Discrimination, Coping, and DNAm Accelerated Aging Among African American Mothers of the InterGEN Study
Abstract
1. Introduction
1.1. Racial Discrimination as a Stressor
1.2. Coping with Stress
1.3. Biological Aging
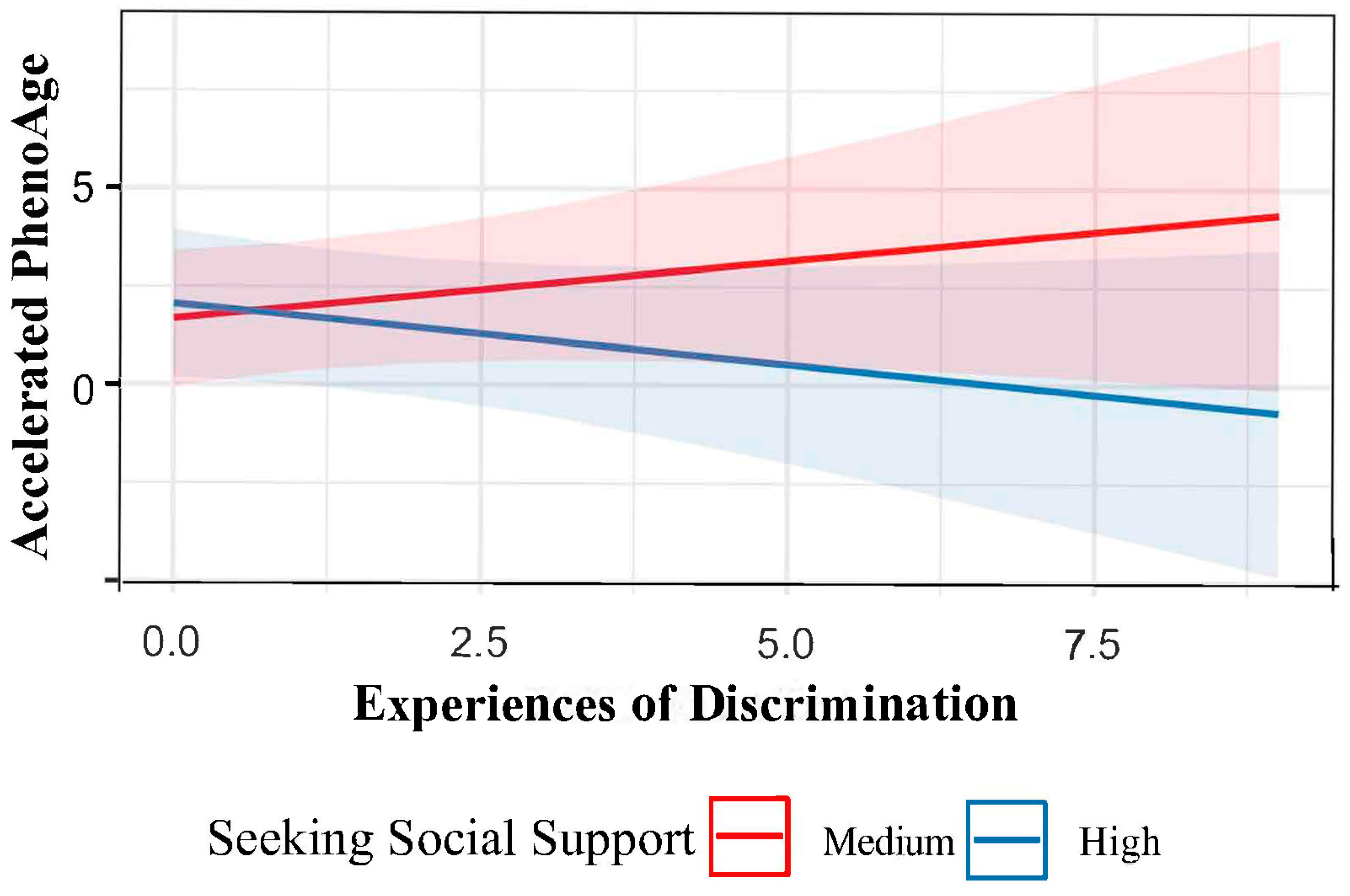
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design and Sample
4.2. Instruments and Measures
4.2.1. Perceived Discrimination
4.2.2. DNA Data Collection
4.3. DNA Methylation Preprocessing and Epigenetic Clock Calculation
DNAm Age Estimation
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACASI | Audio–Computer-Assisted Self-Interview |
| CSI | Coping Strategies Indicator |
| DNAm | DNA methylation |
| EOD | Experiences of Discrimination |
| InterGEN | Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure |
| IRB | Institutional Review Board |
| SD | Standard deviation |
References
- Hall, J.E.; Boulware, L.E. Combating Racism Through Research, Training, Practice, and Public Health Policies. Prev. Chronic Dis. 2023, 20, 230167. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Yu, Y.; Jackson, J.S.; Anderson, N.B. Racial Differences in Physical and Mental Health: Socio-Economic Status, Stress and Discrimination. J. Health Psychol. 1997, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, C.; Hurst, S. Implicit Bias in Healthcare Professionals: A Systematic Review. BMC Med. Ethics 2017, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Mohammed, S.A. Racism and Health I: Pathways and Scientific Evidence. Am. Behav. Sci. 2013, 57, 1152–1173. [Google Scholar] [CrossRef]
- Williams, D.R.; Mohammed, S.A. Discrimination and Racial Disparities in Health: Evidence and Needed Research. J. Behav. Med. 2009, 32, 20–47. [Google Scholar] [CrossRef]
- Alio, A.P.; Lewis, C.A.; Elder, H.; Norwood, W.; Mufhandu, K.; Keefer, M.C. Self-Reported Experiences of Racial Discrimination Among African Americans in Upstate New York. J. Black Stud. 2020, 51, 481–500. [Google Scholar] [CrossRef]
- Carter, R.T.; Forsyth, J. Reactions to racial discrimination: Emotional stress and help-seeking behaviors. Psychol. Trauma Theory Res. Pract. Policy 2010, 2, 183–191. [Google Scholar] [CrossRef]
- Essed, P. Understanding Everyday Racism: An Interdisciplinary Theory; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 1991. [Google Scholar] [CrossRef]
- Lewis, J.A.; Williams, M.G.; Peppers, E.J.; Gadson, C.A. Applying Intersectionality to Explore the Relations between Gendered Racism and Health among Black Women. J. Couns. Psychol. 2017, 64, 475–486. [Google Scholar] [CrossRef]
- Spates, K.; Evans, N.M.; Watts, B.C.; Abubakar, N.; James, T. Keeping Ourselves Sane: A Qualitative Exploration of Black Women’s Coping Strategies for Gendered Racism. Sex Roles 2020, 82, 513–524. [Google Scholar] [CrossRef]
- Black, L.L.; Johnson, R.; VanHoose, L. The Relationship Between Perceived Racism/Discrimination and Health Among Black American Women: A Review of the Literature from 2003 to 2013. J. Racial Ethn. Health Disparities 2015, 2, 11–20. [Google Scholar] [CrossRef]
- Harlow, S.D.; Burnett-Bowie, S.-A.M.; Greendale, G.A.; Avis, N.E.; Reeves, A.N.; Richards, T.R.; Lewis, T.T. Disparities in Reproductive Aging and Midlife Health between Black and White Women: The Study of Women’s Health Across the Nation (SWAN). Women’s Midlife Health 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Millender, E.; Barile, J.P.; Bagneris, J.R.; Harris, R.M.; De Faria, L.; Wong, F.Y.; Crusto, C.A.; Taylor, J.Y. Associations between Social Determinants of Health, Perceived Discrimination, and Body Mass Index on Symptoms of Depression among Young African American Mothers. Arch. Psychiatr. Nurs. 2021, 35, 94–101. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef]
