Simple Summary
The evolutionary history of the order Archaeognatha has long been a subject of considerable scientific interest and debate. In this study, we utilized 14 mitochondrial genomes from Pedetontus and Pedetontinus species to investigate the phylogeny and divergence times within Archaeognatha. Integrated analyses of genetic distances, phylogenetic reconstructions, and divergence time estimations suggest that the current morphological classification system for Pedetontus may require revision. Furthermore, evidence of positive selection was detected in the mitochondrial protein-coding genes of these species under both temperate and tropical environmental conditions.
Abstract
Archaeognatha is phylogenetically positioned as the basal lineage relative to all extant insect orders and comprises approximately 600 described species. The internal phylogenetic relationships and divergence times within this ancient order have long been a subject of scientific debate. In this study, we assembled 14 mitochondrial genomes from species within the genera Pedetontus and Pedetontinus to clarify the phylogenetic relationship and estimate divergence times within Archaeognatha. Phylogenetic analyses revealed that both Machilidae and Machilinae are paraphyletic; Pedetontinus included in this analysis formed a well-supported monophyletic clade, whereas the sampled Pedetontus species were not recovered as a monophyletic clade. Divergence time estimates indicate that Archaeognatha originated during the Late Carboniferous (301.15 Mya, 95% HPD: 298.88–303.67 Mya), with subsequent diversification spanning from the Mesozoic era to the present. The adaptive radiation of epiphytic bryophytes and potential coevolutionary interactions between plants and insects are proposed to have significantly contributed to the diversification of Archaeognatha. Based on multiple lines of evidence, we propose that the current morphological criteria for species delineation within Pedetontus (Pd.) require revision to better reflect its evolutionary history. In the branch-site model analysis, when Pd. silvestrii—collected from temperate regions—was designated as the foreground branch, two positively selected sites were detected at the 66th position of the Cytb and the 34th position of ATP6. When Pd. hainanensis and Pd. bawanglingensis—collected from tropical regions—were used as the foreground branches, six positively selected sites were identified at the 622nd position of Cytb, the 499th position of ATP6, and the 623rd, 873rd, 1106th, and 1141st positions of COI.
1. Introduction
Archaeognatha occupies a pivotal position at the base of the insect phylogenetic tree and comprises approximately 600 extant species, representing the sister group to all other extant insect lineages [,]. The order is currently recognized as comprising two extant families: Machilidae and Meinertellidae []. Additionally, several paleoforms, such as Ditrigoniophthalmus, Charimachilis, and Mesomachilis, have been proposed as early-diverging lineages that predate the split between Machilidae and Meinertellidae []. Although the precise phylogenetic placement of these paleoforms remains unresolved, multiple studies suggest they may retain key plesiomorphic characteristics, providing important insights into early insect evolutionary [,,,]. To date, 41 species of Archaeognatha have been documented in China [,,,,,,,,,,,,,,,,,,,,,], with 40 species belonging to the family Machilidae. These Machilidae species are distributed across the following genera: Pedetontinus (10 species), Pedetontus (17 species), Haslundichilis (2 species), Coreamachilis (2 species), Metamachilis (1 species), Silvestrichilis (3 species), Allopsontus (4 species), and Songmachilis (1 species). The remaining species belongs to the family Meinertellidae and is classified within the genus Machilontus.
Within this taxonomic framework, the family Machilidae is divided into three distinct subfamilies—Machilinae, Petrobiellinae, and Petrobiinae []. The genera Pedetontus and Pedetontinus are classified within the subfamily Petrobiinae. These genera share the following morphological characteristics: an unscaled antennal flagellum, large contiguous compound eyes, paired shoe-shaped ocelli situated subinferior to the compound eyes, mandibles with four apical teeth, scaled maxillary palps and thoracic legs, meso- and metacoxae each bearing a stylus, and an elongate multi-articulate ovipositor. Specifically, Pedetontus is characterized by fore femora that are consistently unswollen, abdominal coxites II–V/VI bearing two pairs of retractile vesicles, and a primary-type ovipositor lacking a terminal claw. In contrast, Pedetontinus typically exhibits unmodified fore femora, each coxite of abdominal segments II–VII bearing only one pair of retractile vesicles, and a tertiary-type ovipositor [,].
Archaeognatha possesses a mitochondrial genome that exhibits structural conservation patterns commonly found in typical insect taxa. Characterized by matrilineal transmission, accelerated evolutionary dynamics, and non-recombining properties, this genomic system has proven to be a valuable tool for molecular studies aimed at testing and refining phylogenetic hypotheses [,,]. Evidence indicates that intergenic regions can serve as homologous characters for genus-level classification and frequently form stem-loop or hairpin secondary structures [,]. These hairpin motifs are widely distributed in the intergenic spacers between mitochondrial PCGs of diverse metazoan taxa, including insects [,,]. Guan et al. were the first to identify long hairpin structures located between the ND1 and 16S rRNA genes in the subfamilies Petrobiinae and Machilinae, revealing a strong correlation between their spatial arrangement and phylogenetic relationships []. These discoveries highlight the functional and evolutionary significance of mitochondrial genome architecture.
In recent years, research on phylogenetic relationships has expanded in both scope and analytical rigor. The majority of phylogenetic studies have consistently supported the monophyly of Hexapoda and Archaeognatha [,,,,,]. However, the internal classification system of Archaeognatha remains complex and continues to be a subject of active scientific debate. Ma et al. demonstrated that both Machilidae and its subfamily Machilinae are paraphyletic, whereas Petrobiellinae forms a monophyletic clade together with Meinertellidae. This finding challenges the previous morphological hypothesis of monophyly and provides critical molecular evidence for revising the phylogenetic framework of Archaeognatha []. Zhang et al. confirmed the monophyly of Meinertellidae through an integrative analysis of morphological and DNA data from 15 Burmese amber specimens []. Montagna subsequently expanded the analysis by incorporating two Triassic fossils and species from Zygentoma, further supporting for the monophyly of Meinertellidae and the paraphyly of the broadly defined Machilidae []. Cen et al. built upon previous work by including sequence data from six Pedetontus species, yielding preliminary support for the monophyly of this genus and revealing its sister-group relationship with Pedetontinus []. Palacios-Martinez et al. conducted a Bayesian phylogenetic analysis integrating COI, 18S rDNA, and ITS2 sequence data across multiple loci, demonstrating that Machilidae and its two subfamilies—Machilinae and Petrobiinae—are non-monophyletic []. Collectively, these findings underscore the need for an integrative taxonomic revision that incorporates multi-locus and multidisciplinary evidence to resolve systematic inconsistencies and clarify cladogenetic patterns within this lineage.
