Harnessing Chitin from Edible Insects for Livestock Nutrition
Simple Summary
Abstract
1. Introduction
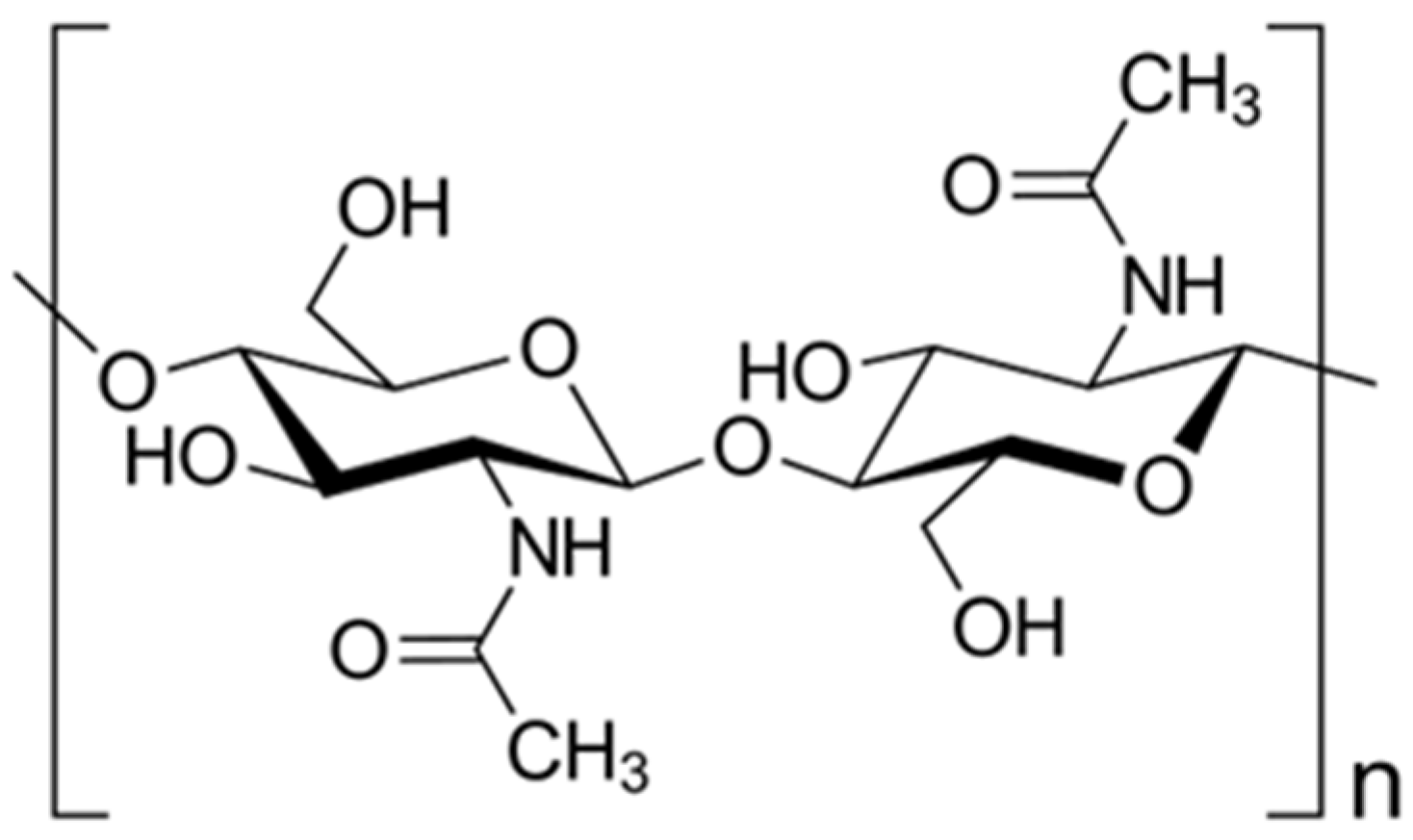
2. Structure and Role of Insect-Derived Chitin
- The cuticle is formed, from outside to inside, by the epicuticle, the outermost, thin layer. It waterproofs the insect and prevents dissection, as it is mainly composed of waxes. It may also contain pigments. The layer beneath the epicuticle is the exocuticle. It is the sclerotised part of the cuticle, often pigmented. It contains a few chitin fibres, which are cross-linked with proteins, providing strength and rigidity to the exoskeleton. The deeper layer of the cuticle is the endocuticle, which is thicker and softer than the exocuticle, as it is composed of chitin and non-sclerotised proteins. It provides flexibility and helps absorb mechanical stress. Chitin microfibres are arranged in a helical or layered pattern to increase strength. The exocuticle and endocuticle constitute the so-called procuticle.
- Below the cuticle is the epidermis, a living, glandular cell layer that secretes all the layers above it. It is responsible for producing chitin, proteins, and wax.
- The basement membrane is a thin layer beneath the epidermis that separates the exoskeleton from the rest of the insect’s body tissues [22].
3. Chitin Content in Edible Insects
4. Importance of Insect-Derived Chitin on Animal Nutrition: Disadvantages and Benefits
4.1. Disadvantages of Insect-Derived Chitin in Animal Nutrition
4.2. Benefits of Insect-Derived Chitin in Animal Nutrition
4.2.1. Prebiotic Effect
4.2.2. Immunostimulatory Effect
4.2.3. Cholesterol and Triglycerides Reduction
4.2.4. Antimicrobial Effect
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAs | Amino acids |
| ADC | Apparent digestibility coefficients |
| CP | Crude proteins |
| EE | Ether extract |
| EU | European Union |
| GE | Gross energy |
| HDL | High density lipoprotein |
| LAB | Lactic acid bacteria |
| LDL | Low density lipoprotein |
| PRRs | Pathogen recognition receptors |
| PUFAs | Polyunsaturated fatty acids |
| SCFAs | Short-chain fatty acids |
| TLRs | Toll-like receptors |
| UDP-Gl Nac | Uridine-Diphosphate -N-acetylglucosamine |
References
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; No. 171. [Google Scholar]
- UN—United Nations General Assembly 2015. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://www.refworld.org/docid/57b6e3e44.html (accessed on 2 July 2025).
- European Commission. Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (2013/0435 (COD). Off. J. Eur. Union 2015, 327, 1–22. [Google Scholar]
- Van Huis, A.; Rumpold, B.A.; Van der Fels-Klerx, H.J.; Tomberlin, J.K. Advancing edible insects as food and feed in a circular economy. J. Insects Food Feed 2021, 7, 935–948. [Google Scholar] [CrossRef]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible insects in a food safety and nutritional perspective: A critical review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Khalifah, A.; Abdalla, S.; Rageb, M.; Maruccio, L.; Ciani, F.; El-Sabrout, K. Could insect products provide a safe and sustainable feed alternative for the poultry industry? A comprehensive review. Animals 2023, 13, 1534. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; Van Loon, J.J. Insects as sources of iron and zinc in human nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2022, 62, 3499–3508. [Google Scholar] [CrossRef]
- Mohan, K.; Rajan, D.K.; Muralisankar, T.; Ganesan, A.R.; Sathishkumar, P.; Revathi, N. Use of black soldier fly (Hermetia illucens L.) larvae meal in aquafeeds for a sustainable aquaculture industry: A review of past and future needs. Aquaculture 2022, 553, 738095. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-origin prebiotics based on chitin: An alternative for the future? a critical review. Foods 2020, 9, 782. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Mlcek, J.; Borkovcova, M.; Rop, O.; Bednarova, M. Biologically active substances of edible insects and their use in agriculture, veterinary and human medicine. J. Cent. Eur. Agric. 2014, 15, 225. [Google Scholar] [CrossRef]
- Gasco, L.; Biasato, I.; Dabbou, S.; Schiavone, A.; Gai, F. Animals fed insect-based diets: State-of-the-art on digestibility, performance and product quality. Animals 2019, 9, 170. [Google Scholar] [CrossRef]
- Gasco, L.; Józefiak, A.; Henry, M. Beyond the protein concept: Health aspects of using edible insects on animals. J. Insects Food Feed 2021, 7, 715–741. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.K.; Cha, J.Y.; Jang, H.W.; Yong, H.I.; Choi, Y.S. How to develop strategies to use insects as animal feed: Digestibility, functionality, safety, and regulation. J. Anim. Sci. Technol. 2022, 64, 409. [Google Scholar] [CrossRef] [PubMed]
- Kępińska-Pacelik, J.; Biel, W. Insects in pet food industry—Hope or threat? Animals 2022, 12, 1515. [Google Scholar] [CrossRef]
- Hasan, I.; Gai, F.; Cirrincione, S.; Rimoldi, S.; Saroglia, G.; Terova, G. Chitinase and insect meal in aquaculture nutrition: A comprehensive overview of the latest achievements. Fishes 2023, 8, 607. [Google Scholar] [CrossRef]
- Kipkoech, C. Beyond proteins—Edible insects as a source of dietary fiber. Polysaccharides 2023, 4, 116–128. [Google Scholar] [CrossRef]
- Schoeller, J.R. Les Insectes, Physiologie et Développement; Masson: New York, NY, USA, 1980. [Google Scholar]
- Odier, A. Mémoire sur la composition chimique des parties cornées des insectes. Mem. Soc. Hist. Paris 1823, 1, 29–42. [Google Scholar]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Mohamed Anuar, N.A.F.; Abu Bakar, M.F. The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef]
- Rehman, K.U.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; Rehman, R.U.; Petrusan, J.I.; Zheng, L.; Zhang, J.; Sohail, S.; et al. Insect-derived chitin and chitosan: A still unexploited resource for the edible insect sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- Kramer, K.J.; Hopkins, T.L.; Schaefer, J. Applications of solids NMR to the analysis of insect sclerotized structures. Insect Biochem. Mol. Biol. 1995, 25, 1067–1080. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Kramer, K.J. Chitin metabolism in insects. In Insect Molecular Biology and Biochemistry; Academic Press: Cambridge, MA, USA, 2012; pp. 193–235. [Google Scholar]
- Conti, B.; Berti, F.; Mercati, D.; Giusti, F.; Dallai, R. The ultrastructure of Malpighian tubules and the chemical composition of the cocoon of Aeolothrips intermedius Bagnall (Thysanoptera). J. Morphol. 2010, 271, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, D.D.; Toprak, U.; Erlandson, M. Peritrophic matrix formation. J. Insect Physiol. 2019, 117, 103898. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, turnover, and functions of chitin in insects. Ann. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Beck, M.; Merzendorfer, H.; Yang, Q. Advances in understanding insect chitin biosynthesis. Insect Biochem. Mol. Biol. 2024, 164, 104058. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2017/893 of 24 May 2017 amending Annexes, I.; IVto Regulation (EC) No 999/2001 of the European Parliament of the Council Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. Off. J. Eur. Union 2017, 138, 92–116. [Google Scholar]
