Possible Fossil Larvae of Staphylinidae from Kachin Amber and a Quantitative Morphological Comparison Indicate That Rove Beetle Larvae Partly Replaced Lacewing Larvae
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Documentation and Preparation Methods
2.3. Description Style for New Specimens
2.4. Shape Analysis
- In addition to publications, some specimens were retrieved from image repositories such as bugguide.com (see Supplementary Table S1).
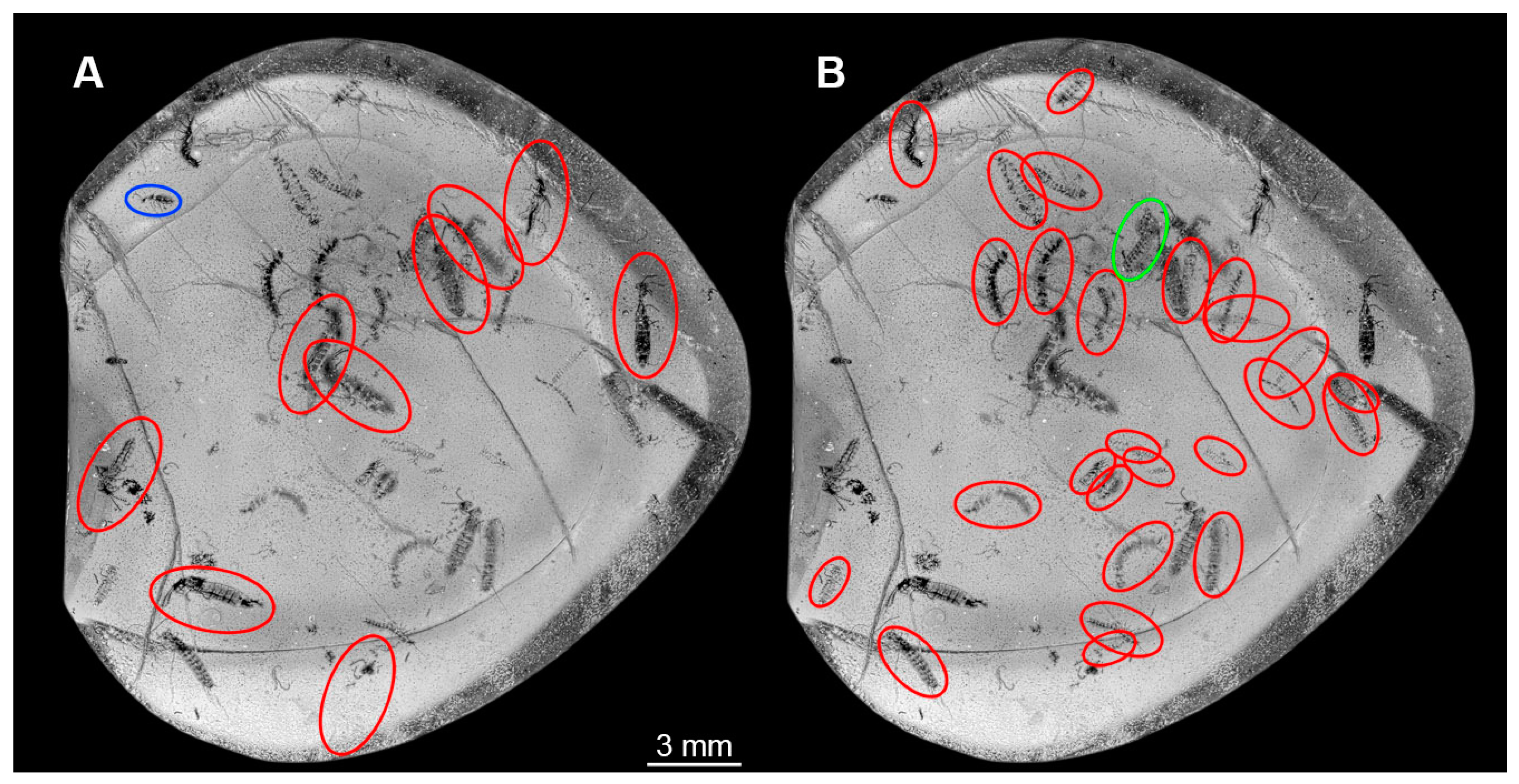
3. Results
3.1. General Description of Body Organisation of the Larvae
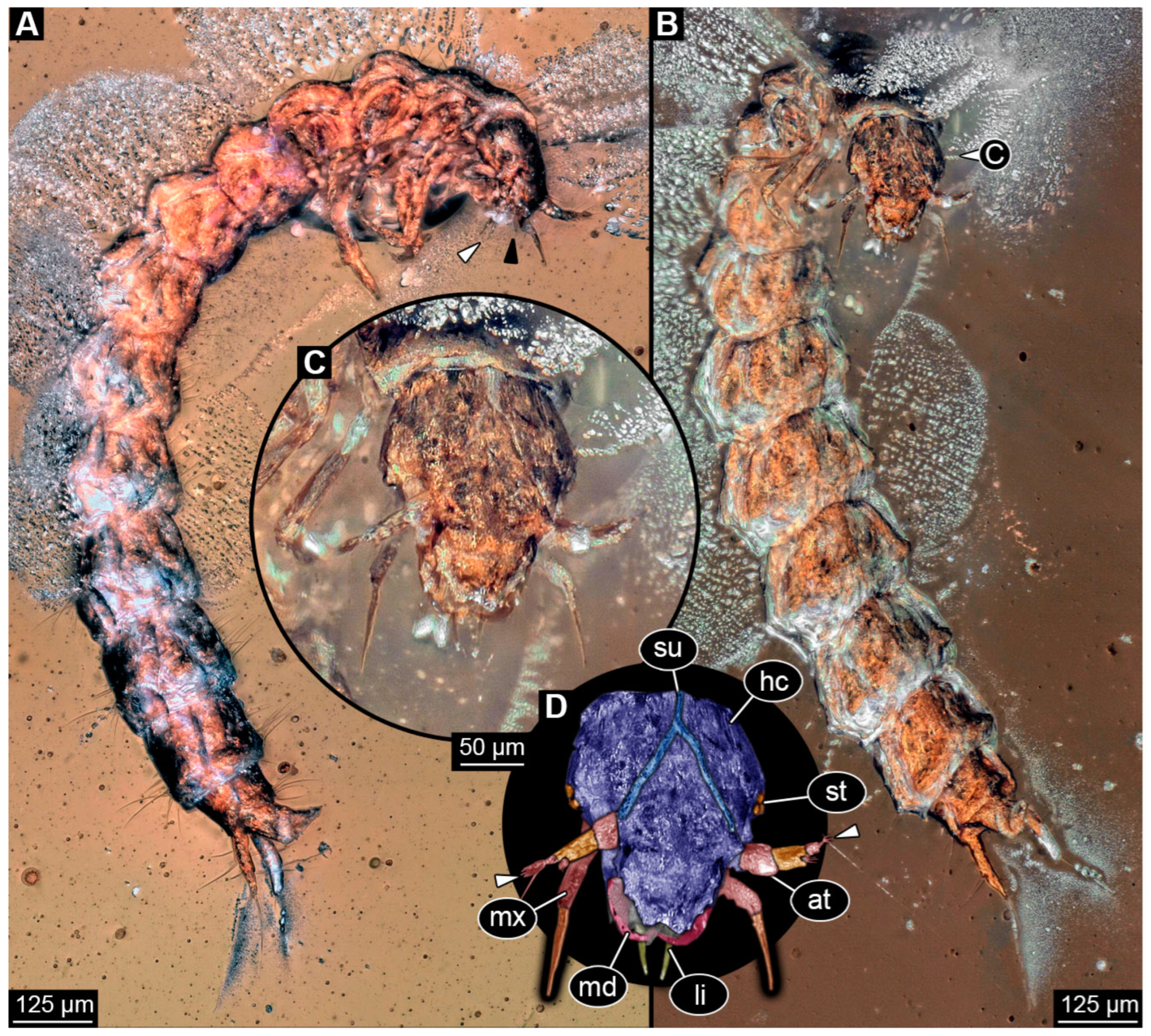
3.2. Description of Specimen PED 3982
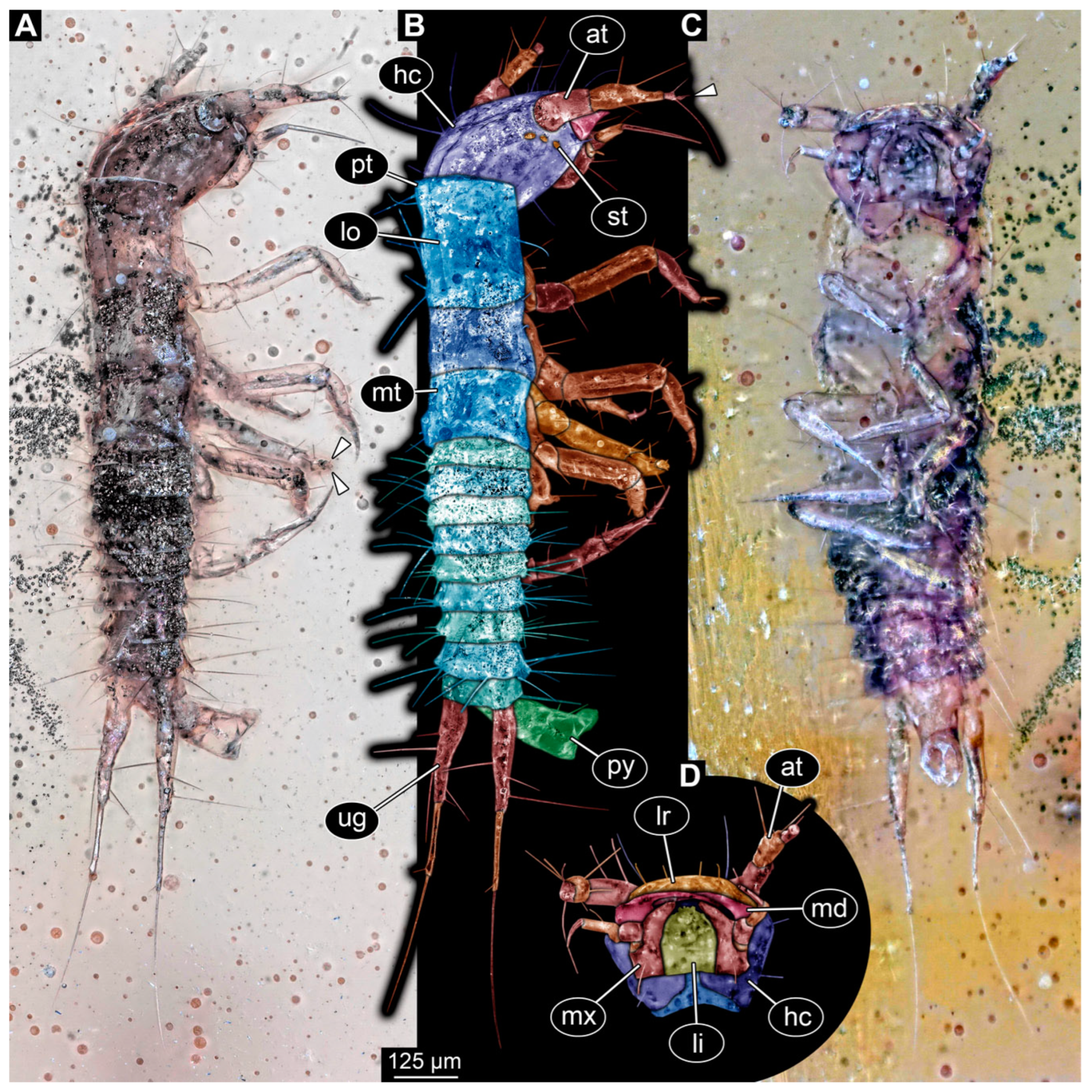
3.3. Description of Specimen PED 0067
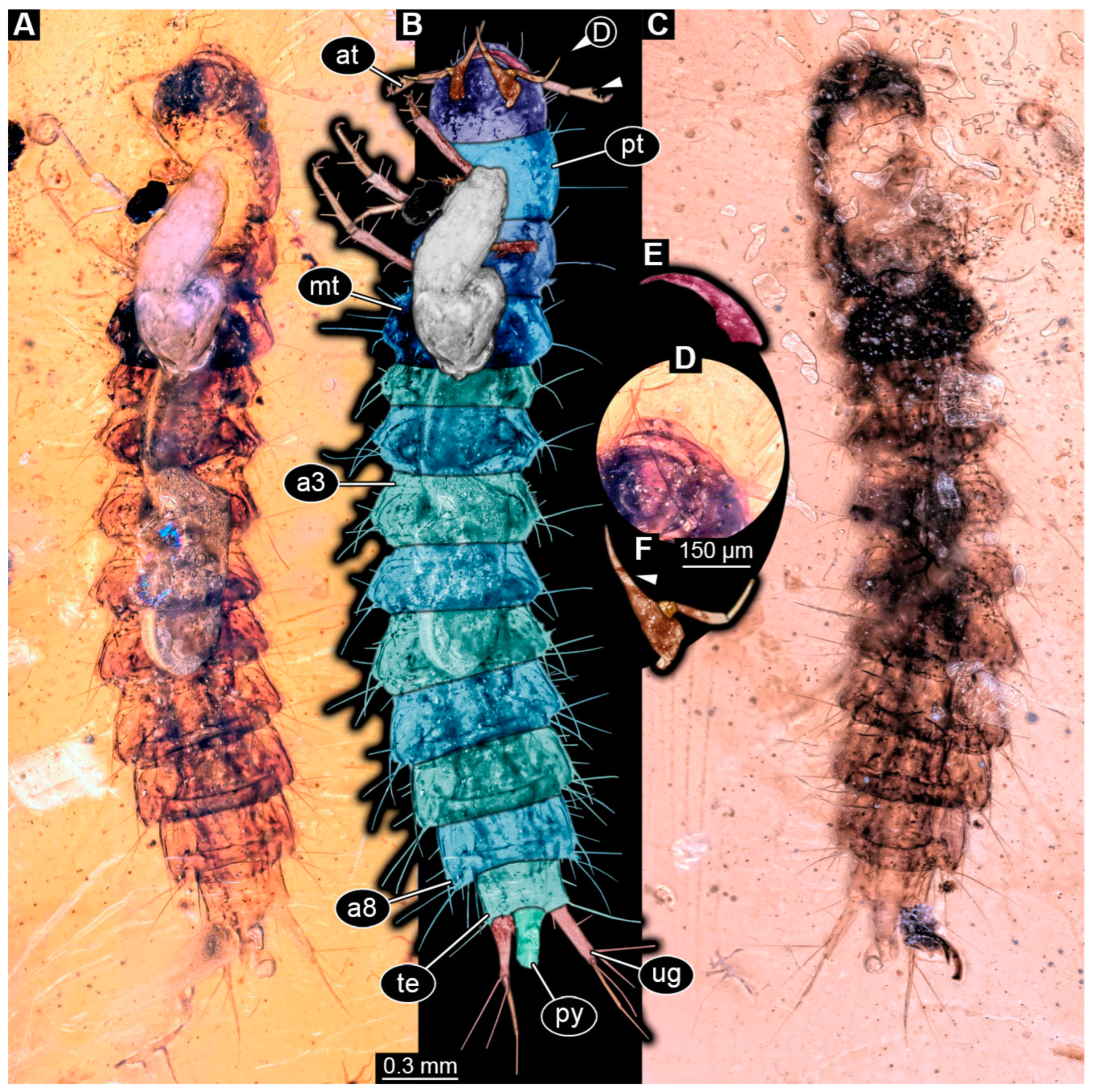
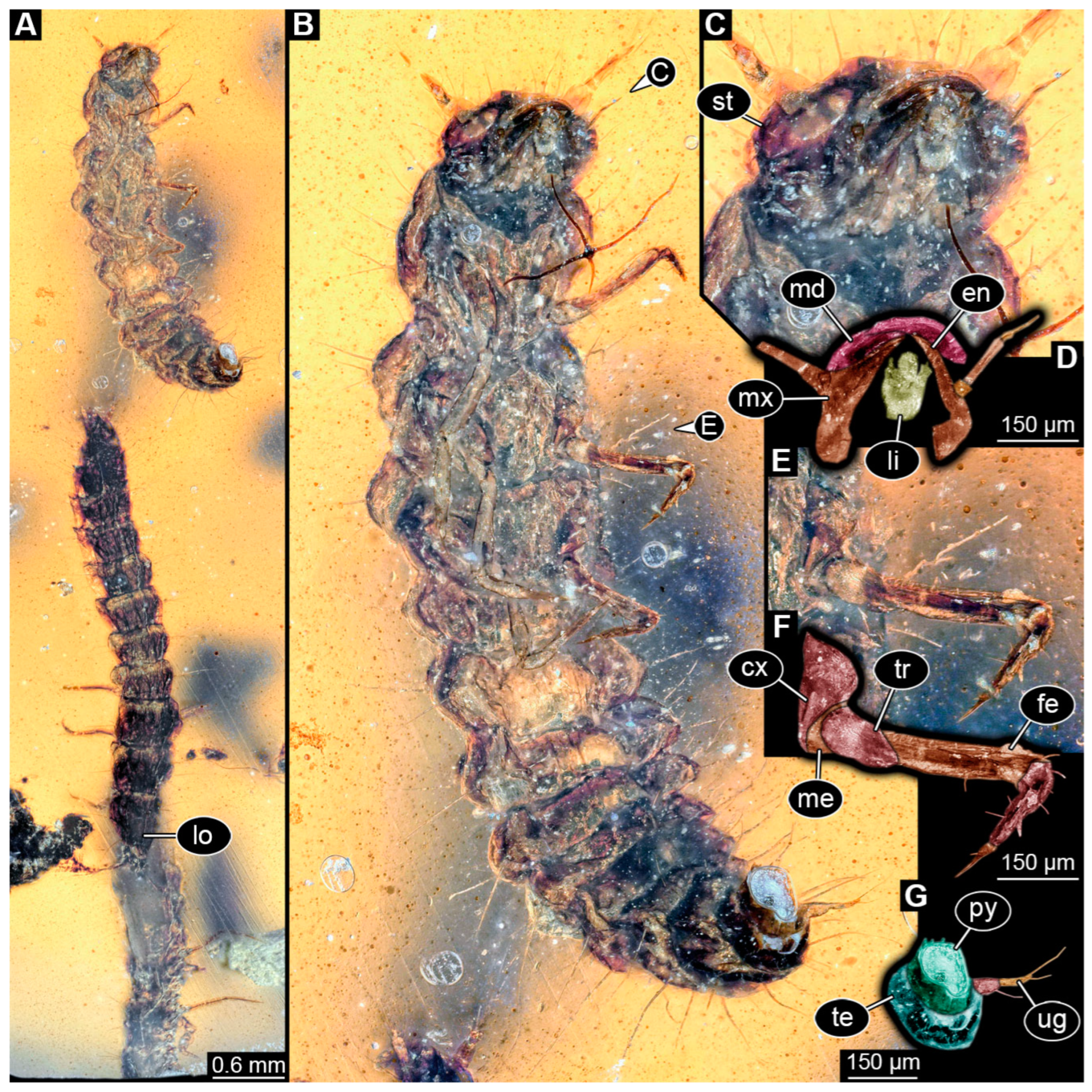
3.4. Description of Specimen PED 2115
3.5. Description of Specimen PED 3368
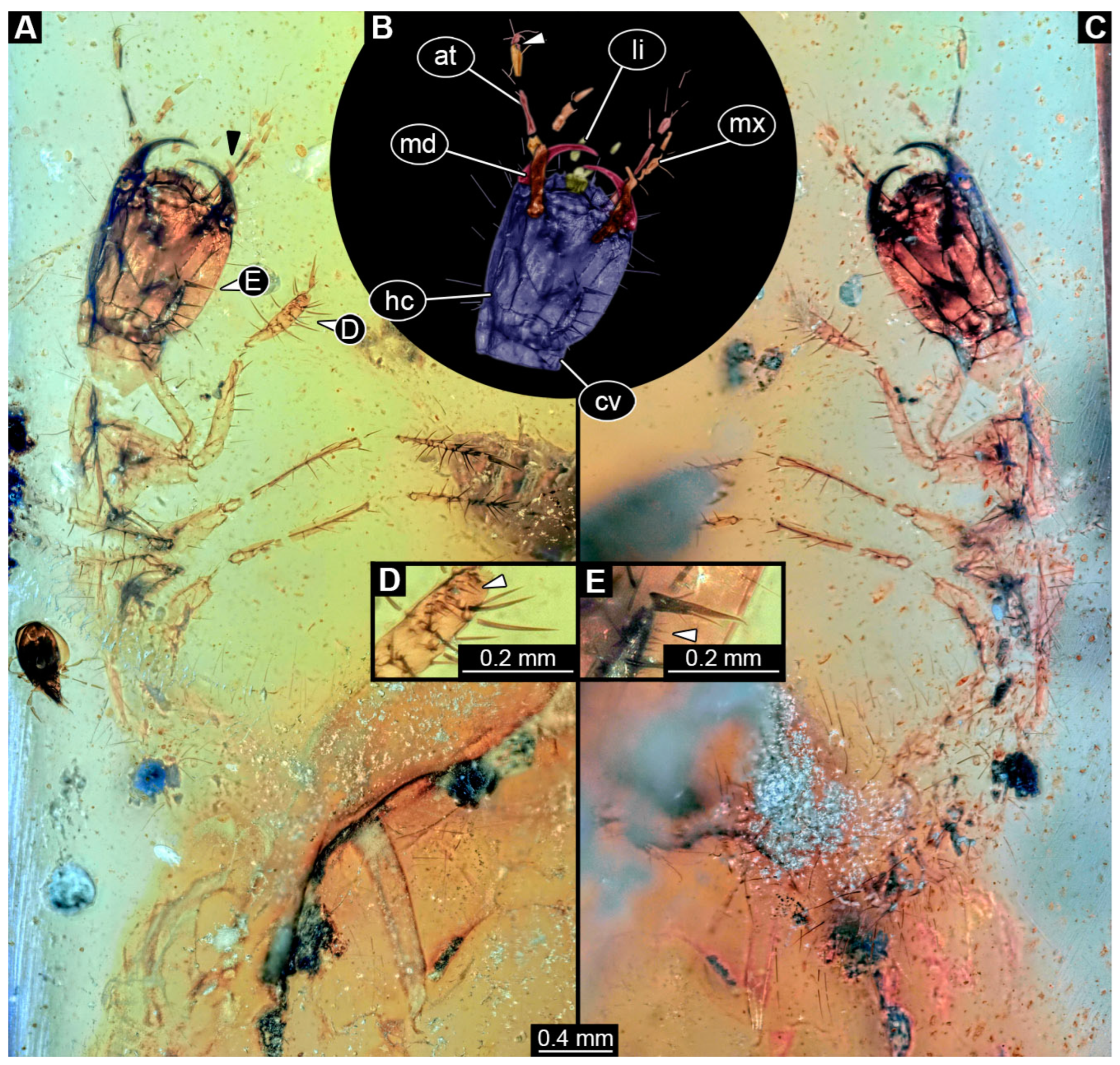
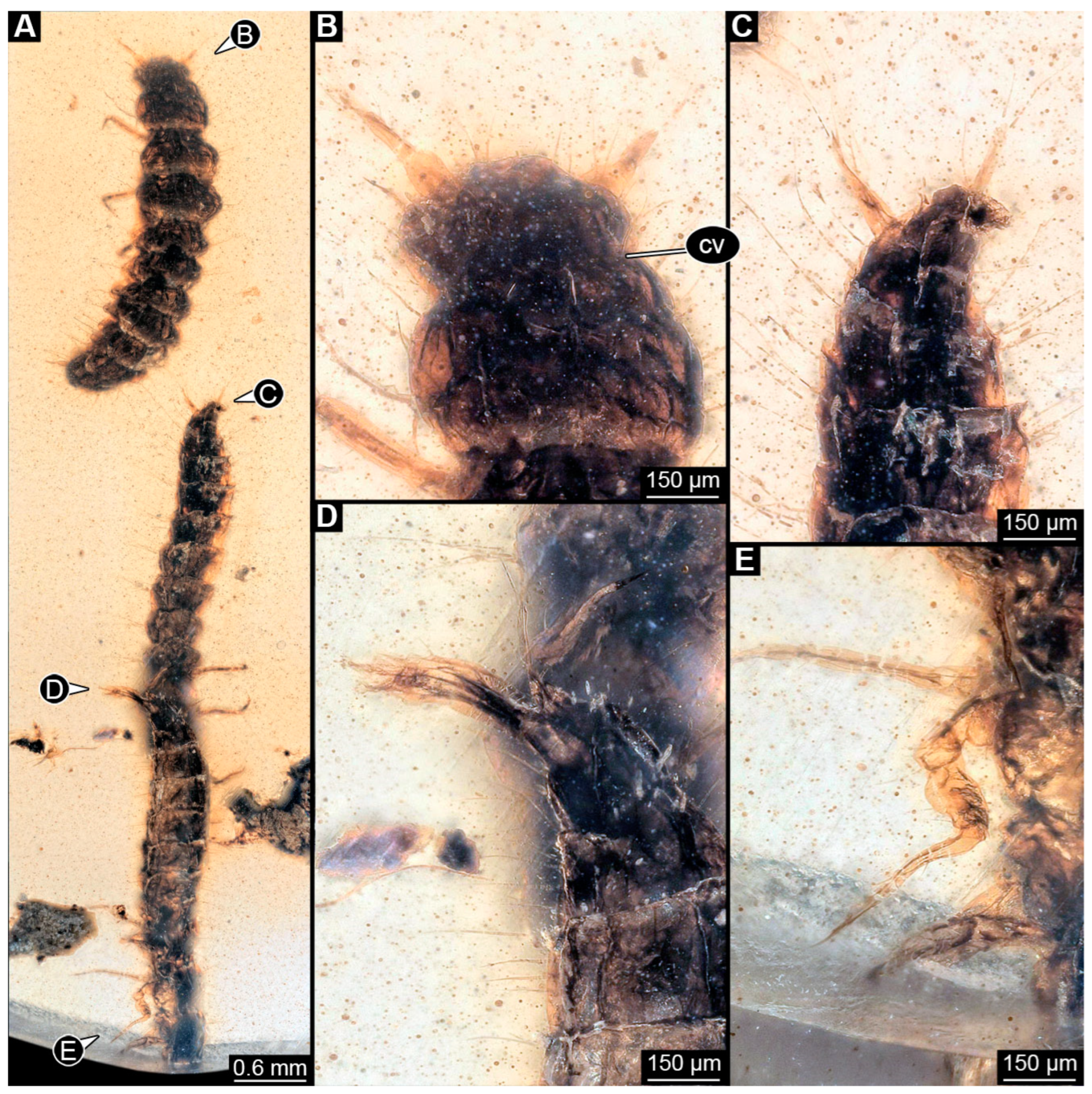
3.6. Description of Specimens in PED 3744
3.7. Description of the Specimen from NIGPAS
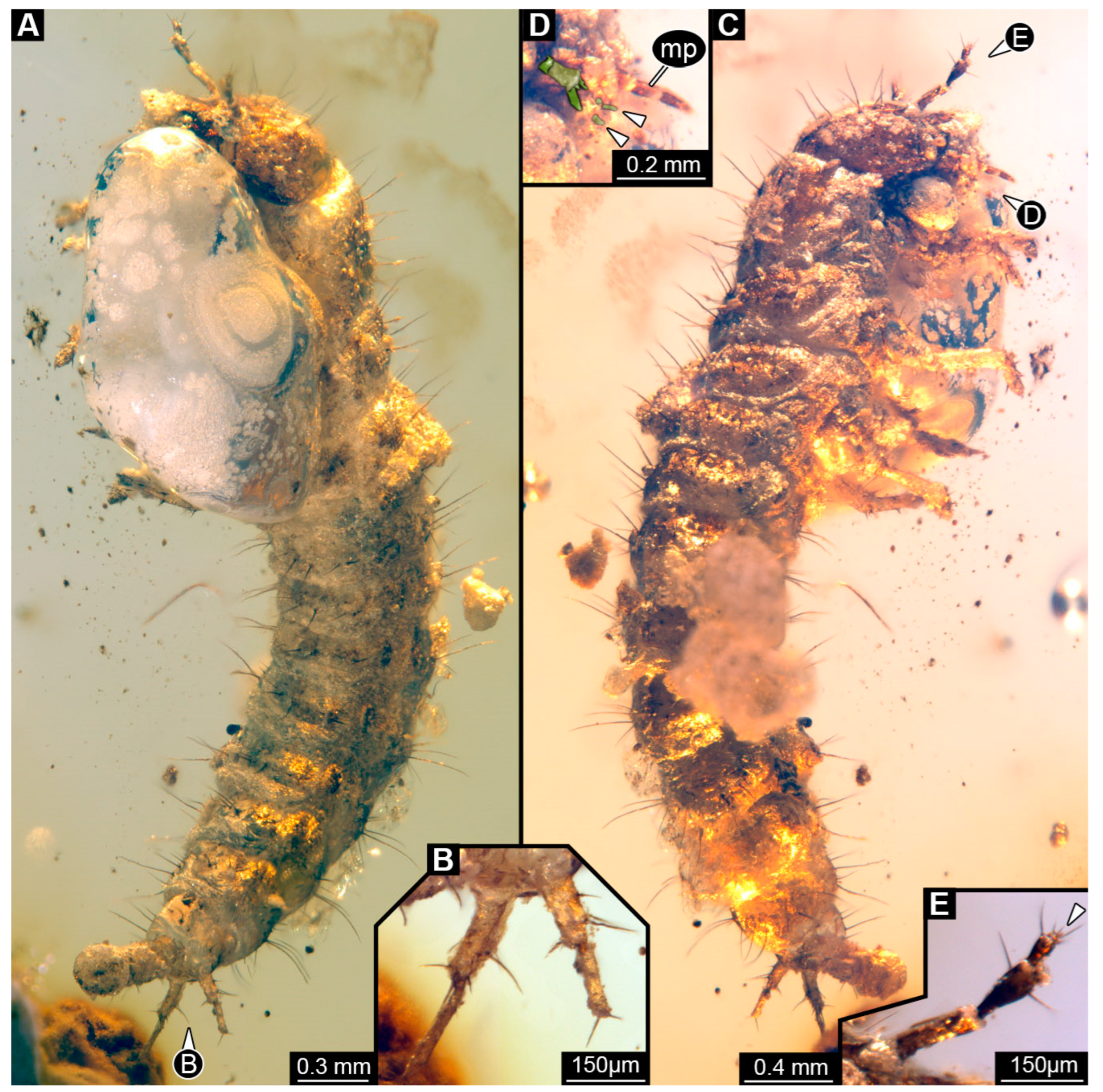
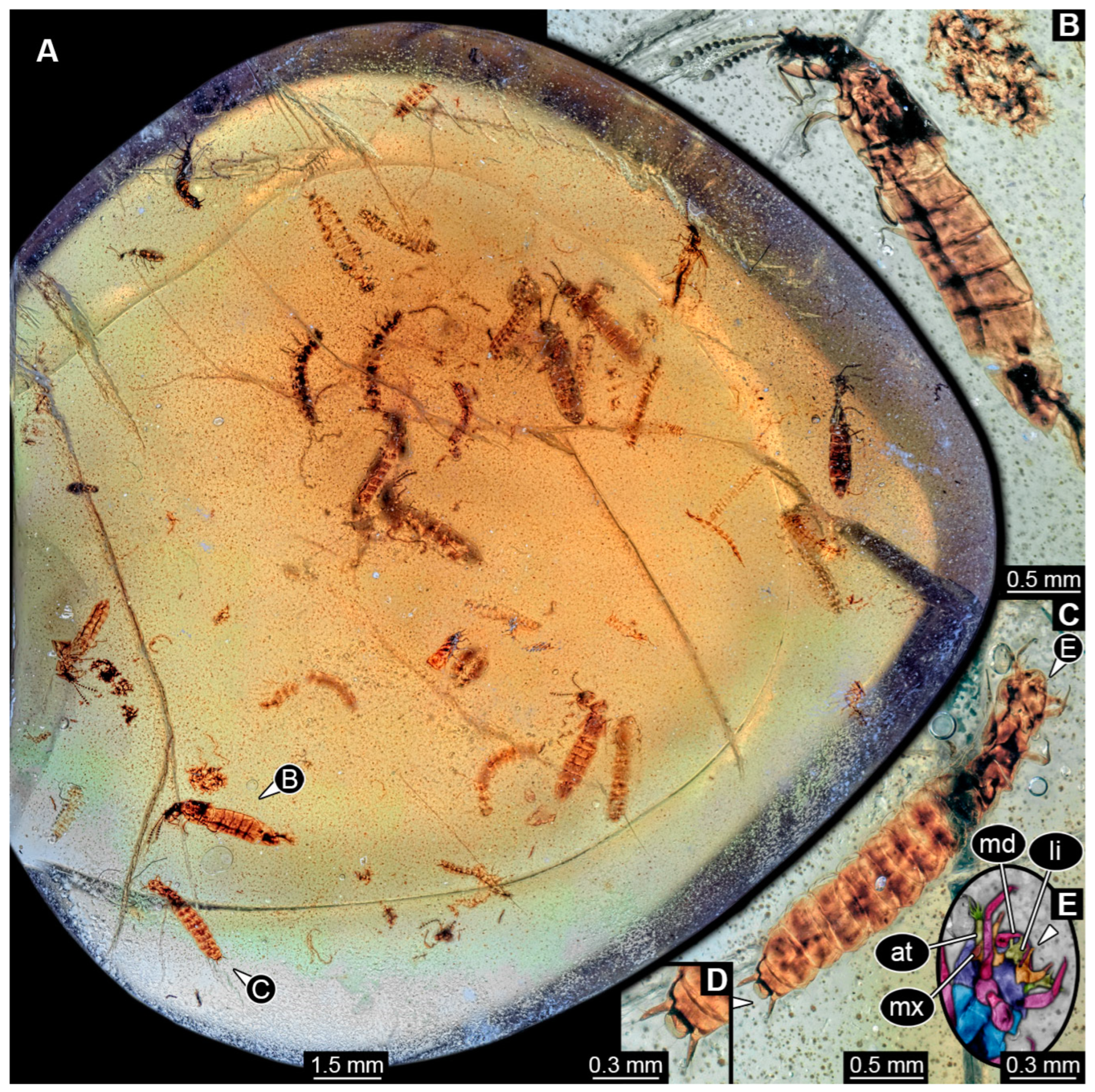
3.8. Description of PED 1302
3.9. Description of Specimen PED 4084
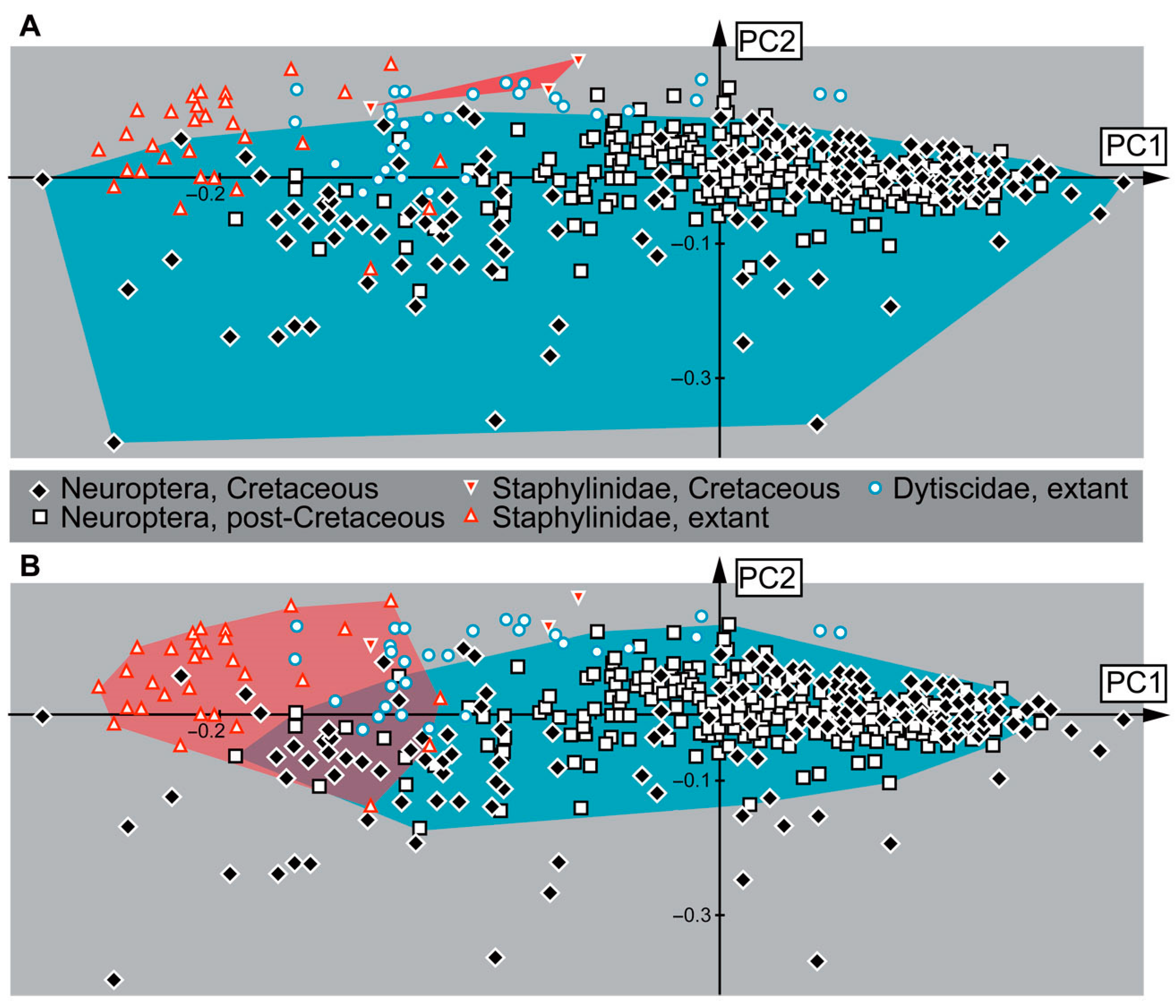
3.10. Results of the Shape Analysis
4. Discussion
4.1. General Identity of the Specimens: Rove Beetle Larvae
4.2. Identity of Specimen PED 3982
4.3. Identity of Specimen PED 0067
4.4. Identity of Specimen PED 2115
4.5. Identity of Specimen PED 3368
4.6. Identity of Specimens in PED 3744
4.7. Identity of the NIGPAS Specimen
4.8. Identity of Specimens in PED 1302
4.9. Identity of Specimen PED 4084
4.10. Life Habits
4.11. Faunal Changes over Time
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bánki, O.; Roskov, Y.; Döring, M.; Ower, G.; Hernández Robles, D.R.; Plata Corredor, C.A.; Stjernegaard Jeppesen, T.; Örn, A.; Pape, T.; Hobern, D.; et al. Catalogue of Life (Version 2025-04-10); Catalogue of Life: Amsterdam, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Ashe, J.S.; Newton, A.F., Jr. Larvae of Trichophya and phylogeny of the tachyporine group of subfamilies (Coleoptera: Staphylinidae) with a review, new species and characterization of the Trichophyinae. Syst. Entomol. 1993, 18, 267–286. [Google Scholar] [CrossRef]
- Solodovnikov, A.Y.; Newton, A.F. Phylogenetic placement of Arrowinini trib. n. within the subfamily Staphylininae (Coleoptera: Staphylinidae), with revision of the relict South African genus Arrowinus and description of its larva. Syst. Entomol. 2005, 30, 398–441. [Google Scholar] [CrossRef]
- Clarke, D.J.; Grebennikov, V.V. Monophyly of Euaesthetinae (Coleoptera: Staphylinidae): Phylogenetic evidence from adults and larvae, review of austral genera, and new larval descriptions. Syst. Entomol. 2009, 34, 346–397. [Google Scholar] [CrossRef]
- Frank, J.H.; Ahn, K.J. Coastal Staphylinidae (Coleoptera): A worldwide checklist, biogeography and natural history. ZooKeys 2011, 107, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Parker, J. Myrmecophily in beetles (Coleoptera): Evolutionary patterns and biological mechanisms. Myrmecol. News 2016, 22, 65–108. [Google Scholar]
- Cai, C.; Huang, D.; Newton, A.F.; Eldredge, K.T.; Engel, M.S. Early evolution of specialized termitophily in Cretaceous rove beetles. Curr. Biol. 2017, 27, 1229–1235. [Google Scholar] [CrossRef]
- Hansen, A.K.; Justesen, M.J.; Kepfer-Rojas, S.; Byriel, D.B.; Pedersen, J.; Solodovnikov, A. Ecogeographic patterns in a mainland-island system in Northern Europe as inferred from the rove beetles (Coleoptera: Staphylinidae) on Læsø island. Europ. J. Entomol. 2018, 115, 256–263. [Google Scholar] [CrossRef]
- Thayer, M.K. 14.7. Staphylinidae Latreille, 1802. In Handbook of Zoology, Arthropoda: Insecta; Beutel, R.G., Kristensen, N.P., Eds.; Arthropoda: Insecta: Coleoptera, Beetles, Volume 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim), 2nd ed.; Beutel, R.G., Leschen, R.A.B., Eds.; Walter de Gruyter: Berlin, Germany, 2016; pp. 394–442. [Google Scholar]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Nat. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef]
- Chatzimanolis, S. A review of the fossil history of Staphylinoidea. In Biology of Rove Beetles (Staphylinidae) Life History, Evolution, Ecology and Distribution; Betz, O., Irmler, U., Klimaszewski, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 27–45. [Google Scholar] [CrossRef]
