Characterization of DNA Viruses in Hindgut Contents of Protaetia brevitarsis Larvae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Sampling
2.2. DNA Preparation and Metagenomic Shotgun Sequencing
2.3. Bioinformatics and Sequencing Data Processing
3. Results
3.1. General Information and Sample Sequencing
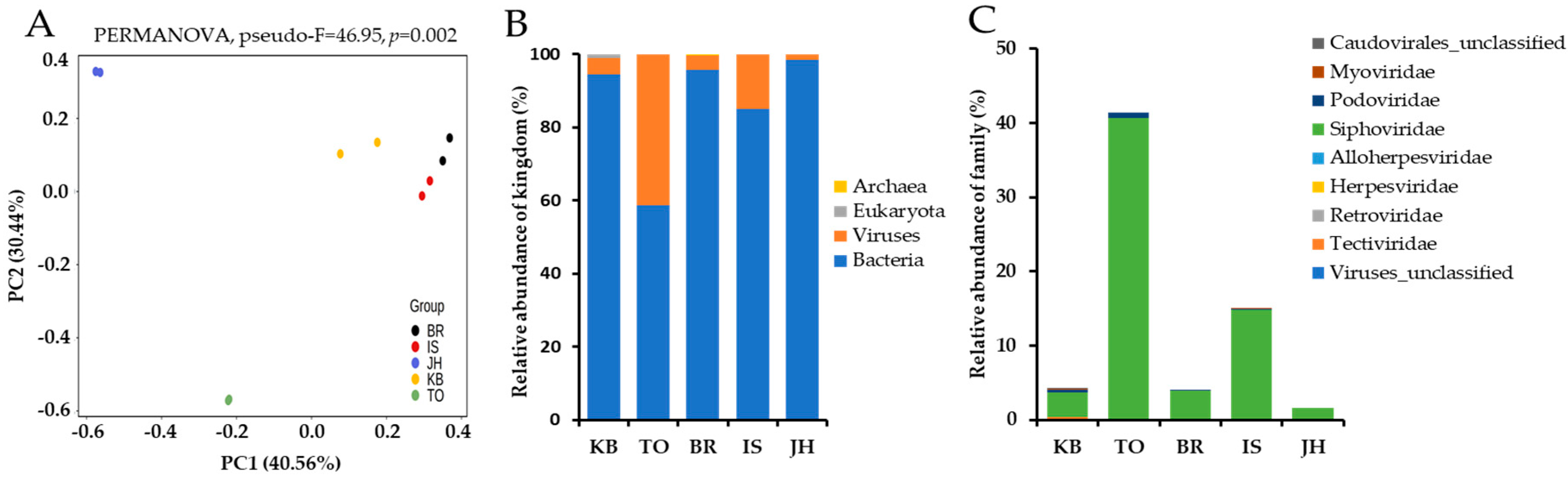
3.2. Analysis of Virus Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, J.; Xu, H.; Cheng, Y.; Mintah, B.K.; Dabbour, M.; Yang, F.; Chen, W.; Zhang, Z.; Dai, C.; He, R.; et al. Recent Insight on Edible Insect Protein: Extraction, Functional Properties, Allergenicity, Bioactivity, and Applications. Foods 2022, 11, 2931. [Google Scholar] [CrossRef]
- Jeong, D.; Min, N.; Kim, Y.; Kim, S.R.; Kwon, O. The effects of feed materials on the nutrient composition of Protaetia brevitarsis larvae. Entomol. Res. 2020, 50, 23–27. [Google Scholar] [CrossRef]
- Kłobukowski, F.; Śmiechowska, M.; Skotnicka, M. Edible Insects from the Perspective of Sustainability—A Review of the Hazards and Benefits. Foods 2025, 14, 1382. [Google Scholar] [CrossRef]
- Park, E.S.; Choi, M.K. Recognition, purchase, and consumption of edible insects in Korean adults. J. Nutr. Health 2020, 53, 190–202. [Google Scholar] [CrossRef]
- Han, R.; Shin, J.T.; Kim, J.; Choi, Y.S.; Kim, Y.W. An overview of the South Korean edible insect food industry: Challenges and future pricing/promotion strategies. Entomol. Res. 2017, 47, 141–151. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Liu, C.; Liu, Y.; Mei, X.; Wang, Z.; Zhang, T. Identification and field verification of an aggregation pheromone from the white-spotted flower chafer, Protaetia brevitarsis Lewis (Coleoptera: Scarabaeidae). Sci. Rep. 2021, 11, 22362. [Google Scholar] [CrossRef]
- Lee, H.L.; Kim, J.M.; Go, M.J.; Lee, H.S.; Kim, J.H.; Kim, I.Y.; Seong, G.-S.; Heo, H.J. Fermented Protaetia brevitarsis Larvae Alleviates High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in C57BL/6 Mice via Regulation of Lipid Accumulation and Inflammation. J. Microbiol. Biotechnol. 2025, 35, e2409025. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Jung, S.; Ha, J.H.; Jeong, Y. Protaetia brevitarsis Hydrolysate Mitigates Muscle Dysfunction and Ectopic Fat Deposition Triggered by a High-Fat Diet in Mice. Nutrients 2025, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Li, Y.; Fu, T.; Geng, L.; Shi, Y.; Chu, H.; Liu, F.; Liu, C.; Song, F.; Zhang, J.; Shu, C. Protaetia brevitarsis larvae can efficiently convert herbaceous and ligneous plant residues to humic acids. Waste Manag. 2019, 83, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, Y.; Zhang, S.; Xu, A.; Meng, Z.; Ge, H.; Li, J.; Liu, Y.; Ma, D. Transformation Capability Optimization and Product Application Potential of Proteatia brevitarsis (Coleoptera: Cetoniidae) Larvae on Cotton Stalks. Insects 2022, 13, 1083. [Google Scholar] [CrossRef]
- Wang, K.; Gao, P.; Geng, L.; Liu, C.; Zhang, J.; Shu, C. Lignocellulose degradation in Protaetia brevitarsis larvae digestive tract: Refining on a tightly designed microbial fermentation production line. Microbiome 2022, 10, 90. [Google Scholar] [CrossRef]
- Kim, J.W.; Bea, S.M.; Park, J.H.; Jang, D.H.; Hwang, Y.H.; Kim, Y.G.; Lee, Y.H. Effect of Protaetia brevitarsis larval frass fertilizer on the growth of ginseng sprout (Panax ginseng) in commercial potting soil. Korean J. Soil Sci. Fert. 2024, 57, 55–62. [Google Scholar] [CrossRef]
