A Systematic Review of Aircraft Disinsection Efficacy
Simple Summary
Abstract
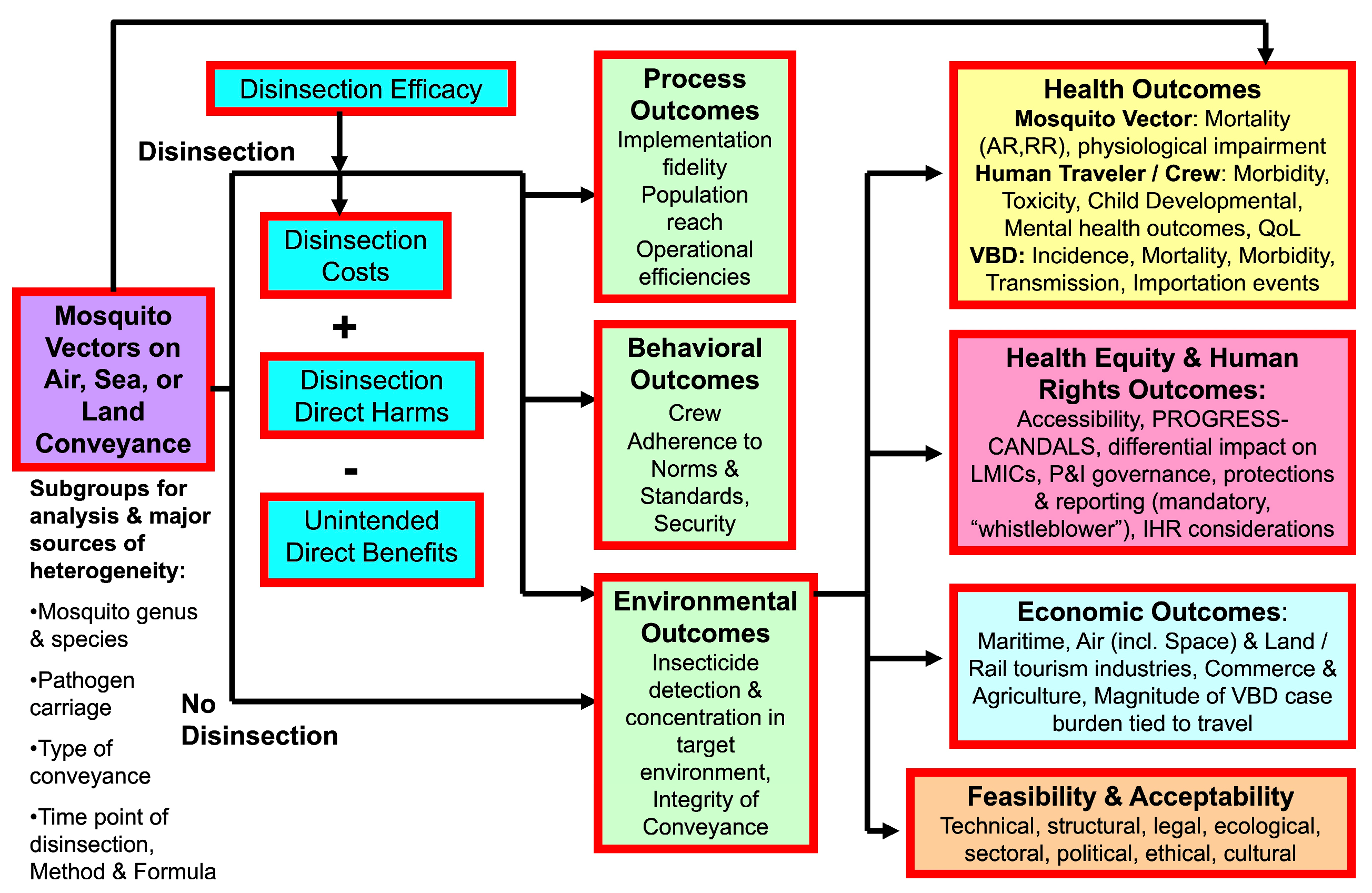
1. Introduction
2. Materials and Methods
| Question: What is the effectiveness of disinsection of international travel carriers versus no disinsection is (passenger chamber, cargo area, cargoes, air-cans or containers) of all modes of transportation (air, water, and land transport) to prevent or reduce the spread of mosquito vectors via international travel? | |
| Population | Mosquitoes (by species) |
| Intervention | Disinsection of international travel carriers (passenger chamber, cargo area, cargoes, air-cans, or containers) of all modes of transportation (air, water, land, and transport) (by chemical [18] or non-chemical agent, method used, and other). |
| Comparator | No disinsection of international travel carriers (passenger chamber, cargo area, cargoes, air-cans, or containers) of all modes of transportation (air, water, and land transport) |
| Outcome | (i) No or reduced number of mosquitoes on aircrafts (passenger chamber, flight deck, cargo area, air-cans, or containers) and cargoes (water or land transport) |
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Outcomes
2.4. Search Strategy
2.5. Data Analysis
2.6. Risk of Bias and Certainty of Evidence
3. Results
3.1. Literature Search
3.2. Included Studies
3.3. Efficacy of Disinsection
3.4. Summary of Methodological Quality and Risk of Bias Assessment

| (a) | ||||||||
| Author (Year) | Study Design | Country Setting | Conveyance | Species * | Sample Size ° | Insecticide Used | Formulation | Disinsection Method |
| Berger-Preiss (2006) [28] | Experimental Trial with comparator arm ‡ | Germany | Grounded passenger aircraft (Airbus A310 and Boeing 747-400) | Ae. aegypti, Anopheles stephensi, Culex pipiens | Total: 9566; Exposed: 8921; Unexposed: 645; Initial: 1849; Residual: 7717; A.a. 4219; A.s. 1177; C.p. 4170 | d-phenothrin | 2% d-phenothrin | Simulated pre-flight and top-of-descent spraying |
| Brooke (1971) [32] | Experimental Trial with comparator arm ‡ | UK | Grounded passenger aircraft (De Havilland Comet 4C) | Ae. aegypti | Total: 8000; Exposed: 7200; Unexposed: 800 | Bioresmethrin, resmethrin, pyrethrins, DDT, bioallethrin, Tropital | Bioresmethrin: 0.05%, 0.075%, 0.10%, 0.25%; resmethrin: 0.10%, 0.25%, 0.50%; 0.40% pyrethrins + 3% DDT; 0.45% pyrethrins 0.45% + 2.7% Tropital | Simulated blocks-away with no passenger present |
| Cawley (1974) [33] | Experimental Trial | US | Commercial passenger aircraft (Boeing 707, Boeing 727) | Culex pipiens fatigans | ND | Bioresmethrin, resmethrin, S-2539 Forte | Bioresmethrin: 2% with 5% ethanol; resmethrin: 0.30%, 1.20%, and 2% with 5% ethanol; S-2539 Forte: 0.30%, 1.20%, and 2% | Blocks-away |
| Jakob (1972) [29] | Experimental Trial | US | Empty trailer trucks and unoccupied propeller-driven passenger aircrafts | Ae. aegypti, Anopheles albimanus, Anopheles quadrimaculatus | ND | Bromophos, carbaryl, chlorpyrifos, DDT, d-trans allethrin, fenitrothion, fenthion, gardona, mobam, Propoxur, pyrethrins, resmethrin, G-1707, G-1729, G-1730, G-1731 | Micronized Dusts: 46.40% bromophos; 10% and 40% chlorpyrifos; 20% chlorpyrifos + 12.80% resmethrin; 13.30% chlorpyrifos + 8.50% resmethrin + 21.30% propoxur; 10% chlorpyrifos + 6.40% resmethrin + 16% propoxur + 20% gardona; 42.50% DDT + 42.50% carbaryl; 14% d-trans allethrin; 26.10% fenitrothion; 20.20% fenthion; 80% gardona; 83.30% mobam; 64% propoxur; 2.80% pyrethrins; 17% and 25.50% resmethrin; Aerosols: G-1707 (2.25% pyrethrins + 2.70% Tropital); G-1729 (2.25% pyrethrins + 2.70% sulfoxide); G-1730 (11% d-trans allethrin); G-1731; 7.50% resmethrin | Simulated trials of residual and pre-flight spraying, without passengers on board |
| Jensen (1965) [44] | Experimental Trial | US | Commercial passenger aircraft (DC-6B) | An. quadrimaculatus | ND | Dichlorvos vapour | Air concentration ranged from 0.13–0.25 ug/L dichlorvos | Disinsection anytime while aircraft is closed, and ventilation system is on |
| Langsford 1976 [34] | Experimental Trial with comparator arm ‡ | Australia | Passenger aircraft (Boeing 747) | Cx. fatigans | Total: 330; Exposed: 260; Unexposed: 70 | Pyrethrins | 0.40% pyrethrins + 1.60% piperonyl butoxide, with 10% iso-paraffin solvents and Freon 11 + 12 as propellants | Blocks-away followed by saturation after disembarking |
| Liljedahl (1976) [35] | Experimental Trial with comparator arm ‡ | US | Commercial passenger aircraft (Boeing 707, Boeing 727) | Ae. aegypti, Aedes taeniorhynchus, An. quadrimaculatus, An. stephensi, Cx. pipiens fatigans | Total: 5773; Exposed: 4677; Unexposed: 1096; A.a. 662; A.t. 2483; A.q. 1757; A.s. 351; C.p.f. 520 | d-phenothrin | 2% (+)-phenothrin in a 3:17 ratio of Freon-11 to 12 (break-off tip cans) and 2% (+)-phenothrin in a 1:1 ratio of Freon-11 to 12 (340 g cans with vertical release valves) | Blocks-away |