- Franz, H. Fossile Scydmaenidae in Baltischem und Dominikanischem Bernstein (Coleoptera: Scydmaenidae). N. Entomol. Nachr. 1983, 7, 25–29. [Google Scholar]
- Jałoszyński, P. Description of Euroleptochromus gen. n. (Coleoptera, Staphylinidae, Scydmaeninae) from Baltic amber, with discussion of biogeography and mouthpart evolution within Clidicini. Syst. Entomol. 2012, 37, 346–359. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Brunke, A.J.; Yamamoto, S.; Takahashi, Y. Evolution of Mastigitae: Mesozoic and Cenozoic fossils crucial for reclassification of extant tribes (Coleoptera: Staphylinidae: Scydmaeninae). Zool. J. Linn. Soc. 2018, 184, 623–652. [Google Scholar] [CrossRef]
- Yin, Z.; Cai, C.Y. A new fossil species of Euroleptochromus Jałoszyński (Coleoptera: Staphylinidae: Scydmaeninae) from Eocene Baltic amber. Zootaxa 2018, 4500, 146–150. [Google Scholar] [CrossRef]
- Shavrin, A.V.; Yamamoto, S. Unexpected palaeodiversity of omaliine rove beetles in Eocene Baltic amber (Coleoptera, Staphylinidae, Omaliinae). ZooKeys 2019, 863, 35–83. [Google Scholar] [CrossRef]
- Jenkins Shaw, J.; Bai, M.; Solodovnikov, A. Evolutionary assessment of the rove beetle subfamily Proteininae Erichson, 1839 (Coleoptera: Staphylinidae) triggered by the X-ray based description of its first fossil in Baltic amber from Denmark. J. Nat. Hist. 2023, 57, 1138–1151. [Google Scholar] [CrossRef]
- O’Keefe, S.; Pike, T.; Poinar, G. Palaeoleptochromus schaufussi (gen. nov., sp. nov.), a new antlike stone beetle (Coleoptera: Scydmaenidae) from Canadian Cretaceous amber. Canad. Entomol. 1997, 129, 379–385. [Google Scholar] [CrossRef]
- Poinar, G.; Brown, A.E. A new subfamily of Cretaceous antlike stone beetles (Coleoptera: Scydmaenidae: Hapsomelinae) with an extra leg segment. Proc. Entomol. Soc. 2004, 106, 789–796. [Google Scholar]
- Chatzimanolis, S.; Engel, M.S.; Newton, A.F.; Grimaldi, D.A. New ant-like stone beetles in mid-Cretaceous amber from Myanmar (Coleoptera: Staphylinidae: Scydmaeninae). Cretac. Res. 2010, 31, 77–84. [Google Scholar] [CrossRef]
- Kirejtshuk, A.G.; Kurbatov, S.A.; Nel, A. A new species of the genus Clidicus from the Lower Cretaceous of France (Coleoptera: Staphylinidae: Scydmaeninae). Proc. Zool. Inst. RAS 2015, 319, 508–514. [Google Scholar] [CrossRef]
- Cai, C.; Huang, D. Cretoleptochromus archaicus gen. et sp. nov., a new genus of ant-like stone beetles in Upper Cretaceous Burmese amber (Coleoptera, Staphylinidae, Scydmaeninae). Cretac. Res. 2016, 63, 7–13. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Peris, D. Cretaceous amber inclusions of Spain and Myanmar demonstrate early diversification and wide dispersal of Cephenniitae (Coleoptera: Staphylinidae: Scydmaeninae). Cretac. Res. 2016, 57, 190–198. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Perkovsky, E.E. The extant genus Eutheia (Coleoptera: Staphylinidae: Scydmaeninae) discovered in Upper Cretaceous Taimyr amber. Cretac. Res. 2016, 66, 6–10. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Perkovsky, E.E. Taimyraphes gen. nov., the first glandulariine ant-like stone beetle from Santonian Taimyr amber (Coleoptera: Staphylinidae: Scydmaeninae). Cretac. Res. 2019, 100, 164–171. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Bai, M.; Wang, B. A new species of the extinct mid-Cretaceous genus Scydmobisetia Jałoszyński & Yamamoto (Coleoptera, Staphylinidae, Scydmaeninae). Zootaxa 2020, 4802, 582–586. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Yamamoto, S.; Takahashi, Y. Scydmobisetia gen. nov., the first definite Glandulariini from Upper Cretaceous Burmese amber (Coleoptera: Staphylinidae: Scydmaeninae). Cretac. Res. 2016, 65, 59–67. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Brunke, A.J.; Metscher, B.; Zhang, W.W.; Bai, M. Clidicostigus gen. nov., the first Mesozoic genus of Mastigini (Coleoptera: Staphylinidae: Scydmaeninae) from Cenomanian Burmese amber. Cretac. Res. 2017, 72, 110–116. [Google Scholar] [CrossRef]
- Yamamoto, S.; Solodovnikov, A. The first fossil Megalopsidiinae (Coleoptera: Staphylinidae) from Upper Cretaceous Burmese amber and its potential for understanding basal relationships of rove beetles. Cretac. Res. 2016, 59, 140–146. [Google Scholar] [CrossRef]