Fossil-calibrated molecular clocks have become a widely accepted and effective method for reconstructing the deep phylogeny of insects [,,,]. However, the evolutionary history of Archaeognatha remains incompletely resolved due to an incomplete and sparse fossil record []. While the earliest potential representatives of Archaeognatha are dated to the Devonian period [,], definitive crown-group fossils have been found exclusively in Late Carboniferous to Early Permian strata from France and North America, supporting the hypothesis that the order originated during the Late Carboniferous [,]. Molecular evidence from Misof et al. suggests that the divergence of Archaeognatha from other insect lineages began approximately 479 million years ago (Mya) []. Zhang et al. further demonstrated, through a comprehensive phylogenetic framework combining fossil morphology with biomolecular evidence from Burmese amber specimens, that the two major families within Archaeognatha diverged well before the Cretaceous []. Notably, Montagna inferred that the extant family Machilidae was already present during the Middle Triassic—approximately 100 million years earlier than previous estimates []. More recently, Cen et al. reported that Machilidae originated in the Middle Triassic (approximately 238.46 Mya), Meinertellidae diverged approximately 127.76 Mya during the Early Cretaceous, and the crown group of Archaeognatha emerged approximately 301.19 Mya during the Late Carboniferous, followed by a prolonged phase of diversification throughout the Mesozoic era []. Despite these advances, the evolutionary history of Archaeognatha remains incompletely resolved, underscoring the importance of integrative analyses that combine fossil evidence with molecular datasets to more accurately reconstruct its diversification dynamics.
Although the prevailing view holds that the mitochondrial genome evolves under neutral or nearly neutral conditions [], accumulating evidence suggests that mitochondrial protein-coding genes (PCGs) associated with environmental adaptation exhibit signatures of significant positive selection [,,]. Given their essential role in energy metabolism during responses to environmental stressors, mitochondrial genomes provide a critical framework for investigating mechanisms of positive selection and adaptive evolution, particularly under conditions of strong natural selection. Xu et al. conducted a positive selection analysis on Heptageniidae taxa inhabiting cold environments and identified 27 positively selected sites across eight protein-coding genes []. Feng et al. performed a similar analysis on Chionea in cold environments and detected significant signals of positive selection in the mitochondrial genes COIII, ND6, and ND5, suggesting that the positive selection of these three genes may represent a key adaptive mechanism enabling Chionea species to survive in low-temperature habitats []. Faddeeva et al. examined selective pressures across Hexapoda lineages and found that 250 genes exhibited significant positive selection, indicating a potential role for these genes in lineage divergence []. Collectively, these studies underscore the importance of comparative mitochondrial genomic analyses across diverse taxa for understanding the functional dynamics of positive selection in adaptation to extreme environments and in shaping evolutionary trajectories.
Building upon the current state of research, this study aims to achieve the following objectives: (1) to compare the mitochondrial genomes of 14 species representing the genera Pedetontus and Pedetontinus; (2) to reconstruct phylogenetic topology and estimate divergence times within Archaeognatha; and (3) to examine whether the mitochondrial genome of Pd. silvestrii from temperate region has undergone positive selection during cold adaptation and whether similar selection signals are present in Pedetontus species from the tropical region in response to high-temperature adaptation.
2. Materials and Methods
2.1. Specimen Acquisition and Genomic DNA Extraction
Detailed sample information is presented in Table 1. For simplicity, the generic name Pedetontus is abbreviated as Pd. and Pedetontinus is abbreviated as Pn. throughout the text. Following sample collection, morphological examination was conducted on fourteen specimens utilizing a Nikon SMZ-1500 stereoscopic microscope (Nikon, Tokyo, Japan). Based on diagnostic morphological characters from established taxonomic references [,,], five species were tentatively assigned to the genus Pedetontinus, and nine species to the genus Pedetontus. After species identification, all tissue samples were preserved in absolute ethanol (−20 °C) and stored under controlled conditions at the Laboratory of Evolution and Molecular Ecology, Zhejiang Normal University. Genomic DNA was extracted from muscle tissues using the Ezup Column Animal Genomic DNA Purification Kit (Sangon Biotech, Shanghai, China), adhering strictly to the manufacturer’s operational guidelines.

Table 1.
Detailed Sample Information for the Fourteen Specimens.
2.2. Sequencing and Assembly of the Mitochondrial Genome
Polymerase chain reaction (PCR) amplification of the COI gene fragment was performed using the universal insect primers LCO1490 and HCO2198 following genomic DNA extraction []. Species identification was conducted through sequence alignment against the NCBI BLAST database (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 30 April 2025). The complete mitochondrial genome was amplified using 13 pairs of species-specific primers designed by Zhang et al. [], combined with Sanger sequencing technology. All PCR products were purified via gel electrophoresis prior to bidirectional sequencing (Sangon Biotech, Shanghai, China). Sequence assembly and verification were performed using the SeqMan Pro module within DNASTAR v6.0 software [] to ensure high data accuracy.
2.3. Sequence Annotation and Analyses
tRNA identification and annotation were conducted using MITOS2 (https://usegalaxy.eu/, accessed on 15 May 2025) []. Comparative analyses of the 12S/16S rRNA and 13 PCGs were conducted based on multiple sequence alignments generated by Clustal W [,]. Genetic distances were calculated utilizing the Kimura 2-parameter model in MEGA version 11 []. PhyloSuite v.1.2.3 [] was utilized to compute AT content and assess relative synonymous codon usage across the fourteen mitochondrial genomes. The complete mitochondrial genomes (GenBank accession numbers: PX391406-PX391419) were visualized using CG View Server V1.0 (http://cgview.ca/, accessed on 18 May 2025) [], and the resulting figures were subsequently refined with Adobe Illustrator 2021 []. RNA secondary structure prediction tools were employed to detect potential hairpin motifs (http://rna.tbi.univie.ac.at/, accessed on 20 May 2025) []. Nucleotide composition bias was quantified using standard deviation-based formulas: AT bias = (A − T)/(A + T) and GC bias = (G − C)/(G + C) [].