- Vila, J. Social Support and Longevity: Meta-Analysis-Based Evidence and Psychobiological Mechanisms. Front. Psychol. 2021, 12, 717164. [Google Scholar] [CrossRef]
- Chida, Y.; Steptoe, A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension 2010, 55, 1026–1032. [Google Scholar] [CrossRef]
- Mariotti, A. The Effects of Chronic Stress on Health: New Insights into the Molecular Mechanisms of Brain–Body Communication. Future Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef]
- McEwen, B.S.; Seeman, T. Protective and Damaging Effects of Mediators of Stress: Elaborating and Testing the Concepts of Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef]
- Geronimus, A.T.; Hicken, M.; Keene, D.; Bound, J. “Weathering” and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States. Am. J. Public Health 2006, 96, 826–833. [Google Scholar] [CrossRef]
- Lim, S.; Nzegwu, D.; Wright, M.L. The Impact of Psychosocial Stress from Life Trauma and Racial Discrimination on Epigenetic Aging—A Systematic Review. Biol. Res. Nurs. 2022, 24, 202–215. [Google Scholar] [CrossRef]
- Belsky, D.W.; Baccarelli, A.A. To Promote Healthy Aging, Focus on the Environment. Nat. Aging 2023, 3, 1334–1344. [Google Scholar] [CrossRef]
- Geronimus, A.T.; Hicken, M.T.; Pearson, J.A.; Seashols, S.J.; Brown, K.L.; Cruz, T.D. Do US Black Women Experience Stress-Related Accelerated Biological Aging?: A Novel Theory and First Population-Based Test of Black-White Differences in Telomere Length. Hum. Nat. 2010, 21, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Polsky, L.R.; Rentscher, K.E.; Carroll, J.E. Stress-Induced Biological Aging: A Review and Guide for Research Priorities. Brain Behav. Immun. 2022, 104, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Graf, G.H.; Crowe, C.L.; Kothari, M.; Kwon, D.; Manly, J.J.; Turney, I.C.; Valeri, L.; Belsky, D.W. Testing Black-White Disparities in Biological Aging Among Older Adults in the United States: Analysis of DNA-Methylation and Blood-Chemistry Methods. Am. J. Epidemiol. 2022, 191, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef]
- Brown, K.M.; Hui, Q.; Huang, Y.; Taylor, J.Y.; Prescott, L.; Barcelona De Mendoza, V.; Crusto, C.; Sun, Y.V. Association Between Stress andCoping with DNA Methylation of Blood Pressure-Related Genes Among African American Women. Chronic. Stress 2019, 3, 2470547019879088. [Google Scholar] [CrossRef]
- Busse, D.; Yim, I.S.; Campos, B.; Marshburn, C.K. Discrimination and the HPA Axis: Current Evidence and Future Directions. J. Behav. Med. 2017, 40, 539–552. [Google Scholar] [CrossRef]
- Nuru-Jeter, A.; Dominguez, T.P.; Hammond, W.P.; Leu, J.; Skaff, M.; Egerter, S.; Jones, C.P.; Braveman, P. “It’s The Skin You’re In”: African-American Women Talk About Their Experiences of Racism. An Exploratory Study to Develop Measures of Racism for Birth Outcome Studies. Matern. Child Health J. 2009, 13, 29–39. [Google Scholar] [CrossRef]
- Bleich, S.N.; Findling, M.G.; Casey, L.S.; Blendon, R.J.; Benson, J.M.; SteelFisher, G.K.; Sayde, J.M.; Miller, C. Discrimination in the United States: Experiences of Black Americans. Health Serv. Res. 2019, 54, 1399–1408. [Google Scholar] [CrossRef]
- Condon, E.M.; Barcelona, V.; Ibrahim, B.B.; Crusto, C.A.; Taylor, J.Y. Racial Discrimination, Mental Health, and Parenting Among African American Mothers of Preschool-Aged Children. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 402–412. [Google Scholar] [CrossRef]
- Donovan, R.A.; West, L.M. Stress and Mental Health: Moderating Role of the Strong Black Woman Stereotype. J. Black Psychol. 2015, 41, 384–396. [Google Scholar] [CrossRef]