- European Commission. Commission Regulation (EU) 2021/1925 of 5 November 2021 amending certain Annexes to Regulation (EU) No 142/2011 as regards the requirements for placing on the market of certain insect products and the adaptation of a containment method. Off. J. Eur. Union 2021, 50, 4–8. [Google Scholar]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A. Edible insects: Challenges and prospects. Entomol. Res. 2022, 52, 161–177. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Francis, F.; Haubruge, E.; Le Gall, P.; Tomberlin, J.K.; Miranda, C.D.; Jordan, H.R.; Picard, C.J.; Pino, M.J.M.; Ramos-Elordy, J.; et al. A worldwide overview of the status and prospects of edible insect production. Entomol. Gen. 2024, 44, 3–27. [Google Scholar] [CrossRef]
- Song, Y.S.; Kim, M.W.; Moon, C.; Seo, D.J.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.K.; Kim, S.A.; Kim, Y.W.; et al. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Black soldier fly Hermetia illucens as a novel source of chitin and chitosan. Int. J. Sci. 2019, 8, 81–86. [Google Scholar] [CrossRef]
- Psarianos, M.; Ojha, S.; Schneider, R.; Schlüter, O.K. Chitin isolation and chitosan production from house crickets (Acheta domesticus) by environmentally friendly methods. Molecules 2022, 27, 5005. [Google Scholar] [CrossRef]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of chitin extracted from black soldier fly in different life stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef]
- Munoz-Seijas, N.; Fernandes, H.; López-Periago, J.E.; Outeirino, D.; Morán-Aguilar, M.G.; Domínguez, J.M.; Salgado, J.M. Characterization of all life stages of Tenebrio molitor: Envisioning innovative applications for this edible insect. Future Foods 2024, 10, 100404. [Google Scholar] [CrossRef]
- Shin, C.S.; Kim, D.Y.; Shin, W.S. Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef]
- Soetemans, L.; Gianotten, N.; Bastiaens, L. Agri-food side-stream inclusion in the diet of Alphitobius diaperinus. Part 2: Impact on larvae composition. Insects 2020, 11, 190. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential extraction and characterisation of lipids, proteins, and chitin from black soldier fly (Hermetia illucens) larvae, prepupae, and pupae. Waste Biomass Valor. 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gargliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.J.; Qin, Q.L.; Zhang, H.; Wang, H.T.; Li, X.; Miao, L.; Wu, Y.J. Preparation and characterisation of food-grade chitosan from housefly larvae. Czech J. Food Sci. 2011, 29, 616–623. [Google Scholar] [CrossRef]
- Kim, M.W.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Kang, S.H.; Kim, S.A.; Jung, W.J. Extraction of chitin and chitosan from housefly, Musca domestica, pupa shells. Entomol. Res. 2016, 46, 324–328. [Google Scholar] [CrossRef]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef] [PubMed]
- Malm, M. Purification and Characterization of Acheta domesticus and Gryllodes sigillatus Cricket Chitin and Chitosan for Bioactive and Biodegradable Food Packaging Applications. Ph.D. Dissertation, Purdue University, West Lafayette, IN, USA, 2021. [Google Scholar]
- Toribio, E.; Correa, M.J.; Medici, S.K.; Ferrero, C.; Arp, C.G. Characterising cricket flour from Gryllus assimilis: An alternative source of nutrients for sustainability. Int. J. Food Sci. Technol. 2024, 59, 7509–7516. [Google Scholar] [CrossRef]
- Stull, V.J.; Weir, T.L. Chitin and omega-3 fatty acids in edible insects have underexplored benefits for the gut microbiome and human health. Nat. Food 2023, 4, 283–287. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.; De Haro, C.; Sanz, A.; Trenzado, C.E.; Villareces, S.; Barroso, F.G. Nutritional evaluation of Tenebrio molitor meal as fishmeal substitute for tilapia (Oreochromis niloticus) diet. Aquac. Nutr. 2016, 22, 943–955. [Google Scholar] [CrossRef]
- Piccolo, G.; Iaconisi, V.; Marono, S.; Gasco, L.; Loponte, R.; Nizza, S.; Bovera, F.; Parisi, G. Effect of Tenebrio molitor larvae meal on growth performance, in vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata). Anim. Feed Sci. Technol. 2017, 226, 12–20. [Google Scholar] [CrossRef]
- Fontes, T.V.; de Oliveira, K.R.B.; Gomes Almeida, I.L.; Orlando, T.M.; Rodrigues, P.B.; da Costa, D.V.; Rosa, P.V.E. Digestibility of insect meals for Nile tilapia fingerlings. Animals 2019, 9, 181. [Google Scholar] [CrossRef]
- Guerreiro, I.; Serra, C.R.; Coutinho, F.; Couto, A.; Castro, C.; Rangel, F.; Peres, H.; Pausao-Ferreira, P.; Matos, E.; Gasco, L.; et al. Digestive enzyme activity and nutrient digestibility in meagre (Argyrosomus regius) fed increasing levels of black soldier fly meal (Hermetia illucens). Aquac. Nutr. 2021, 27, 142–152. [Google Scholar] [CrossRef]
- Eggink, K.M.; Pedersen, P.B.; Lund, I.; Dalsgaard, J. Chitin digestibility and intestinal exochitinase activity in Nile tilapia and rainbow trout fed different black soldier fly larvae meal size fractions. Aquac. Res. 2022, 53, 5536–5546. [Google Scholar] [CrossRef]
- Sándor, Z.J.; Banjac, V.; Vidosavljević, S.; Káldy, J.; Egessa, R.; Lengyel-Kónya, É.; Tömösközi-Farkas, R.; Zalán, Z.; Adányi, N.; Libisch, B.; et al. Apparent digestibility coefficients of black soldier fly (Hermetia illucens), yellow mealworm (Tenebrio molitor), and blue bottle fly (Calliphora vicina) insects for juvenile African catfish hybrids (Clarias gariepinus × Heterobranchus longifilis). Aquac. Nutr. 2022, 18, 4717014. [Google Scholar] [CrossRef]
- Gasco, L.; Caimi, C.; Trocino, A.; Lussiana, C.; Oddon, S.B.; Malfatto, V.; Anedda, R.; Serra, G.; Biasato, I.; Schiafone, A.; et al. Digestibility of defatted insect meals for rainbow trout aquafeeds. J. Insects Food Feed 2022, 8, 1385–1400. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.G.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Coutinho, F.; Castro, C.; Guerreiro, I.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousao-Ferreira, P.; Rawski, M.; Oliva-Teles, A.; et al. Mealworm larvae meal in diets for meagre juveniles: Growth, nutrient digestibility and digestive enzymes activity. Aquaculture 2021, 535, 736362. [Google Scholar] [CrossRef]
- Lock, E.J.; Biancarosa, I.; Gasco, L. Insects as raw materials in compound feed for aquaculture. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 263–276. [Google Scholar]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Oktay, O.; Seong, T.; Kabeya, N.; Morioka, S.L.; Liu, C.M.; Kobayashi, T.; Shimoda, M.; Satoh, S.; Haga, Y. Can black soldier fly meal in diets improve gut microbiota diversity, nutrient digestibility, and growth response of marine fish? A study on red sea bream Pagrus major. Fish. Sci. 2024, 90, 773–786. [Google Scholar] [CrossRef]
- Drosdowech, S.; Chiasson, M.; Ma, D.W.L.; Huyben, D.; Rooney, N. Dietary inclusion of black soldier fly, cricket and superworm in rainbow trout aquaculture: Impacts on growth and nutrient profiles. J. Insects Food Feed 2025, 11, 1305–1321. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Jin, J.N.; Zhu, F.; Roffeis, M.; Zhang, X.Z. A comprehensive evaluation of replacing fishmeal with housefly (Musca domestica) maggot meal in the diet of Nile tilapia (Oreochromis niloticus): Growth performance, flesh quality, innate immunity and water environment. Aquac. Nutr. 2017, 23, 983–993. [Google Scholar] [CrossRef]
- Nandeesha, M.C.; Srikanth, G.K.; Keshavanath, P.; Varghese, T.J.; Basavaraja, N.; Das, S.K. Effects of non-defatted silkworm-pupae in diets on the growth of common carp, Cyprinus carpio. Biol. Wastes 1990, 33, 17–23. [Google Scholar] [CrossRef]
- Zhou, J.S.; Chen, Y.S.; Ji, H.; Yu, E.M. The effect of replacing fish meal with fermented meal mixture of silkworm pupae, rapeseed and wheat on growth, body composition and health of mirror carp (Cyprinus carpio var. Specularis). Aquac. Nutr. 2017, 23, 741–754. [Google Scholar] [CrossRef]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.M.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Ponomarev, S.V.; Fedorovyh, Y.V.; Bastrakov, A.I.; Pavlov, D.S. Physiological basis of the nutritional value of a concentrate of Hermetia illucens larvae in fish diets. Biol. Bull. 2020, 47, 276–282. [Google Scholar] [CrossRef]