2.4. Phylogenetic Analyses
To comprehensively elucidate the evolutionary relationships within Archaeognatha, this study integrated 14 newly sequenced mitochondrial genomes with 20 previously published mitochondrial genomes retrieved from the NCBI database [,,,,,,,,], along with two mitochondrial genomes from Collembola [] used as outgroups. The final dataset comprises a total of 36 species (Table S1), including one representative from Meinertellidae, 33 species from Machilidae and the selected outgroup taxa: Podura aquatica (NC_006075) and Onychiurus orientalis (NC_006074).
A standardized phylogenetic analysis pipeline was applied to the 13 PCGs using PhyloSuite v1.2.3 []. The workflow consisted of three main steps: (1) multiple sequence alignment using MAFFT v7 []; (2) trimming of highly variable regions using Gblocks v0.91b [] to improve alignment accuracy; and (3) sequence assembly using integrated software tools [].
Codon saturation was evaluated using DAMBE v7.3.11 [], confirming the absence of substitution saturation, particularly at the third codon position. Phylogenetic reconstruction was conducted using both Bayesian inference (BI) and maximum likelihood (ML), based on nucleotide sequences from all three codon positions across the 13 PCGs [,]. Model selection was performed in PartitionFinder v2.2.1 [] under the Bayesian Information Criterion (BIC) [], resulting in an optimal data partitioning scheme and corresponding substitution models, as detailed in Table 2. Phylogenetic analyses was performed using two independent approaches: (1) maximum likelihood analysis implemented in IQ-TREE v2.0 [], with 1000 ultrafast bootstrap replicates used to assess nodal support; and (2) Bayesian inference conducted in MrBayes v3.2 [], involving 10 million generations of MCMC sampling, with the first 25% of samples discarded as burn-in to minimize initial state bias. The phylogenetic trees were visualized using FigTree v1.4 [] and further refined in Adobe Illustrator [] to enhance clarity and overall presentation quality.

Table 2.
The best partitioning strategy for phylogenetic analyses, along with the associated alternative models, detailing the model names and the site classifications allocated to each partition.
2.5. Estimation of Divergence Times
The selection of fossil calibration points is a crucial step in divergence times estimation [,,]. To establish robust temporal references, we integrated data from peer-reviewed literature and the Paleobiology Database (https://paleobiodb.org/, accessed on 16 June 2025). According to the insect phylogeny proposed by Misof et al. [], the Archaeognatha-Collembola cladogenic split was dated to approximately 479 million years ago (Mya) during the Early Ordovician radiation, which was implemented as a prior constraint for the root node age in our analysis. Four fossil calibration points were selected for this study: (1) Dasyleptus lucasi Brongniart, 1885 (order Archaeognatha), dated to approximately 298.9–303.7 Mya []. (2) Machilis acuminata (family Machilidae) dated to approximately 33.9–38.0 Mya []. (3) Gigamachilis triassicus (family Machilidae), dated to approximately 235–242 Mya []. (4) Cretaceomachilis libanensis (family Meinertellidae), dated to approximately 125.45–130.00 Mya []. Divergence times were estimated using MCMCTree within the PAML v4.8 software package [], based on the BI phylogenetic tree topology. First, the nucleotide substitution rate was calculated using Baseml, and the gradient of branch lengths along with the associated Hessian matrix was obtained under a Bayesian framework. Subsequently, species divergence times were inferred using the approximate likelihood calculation method (usedata = 2). In the MCMCTree analysis, the following parameters were applied: the burn-in period of 1,000,000 iterations, with sampling every 1000 generations, resulting in the collection of 100,000 effective samples for downstream analyses. Optimization of MCMC chain convergence and mixing was verified using Tracer v1.7.1 []. All model parameters were assessed for effective sample size (ESS), showing that ESS values exceeded the recommended threshold of 200 for each parameter, confirming sufficient sampling efficiency and reliability of the estimates []. Estimated divergence time estimates across phylogenetic branches were visualized using FigTree v1.4 [], and the resulting figures were further refined in Adobe Illustrator [] to improve clarity and presentation quality.
2.6. Selection Pressure Analyses
The EasyCodeML v1.41 software [] was employed to assess selective pressures acting on PCGs within the mitochondrial genomes of Archaeognatha. To investigate molecular evolutionary patterns of PCGs under different thermal regimes, populations of Pd. silvestrii from temperate regions (In proximity to 40° N), characterized by average winter temperatures below 0 °C, were designated as foreground branches representing the temperate group. In contrast, Pd. hainanensis and Pd. bawanglingensis from tropical regions (near 19° N), which experience average summer temperatures exceed 30 °C, were assigned as foreground branches representing the tropical regions group. Selection pressure analyses were carried out using three types of codon models—branch models, branch-site models, and clade models—to detect signatures of selection on specific lineages and sites. The branch model is employed to identify lineage-specific variations in evolutionary rates []. The branch-site model enables comparative analysis between a null model (Model Anull), which assumes only neutral and purifying selection, and an alternative model (Model A), thereby enabling the detection of positive selection on predefined foreground branches []. The clade model framework facilitates the identification of divergent selection pressures across distinct evolutionary lineages []. Additionally, a site model was employed to evaluate overall selective pressures without specifying foreground branches []. Model comparisons were conducted using likelihood ratio tests (LRTs) []. For statistical validation of adaptive evolution signals, the Bayesian empirical Bayes (BEB) approach was applied to estimate posterior probabilities at individual sites, with a threshold of ≥0.95 used to ensure reliable inference [].