- Millender, E.; Harris, R.M.; Bagneris, J.R.; Marks, L.R.; Barcelona, V.; Wong, F.Y.; Crusto, C.A.; Taylor, J.Y. The Cumulative Influence of Perceived Discrimination, Stress, and Coping Responses on Symptoms of Depression Among Young African American Mothers. J. Am. Psychiatr. Nurses Assoc. 2024, 30, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal, and Coping; Springer Publishing Company: New York, NY, USA, 1984. [Google Scholar]
- Pearlin, L.I.; Schooler, C. The Structure of Coping. J. Health Soc. Behav. 1978, 19, 2–21. [Google Scholar] [CrossRef]
- Clark, R.; Anderson, N.B.; Clark, V.R.; Williams, D.R. Racism as a Stressor for African Americans: A Biopsychosocial Model. Am. Psychol. 1999, 54, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Holahan, C.J.; Ragan, J.D.; Moos, R.H. Stress. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Perry, J.S. Resilience in Black Women: Lifeline or Double-Edged Sword? J. Racial Ethn. Health Disparities 2024. [Google Scholar] [CrossRef]
- Pierce, G.R.; Sarason, B.R.; Sarason, I.G.; Joseph, H.J.; Henderson, C.A. Conceptualizing and Assessing Social Support in the Context of the Family. In Handbook of Social Support and the Family; Pierce, G.R., Sarason, B.R., Sarason, I.G., Eds.; Springer: Boston, MA, USA, 1996; pp. 3–23. [Google Scholar] [CrossRef]
- Seawell, A.H.; Cutrona, C.E.; Russell, D.W. The Effects of General Social Support and Social Support for Racial Discrimination on African American Women’s Well-Being. J. Black Psychol. 2014, 40, 3–26. [Google Scholar] [CrossRef]
- Shorter-Gooden, K. Multiple Resistance Strategies: How African American Women Cope with Racism and Sexism. J. Black Psychol. 2004, 30, 406–425. [Google Scholar] [CrossRef]
- Nyembwe, A.; Zhao, Y.; Caceres, B.A.; Hall, K.; Prescott, L.; Potts-Thompson, S.; Morrison, M.T.; Crusto, C.; Taylor, J.Y. Moderating effect of coping strategies on the association between perceived discrimination and blood pressure outcomes among young Black mothers in the InterGEN study. AIMS Public Health 2025, 12, 217–232. [Google Scholar] [CrossRef]
- Dhingra, R.; Nwanaji-Enwerem, J.C.; Samet, M.; Ward-Caviness, C.K. DNA Methylation Age—Environmental Influences, Health Impacts, and Its Role in Environmental Epidemiology. Curr. Environ. Health Rep. 2018, 5, 317–327. [Google Scholar] [CrossRef]
- Cecil, C.A.M.; Zhang, Y.; Nolte, T. Childhood Maltreatment and DNA Methylation: A Systematic Review. Neurosci. Biobehav. Rev. 2020, 112, 392–409. [Google Scholar] [CrossRef]
- De Mendoza, V.B.; Huang, Y.; Crusto, C.A.; Sun, Y.V.; Taylor, J.Y. Perceived Racial Discrimination and DNA Methylation Among African American Women in the InterGEN Study. Biol. Res. Nurs. 2018, 20, 145–152. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.-K.; Klopack, E.; Beach, S.R.H.; Gibbons, F.X.; Philibert, R.A. The Effects of Social Adversity, Discrimination, and Health Risk Behaviors on the Accelerated Aging of African Americans: Further Support for the Weathering Hypothesis. Soc. Sci. Med. 2021, 282, 113169. [Google Scholar] [CrossRef] [PubMed]
- Bakusic, J.; Schaufeli, W.; Claes, S.; Godderis, L. Stress, Burnout and Depression: A Systematic Review on DNA Methylation Mechanisms. J. Psychosom. Res. 2017, 92, 34–44. [Google Scholar] [CrossRef]
- Bayarsaihan, D. Epigenetic Mechanisms in Inflammation. J. Dent. Res. 2011, 90, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wielscher, M.; Mandaviya, P.R.; Kuehnel, B.; Joehanes, R.; Mustafa, R.; Robinson, O.; Zhang, Y.; Bodinier, B.; Walton, E.; Mishra, P.P.; et al. DNA Methylation Signature of Chronic Low-Grade Inflammation and Its Role in Cardio-Respiratory Diseases. Nat. Commun. 2022, 13, 2408. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef]