- Muin, H.; Taufek, N.M. Evaluation of growth performance, feed efficiency and nutrient digestibility of red hybrid tilapia fed dietary inclusion of black soldier fly larvae (Hermetia illucens). Aquac. Fish. 2024, 9, 46–51. [Google Scholar] [CrossRef]
- Owens, C.E.; Powell, M.S.; Gaylord, T.G.; Conley, Z.B.; Sealey, W.M. Investigation of the suitability of 3 insect meals as protein sources for rainbow trout (Oncorhynchus mykiss). J. Econom. Entomol. 2024, 117, 1254–1260. [Google Scholar] [CrossRef]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Chen, H.; Yu, J.; Ran, X.; Wu, J.; Chen, Y.; Tan, B.; Lin, S. Effects of yellow mealworm (Tenebrio molitor) on growth performance, hepatic health and digestibility in juvenile largemouth bass (Micropterus salmoides). Animals 2023, 13, 1389. [Google Scholar] [CrossRef] [PubMed]
- Tabata, E.; Kashimura, A.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; Kino, Y.; Matosk, V.; Bauer, P.O.; Oyama, F. Gastric and intestinal proteases resistance of chicken acidic chitinase nominates chitin-containing organisms for alternative whole edible diets for poultry. Sci. Rep. 2017, 7, 6662. [Google Scholar] [CrossRef] [PubMed]
- Tabata, E.; Kashimura, A.; Kikuchi, A.; Masuda, H.; Miyahara, R.; Hiruma, Y.; Oyama, F. Chitin digestibility is dependent on feeding behaviors, which determine acidic chitinase mRNA levels in mammalian and poultry stomachs. Sci. Rep. 2018, 8, 1461. [Google Scholar] [CrossRef]
- Bovera, F.; Loponte, R.; Marono, S.; Piccolo, G.; Parisi, G.; Iaconisi, V.; Gasco, L.; Nizza, A. Use of Tenebrio molitor larvae meal as protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits. J. Anim. Sci. 2016, 94, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Dourado, L.R.; Lopes, P.M.; Silva, V.K.; Carvalho, F.L.A.; Moura, F.A.; Silva, L.B.; Giannecchini, L.G.; Pinheiro, S.R.I.; Biagiotti, D.; Kimpara, J.M. Chemical composition and nutrient digestibility of insect meal for broiler. An. Acad. Bras. Ciênc. 2020, 92, e20200764. [Google Scholar] [CrossRef]
- Heuel, M.; Sandrock, C.; Leiber, F.; Mathys, A.; Gold, M.; Zurbrügg, C.; Gangnat, I.D.M.; Kreuzer, M.; Terranova, M. Black soldier fly larvae meal and fat can completely replace soybean cake and oil in diets for laying hens. Poult. Sci. 2021, 100, 101034. [Google Scholar] [CrossRef]
- Chobanova, S.; Karkelanov, N.; Mansbridge, S.C.; Whiting, I.M.; Simic, A.; Rose, S.P.; Pirgozliev, V.R. Defatted black soldier fly larvae meal as an alternative to soybean meal for broiler chickens. Poultry 2023, 2, 430–441. [Google Scholar] [CrossRef]
- Hwangbo, J.; Hong, E.C.; Jang, A.; Kang, H.K.; Oh, J.S.; Kim, B.W.; Park, B.S. Utilization of house fly-maggots, a feed supplement in the production of broiler chickens. J. Environ. Biol. 2009, 30, 609–614. [Google Scholar]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Abdulla, N.R.; Foo, H.L.; Zamri, A.N.M.; Shazali, N.; Loh, T.C.; Alshelmani, M.I. Effect of feeding larvae meal in the diets on growth performance, nutrient digestibility and meat quality in broiler chicken. Indian J. Anim. Sci. 2018, 88, 1180–1185. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Rawski, M.; Józefiak, A.; Kozłowski, K.; Jankowski, J.; Józefiak, D. Tenebrio molitor and Zophobas morio full-fat meals in broiler chicken diets: Effects on nutrients digestibility, digestive enzyme activities, and cecal microbiome. Animals 2019, 9, 1128. [Google Scholar] [CrossRef]
- Chu, X.; Li, M.; Wang, G.; Wang, K.; Shang, R.; Wang, Z.; Li, L. Evaluation of the low inclusion of full-fatted Hermetia illucens larvae meal for layer chickens: Growth performance, nutrient digestibility, and gut health. Front. Vet. Sci. 2020, 7, 585843. [Google Scholar] [CrossRef]
- Elangovan, A.V.; Udayakumar, A.; Saravanakumar, M.; Awachat, V.B.; Mohan, M.; Yandigeri, M.S.; Krishnan, S.; Mech, A.; Rao, S.B.N.; Giridhar, K.; et al. Effect of black soldier fly, Hermetia illucens (Linnaeus) prepupae meal on growth performance and gut development in broiler chicken. Int. J. Trop. Insect Sci. 2021, 41, 2077–2082. [Google Scholar] [CrossRef]
- Nascimento Filho, M.A.; Pereira, R.T.; Oliveira, A.B.S.D.; Suckeveris, D.; Burin Junior, A.M.; Soares, C.A.; Menten, J.F.M. Nutritional value of Tenebrio molitor larvae meal for broiler chickens: Metabolizable energy and standardized ileal amino acid digestibility. J. Appl. Poult. Res. 2021, 30, 100102. [Google Scholar] [CrossRef]
- Rahayu, S.; Widiyastuti, T.; Suryapratama, W.; Hartoyo, B.; Rimbawanto, E.A. Performance and feed digestibility of Sentul chicken fed hydrolyzed maggot (Hermetia illucens) meal produced by crude enzymes from Tempeh yeast. Afr. J. Food Agric. Nutr. Dev. 2023, 23, 25006–25023. [Google Scholar] [CrossRef]
- Salahuddin, M.; Abdel-Wareth, A.A.; Hiramatsu, K.; Tomberlin, J.K.; Luza, D.; Lohakare, J. Flight toward sustainability in poultry nutrition with black soldier fly larvae. Animals 2024, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Singh, Y.; Squartini, A.; Stevanato, P.; Cappellozza, S.; Kovitvadhi, A.; Subaneg, S.; Bertelli, D.; Cullere, M. Effect of a dietary inclusion of full-fat or defatted silkworm pupa meal on the nutrient digestibility and faecal microbiome of fattening quails. Animal 2021, 15, 100112. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Zotte, A.D.; Cullere, M.; Chundang, P.; Kongsup, P.; Kovitvadhi, A. Use of an In Vitro Digestibility Approach to Assess Bombyx mori and Camelina sativa as Alternative Feed Ingredients for Poultry Species. Vet. Sci. 2025, 12, 277. [Google Scholar] [CrossRef]
- Kawasaki, K.; Osafune, T.; Tamehira, S.; Yano, K. Piglets can secrete acidic mammalian chitinase from the pre weaning stage. Sci. Rep. 2021, 11, 1297. [Google Scholar] [CrossRef]
- Tabata, E.; Kashimura, A.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; Imamura, Y.; Seki, S.; Ueda, H.; Matoska, V.; et al. Protease resistance of porcine acidic mammalian chitinase under gastrointestinal conditions implies that chitin-containing organisms can be sustainable dietary resources. Sci. Rep. 2017, 7, 12963. [Google Scholar] [CrossRef] [PubMed]
- Phaengphairee, P.; Boontiam, W.; Wealleans, A.; Hong, J.; Kim, Y.Y. Dietary supplementation with full-fat Hermetia illucens larvae and multi-probiotics, as a substitute for antibiotics, improves the growth performance, gut health, and antioxidative capacity of weaned pigs. BMC Vet. Res. 2023, 19, 7. [Google Scholar] [CrossRef]
- Biasato, I.; Renna, M.; Gai, F.; Dabbou, S.; Meneguz, M.; Perona, G.; Martinez, S.; Lajusticia, A.C.B.; Bargagna, S.; Sardi, L.; et al. Partially defatted black soldier fly larva meal inclusion in piglet diets: Effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J.; Li, L.; Duan, Y.; Zhang, X.; Wang, T.; Zang, J.; Piao, X.; Ma, Y.; Li, D. Endogenous chitinase might lead to differences in growth performance and intestinal health of piglets fed different levels of black soldier fly larva meal. Anim. Nutr. 2023, 14, 411–424. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Wang, F.; Ma, X. Evaluation of full-fat Hermetia illucens larvae meal as a fishmeal replacement for weanling piglets: Effects on the growth performance, apparent nutrient digestibility, blood parameters and gut morphology. Anim. Feed Sci. Technol. 2020, 264, 114431. [Google Scholar] [CrossRef]