| Mackie (1938) [43] | Experimental Trial | UK | Passenger aircraft (Imperial flying boat) | ND | ND | Deskito (pyrethrum) | Pyrethrum water-based (1:14) insecticide with paraffin | Immediately after take-off |
| Ong (2018) [30] | Experimental Trial | Australia | Simulated aircraft environment | Ae. aegypti with 996P/1023G kdr mutation | ND | Permethrin | 0.20 g/m2 as target dose of permethrin | Residual treatment |
| Pimentel (1954) [31] | Experimental Trial | US | Commercial aircraft (Convair-240, DC-3) | Ae. aegypti | ~200 | DDT, lindane | Formulation not specified; insecticides were dissolved in methylcyclohexane | Residual treatment |
| Russell (1984) [36] | Experimental Trials ^ | Australia | Passenger aircraft (Boeing 707, Boeing 747) | Culex quinquefasciatus | 1975–1976: ND; 1978: ND; 1980: 1500 | d-phenothrin, pyrethrins | 1975–1976: 0.40% pyrethrins + 1.60% piperonyl butoxide; 1978: 2% d-phenothrin 2%; 0.40% pyrethrins + 1.60% piperonyl butoxide; 0.40% pyrethrins + 1.60% piperonyl butoxide + 0.40% d-phenothrin; 1980: 2% d-phenothrin | Blocks-away |
| Russell (1989) [42] | Experimental Trials # | Australia | Passenger aircraft (Boeing 747, Boeing 767) | Cx. quinquefasciatus | 20 per test site with 10–12 test sites per flight | d-phenothrin | 2% d-phenothrin | Top-of-descent and on-arrival spraying |
| Sullivan (1962) [37] | Experimental Trial with comparator arm ‡ | Italy, Switzerland, UK, US | Passenger aircraft (Boeing 707, Caravelle, Comet 4B, DC-6, DC-8, Viscount) | Ae. aegypti, Anopheles gambiae, An. stephensi, Cx. fatigans | Total: 7855; Exposed: 6574; Unexposed: 1281; A.a. 3157; A.g. 243; A.s.: 1065; C.f. 3390 | Pyrethrum extract(s), pyrethrins, DDT | SRA: 1.60% pyrethrum extract (25% pyrethrins), 3% DDT, 7.50% xylene, 2.90% odourless petroleum distillate, 42.50% Freon-12, 42.50% Freon-11; G-1480: 3.40% pyrethrum extract (20% pyrethrins), 1.17% DDT, 4.50% aromatic petroleum derivative solvents, 63.62% Freon-12, 27.31% Freon-11 | Blocks-away |
| Sullivan (1964) [38] | Experimental Trial | Fiji, New Zealand, Philippines | Passenger aircraft (DC-3, DC-7C, DC-8, Fokker, Viscount,) | Ae. aegypti, Ae. albopictus, Cx. fatigans | ND | DDT, G-1492, pyrethrum extract(s), SRA | SRA: 1.60% pyrethrum extract (25% pyrethrins), 3% DDT, 7.50% xylene, 2.90% odourless petroleum distillate, 42.50% Freon-12, 42.50% Freon-11; G-1492: 6% pyrethrum extract (20% pyrethrins), 2% DDT, 8% xylene, 58.80% Freon-12, 25.20% Freon-11 | Blocks-away |
| Sullivan (1972) [39] | Experimental Trial with comparator arm ‡ | US (WHO) | Commercial jet passenger aircraft (Boeing 747, Boeing 707, BAC 111, CD-8, DC-9) | Ae. aegypti, Anopheles litoralis, An. stephensi, Culex molestus, Cx. pipiens fatigans, Culex pipiens pallens | Total: 5076; Exposed: 4308; Unexposed: 768; A.a. 2035; A.l. 138; A.s. 207; C.m. 198; C.p.f. 2223; C.p.p. 275 | Bioresmethrin, G-1707, resmethrin, pyrethrum extract(s), Tropital, (+)-trans-allethrin | Resmethrin: 1.12% and 2.25% aerosols; bioresmethrin: 1% and 2% aerosols; (+)-trans-allethrin: 1.11% and 2.22% aerosols; G-1707: 2.25% pyrethrum extract (20% pyrethrins, 2.70% Tropital synergist, 10.05% petroleum distillate, 59.90% Freon-12, 25.50% Freon-11 | Blocks-away |
| Sullivan (1974) [27] | Experimental Trial with comparator arm ‡ | US | Tractor trailers and commercial aircraft (Boeing 707, Boeing 727) | Ae. aegypti, An. quadrimaculatus | Total: 1162; Exposed: 602; Unexposed: 560; Tractors: 450; Airplanes: 712; A.a. 701; A.q. 461 | d-phenothrin | 1.20% d-phenothrin and 2% d-phenothrin (both in propellants Freon 11 + 12 50:50) | Blocks-away without passengers |
| Sullivan (1975) [40] | Experimental Trial with comparator arm ‡ | US | Jet passenger aircraft (C-141, Lockheed) | Cx. quinquefasciatus | Total: 378; Exposed: 315; Unexposed: 63 | d-trans-resmethrin, resmethrin | 1.20% resmethrin and 98.66% propellants 11 + 12 (ratios 50:50 and 30:70); 1.20% d-trans resmethrin and 98.67% propellants 11 + 12 (50:50) | Blocks-away |
| Sullivan (1978) [41] | Experimental Trial with comparator arm ‡ | US | Jet aircrafts for pilot training (Lockheed) | Ae. taeniorhynchus, An. quadrimaculatus | Total: 453; Exposed: 285; Unexposed: 168; A.t. 132; A.q. 321 | d-phenothrin | Water-based: 2.03% (+)-phenothrin (98.5%), 0.87% Span 80, 0.03% Tween 60, 30% propellants (80% isobutane, 20% propane), 67.07% deionized water; Freon-based: 2.09% (+)-phenothrin (95.8%), 97.91% propellants (1:1 Freon 11 + 12) | Blocks-away |
| Tew (1951) [45] | Experimental Trial | UK | Grounded Argonaut and Tudor type 2 Aircraft | Ae. aegypti | 200 | DDT, pyrethrins | CMR 1: 0.40% pyrethrins and 3% DD; CMR 2: 1.20% pyrethrins and 2% DDT; CMR 3: 0.40% pyrethrins + 2% DDT + 3% piperonyl butoxide; CMR 4: 0.40% pyrethrins and 3% piperonyl butoxide; Am MS: 1.20% pyrethrins and 2% DDT; Am. IS: 1.20% pyrethrins and 2% DDT | Simulated spraying in grounded aircraft, not specified |
| (b) | ||||||||
| Author (Year) | Insecticide Resistance | Mosquito Mortality | Adherence to WHO Disinsection Guidelines ** | |||||
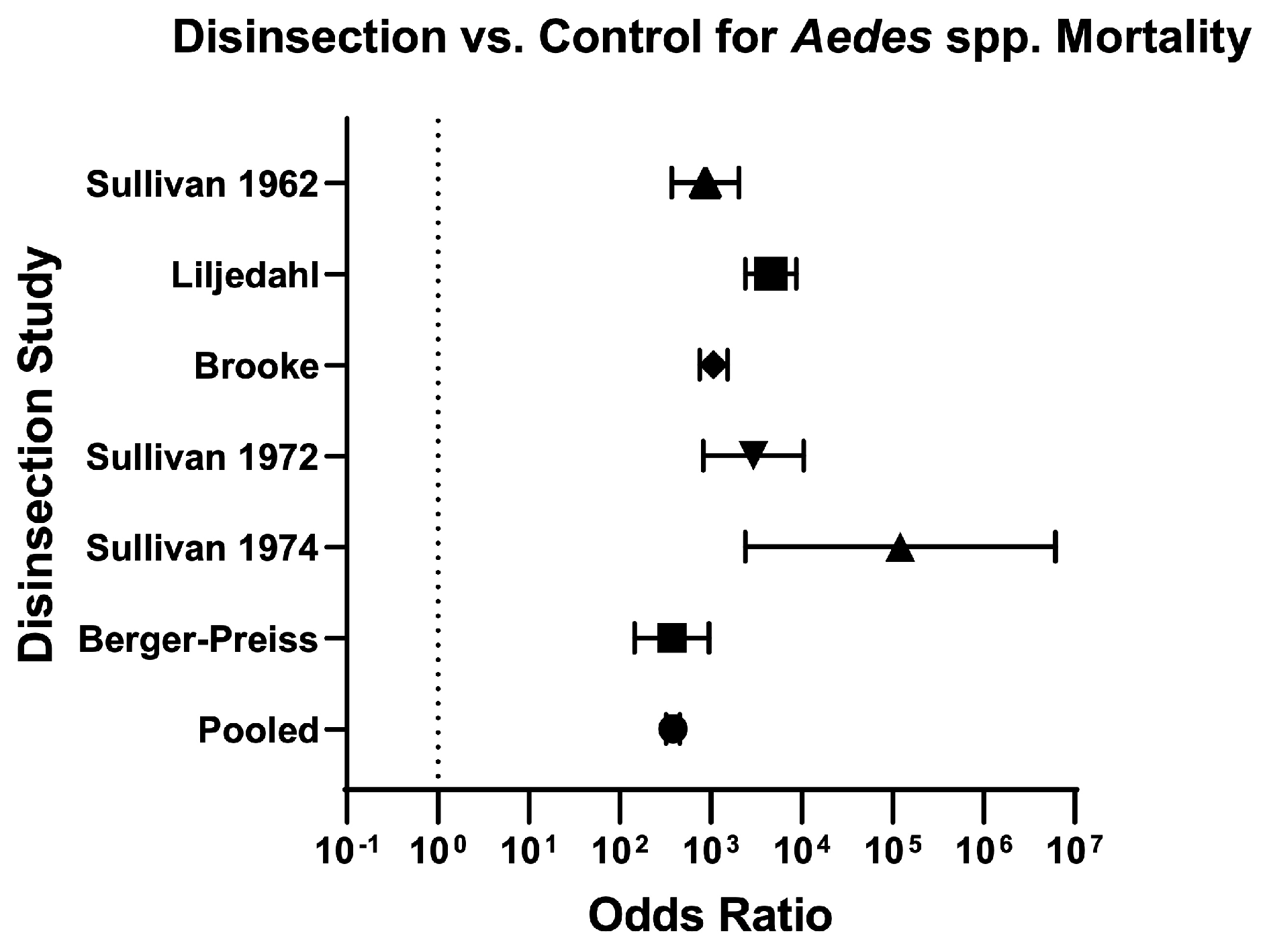
