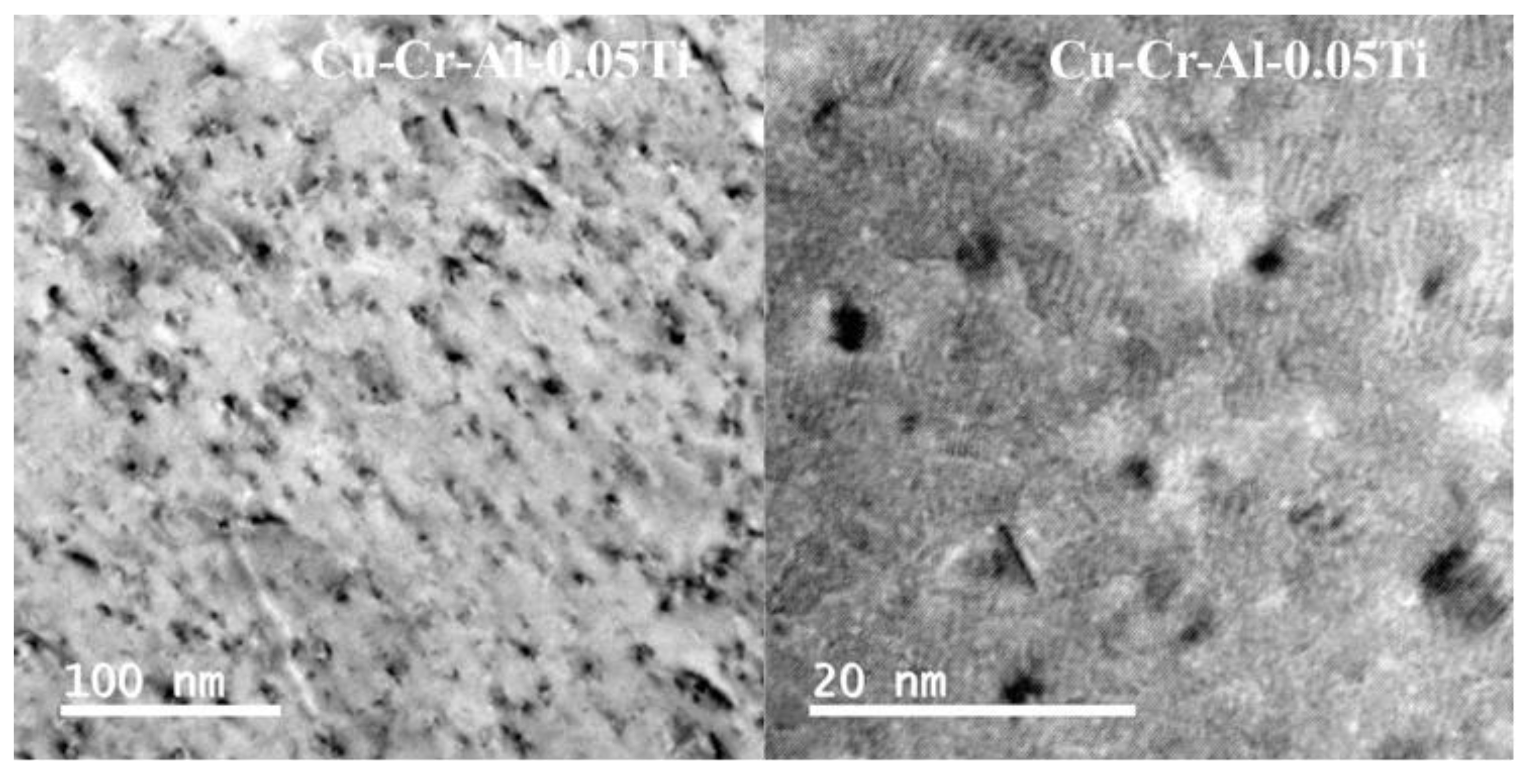
3. Results and Discussions
Experimental Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valiev, R.Z.; Murashkin, M.Y.; Sabirov, I. A nanostructural design to produce high-strength Al alloys with enhanced electrical conductivity. Scr. Mater. 2014, 76, 13–16. [Google Scholar] [CrossRef]
- Sauvage, X.S.; Bobruk, E.V.; Murashkin, M.Y.; Nasedkina, Y.; Enikeev, N.A.; Valiev, R.Z. Optimization of electrical conductivity and strength combination by structure design at the nanoscale in Al–Mg–Si alloys. Acta Mater. 2015, 98, 355–366. [Google Scholar] [CrossRef]
- Zhang, P.; Jie, J.; Gao, Y.; Li, H.; Wang, T.; Li, T. Influence of cold deformation and Ti element on the microstructure and properties of Cu–Cr system alloys. J. Mater. Res. 2015, 30, 2073–2080. [Google Scholar] [CrossRef]
- Fernee, H.; Nairn, J.; Atrens, A. Precipitation hardening of Cu-Fe-Cr alloys part I Mechanical and electrical properties. J. Mater. Sci. 2001, 36, 2711–2719. [Google Scholar] [CrossRef]
- Watanabe, C.; Monzen, R.; Tazaki, K. Mechanical properties of Cu–Cr system alloys with and without Zr and Ag. J. Mater. Sci. 2008, 43, 813–819. [Google Scholar] [CrossRef]
- Gao, N.; Tiainen, T.; Huttunen-Saarivirta, E. Influence of Thermomechanical Processing on the Microstructure and Properties of a Cu-Cr-P Alloy. J. Mater. Eng. Perform. 2002, 11, 376–383. [Google Scholar] [CrossRef]