- Yamamoto, S.; Maruyama, M.; Parker, J. Evidence for social parasitism of early insect societies by Cretaceous rove beetles. Nat. Comm. 2016, 7, 13658. [Google Scholar] [CrossRef]
- Cai, C.; Huang, D.; Newton, A.F.; Eldredge, K.T.; Engel, M.S. Response to “Evidence from amber for the origins of termitophily”. Curr. Biol. 2017, 27, R794–R795. [Google Scholar] [CrossRef]
- Yin, Z.W.; Cai, C.Y.; Huang, D.Y.; Li, L.Z. Specialized adaptations for springtail predation in Mesozoic beetles. Sci. Rep. 2017, 7, 98. [Google Scholar] [CrossRef]
- Yin, Z.; Cai, C.; Huang, D. Last major gap in scydmaenine evolution filled (Coleoptera: Staphylinidae). Cretac. Res. 2018, 84, 62–68. [Google Scholar] [CrossRef]
- Yin, Z.; Cai, C.; Huang, D.Y. A potentially diverse fauna of springtail-hunting scydmaenines during the late Mesozoic (Coleoptera, Staphylinidae, Scydmaeninae). Cretac. Res. 2018, 90, 163–167. [Google Scholar] [CrossRef]
- Yin, Z.; Cai, C.; Huang, D.; Li, L. A second species of the genus Cretoleptochromus Cai & Huang (Coleoptera: Staphylinidae: Scydmaeninae) from mid-Cretaceous Burmese amber. Cretac. Res. 2017, 75, 115–119. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, D.; Cai, C.; Huang, D.; Engel, M.S. Pangusyndicus gen. nov.: A new mid-Cretaceous Scydmaenine with reduced antennae and prothoracic gland (Coleoptera, Staphylindiae: Scydmaeninae). J. Syst. Palaeontol. 2019, 17, 1129–1141. [Google Scholar] [CrossRef]
- Yin, Z.W.; Zhou, D.Y.; Cai, C.Y.; Newton, A.F. Transitional fossils illuminate early evolution of the ant-like stone beetle tribe Leptomastacini (Coleoptera: Staphylinidae: Scydmaeninae). J. Syst. Palaeontol. 2019, 17, 2031–2042. [Google Scholar] [CrossRef]
- Jałoszyński, P. † Praphennium gen. nov.: Extant mite killers meet their Cenomanian relatives (Coleoptera: Staphylinidae: Scydmaeninae: Cephenniini). Cretac. Res. 2018, 90, 142–153. [Google Scholar] [CrossRef]
- Jałoszyński, P. Notes on mid-Cretaceous †Pangusyndicus and its relatives, with description of †Loeblitoides gen. nov. and a †Nuegua-like new species (Coleoptera: Staphylinidae: Scydmaeninae). Cretac. Res. 2020, 107, 104273. [Google Scholar] [CrossRef]
- Yin, Z. Discrimination of species of Hapsomela Poinar & Brown (Coleoptera: Staphylinidae: Scydmaeninae) in mid-Cretaceous Burmese amber. Palaeoentomology 2020, 3, 309–316. [Google Scholar] [CrossRef]
- Yin, Z.W.; Cai, C.Y. A new species of minute Scydmaenini (Coleoptera: Staphylinidae: Scydmaeninae) in mid-Cretaceous amber from Myanmar. Cretac. Res. 2019, 101, 70–75. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, D. Scydmaenus linqibini sp. nov., a new fossil Scydmaenini in mid-Cretaceous amber from northern Myanmar (Coleoptera: Staphylinidae: Scydmaeninae). Palaeoentomology 2020, 3, 036–040. [Google Scholar] [CrossRef]
- Badano, D.; Fratini, M.; Maugeri, L.; Palermo, F.; Pieroni, N.; Cedola, A.; Haug, J.T.; Weiterschan, T.; Velten, J.; Mei, M.; et al. X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Syst. Entomol. 2021, 46, 672–684. [Google Scholar] [CrossRef]
- Mazur, A.; Melke, A. Staphylinina (Coleoptera: Staphylinidae) of Poland; Wydawnictwo Uniwersytetu Przyrodniczego w Poznaniu: Poznań, Poland, 2022; p. 240. [Google Scholar]
- Frank, J.H. Staphylinidae (Staphylinoidea) (including Brathinidae, Empelidae). In Immature Insects, Vol. 2; Stehr, F.W., Ed.; Kendall-Hunt Publishing Co.: Dubuque, IA, USA, 1991; pp. 341–352. [Google Scholar]
- Thayer, M.K. 11.7. Staphylinidae Latreille, 1802. In Handbuch der Zoologie (Handbook of Zoology) Eine Naturgeschichte der Stämme des Tierreiches (A Natural History of the Phyla of the Animal Kingdom) Band IV (Volume IV); Kristensen, N.P., Beutel, R.G., Eds.; Arthropoda: Insecta: Coleoptera, Beetles, Volume 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim); Beutel, R.G., Leschen, R.A.B., Eds.; Walter de Gruyter: Berlin, Germany, 2005; pp. 296–344. [Google Scholar]
- De Marzo, L. Morfologia delle larve e della pupa in Mastigus pilifer Kraatz (Coleoptera, Scydmaenidae). Entomologica 1984, 19, 61–74. [Google Scholar]
- Vit, S.; De Marzo, L. Description of the larva of Leptomastax hypogaeus Pirazzoli (Coleoptera Scydmaenidae). Arch. Sci. 1989, 42, 569–578. [Google Scholar]