- Horvath, S.; Raj, K. DNA Methylation-Based Biomarkers and the Epigenetic Clock Theory of Ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-Wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An Epigenetic Biomarker of Aging for Lifespan and Healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA Methylation GrimAge Strongly Predicts Lifespan and Healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Arseneault, L.; Baccarelli, A.; Corcoran, D.L.; Gao, X.; Hannon, E.; Harrington, H.L.; Rasmussen, L.J.; Houts, R.; et al. Quantification of the Pace of Biological Aging in Humans through a Blood Test, the DunedinPoAm DNA Methylation Algorithm. eLife 2020, 9, e54870. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Corcoran, D.L.; Sugden, K.; Poulton, R.; Arseneault, L.; Baccarelli, A.; Chamarti, K.; Gao, X.; Hannon, E.; et al. DunedinPACE, a DNA Methylation Biomarker of the Pace of Aging. eLife 2022, 11, e73420. [Google Scholar] [CrossRef] [PubMed]
- Kusters, C.D.J.; Horvath, S. Quantification of Epigenetic Aging in Public Health. Annu. Rev. Public Health 2024, 46, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Moqri, M.; Herzog, C.; Poganik, J.R.; Ying, K.; Justice, J.N.; Belsky, D.W.; Higgins-Chen, A.T.; Chen, B.H.; Cohen, A.A.; Fuellen, G.; et al. Validation of Biomarkers of Aging. Nat. Med. 2024, 30, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Raffington, L.; Belsky, D.W. Integrating DNA Methylation Measures of Biological Aging into Social Determinants of Health Research. Curr. Environ. Health Rep. 2022, 9, 196–210. [Google Scholar] [CrossRef]
- Palma-Gudiel, H.; Fañanás, L.; Horvath, S.; Zannas, A.S. Psychosocial stress and epigenetic aging. Int. Rev. Neurobiol. 2020, 150, 107–128. [Google Scholar]
- Zannas, A.S.; Arloth, J.; Carrillo-Roa, T.; Iurato, S.; Röh, S.; Ressler, K.J.; Nemeroff, C.B.; Smith, A.K.; Bradley, B.; Heim, C.; et al. Lifetime Stress Accelerates Epigenetic Aging in an Urban, African American Cohort: Relevance of Glucocorticoid Signaling. Genome Biol. 2015, 16, 266. [Google Scholar] [CrossRef]
- Brody, G.H.; Miller, G.E.; Yu, T.; Beach, S.R.H.; Chen, E. Supportive Family Environments Ameliorate the Link Between Racial Discrimination and Epigenetic Aging: A Replication Across Two Longitudinal Cohorts. Psychol. Sci. 2016, 27, 530–541. [Google Scholar] [CrossRef]
- Higgins-Chen, A.T.; Thrush, K.L.; Wang, Y.; Minteer, C.J.; Kuo, P.-L.; Wang, M.; Niimi, P.; Sturm, G.; Lin, J.; Moore, A.Z.; et al. A Computational Solution for Bolstering Reliability of Epigenetic Clocks: Implications for Clinical Trials and Longitudinal Tracking. Nat. Aging 2022, 2, 644–661. [Google Scholar] [CrossRef]
- Katrinli, S.; Stevens, J.; Wani, A.H.; Lori, A.; Kilaru, V.; Van Rooij, S.J.H.; Hinrichs, R.; Powers, A.; Gillespie, C.F.; Michopoulos, V.; et al. Evaluating the Impact of Trauma and PTSD on Epigenetic Prediction of Lifespan and Neural Integrity. Neuropsychopharmacology 2020, 45, 1609–1616. [Google Scholar] [CrossRef]
- Smith, A.K.; Katrinli, S.; Cobb, D.O.; Goff, E.G.; Simmond, M.; Christensen, G.M.; Prusisz, T.; Garth, S.N.; Brashear, M.; Hüls, A.; et al. Epigenetic Age Acceleration and Disparities in Posttraumatic Stress in Women in Southeast Louisiana: NIMHD Social Epigenomics Program. JAMA Netw. Open 2024, 7, e2421884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- SteelFisher, G.K.; Findling, M.G.; Bleich, S.N.; Casey, L.S.; Blendon, R.J.; Benson, J.M.; Sayde, J.M.; Miller, C. Gender Discrimination in the United States: Experiences of Women. Health Serv. Res. 2019, 54, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Witherspoon, K.M.; Speight, S.L. Gendered Racism, Psychological Distress, and Coping Styles of African American Women. Cult. Divers. Ethn. Minor. Psychol. 