- Kim, K.T.; Cho, H. Cu-Cr-Mg-P-Zr Alloys with High Electrical Conductivity and High Tensile Strength. Republic of Korea 10-2012-0097748, 5 September 2012. [Google Scholar]
- Kim, D.H.; Lee, D.W.; Kim, I.D.; Choi, S.Y.; Lee, J.H.; Jeon, B.M. Copper Alloy Having High Strength and High Conductivity, and Method for Manufacture the Same. Republic of Korea 10-2011-0096941, 31 August 2011. [Google Scholar]
- Shapiro, S.; Shapiro, E.; Mravic, B.; Watson, W.G. Copper Base Alloys with High Strength and High Electrical Conductivity. U.S. Patent No. 4007039A, 8 February 1977. [Google Scholar]
- Li, R.; Kang, H.; Chen, Z.; Fan, G.; Zou, C.; Wang, W.; Zhang, S.; Lu, Y.; Jie, J.; Cao, Z.; et al. A promising structure for fabricating high strength and high electrical conductivity copper alloys. Sci. Rep. 2016, 6, 20799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, H.L.; Volinsky, A.A.; Tian, B.H.; Chai, Z.; Liu, P.; Liu, Y. Characterization of the Hot Deformation Behavior of Cu–Cr–Zr Alloy by Processing Maps. Acta Metall. Sin. 2016, 29, 422–430. [Google Scholar] [CrossRef]
