Simple Summary
Two new species of the genus Philanthaxia were identified in Hainan, China. Morphological comparisons with existing species demonstrated significant taxonomic distinctions, confirming their status as new taxa. The adult host plant of both novelties is Casearia membranacea Hance (Salicaceae).
Abstract
In this paper, we describe two new species of Philanthaxia Deyrolle, 1864, from Hainan, China, namely, P. longicornis Ni & Song, sp. nov., and P. lui Ni & Song, sp. nov., with the adult host plant Casearia membranacea Hance (Salicaceae). The characters of these new species are described with illustrations, comparisons are made with the most similar species, and the COI gene sequence of P. longicornis and its analysis are provided.
1. Introduction
The genus Philanthaxia Deyrolle, 1864, belongs to the tribe Thomassetiini Bellamy, 1987, and was described with Philanthaxia curta Deyrolle, 1864, as the type species [1]. Species of the genus Philanthaxia are characterized by smaller, more oval-shaped eyes and a more convex vertex, which is typically 4.5–6.0 times wider than the eye width; the pronotum being widest at the base, with dense reticulate or punctate sculpturing; and the anal ventrite being regularly rounded, roundly truncated or rarely incurved apically [2].
Before the first revision by Bílý in 1993, Philanthaxia was mainly considered to be distributed in South and Southeast Asia as well as the Greater Sunda Islands [2]. Subsequently, the distribution of this genus expanded, with new records from the Maluku Islands and Irian Jaya (Papua) [3,4]. In China, only two species of the genus have been recorded in the Taiwan region: Philanthaxia sauteri Kerremans, 1912 [5], and Philanthaxia convexifrons Kurosawa, 1954 [6]. In this paper we provide a comparison between these two known species and two species new to science.
At present, records of the biology of the genus Philanthaxia are poorly known. The existing records only report that the adults of Philanthaxia species are found on Castanopsis sp., primarily in the higher level of the canopy, and larvae of this genus are still unknown [7].
Following Bílý’s revision in 1993, Bellamy’s 2008 catalogue documented 61 valid species within Philanthaxia [8]. Taking into account 4 new species described by Bílý & Nakládal [7], 4 new species described by Bílý [9], 1 new species reported by Ohmomo [10], and 2 new species described herein from Hainan, China, the total number of species in this genus has increased to 72 worldwide, including 1 fossil species [11].
2. Materials and Methods
2.1. Insect Collection and Image Processing
The two new species described in this article were captured on the leaves of Casearia membranacea Hance (Salicaceae) in Lizhiling, Sanya, Hainan (Figure 1 and Figure 2). Using standardized aerial sweep nets (Bingqing trading, Model: HL-PW), we implemented systematic sweep netting across all accessible strata of tall-canopied Casearia trees (height > 6 m). Each tree received 20 pendulum sweeps at 3 vertical levels (understory: 0–2 m; mid-canopy: 2–4 m; upper-canopy: 6 m+ via telescopic pole).

Figure 1.
(A) Collecting site photographed at Lizhiling. The arrow indicates the host plant Casearia membranacea. (B,C) Host plant leaves.

Figure 2.
(A,B) Philanthaxia longicornis Ni & Song, sp. nov., feeding on leaves; the arrow indicates the specific feeding site. (C,D) Philanthaxia lui Ni & Song, sp. nov., staying on leaves.
The photos were obtained using a Canon EOS 5DmarkIV (Canon, Beijing, China) with an EF70-200 mm f2.8 IS II USM lens (Canon, Beijing, China) for Figure 1 and an EF 100 mm f2.8L IS USM lens (Canon, Beijing, China) for Figure 2.
The specimens were studied using a Keyence VHX-5000 digital microscope (Keyence, Tokyo, Japan) with a VH-Z20R zoom lens (Keyence, Tokyo, Japan), images were processed and the layers combined into figures using Adobe Photoshop CC 2018. The measurements were followed by Bílý [9] and Qi [12] and made as follows:
Body length: Length between the top of the head and the tip of the elytra.
Body width: Widest part of the body.
Aedeagus length: Length between the base and the tip of the aedeagus.
Aedeagus width: The widest part of the parameres.