2008, 14, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, S.K.; Meyer, I.H.; Overstreet, N.M.; Haile, R.; Hansen, N.B. Exploring Discrimination and Mental Health Disparities Faced by Black Sexual Minority Women Using a Minority Stress Framework. Psychol. Women Q. 2015, 39, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Beydoun, H.A.; Noren Hooten, N.; Maldonado, A.I.; Weiss, J.; Evans, M.K.; Zonderman, A.B. Epigenetic Clocks and Their Association with Trajectories in Perceived Discrimination and Depressive Symptoms among US Middle-Aged and Older Adults. Aging 2022, 14, 5311–5344. [Google Scholar] [CrossRef]
- Carter, S.E.; Ong, M.L.; Simons, R.L.; Gibbons, F.X.; Lei, M.K.; Beach, S.R.H. The Effect of Early Discrimination on Accelerated Aging among African Americans. Health Psychol. 2019, 38, 1010–1013. [Google Scholar] [CrossRef]
- Christian, L.M.; Wilson, S.; Madison, A.A.; Kamp Dush, C.M.; McDade, T.W.; Peng, J.; Andridge, R.R.; Morgan, E.; Manning, W.; Cole, S.W. Sexual Minority Stress and Epigenetic Aging. Brain Behav. Immun. 2025, 126, 24–29. [Google Scholar] [CrossRef]
- Cuevas, A.G.; Cole, S.W.; Belsky, D.W.; McSorley, A.-M.; Shon, J.M.; Chang, V.W. Multi-Discrimination Exposure and Biological Aging: Results from the Midlife in the United States Study. Brain Behav. Immun.—Health 2024, 39, 100774. [Google Scholar] [CrossRef]
- Dhingra, R.; Hillmann, A.R.; Reed, R.G. Major Experiences of Perceived Discrimination across Life and Biological Aging. Psychoneuroendocrinology 2025, 174, 107380. [Google Scholar] [CrossRef]
- Ruiz-Narváez, E.A.; Cozier, Y.; Zirpoli, G.; Rosenberg, L.; Palmer, J.R. Perceived Experiences of Racism in Relation to Genome-Wide DNA Methylation and Epigenetic Aging in the Black Women’s Health Study. J. Racial Ethn. Health Disparitie 2024, 12, 754–763. [Google Scholar] [CrossRef]
- Liu, D.; Aziz, N.A.; Pehlivan, G.; Breteler, M.M. Cardiovascular correlates of epigenetic aging across the adult lifespan: A population-based study. Geroscience 2023, 45, 1605–1618. [Google Scholar] [CrossRef]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.; O’Halloran, A.M.; Fallon, P.; Kenny, R.A.; McCrory, C. Metabolic syndrome accelerates epigenetic ageing in older adults: Findings from The Irish Longitudinal Study on Ageing (TILDA). Exp. Gerontol. 2023, 183, 112314. [Google Scholar] [CrossRef] [PubMed]
- Woods-Giscombé, C.L. Superwoman Schema: African American Women’s Views on Stress, Strength, and Health. Qual. Health Res. 2010, 20, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Wang, Y.; Chae, D.H.; Price, M.M.; Powell, W.; Steed, T.C.; Rose Black, A.; Dhabhar, F.S.; Marquez-Magaña, L.; Woods-Giscombe, C.L. Racial Discrimination, the Superwoman Schema, and Allostatic Load: Exploring an Integrative Stress-coping Model among African American Women. Ann. N. Y. Acad. Sci. 2019, 1457, 104–127. [Google Scholar] [CrossRef]
- Watson, N.N.; Hunter, C.D. “I Had To Be Strong”: Tensions in the Strong Black Woman Schema. J. Black Psychol. 2016, 42, 424–452. [Google Scholar] [CrossRef]
- Beauboeuf-Lafontant, T. You Have to Show Strength: An Exploration of Gender, Race, and Depression. Gend. Soc. 2007, 21, 28–51. [Google Scholar] [CrossRef]
- Albert, M.A.; Slopen, N.; Williams, D.R. Cumulative Psychological Stress and Cardiovascular Disease Risk: A Focused Review with Consideration of Black-White Disparities. Curr. Cardiovasc. Risk Rep. 2013, 7, 318–325. [Google Scholar] [CrossRef]
- Sims, M.; Glover, L.S.M.; Gebreab, S.Y.; Spruill, T.M. Cumulative Psychosocial Factors Are Associated with Cardiovascular Disease Risk Factors and Management among African Americans in the Jackson Heart Study. BMC Public Health 2020, 20, 566. [Google Scholar] [CrossRef]