3. Results
3.1. Composition of Mitogenomes
All 14 mitochondrial genomes possess a double-stranded DNA structure, ranging in size from 14,625 bp (Pn. jinzhaiensis) to 15,808 bp (Pd. bawanglingensis) (Table S1; Figure S1). The control region could not be amplified in Pn. jinzhaiensis, and both the control region and trnI were not successfully amplified in Pd. formosa. Mitochondrial gene lengths, AT content and other relevant information are summarized in Table S2. The initiation and termination codons for each species are detailed in Table S3. The initiation codons are predominantly of the ATN type, however, GTG is also utilized as an initiation codon in several cases, such as in COII and ND2. With regard to stop codons, most PCGs utilize canonical termination signals (TAA or TAG). Incomplete stop codons (T or TA) were detected in COI, COII, COIII, ND5, and ND4. Relative synonymous codon usage (RSCU) is shown in Figure S2, and the corresponding codon usage frequencies are provided in Table S4. Codon usage analysis reveals that UUU, AUU, AUA, and UUA are the most frequently used codons, each occurring more than 200 times. In contrast, codons with G or C at the third nucleotide position exhibit lower usage frequencies. Notably, Long hairpin structures were identified between the ND1 and 16S rRNA genes across the mitochondrial genomes of 14 species within Pedetontus and Pedetontinus. These structures range in length from 42 to 76 bp, with an average of approximately 60 bp (Figure S3).
3.2. Genetic and Intergroup Genetic Distance Analyses
Genetic distances between Pedetontinus and Pedetontus species, based on the COI gene, are summarized in Table S5. Overall genetic divergences range from 1.5% to 23%, with a mean value of 16.98%. Intraspecific genetic divergence within Pedetontinus ranges from 7.4% to 14.2%. However, genetic divergence within Pedetontus is comparatively higher. Genetic divergences between Pedetontus from the southern China and those from the Northeast region all exceed 15%. The pairwise genetic distances between Pd. formosa and Pd. zhoui (collected from Taiwan and Fujian, respectively) and Pedetontus from Zhejiang (excluding Pd. cixiensis) ranged from 11.5% to 13.1%. Genetic divergences between Pd. hainanensis and other Pedetontus species are all consistently high, exceeding 20%. Similarly, genetic distances between Pd. bawanglingensis and other Pedetontus species also exceed 20%. Furthermore, the genetic divergence between Pd. hainanensis and Pd. bawanglingensis is 16.1%. Additionally, genetic distances between Pd. cixiensis and other Pedetontus species range from 17.2% to 21.5%.
Based on the genetic distance analysis, Pd. cixiensis, Pd. hainanensis, and Pd. bawanglingensis exhibited substantial genetic divergence from other species within the same genus. To further assess the intergeneric variation, a stratified genetic distance analysis was conducted. Taxa were reclassified into distinct groups based on two criteria: genus-level taxonomy and geographical distribution. Specifically, species belonging to the same genus and originating from the same geographic region were assigned to the same group, whereas those from different genera or different geographical regions were placed in separate groups. The grouping scheme is as follows: Group 1 included five samples of the genus Pedetontinus and its reference sequence (KJ754502); Group 2 consisted of Pd. hainanensis and Pd. bawanglingensis; Group 3 comprised Pd. cixiensis; Group 4 included six samples of Pedetontus from southern regions of China (excluding Pd. cixiensis, Pd. hainanensis and Pd. bawanglingensis) along with its reference sequence (NC_051491); and Group 5 contained six samples of Pedetontus silvestrii from northeast China and its reference sequence (NC_011717). Genetic distances among these groups were subsequently calculated, and the results are summarized in Table S6.
Following the inter-group genetic distance analysis of the five defined groups, the samples were further categorized into two major genera: Pedetontus and Pedetontinus, and the inter-generic genetic distance between these two genera was calculated. The results revealed that the genetic distance between Group 2 (Pd. hainanensis and Pd. bawanglingensis) and Group 3, 4 and 5 (other Pedetontus groups) all exceeded 20%. In contrast, the genetic divergence between the genera Pedetontus (excluding Pd. hainanensis and Pd. bawanglingensis) and Pedetontinus was 19.8%.
3.3. Phylogenetic Analyses of Archaeognatha
Phylogenetic reconstruction was conducted using both BI and ML approaches, yielding congruent topological structures (Figure 1). The monophyly of Archaeognatha was strongly supported. However, the phylogenetic analyses did not support the monophyly of the family Machilidae, as species previously assigned to the subfamily Petrobiellinae were recovered within Meinertellidae and formed a closely related sister group to this family. Furthermore, the monophyly of the subfamily Machilinae could not be confirmed, as Trigoniophthalmus alternatus, a species taxonomically classified within Machilinae, was positioned outside the clade containing other representative species from the same subfamily.
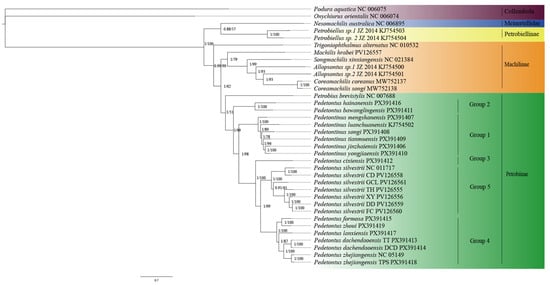
Figure 1.
Phylogenetic relationships within Archaeognatha inferred through the analysis of 13 mitochondrial PCGs using ML and BI methods. Bootstrap values on the right side correspond to each branch, with posterior probabilities on the left.
The phylogenetic tree generated in this study reveals that the genus Pedetontinus included in this analysis forms a robustly supported monophyletic clade, whereas the genus Pedetontus sampled here is not monophyly. Specifically, all sampled individuals of Pd. silvestrii form a distinct and well-supported clade, and the majority of Pedetontus specimens collected from Zhejiang (excluding Pd. cixiensis) also cluster within a separate monophyletic lineage. Pd. formosa and Pd. zhoui from South China formed a distinct clade that is clearly separated from the Zhejiang-derived Pedetontus samples, representing an independent evolutionary lineage. Phylogenetic analysis indicates that Pd. formosa and Pd. zhoui form a sister group to most of the Pedetontus samples from Zhejiang (except Pd. cixiensis), while Pd. silvestrii is recovered as the sister lineage to this clade. Furthermore, Pd. hainanensis and Pd. bawanglingensis are resolved outside the main clade comprising Pedetontus and Pedetontinus, occupying a relatively basal position, suggesting that these two taxa may represent early-diverging lineages within the subfamily.