- Xuan, H.; Gao, P.; Du, B.; Geng, L.; Wang, K.; Huang, K.; Zhang, J.; Huang, T.; Shu, C. Characterization of Microorganisms from Protaetia brevitarsis Larva Frass. Microorganisms 2022, 10, 311. [Google Scholar] [CrossRef]
- Min, N.; Min, J.G.; Cammayo-Fletcher, P.L.T.; Nguyen, B.T.; Yim, D. Comparative Analysis of Hindgut Microbiota Variation in Protaetia brevitarsis Larvae across Diverse Farms. Microorganisms 2024, 12, 496. [Google Scholar] [CrossRef]
- Bertola, M.; Mutinelli, F. A Systematic Review on Viruses in Mass-Reared Edible Insect Species. Viruses 2021, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Zhu, Y.; Liu, Z.; Wei, T.; Wang, P.; Cheng, G. Interaction of Viruses with the Insect Intestine. Annu. Rev. Virol. 2021, 8, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Litov, A.G.; Zueva, A.I.; Tiunov, A.V.; Van Thinh, N.; Belyaeva, N.V.; Karganova, G.G. Virome of Three Termite Species from Southern Vietnam. Viruses 2022, 14, 860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, F.; Liu, X.; Fu, Y.; Fang, W.; Kang, X.; Lu, H.; Li, S.; Liu, B.; Guo, W.; et al. Association of virome dynamics with mosquito species and environmental factors. Microbiome 2023, 11, 101. [Google Scholar] [CrossRef]
- Chen, S.; Fang, Y.; Fujita, R.; Khater, E.I.M.; Li, Y.; Wang, W.; Qian, P.; Huang, L.; Guo, Z.; Zhang, Y.; et al. An Exploration of the Viral Coverage of Mosquito Viromes Using Meta-Viromic Sequencing: A Systematic Review and Meta-Analysis. Microorganisms 2024, 12, 1899. [Google Scholar] [CrossRef]
- Zuo, T.; Lu, X.J.; Zhang, Y.; Cheung, C.P.; Lam, S.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Zhao, R.; Chan, P.K.S.; et al. Gut mucosal virome alterations in ulcerative colitis. Gut 2019, 68, e1. [Google Scholar] [CrossRef]
- Manrique, P.; Bolduc, B.; Walk, S.T.; van der Oost, J.; de Vos, W.M.; Young, M.J. Healthy human gut phageome. Proc. Natl. Acad. Sci. USA 2016, 113, 10400–10405. [Google Scholar] [CrossRef]
- Zhang, Y.; Sharma, S.; Tom, L.; Liao, Y.T.; Wu, V.C.H. Gut Phageome-An Insight into the Role and Impact of Gut Microbiome and Their Correlation with Mammal Health and Diseases. Microorganisms 2023, 11, 2454. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. eBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Li, T.; Zhang, Q.; Zhang, R.; Zhang, Z. Bacteriophage: A Useful Tool for Studying Gut Bacteria Function of Housefly Larvae, Musca domestica. Microbiol. Spectr. 2021, 9, e00599-21. [Google Scholar] [CrossRef] [PubMed]
- Goulet, A.; Spinelli, S.; Mahony, J.; Cambillau, C. Conserved and Diverse Traits of Adhesion Devices from Siphoviridae Recognizing Proteinaceous or Saccharidic Receptors. Viruses 2020, 12, 512. [Google Scholar] [CrossRef]
- Akimkina, T.; Venien-Bryan, C.; Hodgkin, J. Isolation, characterization and complete nucleotide sequence of a novel temperate bacteriophage Min1, isolated from the nematode pathogen Microbacterium nematophilum. Res. Microbiol. 2007, 158, 582–590. [Google Scholar] [CrossRef]