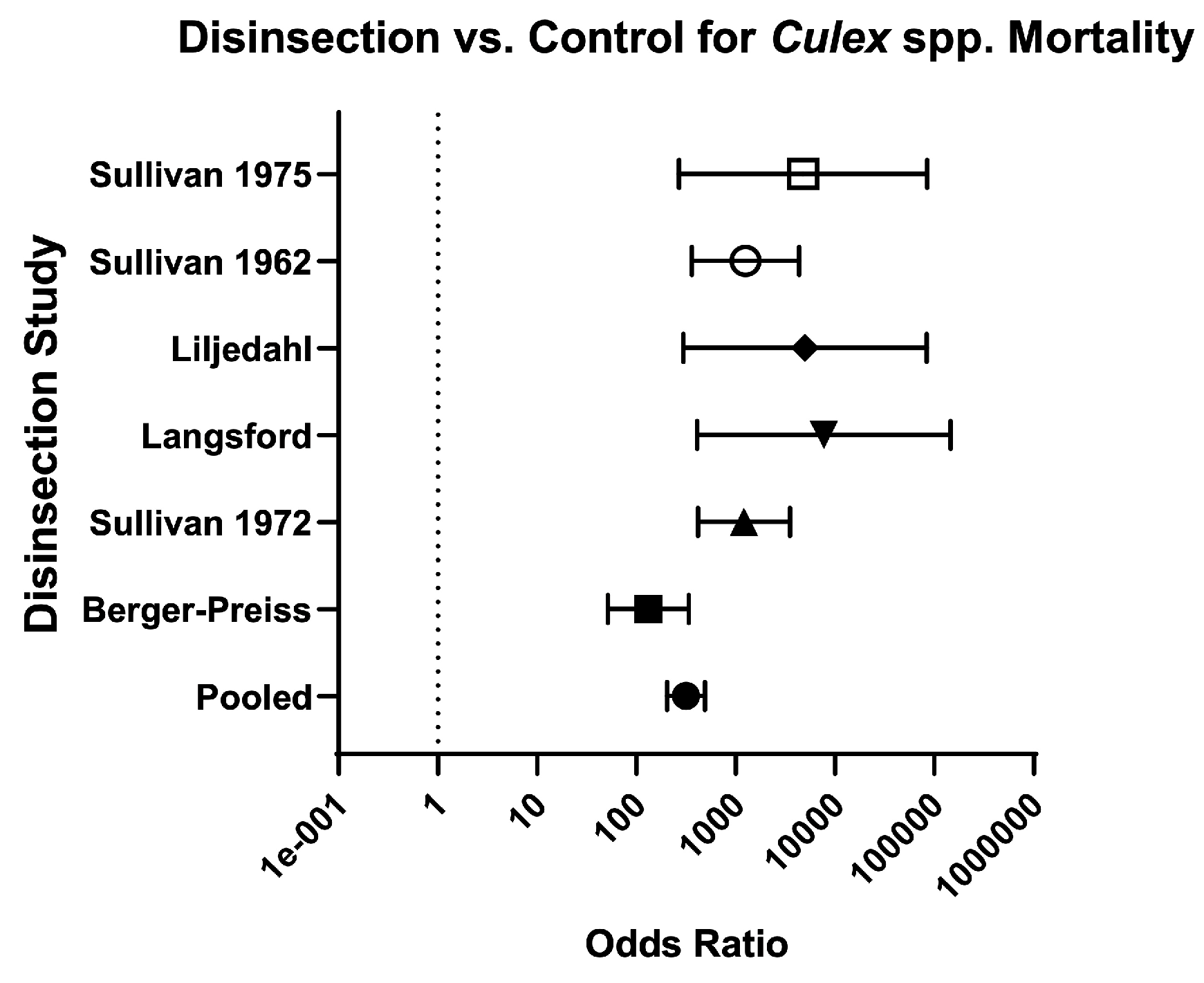
| Berger-Preiss (2006) [28] | Twenty minutes after spraying (pre-embarkation method), mortality was 94–99.50% for Ae. aegypti and 100% for An. Stephensi. Residual efficacy of disinsection, assessed between 7–48 h after spraying, yielded mosquito mortality between 89–100% on horizontal surfaces and 13–100% on vertical surfaces. Mortality was 0–6% in control mosquitoes. | 4/22 (18.18%) | ||||||
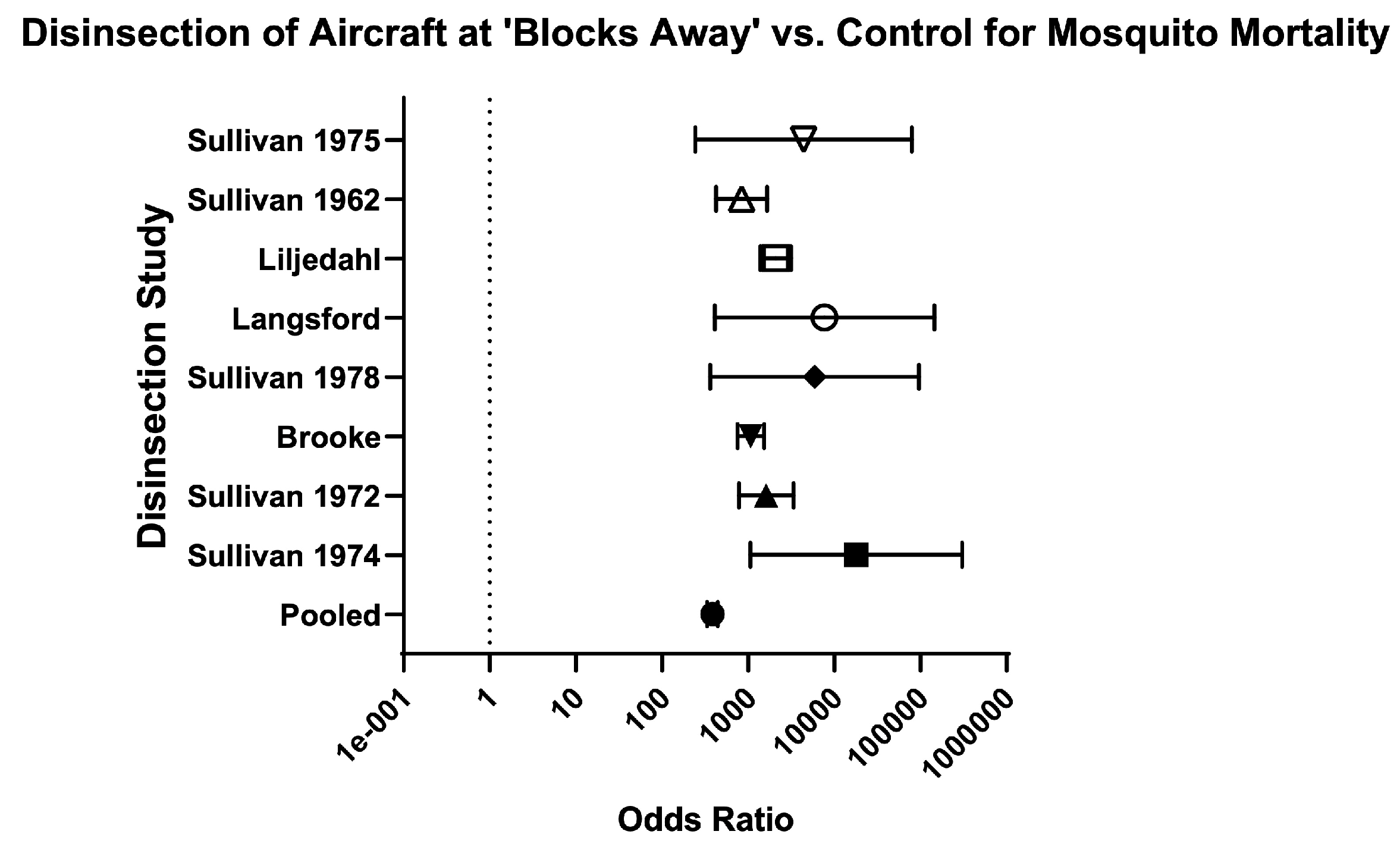
| Brooke (1971) [32] | Disinsection with any insecticide yielded a mean mosquito mortality of 97–100%, compared with 12% in control studies. Mean mosquito mortality by insecticide was as follows: 97% for 0.05% bioresmethrin, 98% for 0.075% bioresmethrin, 99% for 0.10% bioresmethrin, 100% for 0.25% bioresmethrin, 99% for 0.10% resmethrin, 100% for 0.25% resmethrin, 100% for 0.50% resmethrin, 100% for 0.40% pyrethrins + 3% DDT, and 100% for 0.45% pyrethrins + 2.70% Tropital. | 6/16 (37.50%) | ||||||
| Cawley (1974) [33] | Cx. pipiens mortality was tested on seats, floors, and rack positions. Mean mosquito mortality across positions was as follows: 0.30% resmethrin (99.23, 65.42, 29.33), 0.30% S-2539 Forte (86.17, 61.92, 23.95), 1.20% resmethrin (100, 100, 0), 1.20% S-2539 Forte (91.73, 72.47, 71.36), 2% resmethrin (100, 97.69, 96.35), 2% S-2539 Forte (100, 100, 96.81), and 2% bioresmethrin (100, 100, 100). | 5/16 (31.25%) | ||||||
| Jakob (1972) [29] | All aerosol formulations achieved 100% mosquito mortality in both truck trailers and aircrafts. Direct application of micronized dusts 40% chlorpyrifos, 17% and 25.50% resmethrin, and 20% chlorpyrifos + 12.80% resmethrin achieved 100% mosquito mortality in both truck trailers and aircrafts. Tests of 64% propoxur and 2.80% pyrethrins achieved 100% mosquito mortality in truck trailers and 100% mortality in the front and center positions on aircrafts; however, mortality was decreased in rear positions (propoxur achieved 0–95% mortality in rear positions and pyrethrins achieved 46–49% in rear positions). Direct application of micronized dusts 83.30% mobam, 10% chlorpyrifos, 46.40% bromophos, 26.10% fenitrothion, 20.20% fenthion, 14% d-trans allethrin, 42.50% DDT + 42.50% carbaryl, 10% chlorpyrifos + 6.40% resmethrin + 16% propoxur + 20% gardona, and 13.30% chlorpyrifos + 8.50% resmethrin + 21.30% propoxur achieved 100% mosquito mortality in truck trailers. Mobam achieved 99% mortality and gardona achieved 88–100% mortality in truck trailers. Residual treatment with micronized dusts 40% chlorpyrifos, 25.50% resmethrin, 20% chlorpyrifos + 12.80% resmethrin, 10% chlorpyrifos + 6.40% resmethrin + 16% propoxur + 20% gardona, and 83.30% and mobam achieved 100% mosquito mortality in truck trailers. Tests of 64% propoxur achieved 100% mortality of Anopheles but only 80–96% mortality of Aedes, 17% resmethrin achieved 75–86% mortality of Anopheles and 0–70% mortality of Aedes, and 2.80% pyrethrins achieved 0–5% mortality in both species. Mortality was ‘negligible’ except three tests with 10–21% mortality of Ae. albimanus. | 5.5/16 (34.38%) | ||||||
| Jensen (1965) [44] | 100% mortality of An. quadrimaculatus mosquitoes was achieved on all six 30 min flights in all tested compartments (pilot compartment, seat racks, galley, and baggage compartments). No mortalities occurred in control specimens. | 2.5/10 (25%) | ||||||
| Langsford 1976 [34] | Initial inflight spray at the end of the landing roll achieved 100% mortality in all stations at 12 and 24 h. Initial inflight spray followed by a second saturation spray after passengers disembarked also achieved 100% mortality in all stations at 12 and 24 h. Control mosquito mortality was 0% at 12 h and 5.71% at 24 h (four out of 70 control mosquitoes found dead). Authors suggest this was expected as mosquitoes had been in cups for 36 h by this point. | 5/16 (31.25%) | ||||||
| Liljedahl (1976) [35] | In a Boeing-727, application of 2% (+)-phenothrin from the break-off tip can achieved 100% mortality of An. quadrimaculatus and 98–100% mortality of Ae. taeniorhynchus. Application from the 340-g vertical-release can achieved 98–100% mortality of An. quadrimaculatus and 93–100% mortality of Ae. taeniorhynchus. Mortality of control mosquitoes was 12–13% of An. quadrimaculatus and 0–6% of Ae. taeniorhynchus. In a Boeing-707, application from the 340-g vertical-release can achieved 89–100% mortality of An. stephensi, 93–100% mortality of Ae. aegypti, and 82–100% mortality of Cx. pipiens fatigans. Mortality of control mosquitoes was 0% of An. stephensi, 1% of Ae. aegypti, and 0% of Cx. pipiens fatigans. | 6/17 (35.29%) | ||||||
| Mackie (1938) [43] | In one experiment, 100% mosquito mortality was achieved within 2–11 min of spraying. In a second experiment, all but two mosquitoes were dead by 24 h; the two mosquitos alive at 24 h died “an hour or so later.” | 4.5/16 (28.13%) | ||||||