- Newton, A.F. Insecta: Coleoptera: Staphylinidae adults and larvae. In Soil Biology Guide; Dindal, D.L., Ed.; John Wiley and Sons: New York, NY, USA, 1990; pp. 1137–1174. [Google Scholar]
- Schmidt, D.A. Notes on the biology and a description of the egg, third instar larva and pupa of Platydracus tomentosus (Gravenhorst) (Coleoptera: Staphylinidae). Coleopt. Bull. 1994, 48, 310–318. [Google Scholar]
- Thayer, M.K. Glypholoma larvae at last: Phylogenetic implications for basal Staphylinidae? (Coleoptera: Staphylinidae: Glypholomatinae). Invert. Syst. 2000, 14, 741–754. [Google Scholar] [CrossRef]
- Leschen, R.A.; Newton, A.F. Larval description, adult feeding behavior, and phylogenetic placement of Megalopinus (Coleoptera: Staphylinidae). Coleopt. Bull. 2003, 57, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Ashe, J.S. Phylogeny of the tachyporine group subfamilies and ‘basal’ lineages of the Aleocharinae (Coleoptera: Staphylinidae) based on larval and adult characteristics. Syst. Entomol. 2005, 30, 3–37. [Google Scholar] [CrossRef]
- O’Keefe, S.T. 11.5. Scydmaenidae Leach, 1815. In Handbook of Zoology, Vol. IV, Part 38, Coleoptera, Vol. 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Staphyliniformia, Scarabaeiformia, Elateriformia); Beutel, R.G., Leschen, R.A.B., Eds.; De Gruyter: Berlin, Germany, 2005; pp. 280–288. [Google Scholar] [CrossRef]
- Grebennikov, V.V.; Newton, A.F. Good-bye Scydmaenidae, or why the ant-like stone beetles should become megadiverse Staphylinidae sensu latissimo (Coleoptera). Europ. J. Entomol. 2009, 106, 275–301. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Beutel, R.G. Functional morphology and evolution of specialized mouthparts of Cephenniini (Insecta, Coleoptera, Staphylinidae, Scydmaeninae). Arthropod Struct. Dev. 2012, 41, 593–607. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Kilian, A. Larval morphology of Scydmaenus tarsatus and S. hellwigii, with notes on feeding behaviour and a review of the bibliography on the preimaginal stages of ant-like stone beetles (Coleoptera: Staphylinidae: Scydmaeninae). Europ. J. Entomol. 2012, 109, 587–601. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Kilian, A. Description of the second-and third-instar larva of South African Stenomastigus longicornis (Boheman) (Coleoptera: Staphylinidae, Scydmaeninae). Zootaxa 2016, 4158, 151–182. [Google Scholar] [CrossRef]
- Jałoszyński, P.; Kilian, A. Comparative morphology of second and third instar larvae of Palaeostigus bifoveolatus (Boheman, 1851) (Coleoptera: Staphylinidae: Scydmaeninae). Zool. Anz. 2019, 279, 1–25. [Google Scholar] [CrossRef]
- Pietrykowska-Tudruj, E.; Czepiel-Mil, K.; Staniec, B. Larval morphology of selected Quedius Stephens, 1829 (Coleoptera: Staphylinidae: Staphylinini) with comments on their subgeneric affiliation. Zootaxa 2014, 3827, 493–516. [Google Scholar] [CrossRef]
- Staniec, B.; Pietrykowska-Tudruj, E.; Pawlęga, K. First description of the larva of Dinaraea Thomson, 1858, with comments on chaetotaxy, pupa, and life history based on two saproxylic species from Europe (Staphylinidae, Aleocharinae, Athetini). ZooKeys 2018, 752, 99–123. [Google Scholar] [CrossRef] [PubMed]
- Staniec, B.; Pietrykowska-Tudruj, E.; Wagner, G.K.; Mazur, A.; Kowalczyk, M. Synthesis of current knowledge of the morphology of the larval stages of Paederinae (Coleoptera; Staphylinidae), with a first insight into the mature larva of Pseudomedon Mulsant & Rey, 1878, in the light of a new systematic division. Insects 2022, 13, 982. [Google Scholar] [CrossRef]
- Staniec, B.; Sałapa, D.; Pietrykowska-Tudruj, E. Comparative morphology of the larvae of the rove beetles of Paederus, Lathrobium, and Tetartopeus, with notes on its systematic position (Coleoptera: Staphylinidae: Paederinae). J. Insect Sci. 2014, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Jałoszyński, P. Redescription of late-instar larva of Scydmoraphes sparshalli (Denny) Coleoptera: Staphylinidae, Scydmaeninae. Zootaxa 2015, 4032, 582–594. [Google Scholar] [CrossRef]
- Jałoszyński, P. Morphological diversity of immature Scydmaeninae. In Biology of Rove Beetles (Staphylinidae); Betz, O., Irmler, U., Klimaszewski, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 321–333. [Google Scholar] [CrossRef]