- Jin, X.H.; Heo, P.S.; Hong, J.S.; Kim, N.J.; Kim, Y.Y. Supplementation of dried mealworm (Tenebrio molitor larva) on growth performance, nutrient digestibility and blood profiles in weaning pigs. Asian-Australas. J. Anim. Sci. 2016, 29, 979–986. [Google Scholar] [CrossRef]
- Yoo, J.S.; Cho, K.H.; Hong, J.S.; Jang, H.S.; Chung, Y.H.; Kwon, G.T.; Shin, D.G.; Kim, Y.Y. Nutrient ileal digestibility evaluation of dried mealworm (Tenebrio molitor) larvae compared to three animal protein by-products in growing pigs. Asian-Australas. J. Anim. Sci. 2018, 32, 387–394. [Google Scholar] [CrossRef]
- Cho, K.H.; Kang, S.W.; Yoo, J.S.; Song, D.K.; Chung, Y.H.; Kwon, G.T.; Kim, Y.Y. Effects of mealworm (Tenebrio molitor) larvae hydrolysate on nutrient ileal digestibility in growing pigs compared to those of defatted mealworm larvae meal, fermented poultry by-product, and hydrolyzed fish soluble. Asian-Australas. J. Anim. Sci. 2019, 33, 490–500. [Google Scholar] [CrossRef]
- Pereira, J.C.; da Silva, A.R.A.; Rossiti, B.C.O.; da Silva, E.R.; da Silva, L.G.R.; Evangelista, M.Z.; don Santos Ruiz, U.; Tse, M.L.P. Insect Meal (Tenebrio molitor) Has High Nutrient Digestibility for Newly Weaned Piglets. Anim. Sci. J. 2025, 96, e70036. [Google Scholar] [CrossRef]
- Tan, X.; Yang, H.S.; Wang, M.; Yi, Z.F.; Ji, F.J.; Li, J.Z.; Yin, Y.L. Amino acid digestibility in housefly and black soldier fly prepupae by growing pigs. Anim. Feed Sci. Technol. 2020, 263, 114446. [Google Scholar] [CrossRef]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- Tran, N.T.; Li, Z.; Wang, S.; Zheng, H.; Aweya, J.J.; Wen, X.; Li, S. Progress and perspectives of short-chain fatty acids in aquaculture. Rev. Aquac. 2020, 12, 283–298. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gini, E.; Iannini, F.; Gasco, L.; Terova, G. The effects of dietary insect meal from Hermetia illucens prepupae on autochthonous gut microbiota of rainbow trout (Oncorhynchus mykiss). Animals 2019, 9, 143. [Google Scholar] [CrossRef]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]
- Huyben, D.; Vidaković, A.; Hallgren, S.W.; Langeland, M. High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens). Aquaculture 2019, 500, 485–491. [Google Scholar] [CrossRef]
- Józefiak, A.; Nogales-Mérida, S.; Mikołajczak, Z.; Rawski, M.; Kierończyk, B.; Mazurkiewicz, J. The utilization of full-fat insect meal in rainbow trout (Oncorhynchus mykiss) nutrition: The effects on growth performance, intestinal microbiota and gastrointestinal tract histomorphology. Ann. Anim. Sci. 2019, 19, 747–765. [Google Scholar] [CrossRef]
- Rimoldi, S.; Ceccotti, C.; Brambilla, F.; Faccenda, F.; Antonini, M.; Terova, G. Potential of shrimp waste meal and insect exuviae as sustainable sources of chitin for fish feeds. Aquaculture 2023, 567, 739256. [Google Scholar] [CrossRef]
- Józefiak, A.; Nogales-Mérida, S.; Rawski, M.; Kierończyk, B.; Mazurkiewicz, J. Effects of insect diets on the gastrointestinal tract health and growth performance of Siberian sturgeon (Acipenser baerii Brandt, 1869). BMC Vet. Res. 2019, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Leeper, A.; Benhaïm, D.; Smárason, B.Ö.; Knobloch, S.; Òmarsson, K.L.; Bonnafoux, T.; Pipan, M.; Koppe, W.; Björnsdóttir, R.; Øverland, M. Feeding black soldier fly larvae (Hermetia illucens) reared on organic rest streams alters gut characteristics of Atlantic salmon (Salmo salar). J. Insects Food Feed 2022, 8, 1355–1372. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Rocha, S.D.; Øyås, O.; Lagos, L.; Hansen, J.Ø.; Mydland, L.T.; Øverland, M. Modulation of Atlantic salmon (Salmo salar) gut microbiota composition and predicted metabolic capacity by feeding diets with processed black soldier fly (Hermetia illucens) larvae meals and fractions. Anim. Microbiome 2022, 4, 9. [Google Scholar] [CrossRef]
- Rangel, F.; Enes, P.; Gasco, L.; Gai, F.; Hausmann, B.; Berry, D.; Oliva-Teles, A.; Serra, C.R.; Pereira, F.C. Differential modulation of the European sea bass gut microbiota by distinct insect meals. Front. Microbiol. 2022, 13, 831034. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Mikołajczak, Z.; Kierończyk, B.; Skrzypczak, P.; Szymkowiak, P.; Józefiak, D. Black Soldier Fly Meal as a Gastrointestinal Tract Microbiota Remodelling Factor: A New Natural and Sustainable Source of Prebiotic Substances for Fish? Aquac. Res. 2025, 17, 8852384. [Google Scholar] [CrossRef]
- Terova, G.; Gini, E.; Gasco, L.; Moroni, F.; Antonini, M.; Rimoldi, S. Effects of full replacement of dietary fishmeal with insect meal from Tenebrio molitor on rainbow trout gut and skin microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, Y.; Song, K.; Wang, L.; Li, X.; Tan, B.; Lu, K.; Zhang, C. Effects of the replacement of dietary fish meal with defatted yellow mealworm (Tenebrio molitor) on juvenile large yellow croakers (Larimichthys crocea) growth and gut health. Animals 2022, 12, 2659. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cai, M.; Zhong, L.; Yin, Y.; Xie, Y.; Xie, S.; Hu, Y.; Zhang, J. Yellow mealworm (Tenebrio molitor) meal in diets of grass carp (Ctenopharyngodon idellus): Effects on growth performance, antioxidant capacity, immunity, intestinal morphology, and intestinal microbiota. Anim. Nutr. 2025, 21, 70–83. [Google Scholar] [CrossRef]
- Drosdowech, S.; Bezner, S.; Daisley, B.; Chiasson, M.; Easton, A.; Rooney, N.; Huyben, D. Influence of feeding black soldier fly (Hermetia illucens), cricket (Gryllodes sigillatus), and superworm (Zophobas morio) on the gut microbiota of rainbow trout (Oncorhynchus mykiss). J. Appl. Microbiol. 2024, 135, lxae295. [Google Scholar] [CrossRef]
- Busti, S.; Bonaldo, A.; Candela, M.; Scicchitano, D.; Trapella, G.; Brambilla, F.; Guidou, C.; Trespeuch, C.; Sirri, F.; Dondi, F.; et al. Hermetia illucens larvae meal as an alternative protein source in practical diets for gilthead sea bream (Sparus aurata): A study on growth, plasma biochemistry and gut microbiota. Aquaculture 2024, 578, 740093. [Google Scholar] [CrossRef]
- Moutinho, S.; Peres, H.; Martins, N.; Serra, C.; Santos, R.A.; Monroig, Ó.; Oliva-Teles, A. Use of black soldier fly (Hermetia illucens) larvae meal in diets for gilthead seabream juveniles: Effects on growth-related gene expression, intermediary metabolism, digestive enzymes, and gut microbiota modulation. Aquaculture 2024, 580, 740357. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Yong, Y.S.; Chen, Y.; Chen, B.; Cao, J.; Peng, K.; Wang, G.; Huang, H.; Loh, J.Y. Impacts of Black Soldier Fly (Hermetia illucens) Larval Meal on Intestinal Histopathology and Microbiome Responses in Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂): A Comprehensive Analysis. Animals 2024, 14, 3596. [Google Scholar] [CrossRef] [PubMed]
- Józefiak, A.; Kierończyk, B.; Rawski, M.; Mazurkiewicz, J.; Benzertiha, A.; Gobbi, P.; Nogales-Merida, S.; Świątkiewicz, S.; Józefiak, D. Full-fat insect meals as feed additive–the effect on broiler chicken growth performance and gastrointestinal tract microbiota. J. Anim. Feed Sci. 2018, 27, 131–139. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Dabbou, S.; Evangelista, R.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Black soldier fly and gut health in broiler chickens: Insights into the relationship between cecal microbiota and intestinal mucin composition. J. Anim. Sci. Biotechnol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Colombino, E.; Biasato, I.; Ferrocino, I.; Bellezza Oddon, S.; Caimi, C.; Gariglio, M.; Dabbou, S.; Caramori, M.; Battisti, E.; Zanet, S.; et al. Effect of insect live larvae as environmental enrichment on poultry gut health: Gut mucin composition, microbiota and local immune response evaluation. Animals 2021, 11, 2819. [Google Scholar] [CrossRef]