| Ong (2018) [30] | Resistant Aedes aegypti colony at 100% 996P/1023G kdr mutation frequencies was used as a proxy for mosquitoes intercepted at Australian airports. No mortality data reported for resistant mosquito colony. | Bioassays performed on permeable surfaces with 0.20 g/m2 permethrin achieved mortality of <50% in susceptible mosquitoes exposed for 30 min. Patchily treated environments typical of treated aircraft cabins and holds do not result in the universal exposure of mosquitoes in simulated environments. | N/A °° | |||||
| Pimentel (1954) [31] | DDT applied to baggage compartments did not provide satisfactory killing of mosquitoes (75% mortality one week after treatment, down to 18% mortality five weeks after treatment). Lindane, at various concentrations, applied to baggage and passenger compartments achieved 98–100% mortality up to five weeks after treatment and 83–100% mortality up to eight weeks after treatment. | 2.5/9 (27.77%) | ||||||
| Russell (1984) [36] | 1975–1976 trials (0.40% pyrethrins + 1.60% piperonyl butoxide): B707 trial (November 1975, Auckland/Sydney) achieved 100% mosquito mortality. B747 trials (November 1975, Auckland/Sydney, and March 1976, Melbourne/Sydney) achieved less than 100% mortality mosquito (actual percentage not specified); 1978 trials (2% d-phenothrin, 0.40% pyrethrins + 1.60% piperonyl butoxide, and 0.40% pyrethrins + 1.60% piperonyl butoxide + 0.40% d-phenothrin): in a parked B747 in the Sydney airport, 100% mortality was observed after 18 h in all but “one exception.” Authors report “virtually 100% mosquito mortality.” No stratification by insecticide formulation reported; 1980 trials (2% d-phenothrin): in B747 Standard and Combi aircrafts, 99.80% (1497/1500) mosquito mortality was achieved at 24 h at fixed stations. Eleven single “wild cups,” or randomly placed cups aiming to target less accessible locations in the aircraft, achieved 100% mortality (N not specified). Control mosquitoes had 0% mortality in 38 stations and 10–30% mortality in 8 stations (N not specified). | 6/17 (35.29%) | ||||||
| Russell (1989) [42] | February 1986 trials: B747-300 from Singapore to Sydney, disinsected with 2% d-phenothrin via top-of-descent spraying with the air conditioning on, achieved 100% mortality of Culex mosquitoes; 1986 trials: B747-200 from Singapore to an unspecified Australia airport, disinsected with 2% d-phenothrin via on-arrival spraying with the air conditioning on, achieved 100% mortality of Culex mosquitoes; July 1987 trials: B767 from Sydney to Brisbane, disinsected with 2% d-phenothrin via top-of-descent spraying, achieved 100% mortality of mosquitoes. | 11.5/19 (60.50%) | ||||||
| Sullivan (1962) [37] | On London flights, DDT-resistant and susceptible Ae. aegypti were used. SRA achieved 0–33% mortality in DDT-resistant strains (compared to 81–100% mortality in susceptible strains). G-1480 achieved 0% mortality in one cage and 100% mortality in two cages of DDT-resistant mosquitoes (compared to 0% mortality in one cage and 100% mortality in four cages of susceptible strains). In the cage with 0% mortality of both resistant and susceptible mosquitoes, it was placed directly in front of an air inlet; On Rome flights, DDT-resistant Cx. fatigans were used. SRA achieved 0–100% mortality in one flight, 58–100% mortality in another flight, and 33–87% mortality in a training flight. G-1480 achieved 100% mortality in one flight and 27–65% mortality in a training flight (only 3/5 intended dosage used on the training flight). | G-1480 achieved 100% mortality of susceptible and resistant mosquitoes in all but two trials (in one trial, only 3/5 of proper dosage was used; in another trial, the mosquito cages with decreased mortality were placed directly in front of an air inlet). Control mosquito mortality was between 0–5%; SRA achieved 90–100% mortality in most trials of susceptible mosquitoes. SRA failed to achieve adequate mortality of DDT-resistant Aedes or Culex mosquitoes (see Insecticide Resistance column). Control mosquito mortality was between 0–4%. | 8.5/18 (47.22%) | |||||
| Sullivan (1964) [38] | In the Philippines trials (Philippine mosquitos), Aedes had an increased tolerance to DDT (2–3 times the level of normal strains), as determined by susceptibility testing. Cx. fatigans were presumed resistant to DDT. G-1492 was more effective than SRA against resistant Philippine mosquitoes, achieving 100% mortality in 4/5 flights versus 100% mortality in 9/14 flights using SRA. | G-1492 was more effective than SRA against resistant Philippine Aedes and Culex mosquitoes. G-1492 achieved 100% mortality in 4/5 flights (84–94% in remaining one flight). SRA achieved 100% mortality in 9/14 flights, 96–100% mortality in 2/9 flights, and 40–100% mortality in 2/9 flights; G-1492 and SRA both achieved 100% mortality in susceptible Fiji Culex mosquitoes. Controls “in general” had 0–25% mortality, but was reported as high as 36–57% in “very few tests.” | 6.5/17 (38.24%) | |||||