3.4. Divergence Time Calculation
The chronogram, inferred from the BI phylogenetic tree, is presented in Figure 2. Phylogenetic analysis of Archaeognatha based on 13 mitochondrial PCGs reveals that the order originated during the Late Carboniferous period (301.15 Mya, 95% HPD: 298.88–303.67 Mya). Trigoniophthalmus alternatus, a member of the subfamily Machilinae, represents the earliest divergence from the main lineage, occurring in the Middle Triassic period (238.45 Mya, 95% HPD: 235–241.99 Mya). The split between the subfamilies Machilinae and Petrobiinae took place during the Jurassic period (140.64 Mya, 95% HPD: 94.65–222.39 Mya). Pedetontus hainanensis and Pd. bawanglingensis diverged from the main clade comprising the genera Pedetontus and Pedetontinus during the Early Cretaceous (106.93 Mya, 95% HPD: 70.46–170.92 Mya). The divergence between the genera Pedetontus and Pedetontinus occurred in the same geological period (92.1 Mya, 95% HPD: 60.61–145.97 Mya). Pedetontus cixiensis separated from the remaining members of the genus Pedetontus during the Late Cretaceous (73.69 Mya, 95% HPD: 46.24–114.85 Mya), followed by the divergence of Pd. silvestrii and Pedetontus populations from Zhejiang, Fujian, and Taiwan within the same epoch (62.06 Mya, 95% HPD: 38.68–98.34 Mya).
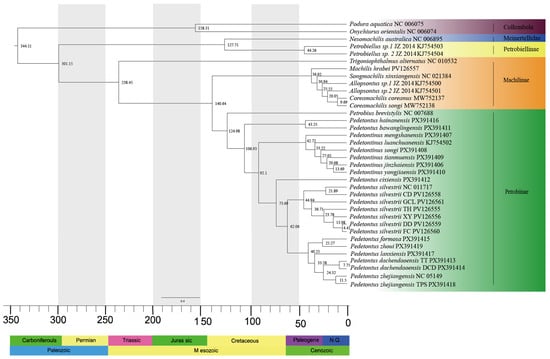
Figure 2.
Time-calibrated phylogenetic tree of Archaeognatha inferred using the MCMCTree algorithm. Median divergence times are indicated at corresponding nodes. Stratigraphic age data are presented along the lower margin of the figure.
Moreover, our findings indicate that Pedetontus diverged earlier than Pedetontinus. The most recent common ancestor (MRCA) of Pedetontus originated during the Late Cretaceous (73.69 Mya, 95% HPD: 46.24–114.85 Mya), whereas the MRCA of Pedetontinus appeared in the Eocene (42.72 Mya, 95% HPD: 25.7–81.83 Mya). Divergence time analysis revealed three major diversification events during the Eocene: the origin of the MRCA of Pedetontus silvestrii at 44.94 Mya (95% HPD: 27.94–77.06 Mya), the divergence of the MRCA between the lineage comprising South China (Pd. formosa and Pd. zhoui) and Zhejiang populations (Pd. lanxiensis, Pd. dachendaoensis TT, Pd. dachendaoensis DCD, Pd. zhejiangensis TPS and Pd. zhejiangensis NC 051491) at 40.25 Mya (95% HPD: 26.17–69.29 Mya), and the emergence of the MRCA of Pedetontus from Zhejiang at 33.58 Mya (95% HPD: 21.28–58.81 Mya). The MRCA of Pd. formosa and Pd. zhoui diverged later during the Oligocene (25.27 Mya, 95% HPD: 11.89–46.44 Mya).
3.5. Positive Selection Analysis
The branch-site model was utilized to investigate selective pressures operating at the amino acid sites, based on aligned sequence data from 13 PCGs derived from 34 Archaeognatha species. When Pedetontus silvestrii were designated as the foreground branch, two amino acid sites were identified as potentially under positive selection, with statistically significant support (p < 0.001, BEB value ≥ 0.95), located at the 66th position of the Cytb gene and the 34th position of ATP6 (Table 3). When Pd. hainanensis and Pd. bawanglingensis were used as foreground branches, six amino acid sites were found to be under positive selection, with statistically significant support (p < 0.001, BEB value ≥ 0.95), located at the 622nd position of the Cytb gene, the 499th position of ATP6, and the 623rd, 873rd, 1106th, and 1141st positions of COI (Table 4). Nevertheless, no significant signals of positive selection were detected using the branch model, site model, or evolutionary distinct branch model.

Table 3.
Codon-based branch site modeling identified adaptive evolution signals by assigning Pedetontus silvestrii as the foreground lineage and utilizing remaining Archaeognatha species as reference clades. The symbol “*” indicates that the posterior probability for this site in the BEB analysis is ≥0.95, providing strong statistical evidence that the site may be under positive selection.

Table 4.
Codon-based branch site modeling identified adaptive evolution signals by assigning Pedetontus hainanensis and Pd. bawanglingensis as the foreground lineage and utilizing remaining Archaeognatha species as reference clades. The symbol “*” indicates that the posterior probability for this site in the BEB analysis is ≥0.95, providing strong statistical evidence that the site may be under positive selection.
4. Discussion
4.1. Special Structure of Mitogenomes from Pedetontus and Pedetontinus
Analysis revealed a long hairpin structure located between the ND1 and 16S rRNA genes across the 14 mitochondrial genomes of Pedetontus and Pedetontinus, which is consistent with the findings reported by Guan et al. []. Notably, such long hairpin structures have so far been observed exclusively in species belonging to the subfamilies Petrobiinae and Machilinae, but not in those within Petrobiellinae or Meinertellidae []. Therefore, we propose that this structural feature may be phylogenetically informative and could represent a shared derived character (synapomorphy) for Petrobiinae and Machilinae. However, its precise biological function and the underlying mechanism of formation remain unclear and require further investigation.
4.2. Analysis of Phylogenetic Tree Topology
Our phylogenetic reconstruction reveals the non-monophyly of Machilidae, challenging the morphological classification system that has been widely used for this taxon []. This incongruence stems from the closer phylogenetic relationship between the subfamily Petrobiellinae and the family Meinertellidae, rather than their affinities with other groups within Machilidae—a pattern consistent with findings from previous phylogenetic studies [,,]. However, the interpretation of Petrobiellinae’s phylogenetic position requires careful consideration of existing taxonomic uncertainties. As critically evaluated by Kaplin [] and Mtow and Machida [], the sequences of Petrobiellus used in our study and in previous analyses are taxonomically problematic. The sequences generated by Ma et al. may represent misidentified members of Meinertellidae rather than true Petrobiellus []. Therefore, while our analysis recovers the non-monophyly of Machilidae, the precise phylogenetic placement of Petrobiellinae should be interpreted not as a definitive conclusion but as a provisional hypothesis awaiting validation through future studies based on taxonomically verified voucher specimens.