- Morton, P.M.; Schafer, M.H.; Ferraro, K.F. Does Childhood Misfortune Increase Cancer Risk in Adulthood? J. Aging Health 2012, 24, 948–984. [Google Scholar] [CrossRef]
- Slopen, N.; Dutra, L.M.; Williams, D.R.; Mujahid, M.S.; Lewis, T.T.; Bennett, G.G.; Ryff, C.D.; Albert, M.A. Psychosocial Stressors and Cigarette Smoking Among African American Adults in Midlife. Nicotine Tob. Res. 2012, 14, 1161–1169. [Google Scholar] [CrossRef]
- Slopen, N.; Kontos, E.Z.; Ryff, C.D.; Ayanian, J.Z.; Albert, M.A.; Williams, D.R. Psychosocial Stress and Cigarette Smoking Persistence, Cessation, and Relapse over 9–10 Years: A Prospective Study of Middle-Aged Adults in the United States. Cancer Causes Control 2013, 24, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Martz, C.D.; Benner, A.D.; Goosby, B.J.; Mitchell, C.; Gaydosh, L. Structural Racism in Primary Schools and Changes in Epigenetic Age Acceleration among Black and White Youth. Soc. Sci. Med. 2024, 347, 116724. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Mode, N.A.; Noren Hooten, N.; Pacheco, N.L.; Ezike, N.; Zonderman, A.B.; Evans, M.K. Association of Race and Poverty Status with DNA Methylation–Based Age. JAMA Netw. Open 2023, 6, e236340. [Google Scholar] [CrossRef]
- Heiss, J.A.; Just, A.C. Identifying Mislabeled and Contaminated DNA Methylation Microarray Data: An Extended Quality Control Toolset with Examples from GEO. Clin. Epigenet. 2018, 10, 73. [Google Scholar] [CrossRef]
- Sugden, K.; Hannon, E.J.; Arseneault, L.; Belsky, D.W.; Corcoran, D.L.; Fisher, H.L.; Houts, R.M.; Kandaswamy, R.; Moffitt, T.E.; Poulton, R.; et al. Patterns of Reliability: Assessing the Reproducibility and Integrity of DNA Methylation Measurement. Patterns 2020, 1, 100014. [Google Scholar] [CrossRef]
- Koch, Z.; Li, A.; Evans, D.S.; Cummings, S.; Ideker, T. Somatic mutation as an explanation for epigenetic aging. Nat. Aging 2025, 5, 709–719. [Google Scholar] [CrossRef]
- Capili, B. Cross-Sectional Studies. AJN Am. J. Nurs. 2021, 121, 59–62. [Google Scholar] [CrossRef]
- Shields, A.E.; Zhang, Y.; Argentieri, M.A.; Warner, E.T.; Cozier, Y.C.; Liu, C.; Dye, C.K.; Kent, B.V.; Baccarelli, A.A.; Palmer, J.R. Stress and Spirituality in Relation to HPA Axis Gene Methylation Among US Black Women: Results from the Black Women’s Health Study and the Study on Stress, Spirituality and Health. Epigenomics 2021, 13, 1711–1734. [Google Scholar] [CrossRef]
- Watkins, S.H.; Testa, C.; Chen, J.T.; De Vivo, I.; Simpkin, A.J.; Tilling, K.; Diez Roux, A.V.; Davey Smith, G.; Waterman, P.D.; Suderman, M.; et al. Epigenetic Clocks and Research Implications of the Lack of Data on Whom They Have Been Developed: A Review of Reported and Missing Sociodemographic Characteristics. Environ. Epigenet. 2023, 9, dvad005. [Google Scholar] [CrossRef]
- Crusto, C.A.; Barcelona De Mendoza, V.; Connell, C.M.; Sun, Y.V.; Taylor, J.Y. The Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure Study (InterGEN): Design and Methods for Recruitment and Psychological Measures. Nurs. Res. 2016, 65, 331–338. [Google Scholar] [CrossRef]
- Taylor, J.Y.; Wright, M.L.; Crusto, C.A.; Sun, Y.V. The Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure (InterGEN) Study: Design and Methods for Complex DNA Analysis. Biol. Res. Nurs. 2016, 18, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.; Smith, K.; Naishadham, D.; Hartman, C.; Barbeau, E.M. Experiences of Discrimination: Validity and Reliability of a Self-Report Measure for Population Health Research on Racism and Health. Soc. Sci. Med. 2005, 61, 1576–1596. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, B.L.; Elm, J.H.L.; Hallgren, K.A. Understanding measures of racial discrimination and microaggressions among American Indian and Alaska Native college students in the Southwest United States. BMC Public Health 2021, 21, 1099. [Google Scholar] [CrossRef] [PubMed]