| Sullivan (1972) [39] | Cx. pipiens fatigans resistant to DDT: 2% d-trans-allethrin achieved 100% mortality in the cabin and lavatory, and 0% mortality in the cockpit. G-1707 achieved 81% mortality in the cabin; Cx. pipiens fatigans resistant to organophosphates: 2% resmethrin achieved 99% mortality in the cabin. | Authors arbitrarily selected morality of 97% as an acceptable level for the cabin. Only three of average mortality levels were lower than 97% (1% resmethrin at 94.20%, 1% d-trans-allethrin at 96.40%, and G-1707 at 94.90%) and in each case the confidence interval contained the 97% point; Mortality in lavatories was acceptable with 1% resmethrin and 2% d-trans-allethrin (100%). Mortality in lavatories was not acceptable with 2% resmethrin (43.8%), 1% d-trans-allethrin (85.2%), 1% bioresmethrin (30%), or G-1707 (75%). There was no acceptable mortality in the cockpit when tested (2% resmethrin achieved 33.30% mortality and 2% d-trans-allethrin and 2% bioresmethrin both achieved 0% mortality). Control mosquito mortality was 0% in all trials except for three, with mortality ranging from 4–8%. In authors’ opinion, 2% resmethrin aerosol at blocks-away appears to be the optimal procedure for disinsecting aircraft. | 6.5/18 (36.11%) | |||||
| Sullivan (1974) [27] | 100% mortality of An. quadrimaculatus was achieved in aircraft and tractor trailers with 1.20% phenothrin; 0% mortality in controls. 100% mortality of Ae. aegypti was also achieved in tractor trailers with 1.20% phenothrin; however, 79% mortality was seen in controls and invalidated the results for Ae. aegypti; 100% mortality of Ae. aegypti and An. quadrimaculatus was achieved in Boeing aircraft (707 and 727) with 2% phenothrin. Control mosquito mortality was 0% for Ae. aegypti and 8% for An. quadrimaculatus. | 5.5/19 (28.94%) | ||||||
| Sullivan (1975) [40] | 100% mortality of Culex mosquitoes was achieved on all three flights with use of 1.20% resmethrin and 1.20% d-trans-resmethrin. Control mosquito mortality ranged from 0–25% (0% on two flights, 8% on one flight, and 25% on one flight). | 7.5/18 (41.66%) | ||||||
| Sullivan (1978) [41] | 100% mortality of Ae. taeniorhynchus and An. quadrimaculatus was achieved in all five trials with mosquitoes (two water-based aerosols at blocks-away, two freon-based aerosols at blocks-away, and one freon-based aerosol on a grounded aircraft). Control mosquito mortality was 6% in Ae. taeniorhynchus and 14% in An. quadrimaculatus. | 5/18 (27.77%) | ||||||
| Tew (1951) [45] | In the Heathrow experiments with caged mosquitoes, Am. MS achieved 71–85% mortality, CMR 1 achieved 83–100% mortality, CMR 2 achieved 85–100% mortality, and Am. IS achieved 85–100% mortality. Control mosquito mortality was observed at 36% in experiments 1–6 and 40% in experiments 7–10. In the Farnborough experiments, both caged and free-flying mosquitoes were used. On the first day of experiments with caged mosquitoes, CMR 1 (dose reduced from 15 g/1000 ft3 to either 5 or 10 g/1000 ft3) achieved 99.50–100% mortality and CMR 3 achieved 100% mortality. On the second day of experiments with caged mosquitoes, CMR 1 (dose reduced to 5 g/1000 ft3) achieved 46% mortality with a closure time of 3 min and 69% mortality with a closure time of 5 min. CMR 4 achieved 65% mortality (closure time 5 min). On both days with free-flying mosquitoes, CMR 1 achieved 100% mortality (four tests), CMR 3 achieved 100% mortality, and CMR 4 achieved 99.50% mortality. Caged control mosquito mortality was observed at 0–2% in mosquitoes exposed in aircraft and 0–7% in mosquitoes in untreated aircraft. Higher mortality was noted with 5-min closure versus 3-min closure. Higher mortality was observed in free-flying mosquitoes versus caged mosquitoes. | 5.5/16 (34.38%) | ||||||
| Insecticide Compared to Control (No Insecticide) During Disinsection of Conveyances | ||||||||||||
| Population: mosquitoes | ||||||||||||
| Setting: aircrafts | ||||||||||||
| Intervention: disinsection | ||||||||||||
| Comparison: no disinsection | ||||||||||||
| Outcome: mosquito mortality | ||||||||||||
| Study Design: experimental trial with non-exposed (control) comparator group | ||||||||||||
| Stratification | No. of Studies | Mortality Exposed (%) | Mortality Unexposed (%) | Relative Risk (95% CI) | Odds Ratio (95% CI) | Risk of Bias | Inc. a | Ind. b | Imp. c | Certainty of Evidence (GRADE) d | Overall Adherence to WHO Guidelines on Disinsection f | References |
| Overall | ||||||||||||
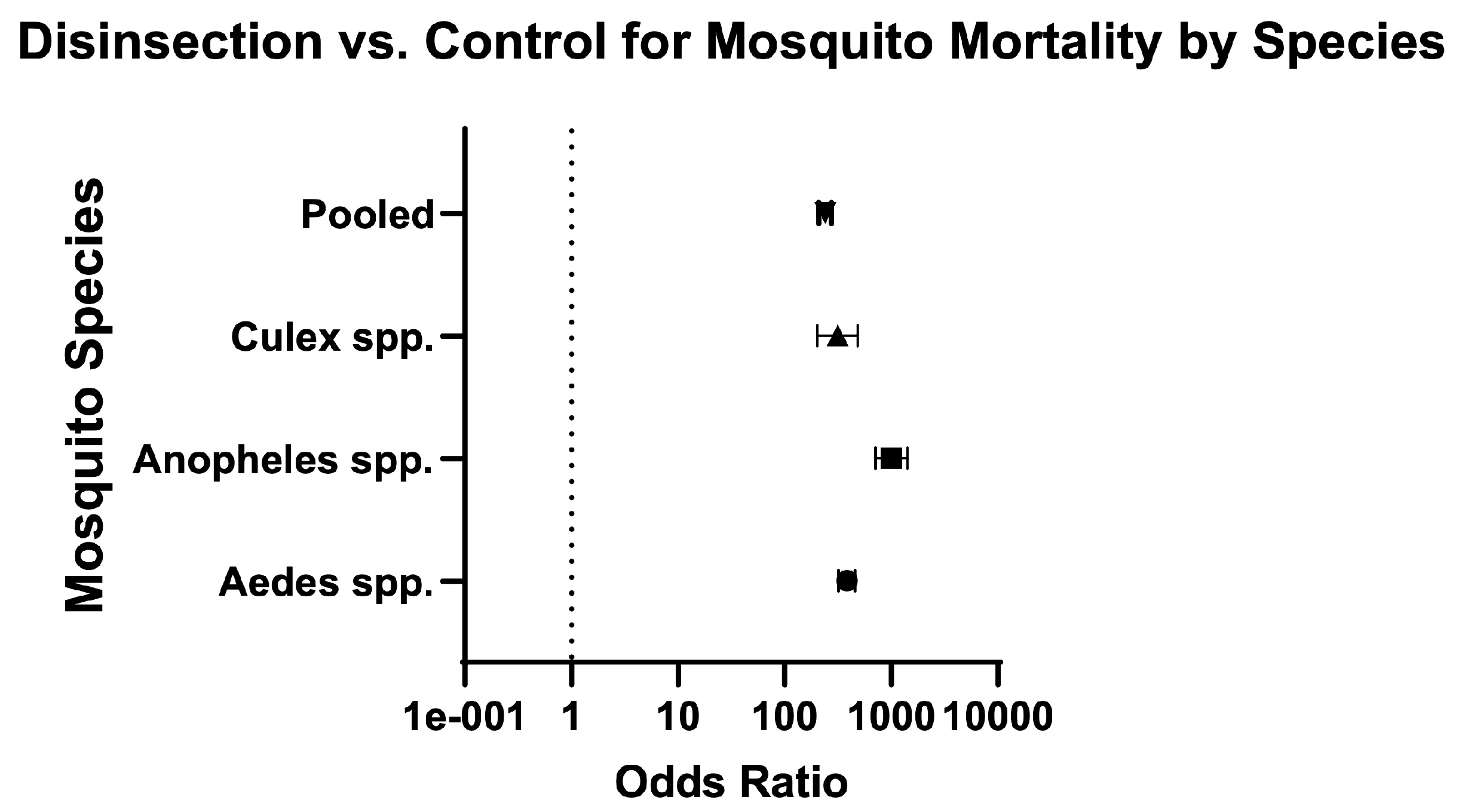
| All insecticides | 9 | 28,819/31,371 (91.90%) | 421/ 6528 (6.50%) | 14.24 (12.99–15.63) | 163.60 (147–182) | Very serious | High risk | High risk | Low risk | Very Low ⨁◯◯◯ | 33.30% (54/162) | Sullivan (1974) [27]; Berger-Preiss (2006) [28]; Brooke (1971) [32]; Langsford (1976) [34]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39]; Sullivan (1975) [40]; Sullivan (1978) [41] |
| Genus of Mosquito | ||||||||||||
| Aedes | 6 | 17,649/18,437 (95.70%) | 154/ 2804 (5.50%) | 17.43 (14.96–20.33) | 384 (321.60–458.40) | Serious | High risk | High risk | Low risk | Low ⨁⨁◯◯ | 33.20% (36.50/110) | Berger-Preiss (2006) [28]; Sullivan (1974) [27]; Brooke (1971) [32]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39] |