- de Souza Vilela, J.; Kheravii, S.K.; Bajagai, Y.S.; Kolakshyapati, M.; Sibanda, T.Z.; Wu, S.B.; Nigel, R.A.; Ruhnke, I. Inclusion of up to 20% Black Soldier Fly larvae meal in broiler chicken diet has a minor effect on caecal microbiota. PeerJ 2023, 11, e15857. [Google Scholar] [CrossRef]
- Fiorilla, E.; Ferrocino, I.; Gariglio, M.; Gai, F.; Zambotto, V.; Ozella, L.; Franciosa, I.; Giribaldi, M.; Antoniazzi, S.; Raspa, F.; et al. Black soldier fly larvae: A one health approach to investigate gut, and organ health and meat quality response in slow-growing chickens. BMC Vet. Res. 2024, 20, 580. [Google Scholar] [CrossRef]
- Martínez Marín, A.L.; Gariglio, M.; Pozzo, S.; Capucchio, M.T.; Ferrocino, I.; Biasato, I.; Schiavone, A. Effects of partially defatted larvae meal of Black Soldier Fly (Hermetia illucens) on caecal microbiota and volatile compounds of Muscovy ducks (Cairina moschata domestica). Ital. J. Anim. Sci. 2023, 22, 1151–1161. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Gut microbiota and mucin composition in female broiler chickens fed diets including yellow mealworm (Tenebrio molitor, L.). Animals 2019, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Józefiak, A.; Benzertiha, A.; Kierończyk, B.; Łukomska, A.; Wesołowska, I.; Rawski, M. Improvement of cecal commensal microbiome following the insect additive into chicken diet. Animals 2020, 10, 577. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 50. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, E.; Wang, Z.; Xiao, M.; Cao, S.; Hu, S.; Wu, S.; Xiong, Y.; Jiang, Z.; Wang, F.; et al. Dietary Hermetia illucens larvae meal improves growth performance and intestinal barrier function of weaned pigs under the environment of enterotoxigenic Escherichia coli K88. Front. Nutr. 2022, 8, 812011. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Gessner, D.K.; Maheshwari, G.; Röhrig, J.; Friedhoff, T.; Most, E.; Zorn, H.; Ringseis, R.; Eder, K. Tenebrio molitor larvae meal affects the cecal microbiota of growing pigs. Animals 2020, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Ferri, I.; Dell’Anno, M.; Quiese, A.; Castiglioni, B.; Cremonesi, P.; Biscarini, F.; Canala, B.; Santoru, M.; Colombini, A.; Ruffo, G.; et al. Microbiota modulation by the inclusion of Tenebrio molitor larvae as alternative to fermented soy protein concentrate in growing pigs diet. Vet. Res. Commun. 2025, 49, 26. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Lim, C.H.; Lee, S.H.; Goo, T.W.; Yun, E.Y. Effect of feed containing Hermetia illucens larvae immunized by Lactobacillus plantarum injection on the growth and immunity of rainbow trout (Oncorhynchus mykiss). Insects 2021, 12, 801. [Google Scholar] [CrossRef]
- Jeong, S.M.; Khosravi, S.; Mauliasari, I.R.; Lee, S.M. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for rainbow trout (Oncorhynchus mykiss) fry. Fish. Aquat. Sci. 2020, 23, 12. [Google Scholar] [CrossRef]
- Sayramoğlu, H.; Öztürk, R.C.; Ustaoglu, D.; Terzi, Y.; Yandi, I.; Kayis, S.; Capkin, E.; Altinok, I. Effects of black soldier fly meal feeding on rainbow trout gut microbiota, immune-related gene expression, and Lactococcus petauri resistance. J. Insects Food Feed 2023, 10, 141–157. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, S.; Jiang, S.; Deng, Y.; Xu, B.; Xiang, F. Effect of full-fat black soldier fly (Hermetia illucens L.) larvae on growth performance, immunological parameters, and gene expressions in zebrafish (Danio rerio). Int. Aquat. Res. 2024, 16, 55–69. [Google Scholar]
- Abd El-Gawad, E.A.; Zahran, E.; Youssuf, H.; Shehab, A.; Matter, A.F. Defatted black soldier fly (Hermetia illucens) diets improved hemato-immunological responses, biochemical parameters, and antioxidant activities in Streptococcus iniae-infected Nile tilapia (Oreochromis niloticus). BMC Vet. Res. 2025, 21, 104. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, S.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Effects of fishmeal replacement by black soldier fly on growth performance, digestive enzyme activity, intestine morphology, intestinal flora and immune response of pearl gentian grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Fish Shellfish Immunol. 2022, 120, 497–506. [Google Scholar] [CrossRef]
- Jiang, B.; Sun, Y.; Li, W.; Liu, C.; Wen, C.; Li, A.; Huang, Y.; Su, Y. Effects of dietary black soldier fly (Hermetia illucens Linnaeus) on the disease resistance of juvenile grouper (Epinephelus coioides). Fish Shellfish Immunol. 2022, 123, 136–141. [Google Scholar] [CrossRef]
- Linh, N.V.; Wannavijit, S.; Tayyamath, K.; Dinh-Hung, N.; Nititanarapee, T.; Sumon, M.A.A.; Srinual, O.; Permpoonpattana, P.; Van Doan, H.; Liu, H.; et al. Black soldier fly (Hermetia illucens) larvae meal: A sustainable alternative to fish meal proven to promote growth and immunity in koi carp (Cyprinus carpio var. Koi). Fishes 2024, 9, 53. [Google Scholar] [CrossRef]
- Henry, M.A.; Gasco, L.; Chatzifotis, S.; Piccolo, G. Does dietary insect meal affect the fish immune system? The case of mealworm, Tenebrio molitor on European sea bass, Dicentrarchus labrax. Dev. Comp. Immunol. 2018, 81, 204–209. [Google Scholar] [CrossRef]
- Su, J.; Gong, Y.; Cao, S.; Lu, F.; Han, D.; Liu, H.; Xie, S. Effects of dietary Tenebrio molitor meal on the growth performance, immune response and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immol. 2017, 69, 59–66. [Google Scholar] [CrossRef]
- Qu, P.; Yuan, J.; Wu, Y.; Tian, S.; Wu, Z.; Chen, P.; Pan, M.; Weng, H.; Mai, K.; Zhang, W. Yellow mealworm (Tenebrio molitor) meal replacing dietary fishmeal alters the intestinal microbiota, anti-oxidation and immunity of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2025, 161, 110272. [Google Scholar] [CrossRef]
- Fawole, F.J.; Shamna, N.; Memudu, H.A.; Abdullahi, N.; Hassaan, M.S.; Gbadamosi, O.K. Housefly maggot meal complement soybean meal in a fish-free diet for hybrid catfish (Clarias gariepinus♀ × Heterobranchus longifilis♂): Effect on growth, body composition, blood biochemistry and antioxidant enzyme activity. Anim. Feed Sci. Technol. 2023, 295, 115543. [Google Scholar] [CrossRef]
- Bagheri, T.; Safari, R.; Bahmani, M.; Hafezieh, M.; Sharifpour, I.; Aghaeimoghadam, A.; Paghe, E.; Mirhashami Rostami, S.A.; Poursoufi, T.; Pajand, Z.; et al. Silkworm pupae (Bombyx mori) substitution with fish meal in fingerling Beluga sturgeon (Huso huso) diets improves growth. J. Insects Food Feed 2025, 1, 1–12. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.M.; Park, Y.K.; Yang, Y.C.; Jung, B.G.; Lee, B.J. Black soldier fly (Hermetia illucens) larvae enhances immune activities and increases survivability of broiler chicks against experimental infection of Salmonella gallinarum. J. Vet. Med. Sci. 2018, 80, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Tykałowski, B.; Koncicki, A.; Kowalczyk, J.; Śmiałek, M.; Bakuła, T.; Murawska, D.; Sobotka, W.; Stenzel, T. The impact of full-fat Hermetia illucens larvae meal on the health and immune system function of broiler chickens. J. Vet. Res. 2023, 67, 197. [Google Scholar] [CrossRef] [PubMed]
- Nassar, F.S.; Alsahlawi, A.M.; Abbas, A.O.; Alaqil, A.A.; Kamel, N.N.; Abdelwahab, A.M. Impact of dietary inclusion of black soldier fly larvae (Hermetia illucens) as a replacement for soybean-corn ingredients on egg production, physiological status, and economic efficiency of laying hens. Adv. Anim. Vet. Sci. 2023, 11, 295–304. [Google Scholar] [CrossRef]