- Hu, F.S.; Bogri, A.; Solodovnikov, A.; Hansen, A.K. Hypogean Quedius of Taiwan and their biogeographic significance (Coleoptera: Staphylinidae: Staphylininae). Europ. J. Taxon. 2020, 664, 1–24. [Google Scholar] [CrossRef]
- Staniec, B.; Bordoni, A. Comparative larval ultramorphology of three endemic Lathrobium (Glyptomerus) species (Coleoptera, Staphylinidae, Paederinae) from the Eastern Alps in Italy. Zootaxa 2022, 5175, 206–230. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Solodovnikov, A. Immature stages of the remarkable and rare West Palaearctic rove beetle Emus hirtus (Coleoptera: Staphylinidae: Staphylinini) in the phylogenetic context of the subtribe Staphylinina. Europ. J. Entomol. 2023, 120, 105–114. [Google Scholar] [CrossRef]
- Zippel, A.; Cao, Q.; Betz, O. Morphology of the abdominal segmental glands and spinning behaviour of Stenus larvae (Coleoptera, Staphylinidae). Arthropod Struct. Dev. 2023, 75, 101286. [Google Scholar] [CrossRef]
- Wu, R.J.C. Secrets of a Lost World: Dominican Amber, and Its Inclusions; Self Publication: Santo Domingo, Dominican Republic, 1996; p. 222. [Google Scholar]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; p. 755. [Google Scholar]
- Poinar, G.O. Hair in Dominican amber: Evidence for tertiary mammals in the Antilles. Experientia 1988, 44, 88–89. [Google Scholar] [CrossRef]
- Haug, C.; Zippel, A.; Müller, P.; Haug, J.T. A modern type of ant-like stone beetle larva preserved in 99-million-year-old Kachin amber. Fragm. Entomol. 2022, 54, 193–200. [Google Scholar] [CrossRef]
- Cruickshank, R.D.; Ko, K. Geology of an amber locality in the Hukawng Valley, northern Myanmar. J. Asian Earth Sci. 2003, 21, 441–455. [Google Scholar] [CrossRef]
- Shi, G.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.; Lei, W.; Li, Q.; Li, X. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Yu, T.; Kelly, R.; Mu, L.; Ross, A.; Kennedy, J.; Broly, P.; Xia, F.; Zhang, H.; Wang, B.; Dilcher, D. An ammonite trapped in Burmese amber. Proc. Nat. Acad. Sci. USA 2019, 116, 11345–11350. [Google Scholar] [CrossRef]
- Haug, J.T.; Azar, D.; Ross, A.; Szwedo, J.; Wang, B.; Arillo, A.; Baranov, V.; Bechteler, J.; Beutel, R.; Blagoderov, V.; et al. Comment on the letter of the Society of Vertebrate Paleontology (SVP) dated April 21, 2020 regarding “Fossils from conflict zones and reproducibility of fossil-based scientific data”, Myanmar amber. PalZ 2020, 94, 431–437. [Google Scholar] [CrossRef]
- Dunne, E.M.; Raja, N.B.; Stewens, P.P.; Zaw, K. Ethics, law, and politics in palaeontological research: The case of Myanmar amber. Commun. Biol. 2022, 5, 1023. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.; Tun, K.L.; Mon, T.L.; Hin, W.W.; Haug, J.T. The strange holometabolan beak larva from about 100 million years old Kachin amber was physogastric and possibly wood-associated. Palaeoentomology 2023, 006, 372–384. [Google Scholar] [CrossRef]
- Haug, J.T.; Kiesmüller, C.; Haug, G.T.; Haug, C.; Hörnig, M.K. A fossil aphidlion preserved together with its prey in 40 million-year-old Baltic amber. Palaeobiodiv. Palaeoenv. 2023, 103, 155–163. [Google Scholar] [CrossRef]
- Tillyard, R.J. Studies in Australian Neuroptera. No. 7. The life-history of Psychopsis elegans (Guérin). Proc. Linn. Soc. 1918, 43, 787–818. [Google Scholar]
- MacLeod, E.G. The Neuroptera of the Baltic Amber. I. Ascalaphidae, Nymphidae, and Psychopsidae. Psyche 1970, 77, 147–180. [Google Scholar] [CrossRef]
- New, T.R. Planipennia, Lacewings. Handbuch der Zoologie, Vol. 4. Arthropoda: Insecta, Part 30; Walter de Gruyter: Berlin, Germany, 1989. [Google Scholar]
- New, T.R. Neuroptera. In The Insects of Australia: A Textbook for Students and Research Workers, Volume 2; Commonwealth Scientific and Industrial Research Organisation, Ed.; Melbourne University Press: Victoria, Australia, 1991; pp. 525–542. [Google Scholar]
- Weitschat, W.; Wichard, W. Atlas of Plants and Animals in Baltic Amber; Dr. Friedrich Pfeil: München, Germany, 2002; p. 256. [Google Scholar]
- Perrichot, V. Environnements Paraliques à Ambre et à Végétaux du Crétacé Nord-Aquitain (Charentes, Sud-Ouest de la France). Ph.D. Thesis, Université Rennes 1, Rennes, France, 2003. Available online: https://tel.archives-ouvertes.fr/tel-00011639/file/Memoire_GS_Web.pdf (accessed on 9 August 2025).