- Du, B.; Xuan, H.; Geng, L.; Li, W.; Zhang, J.; Xiang, W.; Liu, R.; Shu, C. Microflora for improving the Auricularia auricula spent mushroom substrate for Protaetia brevitarsis production. Iscience 2022, 25, 105307. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, M.; Shin, Y.; Subramaniyam, S.; Kim, I.W.; Seo, M.; Kim, M.-A.; Kim, S.H.; Hwang, J.; Choi, E.H.; et al. Draft Genome of the Edible Oriental Insect Protaetia brevitarsis seulensis. Front. Genet. 2020, 11, 593994. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, E.H.; Park, B.; Kim, G.; Shin, C.; Lee, J.H.; Hwang, J.S.; Hwang, U.W. Transcriptome profiling for developmental stages Protaetia brevitarsis seulensis with focus on wing development and metamorphosis. PLoS ONE 2023, 18, e0277815. [Google Scholar] [CrossRef]
| Farm Name | Relative Prevalence (%) | |||||
|---|---|---|---|---|---|---|
| KB | TO | BR | IS | JH | ||
| Family | Viruses_unclassified | 0.14 | - | - | 0.05 | - |
| Tectiviridae | 0.22 | - | - | - | - | |
| Retroviridae | - | - | 0.06 | - | - | |
| Herpesviridae | - | 0.05 | 0.01 | 0.02 | - | |
| Alloherpesviridae | - | 0.05 | - | - | - | |
| Siphoviridae | 3.41 | 40.54 | 3.89 | 14.72 | 1.66 | |
| Podoviridae | 0.30 | 0.74 | 0.13 | 0.20 | - | |
| Myoviridae | 0.19 | - | - | 0.09 | - | |
| Caudovirales_unclassified | 0.10 | - | - | - | - | |
| Other (Bacteria, Archaea, Eukaryota) | 95.64 | 58.61 | 95.92 | 84.91 | 98.34 | |
| Genus | Viruses_unclassified | 0.14 | - | - | 0.05 | - |
| Betatectivirus | 0.22 | - | - | - | - | |
| Retroviridae_unclassified | - | - | 0.06 | - | - | |
| Varicellovirus | - | 0.05 | 0.01 | - | - | |
| Rhadinovirus | - | - | - | 0.02 | - | |
| Cyprinivirus | - | 0.05 | - | - | - | |
| Yuavirus | 0.03 | - | - | - | - | |
| Siphoviridae_unclassified | 2.64 | 33.82 | 2.53 | 6.55 | 0.90 | |
| Send513virus | - | - | - | 0.03 | 0.01 | |
| Phic31virus | 0.32 | - | 0.05 | 0.14 | - | |
| Pbi1virus | 0.22 | 1.04 | 0.43 | 0.21 | 0.15 | |
| Omegavirus | - | - | - | 0.01 | - | |
| L5virus | - | - | 0.61 | 6.79 | 0.15 | |
| Fishburnevirus | - | 1.68 | - | 0.05 | 0.02 | |
| Che9cvirus | 0.12 | 0.70 | 0.10 | 0.63 | 0.14 | |
| Che8virus | 0.08 | 2.38 | 0.16 | 0.24 | 0.21 | |
| Brujitavirus | - | 0.93 | - | 0.07 | 0.08 | |
| Podoviridae_unclassified | - | - | 0.10 | - | - | |
| Bpp1virus | 0.30 | 0.74 | 0.02 | 0.20 | - | |
| Svunavirus | 0.19 | - | - | - | - | |
| P1virus | - | - | - | 0.03 | - | |
| Bxz1virus | - | - | - | 0.06 | - | |
| Caudovirales_unclassified | 0.10 | - | - | - | - | |
| Other (Bacteria, Archaea, Eukaryota) | 95.64 | 58.61 | 95.92 | 84.92 | 98.33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, J.G.; Min, N.; Nguyen, B.T.; Flores, R.A.; Yim, D. Characterization of DNA Viruses in Hindgut Contents of Protaetia brevitarsis Larvae. Insects 2025, 16, 800. https://doi.org/10.3390/insects16080800
Min JG, Min N, Nguyen BT, Flores RA, Yim D. Characterization of DNA Viruses in Hindgut Contents of Protaetia brevitarsis Larvae. Insects. 2025; 16(8):800. https://doi.org/10.3390/insects16080800
Chicago/Turabian StyleMin, Jean Geung, Namkyong Min, Binh T. Nguyen, Rochelle A. Flores, and Dongjean Yim. 2025. "Characterization of DNA Viruses in Hindgut Contents of Protaetia brevitarsis Larvae" Insects 16, no. 8: 800. https://doi.org/10.3390/insects16080800
APA StyleMin, J. G., Min, N., Nguyen, B. T., Flores, R. A., & Yim, D. (2025). Characterization of DNA Viruses in Hindgut Contents of Protaetia brevitarsis Larvae. Insects, 16(8), 800. https://doi.org/10.3390/insects16080800