- Bovera, F.; Piccolo, G.; Gasco, L.; Marono, S.; Loponte, R.; Vassalotti, G.; Mastellone, V.; Lombardi, P.; Attia, Y.A.; Nizza, A. Yellow mealworm larvae (Tenebrio molitor L.) as a possible alternative to soybean meal in broiler diets. Br. Poult. Sci. 2015, 56, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.D.M.; Pereira, R.T.; de Paula, V.R.C.; Junior, H.M.; Menten, J.F.M. Tenebrio meal as a functional ingredient modulates immune response and improves growth performance of broiler chickens. J. Appl. Poult. Res. 2023, 32, 100346. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Wang, G.; Rong, T.; Liu, Z.; Wang, F.; Ma, X. Hermetia illucens larvae as a fishmeal replacement alters intestinal specific bacterial populations and immune homeostasis in weanling piglets. J. Anim. Sci. 2020, 98, skz395. [Google Scholar] [CrossRef] [PubMed]
- Koide, S.S. Chitin-chitosan: Properties, benefits and risks. Nutr. Res. 1998, 18, 1091–1101. [Google Scholar] [CrossRef]
- Dong, L.; Wichers, H.J.; Govers, C. Beneficial health effects of chitin and chitosan. In Chitin and Chitosan: Properties and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 145–167. [Google Scholar]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Haider, R.; Khan, N.; Aihetasham, A.; Shakir, H.A.; Fatima, M.; Tanveer, A.; Ali, W.; Tahir, M.; Asghar, M.; Farooq, A.; et al. Dietary inclusion of black soldier fly (Hermetia illucens) larvae meal, with exogenous protease supplementation, in practical diets for striped catfish (Pangasius hypophthalmus, Sauvage 1878). PLoS ONE 2024, 19, e0313960. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Bastrakov, A.I.; Kozlova, A.A.; Ponomarev, S.V.; Bakaneva, Y.M.; Fedorovykh, Y.V.; Pavlov, D.S. Features of the effect of a complex probiotic with Bacillus bacteria and the larvae of Hermetia illucens biomass on Mozambique tilapia (Oreochromis mossambicus × O. niloticus) and Russian sturgeon (Acipenser gueldenstaedti) fry. Biol. Bull. 2016, 43, 450–456. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Sudha, C.; Ahilan, B.; Felix, N.; Uma, A.; Prabu, E. Effects of dietary protein substitution of fishmeal with black soldier fly larval meal on growth and physiological responses of juvenile striped catfish, Pangasianodon hypophthalmus. Aquac. Res. 2022, 53, 2204–2217. [Google Scholar] [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.O.; Lee, S.M. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture 2018, 496, 79–87. [Google Scholar] [CrossRef]
- Mastoraki, M.; Ferrándiz, P.M.; Vardali, S.C.; Kontodimas, D.C.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture 2020, 528, 735511. [Google Scholar] [CrossRef]
- Bai, N.; Li, Q.; Pan, S.; Qi, Z.; Deng, W.; Gu, M. Effects of defatted yellow mealworm (Tenebrio molitor) on growth performance, intestine, and liver health of turbot (Scophthalmus maximus). Anim. Feed Sci. Technol. 2023, 302, 115672. [Google Scholar] [CrossRef]
- Xu, X.; Ji, H.; Yu, H.; Zhou, J. Influence of replacing fish meal with enzymatic hydrolysates of defatted silkworm pupa (Bombyx mori L.) on growth performance, body composition and non-specific immunity of juvenile mirror carp (Cyprinus carpio var. specularis). Aquac. Res. 2018, 49, 1480–1490. [Google Scholar] [CrossRef]
- Bovera, F.; Loponte, R.; Pero, M.E.; Cutrignelli, M.I.; Calabrò, S.; Musco, N.; Vassalotti, G.; Panettieri, V.; Lombardi, P.; Piccolo, G.; et al. Laying performance, blood profiles, nutrient digestibility and inner organs traits of hens fed an insect meal from Hermetia illucens larvae. Res. Vet. Sci. 2018, 120, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Montalbán, A.; Madrid, J.; Hernández, F.; Schiavone, A.; Ruiz, E.; Sánchez, C.J.; Ayala, L.; Fiorilla, E.; Martínez-Miró, S. The Influence of Alternative Diets and Whole Dry Black Soldier Fly Larvae (Hermetia illucens) on the Production Performance, Blood Status, and Egg Quality of Laying Hens. Animals 2024, 14, 2550. [Google Scholar] [CrossRef]
- Hossain, S.M.; Blair, R. Chitin utilisation by broilers and its effect on body composition and blood metabolites. Br. Poult. sci. 2007, 48, 33–38. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Mikołajczak, Z.; Leciejewska, N.; Józefiak, D. Hermetia illucens fat affects the gastrointestinal tract selected microbial populations, their activity, and the immune status of broiler chickens. Ann. Anim. Sci. 2022, 22, 663–675. [Google Scholar] [CrossRef]
- Kurniawan, D.; Widodo, E.; Susilo, A.; Sjofjan, O. Supplementation of Selenium-enriched Black Soldier Fly (Hermetia illucens) Larvae Meal on Growth Performance, Blood Parameters, and Immune Function in Broiler Ducks. J. Ilmu Ternak Vet. 2024, 29, 227–235. [Google Scholar] [CrossRef]
- Ait-Kaki, A.; Chebli, Y.; El Otmani, S.; Moula, N. Effects of yellow mealworm larvae (Tenebrio molitor) and turmeric powder (curcuma) on laying hens performance, physical and nutritional eggs quality. J. Indones. Trop. Anim. Agric. 2022, 47, 87–96. [Google Scholar] [CrossRef]
- Zacharis, C.; Bonos, E.; Giannenas, I.; Skoufos, I.; Tzora, A.; Voidarou, C.; Tsinas, A.; Fotou, K.; Papadopoulos, G.; Mitsagga, C.; et al. Utilization of Tenebrio molitor Larvae reared with different substrates as feed ingredients in growing pigs. Vet. Sci. 2023, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, M.R.; Siddik, M.A.; Fotedar, R.; Howieson, J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Sci. Rep. 2019, 9, 16703. [Google Scholar] [CrossRef] [PubMed]
- Agbohessou, P.S.; Mandiki, S.N.; Biyong, S.R.M.; Cornet, V.; Nguyen, T.M.; Lambert, J.; Jauniaux, T.; Lalèyè, P.A.; Kestemont, P. Intestinal histopathology and immune responses following Escherichia coli lipopolysaccharide challenge in Nile tilapia fed enriched black soldier fly larval (BSF) meal supplemented with chitinase. Fish Shellfish Immunol. 2022, 128, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Yang, C.J. Efficacy of mealworm and super mealworm larvae probiotics as an alternative to antibiotics challenged orally with Salmonella and E. coli infection in broiler chicks. Poult. Sci. 2017, 96, 27–34. [Google Scholar] [CrossRef]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]


| Order | Species | Instars | Chitin Content (%) | References |
|---|---|---|---|---|
| Coleoptera | Tenebrio molitor (Tenebrionidae) | Larvae | 4.60 | Shin et al. [46] |
| Pupae | 3.90 | |||
| Adults | 8.40 | |||
| Alphitobius diaperinus (Tenebrionidae) | Larvae | 4.2–6.2 | Soetemans et al. [47] | |
| Diptera | Hermetia illucens (Stratiomyidae) | Larvae | 13 | Smets et al. [48]; Triunfo et al. [49] |
| Prepupae | 4.7 | |||
| Exuviae | 31 | |||
| Adults | 9 | |||
| Musca domestica (Muscidae) | Larvae | 9.1 | Zhang et al. [50] Kim et al. [51] | |
| Pupae | 8.02 | |||
| Lepidoptera | Bombyx mori (Bombycidae) | Chrysalides | 15–20 | Zhang et al. [43] |
| Orthoptera | Acheta domesticus (Gryllidae) | Adults | 4.3–7 | Ibitoye et al. [52] |
| Gryllodes sigillatus (Gryllidae) | Adults | 3.4 | Malm [53] | |
| Gryllus assimilis (Gryllidae) | Adults | 2.9–3.3 | Toribio et al. [54] |
| Insect Species | Instars | Fish Species | Inclusion Level (%) | Chitin Content (%) | Effect on Digestibility | References |
|---|---|---|---|---|---|---|
| Hermetia illucens | Larvae | Rainbow trout | 25, 50% | 1, 2% | Dose-dependent low ADC of CP, dry matter and PUFAs | Renna et al. [57] |
| Larvae | 6.6, 13.2, 26.4% | / | No significant differences in the ADC of CP and essential AAs. Dose-dependent low digestibility of lipids, dry matter, and taurine | Dumas et al. [75] | ||