- Li, R.; Guo, E.; Chen, Z.; Kang, H.; Wang, W.; Zou, C.; Li, T.; Wang, T. Optimization of the balance between high strength and high electrical conductivity in CuCrZr alloys through two-step cryorolling and aging. J. Alloy. Compd. 2019, 771, 1044–1051. [Google Scholar] [CrossRef]
- Xu, G.-L.; Peng, L.-J.; Huang, G.-J.; Xie, H.-F.; Yang, Z.; Feng, X.; Yin, X.-Q.; Yang, Z. Microstructural evolution and properties of a Cu–Cr–Ag alloy during continuous manufacturing process. Rare Met. 2019, 1–8, 1–8. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, X.; Qiu, W.; Zhou, L.; Zhao, Z.; Wang, X.; Jiang, Y. Microstructure and properties of Cu-Cr-Nb alloy with high strength, high electrical conductivity and good softening resistance performance at elevated temperature. Mater. Sci. Eng. A 2019, 749, 281–290. [Google Scholar] [CrossRef]
- Peng, L.; Xie, H.; Huang, G.; Xu, G.; Yin, X.; Feng, X.; Mi, X.; Yang, Z. The phase transformation and strengthening of a Cu-0.71 wt% Cr alloy. J. Alloy. Compd. 2017, 708, 1096–1102. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, L.; Huang, G.; Feng, X.; Xie, H.; Mi, X.; Liu, X. Effect of Mg on the stress relaxation resistance of Cu–Cr al-loys. Mater. Sci. Eng. A 2020, 799, 140144. [Google Scholar] [CrossRef]
- Jovanovic, M.T.; Rajkovic, V. High Electrical Conductivity Cu-Based Alloys; Part1; Association of Metallurgical Engineers of Serbia: Belgrade, Serbia, 2009. [Google Scholar]