- Amirkhan, J.H. A Factor Analytically Derived Measure of Coping: The Coping Strategy Indicator. J. Personal. Soc. Psychol. 1990, 59, 1066–1074. [Google Scholar] [CrossRef]
- Nunes, A.P.; Oliveira, I.O.; Santos, B.R.; Millech, C.; Silva, L.P.; González, D.A.; Hallal, P.C.; Menezes, A.M.B.; Araújo, C.L.; Barros, F.C. Quality of DNA Extracted from Saliva Samples Collected with the OrageneTM DNA Self-Collection Kit. BMC Med. Res. Methodol. 2012, 12, 65. [Google Scholar] [CrossRef]
- Bibikova, M.; Barnes, B.; Tsan, C.; Ho, V.; Klotzle, B.; Le, J.M.; Delano, D.; Zhang, L.; Schroth, G.P.; Gunderson, K.L.; et al. High density DNA methylation array with single CpG site resolution. Genomics 2011, 98, 288–295. [Google Scholar] [CrossRef]
- Moran, S.; Martínez-Cardús, A.; Sayols, S.; Musulén, E.; Balañá, C.; Estival-Gonzalez, A.; Moutinho, C.; Heyn, H.; Diaz-Lagares, A.; de Moura, M.C.; et al. Epigenetic profiling to classify cancer of unknown primary: A multicentre, retrospective analysis. Lancet Oncol. 2016, 17, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Graff, J.R.; Myöhänen, S.; Nelkin, B.D.; Baylin, S.B. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. USA 1996, 93, 9821–9826. [Google Scholar] [CrossRef]
- Zinn, R.L.; Pruitt, K.; Eguchi, S.; Baylin, S.B.; Herman, J.G. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007, 67, 194–201. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Triche, T.J.; Hansen, K.D. Preprocessing, Normalization and Integration of the Illumina HumanMethylationEPIC Array with Minfi. Bioinformatics 2017, 33, 558–560. [Google Scholar] [CrossRef]
- Hill, C.V.; Pérez-Stable, E.J.; Anderson, N.A.; Bernard, M.A. The National Institute on Aging Health Disparities Research Framework. Ethn. Dis. 2015, 25, 245–254. [Google Scholar] [CrossRef]


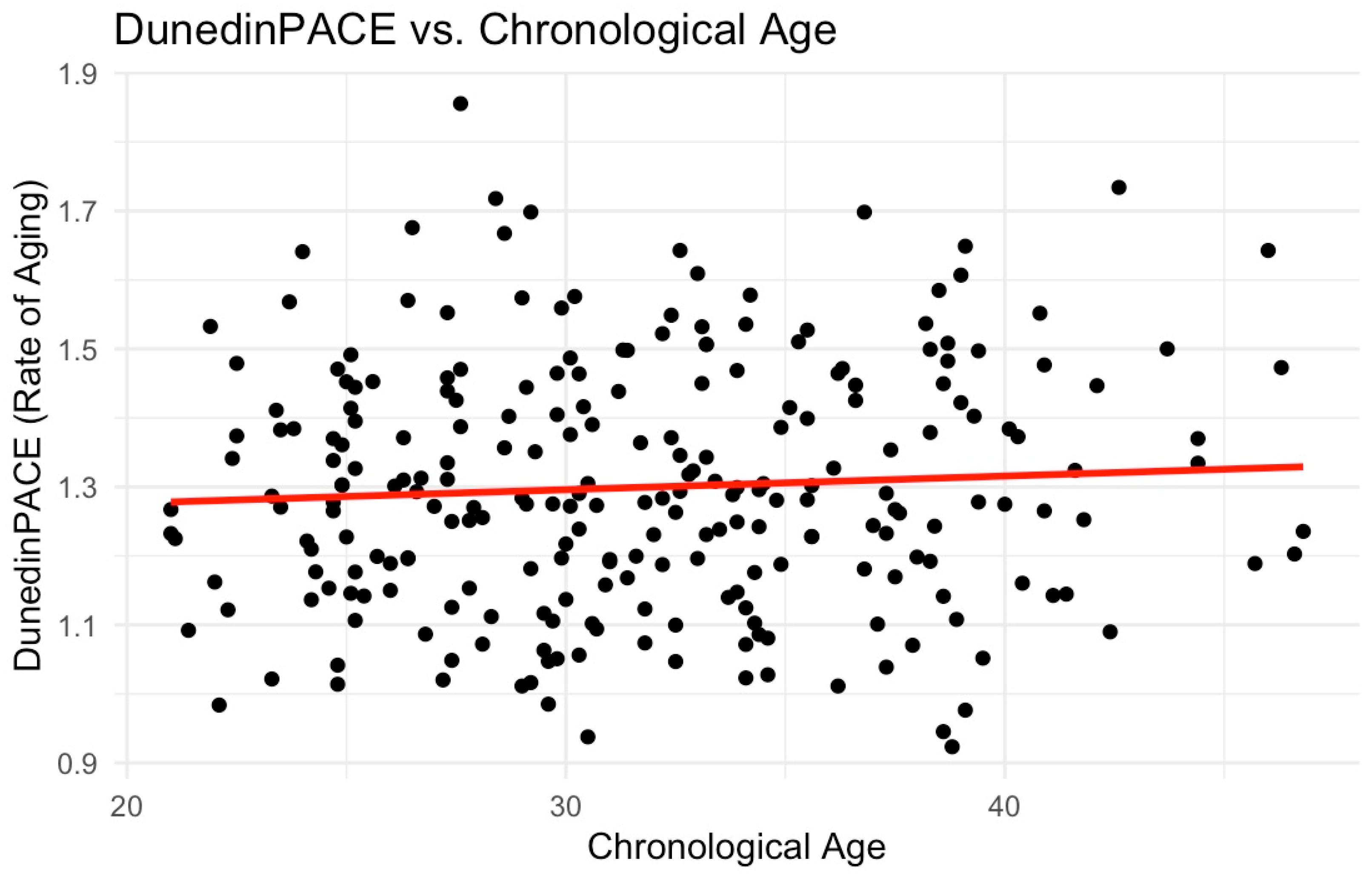
| Sample Characteristics (n = 234) | Mean (SD) n (%) | Median [Min, Max] |
|---|---|---|
| Maternal Age | 31.9 (5.80) | 31.3 [21.0, 46.8] |
| Body Mass Index | 29.6 (8.13) | 28.6 [13.7, 59.0] |
| Systolic Blood Pressure | 14 (13.5) | 113 [81.3, 163] |
| Diastolic Blood Pressure | 73.0 (10.6) | 72.0 [50.0, 110] |
| Smoking Status | ||
| No | 181 (77.4%) | |
| Yes | 53 (22.6%) | |
| Number of children | 2.56 (1.54) | 2.00 [1, 10.0] |
| Child Age | 4.14 (0.78) | 4.10 [3.00, 5.90] |
| Experiences of Discrimination, Situation | 1.45 (1.93) | 1.00 [0, 9.00] |
| Seeking Social Support | 21.7 (7.2) | 20.8 [11, 33] |
| PCHorvath1 | PCHorvath2 | PCHannum | PCPhenoAge | PCGrimAge | DunedinPACE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| (Intercept) | −1.18 (−2.76–0.40) | 0.1 | −1.57 (−3.15–0.00) | 0.5 | −0.46 (−2.01–1.09) | 0.6 | −1.69 (−3.21–−0.17) | 0.03 | −1.39 (−2.83–0.06) | 0.6 | −1.81 (−3.31–−0.31) | 0.02 |
| Experiences of Discrimination | 0.11 (−0.14–0.36) | 0.4 | 0.05 (−0.20–0.31) | 0.7 | 0.21 (−0.04–0.45) | 0.1 | 0.26 (0.02–0.50) | 0.03 | 0.03 (−0.20–0.26) | 0.8 | 0.00 (−0.24–0.24) | 1.0 |
| Social Support | 0.00 (−0.02–0.03) | 0.7 | 0.01 (−0.02–0.03) | 0.6 | −0.00 (−0.03–0.02) | 0.9 | 0.01 (−0.02–0.03) | 0.6 | −0.00 (−0.02–0.02) | 0.9 | 0.00 (−0.02–0.03) | 0.7 |
| Experiences of Discrimination × Social Support | −0.00 (−0.02–0.01) | 0.4 | −0.00 (−0.01–0.01) | 0.5 | −0.01 (−0.02–0.00) | 0.8 | −0.01 (−0.02–−0.00) | 0.03 | −0.00 (−0.01–0.01) | 0.8 | −0.00 (−0.01–0.01) | 0.8 |
| Observations | 234 | 234 | 234 | 234 | 234 | 234 | ||||||
| R2/R2 adjusted | 0.046/−0.011 | 0.049/−0.007 | 0.079/0.024 | 0.115/0.063 | 0.213/0.167 | 0.149/0.099 | ||||||