| Larvae | / | 8% | No significant differences in the ADC of CP, EE, and chitin | Ushakova et al. [76] | ||
| Larvae | 25% with three different types of fractions | 1.8, 2.7, 15.4% | Dose-dependent low ADC of dry matter, CP and nitrogen-free extract | Eggink et al. [62] | ||
| Larvae | 15% | / | No significant differences in the ADC of CP, nitrogen, lipids, and energy, with high levels of lauric and myristic fatty acids | Drosdowech et al. [71] | ||
| Larvae | Nile tilapia | 25% with three different types of fractions | 1.8, 2.7, 15.4% | Dose-dependent low ADC of dry matter, CP and nitrogen-free extract | Eggink et al. [62] | |
| Larvae | Red hybrid tilapia | 30% | 1% | High ADC of proteins, and GE | Muin and Taufek [77] | |
| Larvae | Red tilapia | / | 7.8% | No significant differences in the ADC of CP, EE, and chitin | Ushakova et al. [76] | |
| Larvae | Meagre | 17, 35, 52% | 0.6, 1.1, 1.6% | Dose-dependent low ADC of dry matter, CP, and some essential and non-essential AAs | Guerreiro et al. [61] | |
| Larvae | African catfish hybrid juveniles | 30% | 9% | High ADC of CP, EE, ash and phosphorus, less digestibility of essential AAs | Sándor et al. [63] | |
| Prepupae | Juvenile red sea bream | 15, 30 and 45% | / | No significant differences in the ADC of nutrients except for lipids during complete replacement | Oktay et al. [70] | |
| Larvae | Juvenile turbot | 17, 33, 49, 64 and 76% | From 1.6 to 7.3% | Dose-dependent low ADC of CP and GE | Kroeckel et al. [65] | |
| Larvae | Russian sturgeon | / | 7.8% | No significant differences in the ADC of CP, EE, and chitin | Ushakova et al. [76] | |
| Prepupae | European seabass | 15, 30, 45% | / | No significant differences in the ADC of CP, EE | Magalhães et al. [69] | |
| Larvae | Atlantic salmon | 33, 66, 100% | / | No significant differences in the ADC of CP, EE, AAs and fatty acids, or the digestive enzyme | Belghit et al. [68] | |
| Tenebrio molitor | Larvae | Rainbow trout | 25, 50% | / | Dose-dependent low ADC of CP | Belforti et al. [56] |
| Larvae | 30% | / | No differences in the ADC of CP, lipids, dry matter, and GE | Owens et al. [78] | ||
| Larvae | Nile tilapia | 21, 43% | 1.37, 2.82% | No differences in the ADC of CP | Sánchez-Muros et al. [58] | |
| Larvae | 20% | 3.8% | High ADC of CP, dry matter, and chitin | Fontes et al. [60] | ||
| Larvae | Meagre | 10, 20, 30% | 0, 74, 0, 97, 1.47% | Dose-dependent low ADC of dry matter, GE, CP, and AAs | Coutinho et al. [66] | |
| Larvae | African catfish hybrids | 30% | 6% | Low ADC of dry matter, AAs, fatty acids | Sándor et al. [63] | |
| Larvae | European sea bass | 25, 50% | / | No differences in the ADC of dry matter, CP, and EE | Gasco et al. [79] | |
| Larvae | Gilthead sea bream | 25, 50% | 1.15, 2.31% | Dose-dependent low ADC of CP and EE | Piccolo et al. [59] | |
| Larvae | Juvenile largemouth bass | 12, 24, 36, 48% | / | High ADC of CP, lipids, and dry matter | Chen et al. [80] | |
| Acheta domesticus | Adults | Rainbow trout | 30% | / | No differences in the ADC of CP, lipids, dry matter, and GE | Owens et al. [78] |
| Alphitobius diaperinus | Larvae | Rainbow trout | 30% | 7% | Low ADC of CP, AAs, dry matter, and GE | Gasco et al. [64] |
| Gryllus assimilis | Adults | Nile tilapia | 20% | 5% | Low ADC of dry matter, CP, and GE | Fontes et al. [60] |
| Gryllodes sigillatus | Adults | Rainbow trout | 15% | / | No significant differences in the ADC of CP, nitrogen, lipid, and GE | Drosdowech et al. [71] |
| Musca domestica | Larvae | Nile tilapia | 9, 18, 27, 36% | / | No significant differences in the ADC of dry matter, CP, lipids, GE, and phosphorus | Wang et al. [72] |
| Bombyx mori | Chrysalides | Common carp | 10, 20, 30% | / | No significant differences in the ADC of CP and EE | Nandeesha et al. [73] |
| Chrysalides | Juvenile mirror carp | 4, 8, 12, 16% | / | No significant differences in the ADC of dry matter, CP, and EE | Zhou et al. [74] |
| Insect Species | Instars | Poultry | Inclusion Level (%) | Chitin Content (%) | Effect on Digestibility | References |
|---|---|---|---|---|---|---|
| Hermetia illucens | Larvae | Broilers | 25% | / | High ADC of dry matter, CP, and AAs | De Marco et al. [88] |
| Larvae | 2, 4, 6, 8, 10% | / | No significant differences in the ADC of EE and dry matter, high ADC of CP | Kareem et al. [90] | ||
| Prepupae | 5% | / | No significant differences in the ADC of CP | Elangovan et al. [93] | ||
| Larvae | 100% | 5.5% | Low ADC of fibres and EE | Chobanova et al. [86] | ||
| Larvae | Quails | 10, 15% | / | No significant difference in the ADC of dry matter, CP, and GE, except for EE which was less for 10% inclusion level | Cullere et al. [89] | |
| Larvae | Layers | 3, 6, 9% | / | No significant differences in the ADC of dry matter, CP, EE, calcium, and phosphorous | Chu et al. [92] | |
| Larvae | Laying hens | 15% | 7% | Low ADC of AAs | Heuel et al. [85] | |
| Larvae | Sentul chickens | 2, 4, 6% | / | Dose-dependent high ADC of CP and EE | Rahayu et al. [95] | |
| Tenebrio molitor | Larvae | Broilers | 25% | / | High ADC of dry matter, CP, and AAs | De Marco et al. [88] |
| Larvae | 100% | 4.62% | Low ADC of dry matter and CP | Bovera et al. [83] | ||
| Larvae | 0.2, 0.3% | 8.9% | No significant differences in the ADC of CP and EE | Benzertiha et al. [91] | ||
| Larvae | 20% | / | Low ADC of dry matter and CP, except for EE and GE | Dourado et al. [84] | ||
| Larvae | 30% | / | High ADC of dry matter, GE, and AAs | Nascimento et al. [94] | ||
| Gryllus assimilis | Nymphs | Broilers | 20% | / | Low ADC of dry matter and CP, except for EE and GE | Dourado et al. [84] |
| Musca domestica | Larvae | Broilers | 5, 10, 15, 20% | / | High ADC of CP and AAs | Hwangbo et al. [87] |
| Bombyx mori | Chrysalides | Fattening quails | 12.5% | 2.8–3.5% | Low ADC of dry matter, CP, EE, and GE | Dalle Zotte et al. [97] |
| Chrysalides | Broilers | 4% | / | No significant differences in the ADC of dry matter and CP | Singh et al. [98] |
| Insect Species | Instars | Fish Species | Inclusion Level (%) | Chitin Content (%) | Prebiotic Effect | References |
|---|---|---|---|---|---|---|
| Hermetia illucens | Larvae | Rainbow trout | 30% | / | Increase in richness of microbiota with LAB such as Bacillaceae | Huyben et al. [115] |
| Prepupae | 10, 20, 30% | 0.50, 0.99, 1.51% | Enhancement of diversity and abundance of gut microbiota, with increased mycoplasma linked to LAB production capacity | Rimoldi et al. [117] | ||
| Prepupae | 10, 20, 30% | 0.5, 0.9, 1.5% | Increase in richness and diversity of microbiota and increase in LAB and butyrate production bacteria such as Lactobacillaceae, Bacillaceae, Actinomycetaceae, and Clostridiaceae | Terova et al. [114] | ||
| Larvae | 20% | / | Increase in richness of microbiota with Clostridium and LAB | Józefiak et al. [116] | ||
| Exuviae | 1.6% | / | Increase in richness of gut microbiota with Bacillus, Facklamia, Brevibacterium, and Corynebacterium genera with chitinolytic and lactic acid production activity | Rimoldi et al. [113] | ||
| Larvae | 15% | / | Increase in richness and diversity of microbiota and increase in Peptostreptococcus with prebiotic effect and digestion and fermentation activity | Drosdowech et al. [126] | ||
| Larvae | Siberian sturgeon | 15% | / | Increase in richness of microbiota with Bacillus, Enterococcus, Lactobacillus, and Entrerobacteriaceae | Józefiak et al. [118] | |
| Larvae | Atlantic salmon | 10% | / | Increase in LAB and chitin degrading bacteria of genus Exoguobacterium | Leeper et al. [119] | |