- Engel, M.S.; Grimaldi, D.A. Diverse Neuropterida in Cretaceous amber, with particular reference to the paleofauna of Myanmar (Insecta). Nova Suppl. Entomol. 2008, 20, 1–86. [Google Scholar]
- Ross, A. Amber: The Natural Time Capsule; Firefly Books: Richmond Hill, ON, USA, 2009; p. 112. [Google Scholar]
- Gröhn, C. Einschlüsse im Baltischen Bernstein; Wachholtz Verlag-Murmann Publishers: Kiel, Germany, 2015; p. 424. [Google Scholar]
- Badano, D.; Aspöck, U.; Aspöck, H.; Cerretti, P. Phylogeny of Myrmeleontiformia based on larval morphology (Neuropterida: Neuroptera). Syst. Entomol. 2017, 42, 94–117. [Google Scholar] [CrossRef]
- Makarkin, V.N. Re-description of Grammapsychops lebedevi Martynova, 1954 (Neuroptera: Psychopsidae) with notes on the Late Cretaceous psychopsoids. Zootaxa 2018, 4524, 581–594. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; Pazinato, P.G.; Braig, F.; Perrichot, V.; Gröhn, C.; Müller, P.; Haug, J.T. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontol. Electron. 2020, 23, a39. [Google Scholar] [CrossRef]
- Hassenbach, C.; Buchner, L.; Haug, G.T.; Haug, C.; Haug, J.T. An expanded view on the morphological diversity of long-nosed antlion larvae further supports a decline of silky lacewings in the past 100 million years. Insects 2023, 14, 170. [Google Scholar] [CrossRef]
- Roux, J.L.F.P. Lettre relative à divers Coquilles, Crustacés. Insectes, Reptiles et Oiseaux, observés en Égypte; adressée par M. Roux à M. le Baron de Férussac. Ann. Sci. Natur. 1833, 28, 72–78. [Google Scholar] [CrossRef]
- Schaum, H.R. Necrophilus arenarius Roux, die muthmassliche Larve von Nemoptera. Berlin. Entomol. Z. 1857, 1, 1–9. [Google Scholar] [CrossRef]
- Eltringham, H. On the larva of Pterocroce storeyi, with (Nemopteridae). Trans. Entomol. Soc. 1923, 71, 263–266. [Google Scholar] [CrossRef]
- Imms, A.D. A General Textbook of Entomology; Methuen & Co.: London, UK, 1923; p. 736. [Google Scholar]
- Withycombe, C.L. XIII. Systematic notes on the Crocini (Nemopteridae), with descriptions of new genera and species. Trans. R. Entomol. Soc. 1923, 71, 269–287. [Google Scholar] [CrossRef]
- Pierre, F. Morphologie, milieu biologique et comportement de trois Crocini nouveaux du Sahara nord-occidental (Planipennes, Nemopteridae). Ann. Soc. Entomol. 1952, 119, 1–22. [Google Scholar] [CrossRef]
- Mansell, M.W. The larva of Laurhervasia setacea (Klug), (Neuroptera: Nemopteridae: Crocinae) from southern Africa. J. Entomol. Soc. 1976, 39, 153–158. [Google Scholar]
- Mansell, M.W. A new genus and species in the Crocinae (Neuroptera: Nemopteridae) from Southern Africa. J. Entomol. Soc. 1977, 40, 195–203. [Google Scholar]
- Mansell, M.W. The Crocinae of Southern Africa (Neuroptera: Nemopteridae). 1. The genera Laurhervasia Navás and Thysanocroce Withycombe. J. Entomol. Soc. 1980, 43, 341–365. [Google Scholar]
- Mansell, M.W. The Crocinae of Southern Africa (Neuroptera: Nemopteridae). 2. The genus Concroce Tjeder. J. Entomol. Soc. 1981, 44, 91–106. Available online: https://hdl.handle.net/10520/AJA00128789_2865 (accessed on 17 August 2025).
- Mansell, M.W. New Crocinae (Neuroptera: Nemopteridae) from South America, with descriptions of larvae. J. Entomol. Soc. 1983, 46, 115–130. [Google Scholar]
- Richards, O.W.; Davies, R.G. Imms’ General Textbook of Entomology, 10th ed.; Chapman & Hall: London, UK, 1977; p. 394. [Google Scholar] [CrossRef]
- Monserrat, V.J. Pterocroce capillaris (Klug, 1836) en Europa (Neur., Plan., Nemopteridae). Neuroptera Internat. 1983, 2, 109–128. [Google Scholar]
- Monserrat, V.J. Nuevos datos sobre algunas especies de Nemopteridae y Crocidae (Insecta: Neuroptera). Heteropterus Rev. Entomol. 2008, 8, 1–33. [Google Scholar]
- Miller, A.B.; Stange, L.A. A new species of Moranida Mansell from Venezuela (Neuroptera: Nemopteridae). Insecta Mundi 1989, 3, 65–70. [Google Scholar]
- Hölzel, H. Die Nemopteriden (Fadenhafte) Arabiens. Stapfia 1999, 60, 129–146. [Google Scholar]
- Suludere, Z.; Satar, A.; Candan, S.; Canbulat, S. Morphology and surface structure of eggs and first instar larvae of Dielocroce baudii (Neuroptera: Nemopteridae) from Turkey. Entomol. News 2006, 117, 521–530. [Google Scholar] [CrossRef]
- Aspöck, H.; Aspöck, U. Another neuropterological field trip to Morocco. Lacewing News 2014, 19, 6–7. [Google Scholar]
- Tusun, S.; Satar, A. Morphology, surface structure and sensory receptors of larvae of Dielocroce ephemera (Gerstaecker, 1894) (Neuroptera: Nemopteridae). Entomol. News 2016, 126, 144–149. [Google Scholar] [CrossRef]
- Herrera-Flórez, A.F.; Haug, C.; Burmeister, E.-G.; Haug, J.T. A neuropteran insect with the relatively longest prothorax: The “giraffe” among insects is the larva of a Necrophylus species from Libya. Spixiana 2020, 43, 305–314. [Google Scholar]
- Haug, G.T.; Baranov, V.; Wizen, G.; Pazinato, P.G.; Müller, P.; Haug, C.; Haug, J.T. The morphological diversity of long-necked lacewing larvae (Neuroptera: Myrmeleontiformia). Bull. Geosci. 2021, 96, 431–457. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; Haug, J.T. The morphological diversity of spoon-winged lacewing larvae and the first possible fossils from 99 million-year-old Kachin amber, Myanmar. Palaeodiversity 2021, 14, 133–152. [Google Scholar] [CrossRef]
- Morton, K.J. Life history of Drepanepteryx phalaenoides Linn. Entomol. Mon. Mag. 1910, 46, 54–62. [Google Scholar]
- Smith, R.C. The life histories and stages of some hemerobiids and allied species. Ann. Entomol. Soc. 1923, 16, 129–151. [Google Scholar] [CrossRef]
- Killington, F.J. The life history of Hemerobius simulans Walker (= orotypus Wall.) (Neuroptera, Hemerobiidae). Entomol. Mon. Mag. 1932, 68, 176–180. [Google Scholar]
- Killington, F.J. On Psectra diptera (Burm.) (Neur., Hemerobiidae), including an account of its life-history. Entomol. Mon. Mag. 1946, 82, 161–176. [Google Scholar]
- Genay, A. Contribution à l’étude des Névroptères de Bourgogne. Trav. Lab. Zool. Stat. Aquic. Grimaldi Facul. Sci. Dijon 1953, 3, 1–30. [Google Scholar]
- Nakahara, W. Early stages of some Japanese Hemerobiidae including two new species. Kontyû 1954, 21, 41–46. [Google Scholar]
- MacLeod, E.G. The immature stages of Boriomyia fidelis (Banks) with taxonomic notes on the affinities of the genus Boriomyia (Neuroptera: Hemerobiidae). Psyche 1960, 67, 26–40. [Google Scholar] [CrossRef][Green Version]
- Miller, G.L.; Lambdin, P.L. Redescriptions of the larval stages of Hemerobius stigma Stephens (Neuroptera: Hemerobiidae). Florida Entomol. 1984, 67, 377–382. [Google Scholar] [CrossRef]
- Veenstra, C.; Feichter, F.; Gepp, J. Larval diagnosis of the European genera of Hemerobiidae (Insecta: Neuroptera). In Advances in Neuropterology, Proceedings of the Third International Symposium on Neuropterology Meeting, Berg en Dal, South Africa, 3–4 February 1988; Mansell, M.W., Aspöck, H., Eds.; South African Department of Agricultural Development: Pretoria, South Africa, 1990; pp. 211–213. [Google Scholar]
- Krakauer, A.H.; Tauber, C.A. Larvae of Micromus: Generic characteristics and a description of Micromus subanticus (Neuroptera: Hemerobiidae). Ann. Entomol. Soc. 1996, 89, 203–211. [Google Scholar] [CrossRef]
- Tauber, C.A.; Krakauer, A.H. Larval characteristics and generic placement of endemic Hawaiian hemerobiids (Neuroptera). Pacific Sci. 1997, 51, 413–423. [Google Scholar]
- Reguilón, C. Morfología de los estados inmaduros de Hemerobius bolivari (Neuroptera: Hemerobiidae) [=Morphology of the immature stages of Hemerobius bolivari (Neuroptera: Hemerobiidae)]. Rev. Soc. Entomol. Argent. 2002, 61, 63–68. [Google Scholar]
- Beutel, R.G.; Friedrich, F.; Aspöck, U. The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta). Zool. J. Linn. Soc. 2010, 158, 533–562. [Google Scholar] [CrossRef]
- Monserrat, V.J. Los hemeróbidos de la Península Ibérica y Baleares (Insecta, Neuropterida, Neuroptera: Hemerobiidae). Graellsia 2015, 71, 1–71. [Google Scholar] [CrossRef]
- Brauer, F. Über die Verwandlung verschiedener einheimischer Arten Florfliegen. Ber. Mitt. Freund. Naturwiss. 1851, 7, 125. [Google Scholar]
- Brauer, F.M. Larve von Hypochrysa nobilis Heyd. Verh. Zool.-Bot. Ges. 1867, 17, 27–30. [Google Scholar]
- Withycombe, C.L. XV. Some Aspects of the Biology and Morphology of the Neuroptera. With special reference to the immature stages and their possible phylogenetic significance. Trans. R. Entomol. Soc. 1924, 72, 303–411. [Google Scholar] [CrossRef]
- Townsend, L.H. Lacewings and their allies. Sci. Mon. 1939, 48, 350–357. [Google Scholar]
- Principi, M.M. Contributi allo studio dei „Neurotteri” italiani. IV. Nothochrysa italica Rossi. Boll. Istit. Entomol. Univ. 1946, 15, 85–102. [Google Scholar]
- Principi, M.M. Contributi allo studio dei Neurotteri Italiani. V. Ricerche su Chrysopa formosa Brauer e su alcuni suoi parassiti. Boll. Istit. Entomol. Univ. Stud. 1947, 16, 134–175. [Google Scholar]
- Principi, M.M. Contributi allo studio dei Neurotteri Italiani. XI. Chrysopa viridana Schn. Boll. Istit. Entomol. Univ. Stud. 1954, 20, 359–376. [Google Scholar]
- Principi, M.M. Contributi allo studio dei Neurotteri Italiani. XIII. Studio morfologico, etologico e sistematico di un gruppo omogeneo di specie del Gen. Chrysopa Leach (C. flavifrons Brauer, prasina Burm. e clathrata Schn.). Boll. Istit. Entomol. Univ. Stud. 1956, 21, 319–410. [Google Scholar]
- Adams, P.A. Neuroptera: Myrmeleontidae and Chrysopidae. Insects Micrones. 1959, 8, 13–33. [Google Scholar]
- Mehra, B.P. Biology of Chrysopa madestes Banks (Neuroptera, Chrysopidae). Ind. J. Entomol. 1965, 27, 398–407. [Google Scholar]
- Toschi, C.A. The taxonomy, life histories, and mating behavior of the green lacewings of Strawberry Canyon (Neuroptera, Chrysopidae). Hilgardia 1965, 36, 391–433. [Google Scholar] [CrossRef]
- Rousset, A. Morphologie cephalique des larves de planipennes insectes nevropteroides. Mem. Mus. Nat. Hist. Natur. Sér. A 1966, 42, 1–199. [Google Scholar]
- Tauber, C.A. Systematics of North American chrysopid larvae: Chrysopa carnea group (Neuroptera). Canad. Entomol. 1974, 106, 1133–1153. [Google Scholar] [CrossRef]