2.2. DNA Extraction and Sequence Analysis
Use TIANamp Micro DNA Kit (DP316, Tiangen, Beijing, China) to extract DNA, and refer to the instructions for specific experimental steps. Then use primers [13] (LCO1490: 5′-GGTCAACAAATCATAAAGATATTGG-3′, HCO2198: 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) for PCR amplification and sequence the PCR products.
Use DAMBE (ver. 7.3.32) to calculate ISS and ISS.C values for the target sequence with all 22 species’ sequences of the Bupretidae family on NCBI, and use Mega (ver. 7.0) to align construct evolutionary tree (Maximum Likelihood, ML) based on the Kimura 2-parameter genetic distance model.
All specimens examined herein are housed in the collection of Fujian Academy of Forestry, Fuzhou, China (FAF).
3. Results
Taxonomy
Family Buprestidae Leach, 1815;
Subfamily Buprestinae Leach, 1815;
Tribe Thomassetiini Bellamy, 1987;
Genus Philanthaxia Deyrolle, 1864;
Type species: Philanthaxia curta Deyrolle, 1864.
3.1. Philanthaxia longicornis Ni & Song, sp. nov.
(Figure 3)

Figure 3.
Habitus of Philanthaxia longicornis Ni & Song, sp. nov. (A,B) Male, holotype, 6.9 mm, from Sanya, Hainan. (C) Female, paratype, 8.4 mm, from Sanya, Hainan. (A,C) Dorsal view. (B) Ventral view. Scale bar = 1.0 mm.
Type locality: Mt. Lizhiling, Sanya City, Hainan Province, China.
Type specimen: Holotype (male, FAF): Southern Hainan Province, Sanya City, Mt. Lizhiling, 18°21′21″ N 109°27′20″ E, alt. 390 m, 2021-VI-19, Haitian Song leg.; paratypes (15 males, 9 females, FAF; 1 male (NACRC: IOZ (E)224851), 1 female (NACRC: IOZ (E) 224852): 18°21′21″ N 109°27′20″ E, alt. 390 m, 2021-VI-19, Haitian Song leg.; 2021-VI-22, Lu leg.; 2022-V-23, Jiasheng Lu leg.; 2023-VI-1, Shijie Lu leg.
Diagnosis: Medium-sized, rather convex, dorsal and ventral surfaces golden-green with metallic luster, sides of pronotum slightly depressed, frons with fine white setae and with extremely long antennomeres.
Description of holotype: Length 6.9 mm, width 2.6 mm; the aedeagus measures around 2.4 mm in length and 0.4 mm in width. Spindle-shaped, entirely golden-green with metallic luster.
Head (Figure 5A) golden-green, broader than the lateral side of pronotum, covered with evenly small, reticulate, irregular polygonal sculpturing. Fine white setae present at the frons. The vertex relatively flat, 5.1 times wider than the eye. The eyes large, nearly oval and laterally convex. The antennae (Figure 5E) very slender and long, reaching beyond elytral base and scutellum, consisting of 11 segments, with white setae. The scape rod-shaped, 6.0 times longer than wide. The pedicel short and ovoid, 2.0 times as long as wide. Antennomere 3 almost cylindrical, 2.4 times as long as wide. Antennomeres 4–10 slender and triangular, with a length-to-width ratio of 2.1–3.5; antennomeres 4 and 5 longer and the distal 2.0 times shorter. The terminal antennomere nearly triangular, 1.5 times longer than wide.
Pronotum (Figure 5A) nearly trapezoidal, about 1.6 times wider than long, covered with the same sculpturing as that on the head. Overall golden-green with a metallic sheen. The anterior edge curved, slightly convex in the middle; the posterior edge nearly straight, with the sides distinctly converging forward, slightly curved. The scutellum wide, 2.0 times as wide as long, dark green, subcordiform and depressed near the base of the pronotum.
Elytra (Figure 5C) 1.8 times longer than wide, with yellow coloration on both sides of the elytral suture, tapers sharply near the distal third. Each elytron has eight distinct striae with intervals bearing fine transverse rugosities. The lateral margins with fine serrations along apical third. The humeral callosities do not prominently protrude beyond the elytral lateral edges.