Notably, Petrobiellus from Petrobiellinae exhibits morphological similarities to Petrobius from Petrobiinae, particularly in the underdeveloped distal jaw teeth with fused dental boundaries []. These morphological parallels may reflect either shared ancestry or convergent evolution [,]. However, molecular phylogenetic analyses indicate that these taxa are placed in distinct clades, with the two Petrobiellus species forming a monophyletic clade together with Nesomachilis australica from the family Meinertellidae. This finding, consistent with the observations of Kaplin [] and Mtow and Machida [], further suggests that there may be potential taxonomic inconsistencies or misidentifications within Petrobiellus, which warrant further investigation.
Although the monophyly of Meinertellidae has been supported by the studies of Zhang et al. and Montagna [,], only a single complete mitochondrial genome sequence from this family is currently available in the NCBI database []. Consequently, the present study included only one representative species from Meinertellidae, which limits the ability to comprehensively assess and validate the monophyletic status of this taxon. Nevertheless, several morphological characteristics provide evidence in support of the monophyly of Meinertellidae, including the absence of antennal scales, the lack of accessory reproductive structures, and the reduction in the size of abdominal plates [].
Phylogenetic analyses further support the paraphyly of Machilinae, as evidenced by the placement of T. alternatus within Petrobiinae rather than among other Machilinae species. This finding aligns with the results reported by Guan et al. and Ma et al. [,]. Additionally, it has been demonstrated that the traditional classification of Trigoniophthalmus, which relies on ancestral morphological traits, has limited taxonomic accuracy and leads to inconsistencies between phylogenetic and morphological classifications, as previously noted by Guan et al. []. Therefore, we recommend that the classification of the genus Trigoniophthalmus be reevaluated using an integrative approach that combines phylogenetic data with morphological evidence.
Within the phylogenetic framework, Pedetontinus as sampled here is robustly supported as a monophyletic group, in contrast to the paraphyletic structure observed in Pedetontus as represented here. This non-monophyletic pattern conflicts with the results of previous phylogenetic studies by Shen et al. and Cen et al., which inferred a monophyletic origin for Pedetontus [,]. We propose that this discrepancy may stem from the substantially expanded taxon sampling and broader geographical representation in our study, which includes specimens from multiple regions across southern, eastern, northern, and northeastern China. With regard to Pd. silvestrii, the sampling methodology employed in this study was consistent with that of Cen et al., and the topology of the reconstructed phylogenetic tree showed a high level of agreement with their results []. Notably, Pd. cixiensis collected from Cixi, Zhejiang Province, does not group within other specimens from Zhejiang but instead diverges outside the entire Pedetontus crown group, forming a distinct and elongated branch. Furthermore, the genetic distance between the COI gene of Pd. cixiensis and those of other Pedetontus specimens from Zhejiang exceeds 17%, indicating that this lineage may represent an early divergence event, which merits further investigation.
4.3. Divergence Time Estimation and Evolutionary Node Calibration
Divergence time estimation indicates that the family Machilidae originated during the Middle Triassic period (238.45 Mya, 95% HPD: 235–241.99 Mya), a result consistent with the findings reported by Montagna []. The divergence between the family Meinertellidae and the subfamily Petrobiellinae occurred during the Early Cretaceous (127.71 Mya, 95% HPD: 125.45–130 Mya), a timeframe that aligns with the fossil record of Cretaceomachilis libanensis, discovered in Early Cretaceous strata of Lebanon []. The earlier divergence of Machilidae relative to Meinertellidae is supported by morphological features, including the ovipositor, aedeagus, paragenitalia, coxal sac, and tarsus [,]. Moreover, the divergence time tree constructed by Cen et al. also supports this temporal pattern of diversification []. According to our analysis, the most recent common ancestor (MRCA) of Machilidae and Meinertellidae dates to the Early Cretaceous (127.71 Mya, 95% HPD: 125.45–130 Mya), which is notably later than previous estimates that placed this divergence at the Jurassic-Cretaceous boundary (approximately 146 Mya) [,]. We hypothesize that this discrepancy may stem from insufficient sampling of Meinertellidae in prior analysis, which could have affected the accuracy of the estimated divergence times. Inferred via divergence dating, the subfamilies Machilinae, Petrobiinae, and Petrobiellinae diverged sequentially during the Middle Triassic (approximately 238.45 Mya), Early Cretaceous (approximately 124.98 Mya), and Eocene (approximately 44.26 Mya), respectively. This temporal divergence pattern corresponds to the framework previously proposed by Cen et al. []. Notably, the estimated divergence time of the genus Machilis during the Eocene (36.92 Mya, 95% HPD: 34.33–38.14 Mya) is in close agreement with the fossil record of this genus preserved in Baltic Eocene amber [].
The Mesozoic Era, a critical interval in Earth’s evolutionary history, was characterized by major environmental changes that played a fundamental role in shaping modern insect communities []. Evidence indicates that the substantial rise in atmospheric carbon dioxide levels during the Cretaceous period maintained a prolonged warm climate. Together with the plate tectonic movements driven by the rifting of Gondwana, these factors synergistically promoted the reorganization of global vegetation distribution patterns [,]. Against this climatic and geological background, the adaptive radiation of bryophytes emerged as a pivotal event in ecological evolution during the Cretaceous. Molecular phylogenetic studies indicate that epiphytic moss lineages underwent a phase of rapid diversification, giving rise to a broad spectrum of adaptive strategies []. The intensification of plant-insect coevolutionary interactions during this period further facilitated the diversification of multiple insect groups, including the genus-level differentiation within the order Archaeognatha. During the Cretaceous-Paleogene transition, the rapid diversification of numerous plant, animal, and fungal lineages triggered a major ecological transformation known as the “Cretaceous Terrestrial Revolution”, which drove the evolutionary progression toward more complex and diverse insect-plant symbiotic systems []. Our phylogenetic analysis reveals that Pedetontus and Pedetontinus diverged from a common ancestral lineage in the late Mesozoic, a timeframe that closely aligns with a major phase of reorganization in the global biogeographic patterns of bryophytes []. Collectively, these findings highlight the profound impact of Mesozoic environmental changes on the evolutionary assembly of contemporary insect communities.