- Pang, Y.; Xia, C.; Wang, M.; Li, Z.; Xiao, Z.; Wei, H.; Sheng, X.; Jia, Y.; Chen, C. Effects of Zr and (Ni, Si) additions on properties and microstructure of Cu–Cr alloy. J. Alloy. Compd. 2014, 582, 786–792. [Google Scholar] [CrossRef]
- Gao, N.; Tiainen, T.; Laakso, L.; Ji, Y. Control of Microstructures and Properties of a Phosphorus-Containing Cu-0.6 Wt.% Cr Alloy through Precipitation Treatment. J. Mater. Eng. Perform. 2000, 9, 623–629. [Google Scholar] [CrossRef]
- Han, S.J.; Ahn, J.H. A Copper Alloy having High Strength and High Electrical Conductivity. Republic of Korea 10-1468959, 28 November 2014. [Google Scholar]
- European Copper Institute. Copper as Electrical Conductive Material with Above-Standard Performance Properties; European Copper Institute: New York, NY, USA, 2020. [Google Scholar]
- Zhang, P.C.; Jie, J.C.; Gao, Y.; Wang, T.M.; Li, T.J. Effect of Ti Element on Microstructure and Properties of Cu-Cr Alloy. Mater. Sci. Forum 2015, 817, 307–311. [Google Scholar] [CrossRef]
- Ardell, A.J. Precipitation hardening. Met. Mater. Trans. A 1985, 16, 2131–2165. [Google Scholar] [CrossRef]

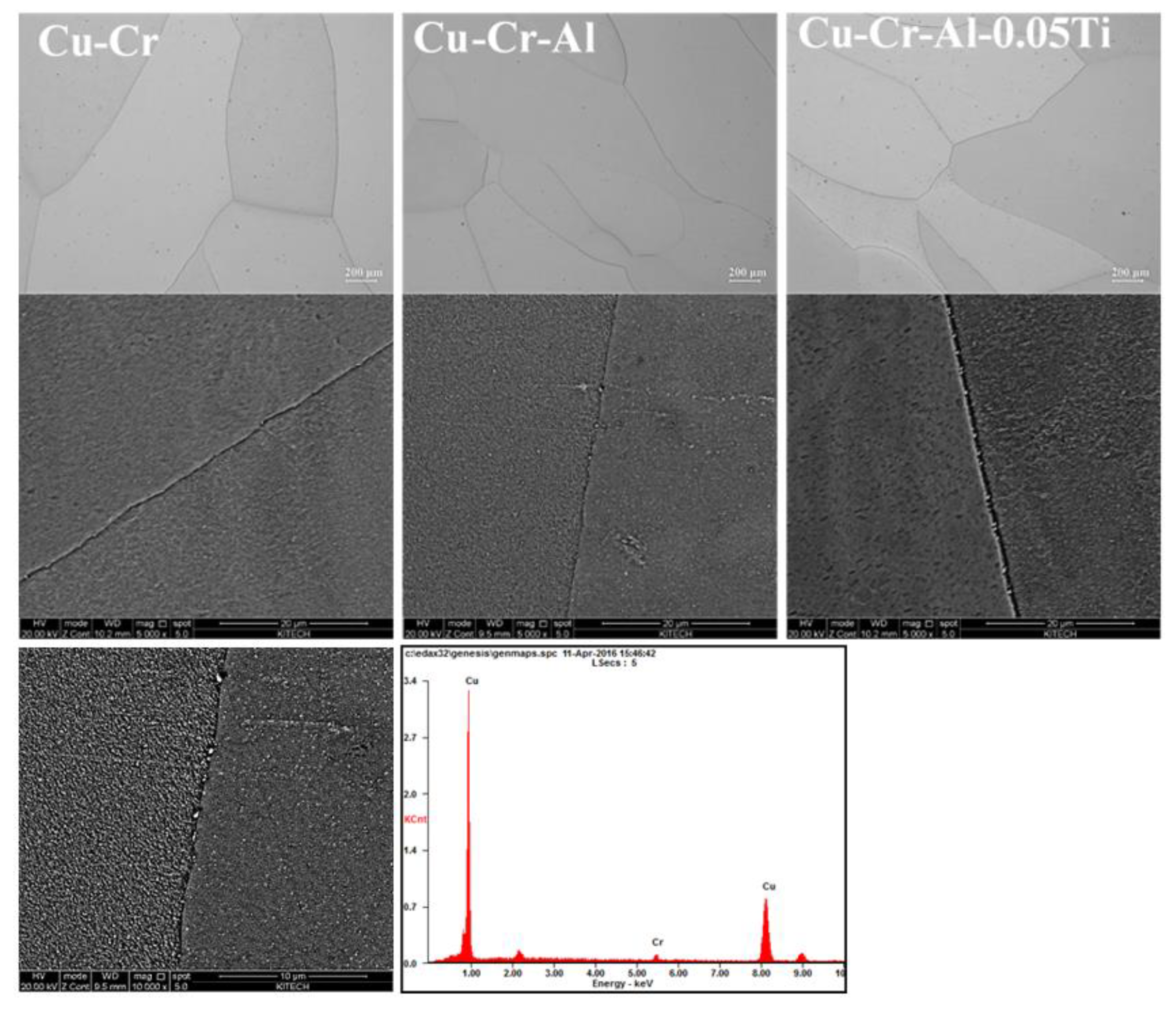

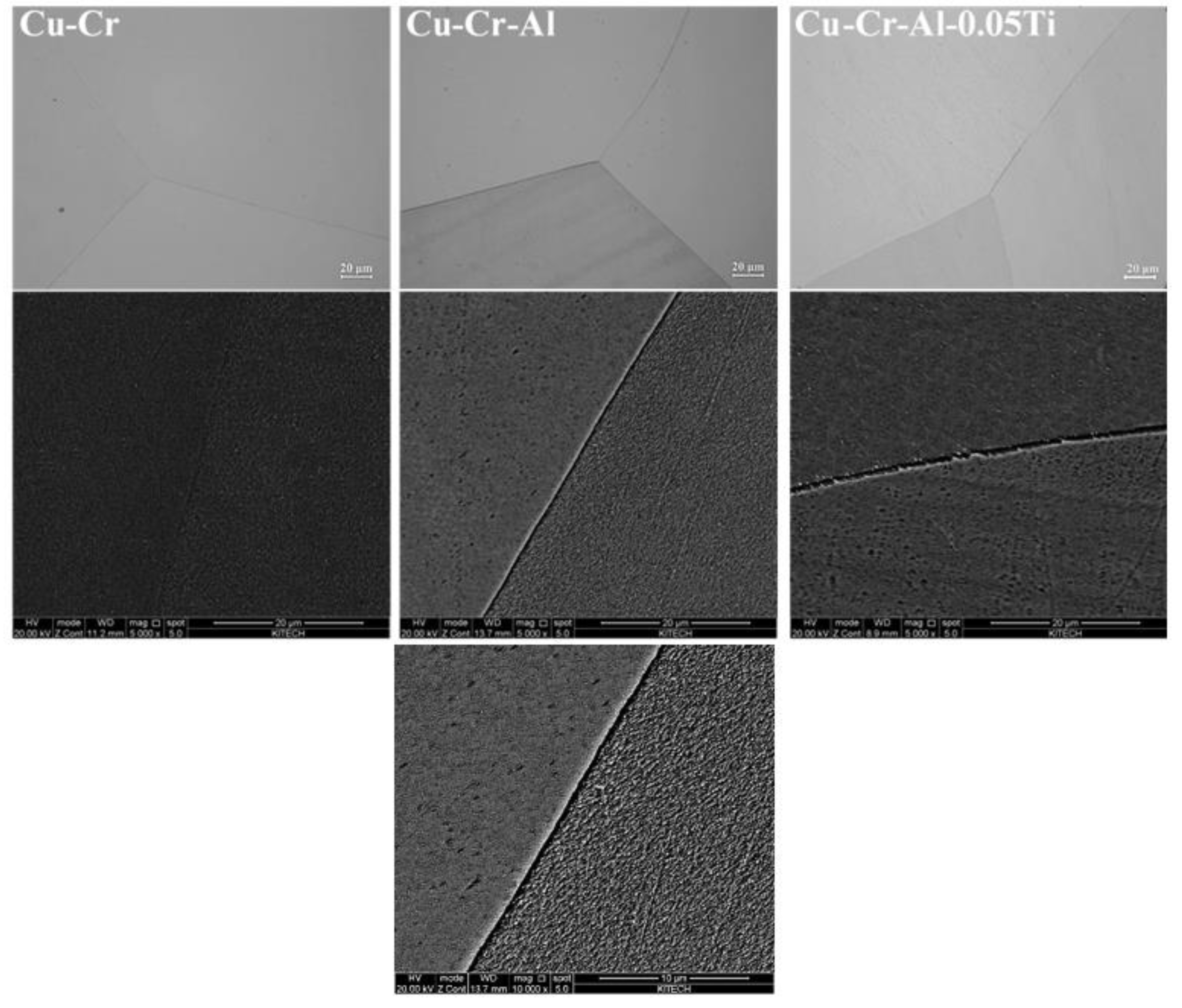




| Wt.% | Cr | Al | Ti | Cu |
|---|---|---|---|---|