| Anopheles | 4 | 4461/4523 (98.60%) | 69/ 1047 (6.60%) | 14.97 (11.94–18.82) | 1005 (709.20–1424) | Very serious | High risk | High risk | Low risk | Very Low ⨁◯◯◯ | 33.30% (25/75) | Berger-Preiss (2006) [28]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39] |
| Culex | 6 | 8004/9787 (81.80%) | 20/ 1452 (1.40%) | 59.37 (38.61–91.55) | 313.60 (202.2–486.5) | Very serious | High risk | High risk | Low risk | Very Low ⨁◯◯◯ | 34.40% (37.50/109) | Berger-Preiss (2006) [28]; Langsford (1976) [34]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39]; Sullivan (1975) [40] |
| Pooled: genus | 9 | 30,114/32,747 (92%) | 243/ 5303 (4.60%) | 20.07 (17.76–22.70) | 237.60 (207.70–271.90) | Very serious | High risk | High risk | Low risk | Very Low ⨁◯◯◯ | 33.30% (54/162) | Sullivan (1974) [27]; Berger-Preiss (2006) [28]; Brooke (1971) [32]; Langsford (1976) [34]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39]; Sullivan (1975) [40]; Sullivan (1978) [41] |
| Method of Disinsection | ||||||||||||
| Pre-embarkation (aerosol) | 1 | 1595/1612 (99%) | 0/ 237(0%) | ∞ (2–∞) | 43,306 (2596–722,509) | Serious | N/A | High risk | High risk | Very Low e ⨁◯◯◯ | 18.20% (4/22) | Berger-Preiss (2006) [28] |
| Pre-embarkation (residual) | 1 | 5781/7309 (79.10%) | 8/ 408 (2%) | 40.34 (20.70–79.35) | 178.20 (90.14–352.40) | Serious | N/A | High risk | High risk | Very Low e ⨁◯◯◯ | 18.20% (4/22) | Berger-Preiss (2006) [28] |
| Blocks-away | 8 | 21,667/22,674 (95.60%) | 235/ 4451 (5.30%) | 18.10 (15.99–20.50) | 385.10 (332.90–445.40) | Very serious | High risk | High risk | High risk | Very Low ⨁◯◯◯ | 35.70% (50/140) | Sullivan (1974) [27]; Brooke (1971) [32]; Langsford (1976) [34]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39]; Sullivan (1975) [40]; Sullivan (1978) [41] |
| Pooled: method of disinsection | 9 | 29,043/31,595 (91.90%) | 243/ 5096 (4.80%) | 19.28 (17.06–21.80) | 226.80 (198.20–259.60) | Very serious | High risk | High risk | High risk | Very Low ⨁◯◯◯ | 33.30% (54/162) | Sullivan (1974) [27]; Berger-Preiss (2006) [28]; Brooke (1971) [32]; Langsford (1976) [34]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39]; Sullivan (1975) [40]; Sullivan (1978) [41] |
| Insecticide | ||||||||||||
| Allethrin | 1 | 328/378 (86.80%) | 2/ 103 (1.90%) | 44.69 (12.74–162.50) | 264.10 (72.75–958.70) | Very serious | N/A | High risk | High risk | Very Low e ⨁◯◯◯ | 36.10% (6.50/18) | Sullivan (1972) [39] |
| Bioresmethrin | 2 | 4435/4570 (97.10%) | 102/ 1057 (9.70%) | 10.06 (8.38–12.11) | 305.10 (233.90–398.10) | Serious | High risk | High risk | High risk | Very Low e ⨁◯◯◯ | 36.80% (12.50/34) | Brooke (1971) [32]; Sullivan (1972) [39] |
| DDT-containing | 3 | 5639/6319 (89.20%) | 112/ 1940 (5.80%) | 15.46 (12.93–18.52) | 134.70 (109.60–165.60) | Serious | Low risk | High risk | Low risk | Low ⨁⨁◯◯ | 39% (19.50/50) | Brooke (1971) [32]; Langsford (1976) [34]; Sullivan (1962) [37] |
| d-phenothrin | 4 | 12,687/14,274 (88.90%) | 96/ 2169 (4.40%) | 20.08 (16.53–24.43) | 171.70 (139.10–212) | Serious | High risk | High risk | Low risk | Low ⨁⨁◯◯ | 27% (20.50/76) | Sullivan (1974) [27]; Berger-Preiss (2006) [28]; Liljedhal (1976) [35]; Sullivan (1978) [41] |
| Pyrethrins | 4 | 6820/7523 (90.70%) | 114/ 2044 (5.60%) | 16.25 (13.61–19.44) | 163.50 (133.30–200.40) | Serious | High risk | High risk | Low risk | Low ⨁⨁◯◯ | 38.20% (26/68) | Brooke (1971) [32]; Langsford (1976) [34]; Sullivan (1962) [37]; Sullivan (1972) [39] |
| Resmethrin | 3 | 4549/4626 (98.30%) | 107/ 1155 (9.30%) | 10.60 (8.68–12.73) | 572.60 (424.30–772.60) | Very serious | Low risk | High risk | High risk | Very Low ⨁◯◯◯ | 38.50% (20/52) | Brooke (1971) [32]; Sullivan (1972) [39]; Sullivan (1975) [40] |
| Pooled: insecticide | 9 | 28,819/31,371 (91.90%) | 421/ 6528 (6.50%) | 14.24 (12.99–15.63) | 163.60 (147–182) | Very serious | High risk | High risk | Low risk | Very Low ⨁◯◯◯ | 33.30% (54/162) | Sullivan (1974) [27]; Berger-Preiss (2006) [28]; Brooke (1971) [32]; Langsford (1976) [34]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39]; Sullivan (1975) [40]; Sullivan (1978) [41] |
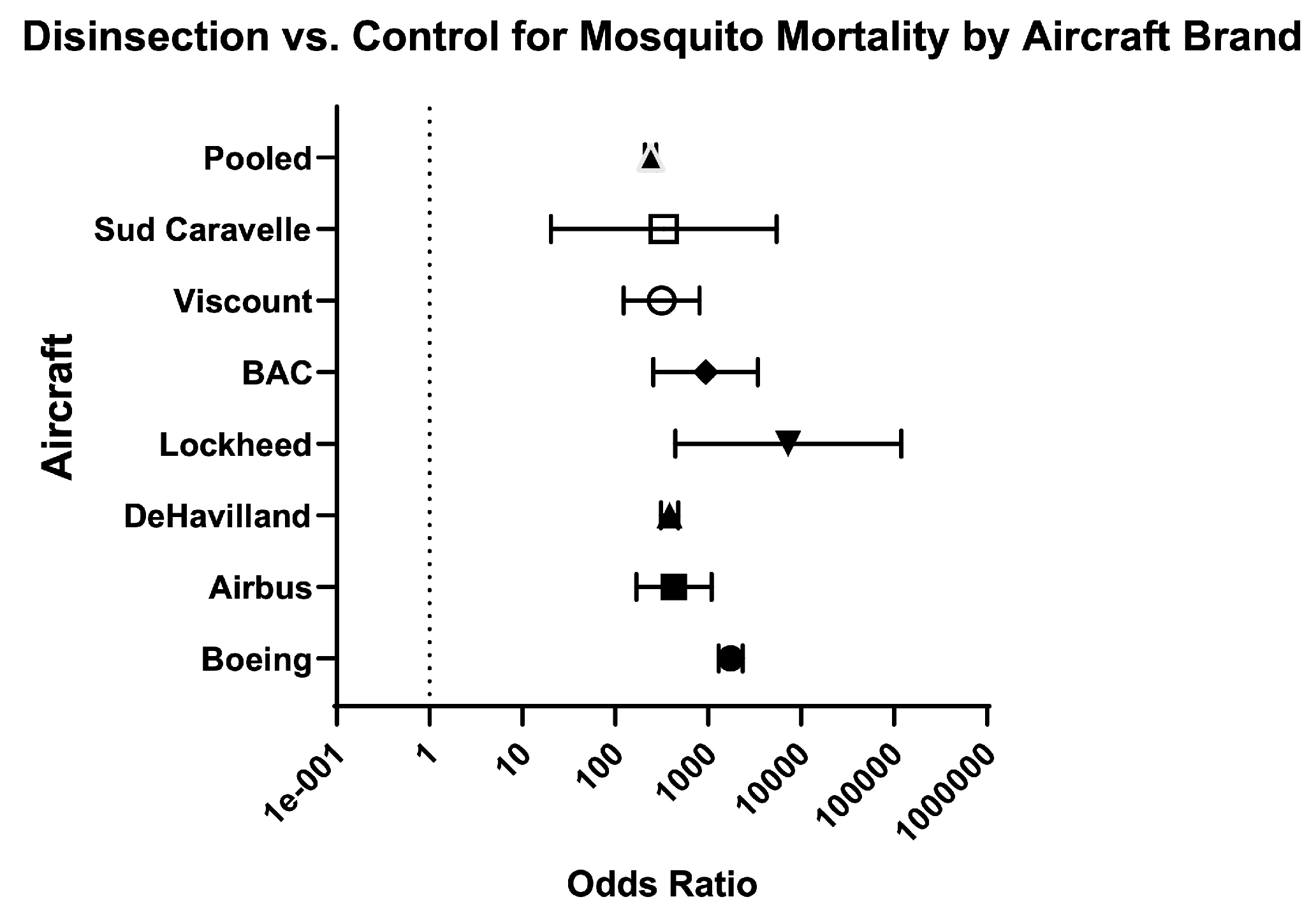
| Aircraft | ||||||||||||
| Airbus | 1 | 5281/6826 (77.40%) | 4/ 565 (0.70%) | 109.30 (42.83–280.7) | 426.40 (168.30–1080) | Serious | N/A | High risk | High risk | Very Low e ⨁◯◯◯ | 18.20% (4/22) | Berger-Preiss (2006) [28] |
| BAC | 1 | 566/601 (94.20%) | 2/148 (1.40%) | 69.69 (19.65–253.60) | 935.10 (256.10–3415) | Very serious | N/A | High risk | High risk | Very Low ⨁◯◯◯ | 36.10% (6.50/18) | Sullivan (1972) [39] |
| Boeing | 5 | 8728/8839 (98.70%) | 76/ 1784 (4.30%) | 23.18 (18.63–28.90) | 1748 (1301–2350) | Serious | High risk | High risk | High risk | Low ⨁⨁◯◯ | 29.30% (27/92) | Sullivan (1974) [27]; Berger-Preiss (2006) [28]; Langsford (1976) [34]; Liljedhal (1976) [35]; Sullivan 1972 [39] |