The MRCA of Pd. formosa and Pd. zhoui diverged during the late Oligocene (25.27 Mya, 95% HPD: 11.89–46.44 Mya). This molecular dating estimate appears inconsistent with the geological timeline of Taiwan Island’s formation. Geological studies indicate that the fundamental topographic framework of Taiwan Island was established approximately 6.5 Mya, resulting from oblique plate interactions along the Luzon-Eurasian tectonic boundary []. Prior to this island formation, however, the region underwent significant tectonic evolution: continental rifting occurred during the Paleogene (39–50 Mya), followed by the opening of the South China Sea during the Oligocene to Middle Miocene (16–33 Mya) []. These tectonic processes likely contributed to the opening of the Taiwan Strait and induced localized crustal movements, which may have influenced biogeographic patterns and speciation events. Such dynamic geological settings could have provided diverse ecological niches for the ancestral populations of Pd. formosa and Pd. zhoui, thereby facilitating their divergence and adaptive evolutionary trajectories.
4.4. Genetic-Morphological Discrepancy in Pedetontus Taxonomy
Morphological methods have long served as the primary tools for species identification. Nevertheless, evolutionary relationships inferred from these approaches often exhibit substantial inconsistencies when compared with genetic evidence [,,]. Due to the inherent limitations of traditional morphological identification systems in achieving accurate taxonomic classification, there is an increasing need for integrative frameworks that enable the systematic identification, refinement, and validation of diagnostic traits with improved discriminatory power. Phylogenetic and genetic distance analyses of Pedetontus have uncovered substantial taxonomic discrepancies within the genus. Comparative data indicate that the intergeneric genetic distance between Pedetontus and Pedetontinus was 19.7%, whereas the interspecific genetic distances between Group 2 (Pd. hainanensis and Pd. bawanglingensis) and other conspecific species exceeded 20%, ranging from 20.2% to 23.0%. Notably, the level of intrageneric genetic differentiation exceeds that typically observed between genera, providing strong support the hypothesis of a potential misclassification of Pd. hainanensis and Pd. bawanglingensis at the generic level. Phylogenetic reconstruction corroborates this conclusion, showing their phylogenetic divergence from congeneric Pedetontus species. Divergence time estimation further indicates that the lineage containing the Hainan population diverged approximately 106.93 Mya, significantly earlier than the divergence times of other Pedetontus species. Based on the molecular phylogenetic findings presented above, we propose an integrative taxonomic revision of Pedetontus to identify key morphological diagnostic characters suitable for genus-level classification. Furthermore, we recommend the development of a standardized and operationally robust framework for species identification that integrates morphological, molecular phylogenetic, and biogeographic evidence.
4.5. Positive Selection Pressure Analysis
Branch-site model analysis identified one site under positive selection in Cytb and an additional site in ATP6 when Pd. silvestrii was designated as the foreground branch. Cytb, recognized as the major transmembrane component of complex III, is essential for ATP production []. As a core subunit of the mitochondrial respiratory chain, Cytb primarily mediates electron transfer from the substrate side to cytochrome c1. Its specialized energy-transducing form, cytochrome bT, is critically involved in coupling electron transport with proton gradient formation through the inner mitochondrial membrane, thereby serving as a key functional unit in oxidative phosphorylation and cellular energy conservation []. ATP synthase is also a core component of the mitochondrial respiratory chain, utilizes the proton motive force across the inner mitochondrial membrane to catalyze ATP synthesis from ADP and inorganic phosphate []. As a critical subunit of the enzyme, ATP6 plays an essential role in oxidative phosphorylation [].
Additionally, branch-site model analysis with Pd. hainanensis and Pd. bawanglingensis designated as foreground branches identified six positively selected sites: four in COI, one in ATP6, and one in Cytb. Complex IV catalyzes the final step of the electron transport chain by transferring electrons from reduced cytochrome c to oxygen, thereby reducing oxygen and generating a proton gradient []. The COI subunit is essential for initiating complex IV assembly and constitutes one of its catalytic core components [,].
It is noteworthy that in the analysis of positive selection sites under two distinct environmental conditions, both the Cytb and ATP6 genes were identified. This finding indicates that these genes may have been subject to natural selection during both temperate and tropical environmental adaptation processes, suggesting their potential role in a shared adaptive mechanism in response to temperature-related selective pressures. In studies investigating other species’ responses to temperate environments, mitochondrial genes such as Cytb and ATP6 have exhibited significant signals of positive selection. Xu et al. identified 27 positively selected sites in Heptageniidae species in their study on cold adaptation, including two sites in the Cytb gene []. Hong et al. reported five amino acid sites under positive selection in Hylidae species inhabiting cold environments, including residues within the Cytb gene []. Ngatia et al. demonstrated evidence of positive selection acting on six genes—including Cytb and ATP6—in Mammuthus primigenius under cold stress conditions []. Collectively, these findings demonstrate that key mitochondrial genes associated with energy metabolism have undergone adaptive evolution during biological responses to low-temperature environments. Notably, while existing studies on positive selection have predominantly focused on temperate species, the present investigation into the adaptive evolutionary patterns of tropical species provides novel insights.
Overall, the PCGs of Pedetontus are functionally associated with energy metabolism and are subject to positive selection, potentially reflecting adaptive mechanisms that enable the species to meet energy demands and maintain normal physiological functions under temperate and tropical environmental stress.