Legs (Figure 3) slender, covered with setae; fore tibiae slightly bent outward, without serrations, and tarsal segments 2–4 enlarged. The tarsal segments covered with setae.
Ventral side (Figure 3B) of the abdomen sparsely covered with short white setae. Prosternal process (Figure 5B) elongated, angularly tapering at apex. Abdominal apex lighter, changing from green to gold, with darker sides changing from green to black. The ventral surface of the last visible ventrite (Figure 5D) more or less black, covered with relatively denser and longer white setae, apex rounded.
Aedeagus (Figure 5F) widest at the middle, tapering at the posterior half; apices of parameres sharp, not distinctly expanded, the sides of the terminal portion covered with setae; apex of median lobe sharp.
Sexual dimorphism: Female (Figure 3C) clearly differs from male by the much shorter antennae and somewhat more robust body.
Etymology: This species is named after its morphological character of long antennae.
Differential diagnosis: The differential diagnosis is provided in Table 1.

Table 1.
Differential diagnosis of Philanthaxia longicorna Ni & Song, sp. nov., with two morphologically similar congeners.
Distribution: China (Hainan).
3.2. Philanthaxia lui Ni & Song, sp. nov.
(Figure 4)

Figure 4.
Habitus of Philanthaxia lui Ni & Song, sp. nov. (A,B) Male, holotype, 5.6 mm, from Sanya, Hainan. (C) Female, paratype, 8.5 mm, from Sanya, Hainan. (A,C) Dorsal view. (B) Ventral view. Scale bar = 1.0 mm.
Type locality: Mt. Lizhiling, Sanya City, Hainan Province, China.
Type specimen: Holotype (male, FAF): Southern Hainan Province, Sanya City, Mt. Lizhiling, 18°21′21″ N 109°27′20″ E, alt. 390 m, 2021-VI-19, Haitian Song leg.; paratypes (1 female, FAF; 1 female (NACRC: IOZ (E) 224853.) same data as holotype.
Diagnosis: Medium-sized, dorsally green, ventrally bronze, with metallic luster, pronotum evenly convex, frons green with fine setae.
Description of holotype: Length 5.6 mm, width 2.1 mm; the aedeagus measures around 2.2 mm in length and 0.3 mm in width. Fusiform with most of the anterior part of the body copper-colored with metallic luster.
Head (Figure 5G) large and broad, significantly wider than the pronotum. Vertex flat without noticeable convexity, and width about 4.8 times the eye width. Eyes large, oval and prominently protruding beyond the head sides. The sculpture consists of small, irregular polygonal cells with clearly defined boundaries and a metallic copper-green sheen. A fine groove runs along the middle, and the apex is covered with extremely fine white setae. Antennae (Figure 5K) long, almost reaching the base of the pronotum; scape nearly clavate, slightly curved and 5.7 times longer than wide; pedicel ovoid, about twice as long as wide. Antennomere 3 slender and ovoid, about 5.0 times as long as wide. Antennomeres 4–10 transitional from conical to nearly triangular in shape, shorter and thicker, with lengths 2.0–2.4 times their widths. The terminal flagellomere nearly triangular, 2.0 times as long as wide.
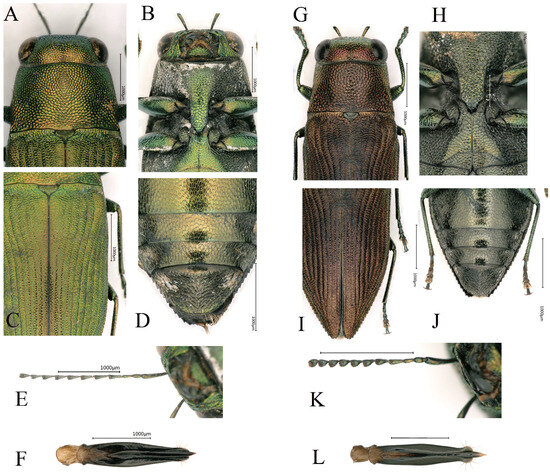
Figure 5.