| Caravelle | 1 | 230/330 (69.70%) | 0/72 (0%) | ∞ (2–∞) | 332.60 (20.40–5421) | Very serious | N/A | High risk | High risk | Very Low ⨁◯◯◯ | 47.20% (8.5/18) | Sullivan (1962) [37] |
| De Havilland | 3 | 12,145/12,667 (95.90%) | 107/ 1882 (5.70%) | 16.86 (14.05–20.29) | 383.90 (310.10–475.30) | Very serious | Low risk | High risk | Low risk | Very Low ⨁◯◯◯ | 40.40% (21/52) | Brooke (1971) [32]; Sullivan (1962) [37]; Sullivan (1972) [39] |
| Lockheed | 2 | 539/539 (100%) | 28/219 (12.80%) | 7.82 (5.71–9.44) | 7250 (440.50–119,330) | Very serious | Low risk | High risk | High Risk | Very Low ⨁◯◯◯ | 34.7% (12.50/36) | Sullivan (1975) [40]; Sullivan (1978) [41] |
| Viscount | 1 | 1330/1569 (84.80%) | 4/ 258 (1.60%) | 54.67 (21.63–140.20) | 314.20 (122.50–806.10) | Very serious | N/A | High risk | High risk | Very Low ⨁◯◯◯ | 47.20% (8.50/18) | Sullivan (1962) [37] |
| Pooled: aircraft | 9 | 28,769/31,321 (91.90%) | 220/ 4913 (4.50%) | 20.48 (18.02–23.35) | 240 (208.50–276.20) | Very serious | High risk | High risk | Low risk | Very Low ⨁◯◯◯ | 33.30% (54/162) | Sullivan (1974) [27]; Berger-Preiss (2006) [28]; Brooke (1971) [32]; Langsford (1976) [34]; Liljedhal (1976) [35]; Sullivan (1962) [37]; Sullivan (1972) [39]; Sullivan (1975) [40]; Sullivan (1978) [41] |
3.5. Quantitative and Qualitative Synthesis: Summary of Findings—Efficacy










4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Aircraft Disinsection Methods and Procedures, 2nd ed.; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/handle/10665/374318 (accessed on 3 October 2024).
- Report of the Informal Consultation on Aircraft Disinsection, Geneva. 6–10 November 1995. Available online: https://iris.who.int/handle/10665/59700 (accessed on 3 October 2024).
- Gratz, N.G.; Steffen, R.; Cocksedge, W. Why aircraft disinsection? Bull. World Health Organ. 2000, 78, 995–1004. [Google Scholar] [PubMed]
- World Health Organization. International Health Regulations, 3rd ed.; World Health Organization: Geneva, Switzerland, 2005; Available online: https://apps.who.int/iris/handle/10665/246107 (accessed on 3 October 2024).
- Airport vector control register. In Crises and Rapid Response Programme; International Civil Aviation Organization, Uniting Aviation: Montreal, QC, Canada, 2020; Available online: https://www.icao.int/crr/Pages/Airport-Vector-Control-Register.aspx (accessed on 3 October 2024).
- WHO Ad-Hoc Advisory Group on Aircraft Disinsection for Controlling the International Spread of Vector-Borne Diseases, Geneva, Switzerland. 21–22 April 2016. Available online: https://iris.who.int/handle/10665/205795 (accessed on 3 October 2024).
- Methods and Operating Procedures for Aircraft Disinsection. Report of a WHO Consultation, Geneva. 3–4 July 2018. Available online: https://iris.who.int/handle/10665/279702 (accessed on 3 October 2024).
- World Health Organization. WHO Aircraft Disinsection Methods and Procedures; World Health Organization: Geneva, Switzerland, 2021. Available online: https://iris.who.int/handle/10665/339863 (accessed on 3 October 2024).
- Dengue-Global Situation. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498 (accessed on 3 October 2024).
- Chikungunya Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/chikungunya (accessed on 3 October 2024).
- Public Health Risk Assessment Related to Western Equine Encephalitis (WEE) Virus in the Region of the Americas: 23 February 2024 Update. Available online: https://www.paho.org/en/documents/public-health-risk-assessment-related-western-equine-encephalitis-wee-virus-region (accessed on 3 October 2024).
- Zika Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/zika-virus (accessed on 3 October 2024).
- Zika Epidemiology Update—February 2022. Available online: https://www.who.int/publications/m/item/zika-epidemiology-update---february-2022 (accessed on 3 October 2024).
- WHO Initiative to Stop the Spread of Anopheles Stephensi in Africa 2023 Update. Available online: https://iris.who.int/bitstream/handle/10665/372259/WHO-UCN-GMP-2023.06-eng.pdf?sequence=1 (accessed on 3 October 2024).
- Pang, A.M.; Gay, S.; Yadav, R.; Dolea, C.; Ponce, C.; Velayudhan, R.; Grout, A.; Fehr, J.; Plenge-Boenig, A.; Schlagenhauf, P. The safety and applicability of synthetic pyrethroid insecticides for aircraft disinsection: A systematic review. Travel Med. Infect. Dis. 2020, 33, 101570. [Google Scholar] [CrossRef] [PubMed]
- WHO. Effectiveness of Disinsection of Conveyances to Prevent or Reduce the Spread of Mosquito Vectors via International Travel: Evidence Review; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Aircraft Disinsection Insecticides. Available online: https://iris.who.int/handle/10665/100023 (accessed on 3 October 2024).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Flack-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [PubMed]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490–1494. [Google Scholar] [CrossRef]
- OCR Space. Available online: https://ocr.space/ (accessed on 3 October 2024).