- Tsukaguchi, S. Descriptions of the larvae of Chrysopa Leach (Neuroptera, Chrysopidae) of Japan. Kontyû 1978, 46, 99–122. [Google Scholar]
- Tsukaguchi, S. Taxonomic notes on Brinckochrysa kintoki (Okamoto) (Neuroptera: Chrysopidae). Kontyû 1979, 47, 358–366. [Google Scholar]
- Monserrat, V.J. Sobre los Neurópteros de las Islas Canarias, III: Chrysopa flaviceps (Brullé, 1838) (Neur., Plan., Chrysopidae). Bol. Asoc. Españ. Entomol. 1982, 6, 113–119. [Google Scholar]
- Monserrat, V.J. Nuevos datos sobre algunas especies de crisópidos (Insecta: Neuroptera: Chrysopidae). Heteropterus Rev. Entomol. 2008, 8, 171–196. [Google Scholar]
- Díaz Aranda, L.M.; Monserrat, V.J. On the larval stages of genus Suarius Navás, 1914 in Europe (Neuroptera: Chrysopidae). Dt. Entomol. Z. Berlin (N.F.) 1996, 43, 89–97. [Google Scholar] [CrossRef]
- Tauber, C.A.; de León, T.; Arroyo, J.I.L.; Tauber, M.J. Ceraeochrysa placita (Neuroptera: Chrysopidae): Generic characteristics of larvae, larval descriptions, and life cycle. Ann. Entomol. Soc. 1998, 91, 608–618. [Google Scholar] [CrossRef]
- Tauber, C.A.; Tauber, M.J.; Albuquerque, G.S. Berchmansus elegans (Neuroptera: Chrysopidae): Larval and adult characteristics and new tribal affiliation. Europ. J. Entomol. 2006, 103, 221–231. [Google Scholar] [CrossRef]
- Hölzel, H.; Ohm, P.; Duelli, P. Contribution to the knowledge of the Neuroptera of Ethiopia. Entomofauna 1999, 20, 345–372. [Google Scholar]
- Tauber, C.A. Generic characteristics of Chrysopodes (Neuroptera: Chrysopidae), with new larval descriptions and a review of species from the United States and Canada. Ann. Entomol. Soc. 2003, 96, 472–490. [Google Scholar] [CrossRef]
- Tauber, C.A. Apochrysinae (Neuroptera: Chrysopidae): New larval description and subfamilial comparisons. Zootaxa 2014, 3835, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Rojht, H.; Budija, F.; Trdan, S. Effect of temperature on cannibalism rate between green lacewings larvae (Chrysoperla carnea [Stephens], Neuroptera, Chrysopidae). Acta Agricult. Sloven. 2009, 93, 5–9. [Google Scholar] [CrossRef]
- Tauber, C.A.; Tauber, M.J. An unusual chrysopid larva: Identification, description, and taxonomic implications. Ann. Entomol. Soc. 2013, 106, 729–740. [Google Scholar] [CrossRef]
- Zhao, C.; Ang, Y.; Wang, M.; Gao, C.; Zhang, K.; Tang, C.; Liu, X.; Li, M.; Yang, D.; Meier, R. Contribution to understanding the evolution of holometaboly: Transformation of internal head structures during the metamorphosis in the green lacewing Chrysopa pallens (Neuroptera: Chrysopidae). BMC Evol. Biol. 2020, 20, 79. [Google Scholar] [CrossRef]
- Weitschat, W. Jäger, Gejagte, Parasiten und Blinde Passagiere–Momentaufnahmen aus dem Bernsteinwald. Denisia 2009, 26, 243–256. [Google Scholar]
- Makarkin, V.N.; Wedmann, S.; Weiterschan, T. First record of a fossil larva of Hemerobiidae (Neuroptera) from Baltic amber. Zootaxa 2012, 3417, 53–63. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Speranza, M.; Wierzchos, J.; Ascaso, C.; Engel, M.S. Early evolution and ecology of camouflage in insects. Proc. Nat. Acad. Sci. USA 2012, 109, 21414–21419. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Peñalver, E.; Azar, D.; Engel, M.S. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Sci. Rep. 2018, 8, 16663. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Engel, M.S.; Azar, D.; Peñalver, E. The hatching mechanism of 130-million-year-old insects: An association of neonates, egg shells and egg bursters in Lebanese amber. Palaeontology 2019, 62, 547–559. [Google Scholar] [CrossRef]
- Xia, F.; Yang, G.; Zhang, Q.; Shi, G.; Wang, B. Amber: Life Through Time and Space; Science Press: Beijing, China, 2015; p. 196. [Google Scholar]
- Liu, H.; Luo, C.; Jarzembowski, E.A.; Xiao, C. Acanthochrysa langae gen. et sp. nov., a new lacewing larva (Neuroptera: Chrysopoidea) from mid-Cretaceous Kachin amber. Cretac. Res. 2022, 133, 105146. [Google Scholar] [CrossRef]
- Liu, X.; Shi, G.; Xia, F.; Lu, X.; Wang, B.; Engel, M.S. Liverwort mimesis in a Cretaceous lacewing larva. Curr. Biol. 2018, 28, 1475–1481. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Winterton, S.L.; Breitkreuz, L.C.; Engel, M.S. Early morphological specialization for insect-spider associations in Mesozoic lacewings. Curr. Biol. 2016, 26, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, G.; Xu, C.; Spicer, R.A.; Perrichot, V.; Schmidt, A.R.; Feldberg, K.; Heinrichs, J.; Chény, C.; Pang, H.; et al. The mid-Miocene Zhangpu biota reveals an outstandingly rich rainforest biome in East Asia. Sci. Adv. 2021, 7, eabg0625. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xia, F.; Engel, M.S.; Perrichot, V.; Shi, G.; Zhang, H.; Chen, J.; Jarzembowski, E.A.; Wappler, T.; Rust, J. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Sci. Adv. 2016, 2, e1501918. [Google Scholar] [CrossRef]
- Haug, J.T.; Baranov, V.; Müller, P.; Haug, C. New extreme morphologies as exemplified by 100 million-year-old lacewing larvae. Sci. Rep. 2021, 11, 20432. [Google Scholar] [CrossRef]
- Haug, C.; Haug, G.T.; Baranov, V.A.; Solórzano-Kraemer, M.M.; Haug, J.T. An owlfly larva preserved in Mexican amber and the Miocene record of lacewing larvae. Bol. Soc. Geol. Mex. 2021, 73, A271220. [Google Scholar] [CrossRef]
- Haug, J.T.; Linhart, S.; Haug, G.T.; Gröhn, C.; Hoffeins, C.; Hoffeins, H.-W.; Müller, P.; Weiterschan, T.; Wunderlich, J.; Haug, C. The diversity of aphidlion-like larvae over the last 130 million years. Insects 2022, 13, 336. [Google Scholar] [CrossRef]
- Froggatt, W.W. Australian Insects; William Brooks & Company Ltd.: Sydney, Australia, 1907; p. 449. [Google Scholar]
- Withycombe, C.L. Notes on the biology of some British Neuroptera (Planipennia). Trans. R. Entomol. Soc. 1922, 70, 501–594. [Google Scholar] [CrossRef]
- Killington, F.J. A Monograph of the British Neuroptera, Vol. 1 [of 2]; Ray Society: London, UK, 1936; p. xix + 269. [Google Scholar]
- MacLeod, E.G. A Comparative Morphological Study of the Head Capsule and Cervix of Larv al Neuroptera (Insecta). Doctoral Dissertation, Harvard University, Cambridge, MA, USA, 1964; p. 528. [Google Scholar]
- Riek, E.F. Neuroptera (Lacewings). In Insects of Australia: A Textbook for Students and Research Workers; CSIRO, Ed.; Melbourne University Press: Melbourne, Australia, 1970; pp. 472–494. [Google Scholar]
- Aspöck, H.; Aspöck, U. Synopsis der Systematik, Ökologie und Biogeographie der Neuropteren Mitteleuropas im Spiegel der Neuropteren-Fauna von Linz und Oberösterreich, sowie Bestimmungs-Schlüssel für die mitteleuropäischen Neuropteren und Beschreibung von Coniopteryx lentiae nov. spec. Naturkundl. Jahrb. 1964, 1964, 127–282. [Google Scholar]
- Aspöck, U.; Aspöck, H. Kamelhälse, Schlammfliegen, Ameisenlöwen. Wer sind sie? (Insecta: Neuropterida: Raphidioptera, Megaloptera, Neuroptera). Stapfia 1999, 60, 1–34. [Google Scholar]
- Aspöck, U.; Aspöck, H. Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia 2007, 20, 451–516. [Google Scholar]
- Gepp, J. Erforschungsstand der Neuropteren. Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 beschriebenen Larven und 600 Literaturzitaten). In Progress in World’s Neuropterology; Gepp, J., Aspöck, H., Hölzel, H., Eds.; Graz, Austria, 1984; pp. 183–239. [Google Scholar]
- Gepp, J. Neuropteren als Indikatoren der Naturraumbewertung. Eignung als Modellgruppe, Methodenwahl, Fallbeispiele sowie Diskussion möglicher Fragestellungen (Neuropterida). Neuropterida: Raphidioptera, Megaloptera, Neuroptera. Kamelhälse, Schlammfliegen, Ameisenlöwen. Stapfia 1999, 60, 167–208. [Google Scholar]
- Zhang, W.W. Frozen Dimensions. The Fossil Insects and Other Invertebrates in Amber; Chongqing University Press: Chongqing, China, 2017; p. 692. [Google Scholar]
- Badano, D.; Engel, M.S.; Basso, A.; Wang, B.; Cerretti, P. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nat. Comm. 2018, 9, 3257. [Google Scholar] [CrossRef]
- Wise, K.A.J. Larvae of three aquatic beetles (Coleoptera: Dytiscidae). New Zeal. Entomol. 1961, 2, 18–22. [Google Scholar] [CrossRef]
- Spangler, P.J. Description of the larva of Hydrovatus cuspidatus pustulatus Melsheimer (Coleoptera: Dytiscidae). J. Kansas Entomol. Soc. 1962, 35, 278–280. [Google Scholar]
- Spangler, P.J. A description of the larva of Macrovatellus mexicanus Sharp (Coleoptera: Dytiscidae). Coleopt. Bull. 1963, 17, 97–100. [Google Scholar] [CrossRef]
- Spangler, P.J. A new species of Derovatellus from Guatemala and a description of its larva (Coleoptera: Dytiscidae). Coleopt. Bull. 1966, 20, 11–18. [Google Scholar] [CrossRef]
- Galewski, K. Descriptions of larvae of Agabus uliginosus (L.) and A. congener (THUNB.) (Coleoptera, Dytiscidae). Ann. Zool. 1968, XXVI, 323–332. [Google Scholar]
- Galewski, K. The descriptions of larvae of Colymbetes dolabratus (PAYK.) with keys to the identification of larvae of the European species of Colymbetes CLAIRV. (Coleoptera, Dytiscidae). Ann. Zool. 1968, XXVI, 227–238. [Google Scholar]
- Galewski, K. Descriptions of the unknown larvae of the genera Hydaticus Leach and Graphoderus Dejean (Coleoptera, Dytiscidae) with some data on their biology. Ann. Zool. 1975, XXXII, 249–268. [Google Scholar]
- Galewski, K. Diagnostic characters of the second stage larvae of Central European species of Agabus LEACH (Coleoptera, Dytiscidae). Ann. Zool. 1986, 40, 397–415. [Google Scholar]
- Longley, G.; Spangler, P.J. The larva of a new subterranean water beetle, Haideoporus texanus (Coleoptera: Dytiscidae: Hydroporinae). Proc. Biol. Soc. 1977, 90, 532–535. [Google Scholar]
- Nilsson, A.N. Second and third stage larvae of Agabus serricornis Paykull (Coleoptera: Dytiscidae). Insect Syst. Evol. 1979, 10, 317–320. [Google Scholar] [CrossRef]
- Nilsson, A.N. The larvae of the predaceous diving beetles Bidessus grossepunctatus, Graptodytes granularis and G. pictus (Coleoptera: Dytiscidae). Aquat. Insects 1985, 7, 165–172. [Google Scholar] [CrossRef]
- Wolfe, G.W. The larva and pupa of Acilius fraternus fraternus (Coleoptera: Dytiscidae) from the Great Smoky Mountains, Tennessee. Coleopt. Bull. 1980, 34, 121–126. [Google Scholar]