| Larvae | Atlantic salmon | 20% | 1.44% | Increase in LAB such as Actinomycetaceae, Lactobacillaceae, and chitinolytic bacteria such as Bacillaceae and Actinomycetaceae | Weththasinghe et al. [120] | |
| Larvae | Atlantic salmon | 5, 10, 15, 20% | / | Increase in Bacillus, Enterococcus and Lactobacillus with chitinolytic and prebiotic effect | Rawski et al. [122] | |
| Larvae | European sea bass | 25% | 1.8% | Increase in Firmicutes, Bacillaceae, Enterococcaceae, Lachnospiraceae, and Actinomycetaceae with chitinolytic activity and prebiotic effect | Rangel et al. [121] | |
| Larvae | Gilthead sea bram | 5, 10, 15% | / | Increase and shift in microbiota with abundance of Bacillaceae and Paenibacillaceae involved in chitin degradation and prebiotic effect | Busti et al. [127] | |
| Larvae | Gilthead seabream juveniles | 15, 30, 45% | / | Increase in richness and diversity of gut microbiota promoting beneficial digesta bacteria | Moutinho et al. [128] | |
| Larvae | Hybrid grouper | 10, 30, 50% | / | Increase in gut microbiota diversity with Thiobacillus, Sutterella, Veillonella, Dialister and Biophila genera, and Hydrogenophilaceae family associated with beneficial metabolic functions | Chen et al. [129] | |
| Tenebrio molitor | Larvae | Rainbow trout | 20% | / | Increase in richness of microbiota with Clostridium and LAB | Józefiak et al. [116] |
| Larvae | 100% | 1.49% | Increase in richness and diversity of microbiota with abundance of Lactobacillales | Terova et al. [123] | ||
| Juvenile large yellow croakers | 15, 30, 45% | / | Increase in relative abundance of Bacillus and Lactobacillus | Zhang et al. [124] | ||
| Larvae | Grass carp | 25, 50, 75, 100% | / | 25% inclusion positively affected beneficial intestinal bacteria, while higher levels disrupted gut microbiota increasing harmful bacteria like Brevinema | Yang et al. [125] | |
| Larvae | Siberian sturgeon | 15% | / | Increase in richness of microbiota with Bacillus, Enterococcus, and Lactobacillus | Józefiak et al. [118] | |
| Larvae | European sea bass | 25% | / | Increase in the relative abundance of beneficial and chitinolytic bacteria | Rangel et al. [121] | |
| Gryllodes sigillatus | Nymphs | Rainbow trout | 20% | / | Increase in richness of microbiota with Clostridium and LAB | Józefiak et al. [116] |
| Insect Species | Instars | Poultry | Inclusion Level (%) | Chitin Content (%) | Prebiotic Effect | References |
|---|---|---|---|---|---|---|
| Hermetia illucens | Larvae | Broiler | 0.1, 0.2% | / | Increase in Bacteroides, Prevotella, Clostridium coccoides, Eubacterium, Streptococcus spp. and Lactococcus spp., with prebiotic effect and beneficial effect | Józefiak et al. [130] |
| Larvae | 5, 10, 15% | / | Moderate inclusion level increases beneficial microbiota such as L-Ruminococcus, Lactobacillus, Faecalibacterium, Blautia, Roseburia, and Clostridium with lactic acid activity and SCFA production | Biasato et al. [131] | ||
| Larvae | 5% | / | Increase in Victivillaceae, Saccharibacteri, Clostridium, and Eubacterium involved in polysaccharide fermentation and SCFA production | Colombino et al. [132] | ||
| Larvae | 5, 10, 15, 20% | / | Abundance of the bacterial group Roseburia, known for the SCFA production | De Souza Vilela et al. [133] | ||
| Larvae | Slow-growing chickens | 5% | / | Increase in beneficial bacteria, such as Faecalibacterium, known to produce SCFA | Fiorilla et al. [134] | |
| Larvae | Muscovy ducks | 3, 6, 9% | / | Increase in Faecalibacterium, Megamonas, and Ruminococcus, known for the SCFA production and beneficial effect | Martínez Marín et al. [135] | |
| Tenebrio molitor | Larvae | Broiler | 5, 10, 15% | / | Increase in abundance of Clostridium, Alistipes, and Sutterella with beneficial effect and butyric acid production | Biasato et al. [136] |
| Larvae | 0.1, 0.2% | / | Increase in Lactobacillus spp., Enterococcus | Józefiak et al. [130] | ||
| Larvae | 0.2, 0.3% | / | Increase in abundance of Ruminococcaceae and Lactobacillus | Józefiak et al. [137] | ||
| Larvae | 5% | / | Increase in Collinsella and Eubacterium with beneficial effect and SCFA production | Colombino et al. [132] | ||
| Bombyx mori | Chrysalides | Fattening quails | 12.5% | 1–1.40% | Increase in Streptococcaceae, Rikenellaceae, Eubacteriaceae, Lactobacillus, and bacillus involved in polysaccharide fermentation, SCFA production, and prebiotic effect | Dalle Zotte et al. [97] |
| Insect Species | Instars | Fish Species | Inclusion Level (%) | Immunostimulatory Effect | References |
|---|---|---|---|---|---|
| Hermetia illucens | Larvae | Rainbow trout | 25, 50% | Inhibitory activity of pathogen bacterial, aspartate blood aminotransferase lower and lysozyme content higher compared to the control | Hwang et al. [142] |
| Larvae | 25, 50, 75, 100% | Increase in the expression of cytokines (TGF, IL-10, IL-1β, TNF−α, and IL-8) and immune-related genes (IgM, IgT, MHC-II, and TRL-5) in all insect meals compared to the control | Sayramoğlu et al. [144] | ||
| Larvae | Nile tilapia | 33, 100% | Increase in lysozyme activity, white blood cell count, lymphocyte count at 100% inclusion level compared to the control. Even globulin and albumin increased in both treated groups | Abd El-Gawad et al. [146] | |
| Larvae | Koi carp | 5, 10, 15, 20% | Increase in mRNA transcripts of immune-related genes such as TNF-α, TGF-β, IL1, IL10, and hsp70 | Linh et al. [149] | |
| Larvae | Zebrafish | 2,5, 3, 10% | Increase in TNF-α, IL-10, and hsp70 genes | Zhang et al. [145] | |
| Larvae | Pearl gentian grouper | 10, 20, 30% | Increase in immune response as shown by the increase in key genes NF-κB, TLR, MyD88, IL-10 | Huang et al. [147] | |
| Larvae | Juvenile grouper | 2.5, 5, 10% | Increase in lysozyme activity, TNF-α, hsp70, and IL-1β genes | Jian et al. [148] | |
| Tenebrio molitor | Larvae | Rainbow trout | 7, 14, 21, 28% | Increase in lysozyme activity and myeloperoxidase | Jeong et al. [143] |
| Larvae | European sea bass | 36% | Increase in lysozyme antibacterial activity with inhibition of serum trypsin inhibition | Henry et al. [150] | |
| Larvae | Juvenile largemouth Bass | 12, 24, 36, 48% | Increase in anti-inflammatory genes such as IL-10 and TGF and pro-inflammatory genes such as IL-8 and IL-1β | Chen et al. [80] | |
| Larvae | Grass carp | 25, 50, 75, 100% | Increase in cytokines with anti-inflammatory responses such as NF-kB, IL- β, IL-6 and TNF-α | Yang et al. [125] | |
| Larvae | Large yellow croaker | 15, 30, 45, 60, 75, 100% | Increase in Keap-1, NF-kB, and TNF-α in intestine and liver | Qu et al. [152] | |
| Larvae | Yellow catfish | 25, 50, 75% | Increase in immune-related genes as MHC II, IL-1, CypA, IgM, HEq | Su et al. [151] | |
| Musca domestica | Larvae | Hybrid catfish | 14, 21% | Improving immune physiology with white blood cell, lymphocyte count and globulin increase | Fawole et al. [153] |
| Bombyx mori | Chrysalides | Beluga sturgeon | 5, 10, 15% | Increase in lysozyme and IgM activity | Bagheri et al. [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abenaim, L.; Conti, B. Harnessing Chitin from Edible Insects for Livestock Nutrition. Insects 2025, 16, 799. https://doi.org/10.3390/insects16080799
Abenaim L, Conti B. Harnessing Chitin from Edible Insects for Livestock Nutrition. Insects. 2025; 16(8):799. https://doi.org/10.3390/insects16080799
Chicago/Turabian StyleAbenaim, Linda, and Barbara Conti. 2025. "Harnessing Chitin from Edible Insects for Livestock Nutrition" Insects 16, no. 8: 799. https://doi.org/10.3390/insects16080799
APA StyleAbenaim, L., & Conti, B. (2025). Harnessing Chitin from Edible Insects for Livestock Nutrition. Insects, 16(8), 799. https://doi.org/10.3390/insects16080799