- Deep L Translator. Available online: https://www.deepl.com/translator (accessed on 3 October 2024).
- PDF Translator (GPT-4). Available online: https://chatgpt.com/g/g-DTk1KpYjg-pdf-translator (accessed on 3 October 2024).
- Joanna Briggs Institute. Checklist for Systematic Reviews and Research Syntheses. Available online: https://jbi.global/critical-appraisal-tools (accessed on 3 October 2024).
- Guidelines for Testing the Efficacy of Insecticide Products Used in Aircraft. Available online: https://www.who.int/publications/i/item/9789241503235 (accessed on 3 October 2024).
- Sullivan, W.N.; Schoof, H.F.; Maddock, D.R.; Amyx, C.M.; Porter, J.E. D-Phenothrin, a promising new pyrethroid for disinsecting aircraft. J. Med. Entomol. 1974, 11, 231–233. [Google Scholar]
- Berger-Preiss, E.; Koch, W.; Gerling, S.; Kock, H.; Klasen, J.; Hoffman, G.; Appel, K.E. Aircraft disinsection: Exposure assessment and evaluation of a new pre-embarkation method. Int. J. Hyg. Environ. Health 2006, 209, 41–56. [Google Scholar] [CrossRef]
- Jakob, W.L.; Maddock, D.R.; Schoof, H.F.; Porter, J.E. Gas-propelled aerosols and micronized dusts for control of insects in aircraft. 5. Effectiveness against insects of public health importance. J. Econ. Entomol. 1972, 65, 1454–1458. [Google Scholar] [CrossRef]
- Ong, O.; Rigby, L.; Rasic, G.; Sly, A.; Devine, G. An evaluation of the efficacy of aircraft disinsection procedures at australian airports. Am. J. Trop. Med. Hyg. 2018, 99, 265–266. [Google Scholar]
- Pimentel, D.; Klock, J.W. Disinsectization of aircraft by residual deposits of insecticides. Am. J. Trop. Med. Hyg. 1954, 3, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J.P.; Evans, M. Disinsection of aircraft with pressure packs containing the pyrethroids, resmethrin and bioresmethrin. Pestic. Sci. 1971, 2, 133–137. [Google Scholar] [CrossRef]
- Cawley, B.M.; Sullivan, W.N.; Schechter, M.S.; McGuire, J.U. Desirability of three synthetic pyrethroid aerosols for aircraft disinsection. Bull. World Health Organ. 1974, 51, 537–540. [Google Scholar]
- Langsford, W.A. A trial to assess the efficacy of inflight disinsection of a Boeing 747 aircraft on the Singapore/Sydney sector. Pyrethrum Post. 1976, 13, 137–142. [Google Scholar]
- Liljedahl, L.A.; Rtzer, H.J.; Sullivan, W.N.; Schechter, M.S.; Cawley, B.M.; Morgan, N.O.; Amyx, C.M.; Schiefer, B.A.; Gerberg, E.J.; Pal, R. Aircraft disinsection: The physical and insecticidal characteristics of (+)-phenothrin applied by aerosol at “blocks away”. Bull. World Health Organ. 1976, 54, 391–396. [Google Scholar]
- Russell, R.C.; Rajapaksa, N.; Whelan, P.I.; Langsford, W.A. Mosquito and other insect introductions to Australia aboard international aircraft, and the monitoring of disinsection procedures. In Commerce and the Spread of Pests and Disease Vectors; Laird, M., Ed.; Praegar Scientific: New York, NY, USA, 1984; pp. 109–141. [Google Scholar]
- Sullivan, W.N.; Keiding, J.; Wright, J.W. Studies on aircraft disinsection at ‘blocks away’. Bull. World Health Organ. 1962, 27, 263–273. [Google Scholar] [PubMed]
- Sullivan, W.N.; Azurin, J.C.; Wright, J.W.; Gratz, N.G. Studies on Aircraft Disinsection at “Blocks Away” in Tropical Areas. Bull. World Health Organ. 1964, 30, 113–1188. [Google Scholar]
- Sullivan, W.N.; Pal, R.; Wright, J.W.; Azurin, J.C.; Okamoto, R.; McGuire, J.U.; Waters, R.M. Worldwide studies on aircraft disinsection at “blocks away”. Bull. World Health Organ. 1972, 46, 485–491. [Google Scholar]
- Sullivan, W.N.; Hewing, A.N.; Schechter, M.S.; McGuire, J.U.; Waters, R.M.; Fields, E.S. Further studies of aircraft disinsection and odor characteristics of aerosols containing resmethrin and d-trans-resmethrin. Pest. Control. Sci. 1975, 40, 5–13. [Google Scholar]
- Sullivan, W.N.; Cawley, B.M.; Schechter, M.S.; Hayes, D.K.; Staker, K.; Pal, R. A comparison of Freon- and water-based insecticidal aerosols for aircraft disinsection. Bull. World Health Organ. 1978, 56, 129–132. [Google Scholar]
- Russell, R.C.; Paton, R. In-flight disinsection as an efficacious procedure for preventing international transport of insects of public health importance. Bull. World Health Organ. 1989, 67, 543–547. [Google Scholar] [PubMed]
- Mackie, F.P.; Crabtree, H.S. The destruction of mosquitoes in aircraft. Lancet 1938, 235, 447–450. [Google Scholar] [CrossRef]
- Jensen, J.A.; Flury, V.P.; Schoof, H.F. Dichlorvos vapour disinsection of aircraft. Bull. World Health Organ. 1965, 32, 175–180. [Google Scholar]
- Tew, R.P.; David, W.A.; Busvine, J.R. Factors affecting the efficiency of aircraft disinsectisation procedures. Month Bull. Min. Health Emerg. Pub Health Lab. Serv. 1951, 10, 30–38. [Google Scholar]
- Aircraft Disinsection Requirements|US Department of Transportation. Available online: https://www.transportation.gov/airconsumer/spray (accessed on 3 October 2024).
- Reviewing the Topic of Aircraft Disinsection. Association of Canadian Travel Agencies and Travel Advisors. (n.d.). Available online: https://www.acta.ca/news-releases/cb0206 (accessed on 3 October 2024).
- Air Canada—Health and Travel Tips. Air Canada. (n.d.). Available online: https://www.aircanada.com/ca/en/aco/home/plan/peace-of-mind/travel-tips.html#/ (accessed on 3 October 2024).
- Subiakto, Y. Aviation Medicine Capacity on Facing Biological Threat in Indonesia Airports. Infect. Dis. Rep. 2020, 12 (Suppl. S1), 8738. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. ARCHIVED—Ship Sanitation Certificate Program. 4 February 2009. Available online: https://www.canada.ca/en/health-canada/services/healthy-living/travel-health/general-advice/ship-sanitation-certificate-program-health-canada.html (accessed on 3 October 2024).
- China: Yellow Fever Prevention Measures—The Swedish Club. 12 February 2018. Available online: https://www.swedishclub.com/news/loss-prevention/china-yellow-fever-prevention-measures/ (accessed on 3 October 2024).
- Zika Virus: South Korea Requires Self-Disinfection Certificates for Vessels—The Swedish Club. 24 March 2016. Available online: https://www.swedishclub.com/news/loss-prevention/zika-virus-south-korea-requires-self-disinfection-certificates-for-vessels/ (accessed on 3 October 2024).
- Interim Guidance on Maritime Transport and Zika Virus Disease. (n.d.). Available online: https://www.shipsan.eu/Portals/0/docs/MaritimeZika_EUSHIPSAN_UPDATE_13.4.2016.pdf (accessed on 3 October 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawley, G.; Klowak, M.; Ahmad, S.Z.; Madakadze, C.; Hewitt, J.; Reid-John, A.; Adawi, A.; Boggild, A.K. A Systematic Review of Aircraft Disinsection Efficacy. Insects 2025, 16, 911. https://doi.org/10.3390/insects16090911
Hawley G, Klowak M, Ahmad SZ, Madakadze C, Hewitt J, Reid-John A, Adawi A, Boggild AK. A Systematic Review of Aircraft Disinsection Efficacy. Insects. 2025; 16(9):911. https://doi.org/10.3390/insects16090911
Chicago/Turabian StyleHawley, Gregory, Michael Klowak, Syed Zain Ahmad, Candice Madakadze, Jahmar Hewitt, Aquilla Reid-John, Asal Adawi, and Andrea K. Boggild. 2025. "A Systematic Review of Aircraft Disinsection Efficacy" Insects 16, no. 9: 911. https://doi.org/10.3390/insects16090911
APA StyleHawley, G., Klowak, M., Ahmad, S. Z., Madakadze, C., Hewitt, J., Reid-John, A., Adawi, A., & Boggild, A. K. (2025). A Systematic Review of Aircraft Disinsection Efficacy. Insects, 16(9), 911. https://doi.org/10.3390/insects16090911