- Drummond, H.; Wolfe, G.W. An observation of a diving beetle larva (Insecta: Coleoptera: Dytiscidae) attacking and killing a garter snake, Thamnophis elegans (Reptilia: Serpentes: Colubridae). Coleopt. Bull. 1981, 35, 121–124. [Google Scholar]
- Dettner, K. Description of the larvae of Acilius duvergeri (Col., Dytiscidae), with keys to larvae of European species of genus Acilius and of the European genera of subfamily Dytiscinae. Aquat. Ins. 1982, 4, 81–88. [Google Scholar] [CrossRef]
- Cuppen, J.G.; Nilsson, A.N. The second- and third-instar larvae of Hygrotus decoratus (Gyllenhal) (Coleoptera: Dytiscidae). Insect Syst. Evol. 1984, 15, 65–69. [Google Scholar] [CrossRef]
- Matta, J.F.; Peterson, D.E. The larvae of six Nearctic Hydroporus of the subgenus Neoporus (Coleoptera: Dytiscidae). Proc. Acad. Nat. Sci. Philadelphia 1985, 137, 53–60. [Google Scholar]
- Wolfe, G.W.; Roughley, R.E. Description of the pupa and mature larva of Matus ovatus ovatus Leech (Coleoptera: Dytiscidae) with a chaetotaxal analysis emphasizing mouthparts, legs, and urogomphus. Proc. Acad. Nat. Sci. USA 1985, 137, 61–79. [Google Scholar]
- Arndt, E.; Kirmse, S.; Erwin, T.L. Arboreal beetles of Neotropical forests: Agra Fabricius, larval descriptions with notes on natural history and behaviour (Coleoptera, Carabidae, Lebiini, Agrina). Coleopt. Bull. 2001, 55, 297–310. [Google Scholar] [CrossRef]
- Alarie, Y.; Bilton, D.T. Larval morphology of Aspidytidae (Coleoptera: Adephaga) and its phylogenetic implications. Ann. Entomol. Soc. Amer. 2005, 98, 417–430. [Google Scholar] [CrossRef]
- Costa, C.; Lawrence, J.F.; Rosa, S.P. 4.7. Elateridae Leach, 1815. In Handbook of Zoology, Coleoptera, Beetles, Volume 2 Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim); Leschen, R.A.B., Beutel, R.G., Lawrence, J.F., Eds.; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 2010; pp. 75–103. [Google Scholar]
- Kasule, F.K. The subfamilies of the larvae of Staphylinidae (Coleoptera) with keys to the larvae of the British genera of Steninae and Proteininae. Trans. R. Entomol. Soc. 1966, 118, 261–283. [Google Scholar] [CrossRef]
- Yamamoto, S. Tachyporinae revisited: Phylogeny, evolution, and higher classification based on morphology, with recognition of a new rove beetle subfamily (Coleoptera: Staphylinidae). Biology 2021, 10, 323. [Google Scholar] [CrossRef]
- Steel, W.O. The larvae of the genera of the Omaliinae (Coleoptera: Staphylinidae) with particular reference to the British fauna. Trans. R. Entomol. Soc. 1970, 122, 1–47. [Google Scholar] [CrossRef]
- Gusarov, V.I. Phylogeny of the family Staphylinidae based on molecular data: A review. In Biology of Rove Beetles (Staphylinidae) Life History, Evolution, Ecology and Distribution; Betz, O., Irmler, U., Klimaszewski, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 7–25. [Google Scholar]
- Kasule, F.K. The larvae of Paederinae and Staphylininae (Coleoptera: Staphylinidae) with keys to the known British genera. Trans. R. Entomol. Soc. 1970, 122, 49–80. [Google Scholar] [CrossRef]
- Pietrykowska-Tudruj, E.; Staniec, B. Description of the egg and larva of Philonthus punctus (Gravenhorst, 1802) (Coleoptera, Staphylinidae, Staphylininae). Dt. Entomol. Z. 2006, 53, 179–192. [Google Scholar] [CrossRef]
- Amaral, A.P.; Gombos, D.; Haug, G.T.; Haug, C.; Gauweiler, J.; Hörnig, M.K.; Haug, J.T. Expanding the fossil record of soldier fly larvae—An important component of the Cretaceous amber forest. Diversity 2023, 15, 247. [Google Scholar] [CrossRef]
- Amaral, A.P.; Haug, J.T.; Haug, C.; Linhart, S.; Müller, P.; Hammel, J.U.; Baranov, V. Expanding the Mesozoic record of early brachyceran fly larvae, including new larval forms with chimera-type morphologies. Diversity 2024, 15, 270. [Google Scholar] [CrossRef]
- Baranov, V.A.; Wang, Y.; Gašparič, R.; Wedmann, S.; Haug, J.T. Eco-morphological diversity of larvae of soldier flies and their closest relatives in deep time. PeerJ 2020, 8, e10356. [Google Scholar] [CrossRef]
- Whiting, M.F. Phylogeny of holometabolous insects: The most successful group of terrestrial organisms. In Assembling the Tree of Life; Cracraft, J., Donoghue, M., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 345–364. [Google Scholar]
- Salnitska, M.; Solodovnikov, A.; Orlov, I. Sampling and curation of rove beetles (Insecta, Coleoptera, Staphylinidae) for comprehensive and DNA-grade collections to enhance biodiversity exploration in Northern Eurasia. Biodiv. Data J. 2022, 10, e96080. [Google Scholar] [CrossRef]
- Dettner, K.; Reissenweber, F. The defensive secretion of Omaliinae and Proteininae (Coleoptera: Staphylinidae): Its chemistry, biological and taxonomic significance. Biochem. Syst. Ecol. 1991, 19, 291–303. [Google Scholar] [CrossRef]
- Haupt, J.; Müller, F. New products of defense secretion in South East Asian whip scorpions (Arachnida: Uropygi: Thelyphonida). Z. Naturforsch. C 2004, 59, 579–581. [Google Scholar] [CrossRef]
- Freeman, J.; Hochberg, R. Structure and functional morphology of the acid-secreting pygidial glands in the whipscorpion Mastigoproctus giganteus (Lucas, 1835) (Arachnida: Thelyphonida). Zoomorphology 2018, 137, 85–103. [Google Scholar] [CrossRef]
- Cavill, G.W.K.; Robertson, P.L. Ant Venoms, Attractants, and Repellents: Secretions are used by ants in attack and defense and as chemical messengers in their social organization. Science 1965, 149, 1337–1345. [Google Scholar] [CrossRef]
- Scheffrahn, R.H.; Gaston, L.K.; Sims, J.J.; Rust, M.K. Defensive ecology of Forelius foetidus and its chemosystematic relationship to F.(=Iridomyrmex) pruinosus (Hymenoptera: Formicidae: Dolichoderinae). Environ. Entomol. 1984, 13, 1502–1506. [Google Scholar] [CrossRef]
- Grimaldi, D.A.; Engel, M.S.; Nascimbene, P.C. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Amer. Mus. Nov. 2002, 3361, 1–71. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Engel, M.S.; Delclòs, X.; Peñalver, E. Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretac. Res. 2020, 106, 104200. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; van der Wal, S.; Müller, P.; Haug, J.T. Split-footed lacewings declined over time: Indications from the morphological diversity of their antlion-like larvae. PalZ 2022, 96, 29–50. [Google Scholar] [CrossRef]
- Haug, C.; Braig, F.; Haug, J.T. Quantitative analysis of lacewing larvae over more than 100 million years reveals a complex pattern of loss of morphological diversity. Sci. Rep. 2023, 13, 6127. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, H.; Jarzembowski, E.A. High morphological disparity of neuropteran larvae during the Cretaceous revealed by a new large species. Geol. Mag. 2022, 159, 954–962. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. 100 million-year-old straight-jawed lacewing larvae with enormously inflated trunks represent the oldest cases of extreme physogastry in insects. Sci. Rep. 2022, 12, 12760. [Google Scholar] [CrossRef] [PubMed]
- Haug, J.T.; Haug, C. Oldest record of a dustywing-type larva in about 100-million-year-old amber. Palaeodiversity 2023, 16, 141–150. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. New details of the enigmatic 100 million years old antlion-like larvae of Ankyloleon (Myrmeleontiformia, Neuroptera). Europ. J. Taxon. 2023, 908, 135–154. [Google Scholar] [CrossRef]
- Braig, F.; Popp, T.; Zippel, A.; Haug, G.T.; Linhart, S.; Müller, P.; Weiterschan, T.; Haug, J.T.; Haug, C. The diversity of larvae with multi-toothed stylets from about 100 million years ago illuminates the early diversification of antlion-like lacewings. Diversity 2023, 15, 1219. [Google Scholar] [CrossRef]
- Buchner, L.; Linhart, S.; Kalmar, G.; Arce, S.; Haug, G.T.; Haug, J.T.; Haug, C. New fossil lacewing larvae with trumpet-shaped elongate empodia provide insight into the evolution of this attachment structure. Riv. Ital. Paleo. Stratigr. 2024, 130, 67–80. [Google Scholar] [CrossRef]
- Mengel, L.; Linhart, S.; Haug, G.T.; Weiterschan, T.; Müller, P.; Hoffeins, C.; Hoffeins, H.-W.; Baranov, V.; Haug, C.; Haug, J.T. The morphological diversity of dragon lacewing larvae (Nevrorthidae, Neuroptera) changed more over geological time scales than anticipated. Insects 2023, 14, 749. [Google Scholar] [CrossRef]
- van Eldijk, T.J.; Wappler, T.; Strother, P.K.; van der Weijst, C.M.; Rajaei, H.; Visscher, H.; van de Schootbrugge, B. A Triassic-Jurassic window into the evolution of Lepidoptera. Sci. Adv. 2018, 4, e1701568. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C. A 100 million-year-old armoured caterpillar supports the early diversification of moths and butterflies. Gondwana Res. 2021, 93, 101–105. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, C.; Wang, Y.; Baranov, V.A. The fossil record of lepidopteran caterpillars in Dominican and Mexican amber. Lethaia 2022, 55, 1–14. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haug, J.T.; Zippel, A.; Haug, G.T.; Haug, C. Possible Fossil Larvae of Staphylinidae from Kachin Amber and a Quantitative Morphological Comparison Indicate That Rove Beetle Larvae Partly Replaced Lacewing Larvae. Insects 2025, 16, 910. https://doi.org/10.3390/insects16090910
Haug JT, Zippel A, Haug GT, Haug C. Possible Fossil Larvae of Staphylinidae from Kachin Amber and a Quantitative Morphological Comparison Indicate That Rove Beetle Larvae Partly Replaced Lacewing Larvae. Insects. 2025; 16(9):910. https://doi.org/10.3390/insects16090910
Chicago/Turabian StyleHaug, Joachim T., Ana Zippel, Gideon T. Haug, and Carolin Haug. 2025. "Possible Fossil Larvae of Staphylinidae from Kachin Amber and a Quantitative Morphological Comparison Indicate That Rove Beetle Larvae Partly Replaced Lacewing Larvae" Insects 16, no. 9: 910. https://doi.org/10.3390/insects16090910
APA StyleHaug, J. T., Zippel, A., Haug, G. T., & Haug, C. (2025). Possible Fossil Larvae of Staphylinidae from Kachin Amber and a Quantitative Morphological Comparison Indicate That Rove Beetle Larvae Partly Replaced Lacewing Larvae. Insects, 16(9), 910. https://doi.org/10.3390/insects16090910