| Cu-0.25Cr | 0.2677 | 0.0011 | 0.0004 | Bal. |
| Cu-0.25Cr-0.06Al | 0.2655 | 0.0548 | <0.0004 | Bal. |
| Cu-0.25Cr-0.06Al-0.015Ti | 0.253 | 0.0565 | 0.0189 | Bal. |
| Cu-0.25Cr-0.06Al-0.03Ti | 0.2604 | 0.0577 | 0.0264 | Bal. |
| Cu-0.25Cr-0.06Al-0.05Ti | 0.2438 | 0.0620 | 0.0507 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, C.-H.; Shin, J.; Kim, D.; Cho, H. Influence of Addition of Al and Ti Solutes and Variable Processing Conditions on Mechanical and Electrical Properties of Cu-Cr Alloys. Metals 2021, 11, 39. https://doi.org/10.3390/met11010039
Cho C-H, Shin J, Kim D, Cho H. Influence of Addition of Al and Ti Solutes and Variable Processing Conditions on Mechanical and Electrical Properties of Cu-Cr Alloys. Metals. 2021; 11(1):39. https://doi.org/10.3390/met11010039
Chicago/Turabian StyleCho, Chang-Hee, Jesik Shin, Dongearn Kim, and Hoon Cho. 2021. "Influence of Addition of Al and Ti Solutes and Variable Processing Conditions on Mechanical and Electrical Properties of Cu-Cr Alloys" Metals 11, no. 1: 39. https://doi.org/10.3390/met11010039
APA StyleCho, C.-H., Shin, J., Kim, D., & Cho, H. (2021). Influence of Addition of Al and Ti Solutes and Variable Processing Conditions on Mechanical and Electrical Properties of Cu-Cr Alloys. Metals, 11(1), 39. https://doi.org/10.3390/met11010039