(A–F) Philanthaxia longicornis Ni & Song, sp. nov. (holotype). (G–L) Philanthaxia lui Ni & Song, sp. nov. (holotype). (A,G) Details of pronotum. (B,H) Prosternal process. (C,I) Elytra. (D,J) Abdomen. (E,K) Antennae. (F,L) Aedeagus. (A,C,F,G,I,L) Dorsal view. (B,D,E,H,J,K) Ventral view. Scale bar = 1.0 mm, except scale bar of (H) = 0.5 mm.
Pronotum (Figure 5G) evenly convex, 1.4–1.7 times as wide as long; anterior margin convex at the center, posterior margin slightly curves inward at middle, lateral margins mostly straight. The sides of the pronotum bear paired symmetrical shallow depressions. The posterolateral edges slightly expand outward, marking the widest point of the pronotum. The pronotum sculpture resembles that of the head but with larger individual sculptural cells. Scutellum large, darker in color than the pronotum, subcordiform, depressed at the anterior, 1.6 times as wide as long.
Elytra (Figure 5I) flat, slightly raised along the suture, 2.0 times as long as wide; two-thirds of the anterior part nearly parallel, with fine serrations along the sides, while the posterior third tapers sharply, with serrations larger than those on the anterior two-thirds. The humeral callosities distinct, with the apex slightly projecting beyond the sides of the elytra. The basal transverse area broadly and shallowly depressed, almost connecting with the scutellum, and aligns with the pronotum without notable indentations or protrusions. Each elytron has eight deep and distinct longitudinal striae, with intervals bearing fine transverse rugosities.
Ventral side (Figure 5E) overall dark green, with the abdominal ventrites being the lightest in color. The entire abdomen flat and covered with fine setae. Prosternal process (Figure 5H) broad, nearly parallel and has an obtusely pointed apex. The anal ventrite (Figure 5J) nearly circular apically, lacks serrations and covered with setae.
Legs (Figure 5D,E) slender, covered with setae, without serration, fore tibiae with gentle bent outward and the anterior tarsal claws hooked.
Aedeagus (Figure 5L) slender, subparallel. Parameres widest at the middle, converging from basal 2/3 of length, apical everted and bearing setae. Apex of median lobe transparent and needle-shaped.
Sexual dimorphism: Female differs from male by its much larger size and reddish frons and lateral sides of pronotum.
Etymology: This new species is named after Mr. Jiasheng Lu (Sanya, Hainan), who is a Lepidoptera enthusiasts, leading the way for us during the collection process.
Differential diagnosis: The differential diagnosis against two congeners is provided in Table 2.

Table 2.
Differential diagnosis of Philanthaxia lui Ni & Song, sp. nov., with two morphologically similar congeners.
Distribution: China (Hainan).
3.3. Molecular Identification of Philanthaxia longicornis Ni & Song, sp. nov.
3.3.1. DAMBE Base Sequence Analysis
Sequence evolutionary analysis was performed using DAMBE v7.3.32 on the 23 obtained sequences. The calculated index of ISS = 0.3632 was significantly lower than the ISS.C = 0.7850 (p < 0.001). This confirms minimal substitution saturation, validating the suitability of these sequences for robust phylogenetic reconstruction.
3.3.2. Genetic Distance Analysis
The genetic distances among the 23 sequences were analyzed using the Kimura two-parameter model with bootstrap testing (1000 replicates). As shown in Table 3, interspecific genetic distances ranged from 0.137 to 0.308, with a mean value of 0.2504. The smallest genetic distance (0.196) was observed between P. longicornis Ni & Song, sp. nov. and Chrysochroa fulgidissima, whereas the largest distance (0.308) was recorded between P. longicornis Ni & Song, sp. nov. and Endelus continentalis, indicating significant differences in genetic distance among different species. This sequence can be used as a basis for analyzing and identifying species.

Table 3.
Inter-species genetic distance of Buprestidae family insects and P. longicornis Ni & Song, sp. nov. based on Kimura 2-parameter model.