| PCHorvath1 | PCHorvath2 | PCHannum | PCPhenoAge | PCGrimAge | DunedinPACE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| (Intercept) | −1.14 (−2.59–0.31) | 0.12 | −1.46 (−2.90–−0.01) | 0.05 | −0.68 (−2.12–0.76) | 0.35 | −1.73 (−3.15–−0.32) | 0.02 | −1.44 (−2.76–−0.11) | 0.03 | −1.71 (−3.08–−0.33) | 0.02 |
| Experiences of Discrimination | −0.01 (−0.08–0.06) | 0.85 | −0.03 (−0.10–0.04) | 0.46 | −0.01 (−0.08–0.06) | 0.77 | 0.00 (−0.06–0.07) | 0.92 | 0.00 (−0.06–0.07) | 0.95 | −0.03 (−0.09–0.04) | 0.45 |
| Observations | 234 | 234 | 234 | 234 | 234 | 234 | ||||||
| R2/R2 adjusted | 0.042/−0.006 | 0.047/0.000 | 0.058/0.011 | 0.093/0.049 | 0.213/0.174 | 0.148/0.106 | ||||||
| PCHorvath1 | PCHorvath2 | PCHannum | PCPhenoAge | PCGrimAge | DunedinPACE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| (Intercept) | −1.10 (−2.66–0.46) | 0.17 | −1.54 (−3.10–0.02) | 0.05 | −0.30 (−1.85–1.24) | 0.70 | −1.49 (−3.01–0.03) | 0.06 | −1.36 (−2.79–0.06) | 0.06 | −1.82 (−3.30–−0.33) | 0.02 |
| Seeking Social Support | −0.00 (−0.02–0.02) | 0.86 | 0.00 (−0.02–0.02) | 0.88 | −0.01 (−0.03–0.01) | 0.19 | −0.01 (−0.03–0.01) | 0.41 | −0.00 (−0.02–0.02) | 0.79 | 0.00 (−0.02–0.02) | 0.79 |
| Observations | 234 | 234 | 234 | 234 | 234 | 234 | ||||||
| R2/R2 adjusted | 0.042/−0.006 | 0.045/−0.002 | 0.065/0.019 | 0.096/0.051 | 0.213/0.174 | 0.147/0.104 | ||||||
| PCHorvath1 | PCHorvath2 | PCHannum | PCPhenoAge | PCGrimAge | DunedinPACE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p | Estimates | p |
| (Intercept) | −1.09 (−2.66–0.48) | 0.17 | −1.51 (−3.07–0.05) | 0.06 | −0.29 (−1.84–1.26) | 0.71 | −1.49 (−3.02–0.03) | 0.55 | −1.36 (−2.79–0.07) | 0.06 | −1.79 (−3.28–−0.30) | 0.02 |
| Experiences of Discrimination | −0.01 (−0.08–0.06) | 0.85 | −0.03 (−0.10–0.04) | 0.46 | −0.01 (−0.08–0.06) | 0.81 | 0.00 (−0.06–0.07) | 0.89 | 0.00 (−0.06–0.07) | 0.94 | −0.03 (−0.09–0.04) | 0.45 |
| Seeking Social Support | −0.00 (−0.02–0.02) | 0.87 | 0.00 (−0.02–0.02) | 0.85 | −0.01 (−0.03–0.01) | 0.19 | −0.01 (−0.03–0.01) | 0.41 | −0.00 (−0.02–0.02) | 0.78 | 0.00 (−0.02–0.02) | 0.76 |
| Observations | 234 | 234 | 234 | 234 | 234 | 234 | ||||||
| R2/R2 adjusted | 0.042/−0.010 | 0.047/−0.004 | 0.065/0.015 | 0.096/0.047 | 0.213/0.170 | 0.149/0.103 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyembwe, A.; Zhao, Y.; Caceres, B.A.; Belsky, D.W.; Ryan, C.P.; Taylor, B.; Morrison, M.T.; Prescott, L.; Potts-Thompson, S.; Aziz, A.; et al. Discrimination, Coping, and DNAm Accelerated Aging Among African American Mothers of the InterGEN Study. Epigenomes 2025, 9, 14. https://doi.org/10.3390/epigenomes9020014
Nyembwe A, Zhao Y, Caceres BA, Belsky DW, Ryan CP, Taylor B, Morrison MT, Prescott L, Potts-Thompson S, Aziz A, et al. Discrimination, Coping, and DNAm Accelerated Aging Among African American Mothers of the InterGEN Study. Epigenomes. 2025; 9(2):14. https://doi.org/10.3390/epigenomes9020014
Chicago/Turabian StyleNyembwe, Alexandria, Yihong Zhao, Billy A. Caceres, Daniel W. Belsky, Calen Patrick Ryan, Brittany Taylor, Morgan T. Morrison, Laura Prescott, Stephanie Potts-Thompson, Arezo Aziz, and et al. 2025. "Discrimination, Coping, and DNAm Accelerated Aging Among African American Mothers of the InterGEN Study" Epigenomes 9, no. 2: 14. https://doi.org/10.3390/epigenomes9020014
APA StyleNyembwe, A., Zhao, Y., Caceres, B. A., Belsky, D. W., Ryan, C. P., Taylor, B., Morrison, M. T., Prescott, L., Potts-Thompson, S., Aziz, A., Aruleba, F., Matute-Arcos, E., Williams, O., Crusto, C., & Taylor, J. Y. (2025). Discrimination, Coping, and DNAm Accelerated Aging Among African American Mothers of the InterGEN Study. Epigenomes, 9(2), 14. https://doi.org/10.3390/epigenomes9020014