3.3.3. Phylogenetic Analysis
The phylogenetic tree was constructed using the Maximum Likelihood method with the Kimura two-parameter genetic distance model, and branch confidence was assessed via 1000 bootstrap replicates. As illustrated in Figure 6, P. longicornis Ni & Song, sp. nov. exhibits significantly lower bootstrap values compared to other species, indicating greater genetic divergence. In contrast, conspecific specimens within the same genus display bootstrap values approaching unity, consistent with closer genetic relationships.
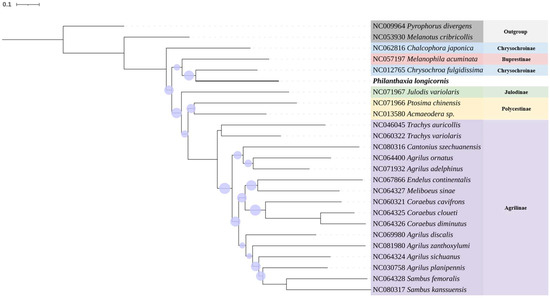
Figure 6.
Maximum Likelihood tree based on the analysis of COⅠ gene sequences of Philanthaxia longicornis Ni & Song, sp. nov. and Buprestidae species. The purple dots represent the bootstrap value (15–95),the larger the dots, the higher the value. Outgroup: Pyrophorus divergens (Elateridae) and Melanotus cribcollis (Elateridae). Rooted at P. divergens clade.
4. Discussion
Philanthaxia longicornis Ni & Song, sp. nov. and P. lui Ni & Song, sp. nov. represent the first record of this genus on the Chinese mainland. We have further extracted the COI gene sequence from P. longicornis and conducted evolutionary analyses through comparative sequencing with COI genes from 22 other species within the family Buprestidae and 2 outgroup species. The genetic distance and bootstrap value indicate that the molecular identification results corroborated the morphological identification findings, and added a COI sequence to the genus Philanthaxia, which is the first record of molecular data in this genus. The results of the COI phylogenetic analysis show that P. longicornis is closer to the species of subfamily Buprestidae and Chrysochroinae compared to Julodinae and Polycestinae, while compare to subfamily Buprestidae, P. longicornis is closer to the species of subfamily Chrysochroinae, and Melanophila acuminata is predicted between two species of Chrysochroinae, we speculate that subfamily Buprestidae and Chrysochroinae have a highly homologous, it is similar to previous research results [14]. The other subfamilies showed clear clustering relationships within the same subfamily and distinct species segregation. Most of the analysis based on the COⅠ gene sequence is consistent with the results of genome analysis in previous studies, such as Julodinae being closest to Polycestinae with high bootstrap values, which is consistent with the results of a previous study [14], and Agrilinae has a significant genetic distance from other subfamilies without species crossing, which is consistent with previous studies [14,15,16]. There are also differences from previous studies, such as the fact that the two species of the genus Trachys did not cluster in the closer clade; these differences only appear in the subfamily Agrilinae [17].
Owing to the limited number of P. lui paratype specimens collected, molecular analysis was not performed on this species in the present study. Based on the concordant morphological and molecular identification results obtained for P. longicornis, we consider the morphological identification of P. lui to be reliable. Future collection of additional specimens will facilitate the acquisition of complementary molecular data.
Additionally, given comparable climatic conditions and historically limited surveys, we hypothesize potential occurrences of Philanthaxia in other southern Chinese provinces (e.g., Yunnan, Fujian, Guangdong). Verification requires systematic long-term field investigations across these regions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16080839/s1.
Author Contributions
Conceptualization, H.S.; methodology, R.W. and H.S.; software, T.N.; formal analysis, T.N. and Z.Q.; investigation, T.N., Z.Q. and X.P.; writing—original draft preparation, T.N.; writing—review and editing, T.N., Z.Q., H.S. and R.W.; supervision, H.S. and R.W.; funding acquisition, R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 31900354, 31600522) and the Natural Science Foundation of Fujian Province (grant number: 2017J0106, 2019J05132).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We are grateful to Jiasheng Lu and Shijie Lu, who led the way for us during the collection process. We also thank Xueliang Hou and Zhongsheng He for identifying the host plant.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FAF | Fujian Academy of Forestry, Fuzhou, China |
| NACRC-IOZ | National Animal Collection Resource Center, Institute of Zoology, Chinese Academy of Sciences, Beijing, China |
References
- Deyrolle, H. Description des Buprestides de la Malaisie recueillés par M. Wallace pendant son voyage dans cet Archipel. In Annales de la Société Entomologique de Belgique; 4 plates, i–iii in color; Nabu Press: Charleston, SC, USA, 1864; Volume 8, pp. 1–280. [Google Scholar]
- Bílý, S. A revision of the genera Philanthaxia Deyrolle and Pagdeniella Théry (Coleoptera, Buprestidae). Folia Heyrovskyana 1993, 1, 84–115. [Google Scholar]
- Bílý, S. Comments on the genus Philanthaxia, with descriptions of new species (Coleoptera: Buprestidae). Folia Heyrovskyana 2001, 9, 105–129. [Google Scholar]
- Bílý, S. Philanthaxia nelsoni sp. nov. from Indonesia (Coleoptera: Buprestidae). Pan-Pac. Entomol. 2006, 82, 144–146. [Google Scholar]
- Ong, U.; Hattori, T. Jewel Beetles of Taiwan; Kuá Series; Ministry of Beetles: Tainan, Taiwan, 2019; Volume 1, p. 235. [Google Scholar]
- Ong, U.; Curletti, G.; Hattori, T. Jewel Beetles of Taiwan; Buprestidae, Kud Series; Ministry of Beetles: Tainan, Taiwan, 2023; Volume 2, p. 219. [Google Scholar]
- Bílý, S.; Nakládal, O. Four new species of the genus Philanthaxia Deyrolle, 1864 from Southeast Asia and comments on P. iris Obenberger, 1938 (Coleoptera, Buprestidae, Thomassetiini). ZooKeys 2011, 116, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, C.L. A World Catalogue and Bibliography of the Jewel Beetles (Coleoptera: Buprestoidea); Buprestinae: Pterobothrini through Agrilinae: Rhaeboscelina; Pensoft Series Faunistica: Bulgaria, Sofia, 2008; Volume 3, No. 76; pp. 1265–1931. [Google Scholar]
- Bílý, S. Four new species of the genus Philanthaxia Deyrolle, 1864 (Coleoptera: Buprestidae: Thomassetiini). Zootaxa 2016, 4205, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ohmomo, S. Notes on Buprestid Beetles (Coleoptera, Buprestidae) from Thailand, IX. A Key to the Species of the Genus Philanthaxia DEYROLLE, 1865 from Thailand, with Description of a New Species. Elytra Tokyo New Ser. 2011, 1, 237–243. [Google Scholar]
- Weidlich, M. Systematik und Taxonomie der Buprestidae des mitteleozänen Geiseltales (Insecta, Coleoptera). Hallesches Jahrb. Geowissenschafen 1987, 12, 29–52. [Google Scholar]
- Qi, Z.; Ai, H.; He, X.; Su, R.; Cai, S.; Song, H. A study of Anthaxia subgen. Thailandia Bílý, 1990 from China (Coleoptera, Buprestidae, Buprestinae). ZooKeys 2023, 1154, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Evans, A.; Mckenna, D.; Bellamy, C.; Farrell, B. Large-scale molecular phylogeny of metallic wood-boring beetles (Coleoptera: Buprestoidea) provides new insights into relationships and reveals multiple evolutionary origins of the larval leaf-mining habit. Syst. Entomol. 2015, 40, 385–400. [Google Scholar] [CrossRef]
- Volkovitsh, M. The comparative morphology of antennal structures in Buprestidae (Coleoptera): Evolutionary trends, taxonomic and phylogenetic implications. Part 1. Acta Musei Morav. Sci. Biol. [Bron] 2001, 86, 43–169. [Google Scholar]
- Huang, X.; Chen, B.; Wei, Z.; Shi, A. First report of complete mitochondrial genome in the tribes Coomaniellini and Dicercini (Coleoptera: Buprestidae) and phylogenetic implications. Genes 2022, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, X.; Shi, A. First mitochondrial genome of subfamily Julodinae (Coleoptera, Buprestidae) with its phylogenetic implications. ZooKeys 2023, 1139, 165–182. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).