5. Conclusions
This study involved the analysis of mitogenomes from 14 species representing the genera Pedetontus and Pedetontinus. Phylogenetic assessments employing ML and BI methodologies yielded the following findings: (1) the family Machilidae is non-monophyletic; (2) the subfamily Petrobiellinae and the family Meinertellidae form a single clade, and the subfamily Machilinae is also non-monophyletic; (3) within the subfamily Petrobiinae, Pedetontinus is monophyletic, whereas Pedetontus is non-monophyletic, with Pd. hainanensis and Pd. bawanglingensis diverging early from the main lineage. Divergence time estimation indicates that the diversification of Petrobiinae occurred during the Mesozoic, leading to the emergence of the genera Pedetontus and Pedetontinus, a process potentially driven by the co-evolution between organisms and their environment. By integrating analyses of genetic distance, phylogenetic topology, and estimations of divergence timing, we propose a re-evaluation of the species status of Pd. hainanensis and Pd. bawanglingensis. Future research should aim to identify genus-level morphological diagnostic characters to refine the current taxonomic framework. Selection pressure analysis reveals that the mitochondrial PCGs of Pedetontus have undergone positive selection under both temperate and tropical environmental stress, likely reflecting adaptive responses to altered energy demands.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16121194/s1, Figure S1: Circular genome maps illustrate the mitochondrial genomes of fourteen specimens representing the genera Pedetontus and Pedetontinus. Figure S2: Relative Synonymous Codon Usage (RSCU) patterns in 14 mitochondrial genomes. Figure S3: Inferred hairpin structures of 14 mitogenomes. The circle means the length between the NDI gene and 16S RNA gene. Table S1: Species data employed in the construction of the phylogenetic tree. Table S2: The lengths of mitochondrial genes, AT content, AT skew and GC skew of 14 mitochondrial genomes. Table S3: Location of features of fourteen mitogenomes. Table S4: Comparative analysis of codon usage frequency profiles among 14 mitochondrial genomes. Table S5: Genetic distance analysis of the COI gene among species of Pedetontinus and Pedetontus is included in this study. Table S6: Pairwise genetic distance values among Pedetontus and Pedetontinus groups.
Author Contributions
Conceptualization, J.-Y.Z., D.-N.Y., W.C., K.B.S. and T.Y.; Methodology, W.C., T.Y. and J.-W.L.; Statistical analysis, W.C., T.Y. and J.-W.L.; Investigation, J.-Y.Z. and D.-N.Y.; Data curation, W.C., T.Y., J.-Y.Z., K.B.S. and D.-N.Y.; Writing—original draft preparation, W.C., J.-Y.Z. and D.-N.Y.; Writing—review and editing, W.C., T.Y., J.-Y.Z., K.B.S. and D.-N.Y.; Maps and graphics, W.C., J.-W.L. and T.Y.; Project administration, J.-Y.Z. and D.-N.Y.; funding acquisition, J.-Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (LY23C040002) and by the Natural Science Foundation of China (32470475). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The publication costs of this study were supported by the National Undergraduate Training Program on Innovation and Entrepreneurship (202510345032).
Institutional Review Board Statement
All samples belong to non-protected invertebrate species, so no animal care protocol was needed.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data to support this study are available from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov) (accessed on 25 July 2025). The GenBank numbers are PX391406-PX391419.
Acknowledgments
We gratefully acknowledge Jie-Hong Ji and Chen-Yang Shen for their invaluable assistance in sample acquisition. We also thank Chen-Yang Shen for providing essential morphological literature. Additionally, we are deeply grateful to three reviewers for their insightful comments and constructive feedback on this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Palacios–Martinez, I.; Jiménez-Ruiz, Y.; Otero-Ferre, P.; García-París, M. Systematics of the genus Dilta Strand, 1911 (Machilidae) in the Canary Islands (NW Africa, Spain) with comments on the phylogeny of Microcoryphia (Insecta). Zool. Anz. 2025, 317, 1–18. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Shih, C.; Zhang, A.; Ren, D. Phylogenetic analyses with four new Cretaceous bristletails reveal inter-relationships of Archaeognatha and Gondwana origin of Meinertellidae. Cladistics 2018, 34, 384–406. [Google Scholar] [CrossRef]
- Ma, Y.; He, K.; Yu, P.P.; Yu, D.N.; Cheng, X.F.; Zhang, J.Y. The complete mitochondrial genomes of three bristletails (Insecta: Archaeognatha): The paraphyly of Machilidae and insights into Archaeognathan phylogeny. PLoS ONE 2015, 10, e0117669. [Google Scholar] [CrossRef]
- Sturm, H.; Bach, d.R.C. On the systematics of the Archaeognatha (Insecta). Entomol. Gen. 1993, 18, 55–90. [Google Scholar] [CrossRef]
- Koch, M. Character evolution in the Archaeognatha: Consensus and conflict. Entomol. Abh. 2003, 61, 120–122. [Google Scholar]
- Mendes, L.F.; Foottit, R.; Adler, P. Biodiversity of the thysanurans (Microcoryphia and Zygentoma). Insect Biodivers. Sci. Soc. 2018, 2, 155–198. [Google Scholar]
- Matushkina, N.A.; Klass, K.-D. Male genitalia of Charimachilis (Insecta: Archaeognatha) and the status of archaeognathan “paleoforms”. Org. Divers. Evol. 2020, 20, 253–266. [Google Scholar] [CrossRef]
- Klass, K.; Matushkina, N. The exoskeleton of the male genitalic region in Archaeognatha, with hypotheses on the early evolution and the morphological interpretation of genitalia in insects. Arthropod Syst. Phylogeny 2018, 76, 235–294. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Song, D.X.; Zhou, K.Y. A new species of the genus Pedetontinus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sin. 2005, 30, 549–554. [Google Scholar]
- Yu, D.N.; Zhang, W.W.; Zhang, J.Y. Two new species of the genus Pedetontus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sin. 2010, 35, 444–450. [Google Scholar]
- Xue, L.; Yin, W. Two new species of Machilidae from the Tianmu Mountain, China (Microcoryphia). Contrib. Shanghai Inst. Entomol. 1991, 10, 77–86. [Google Scholar]
- Zhang, J. A new record genus and species of Machilinae from China (Microcoryphia: Machilidae). Jiangxi Plant Prot. 2010, 33, 57–59. [Google Scholar]
- Uchida, H. Pedetontus from Formosa: Thysanura: Machilidae. Spec. Bull. Lepidopterol. Soc. Jpn. 1965, 1, 249–252. [Google Scholar]
- Zhang, J.Y.; Li, T. A new bristletail species of the genus Pedetontinus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sin. 2009, 34, 203–206. [Google Scholar]
- Zhang, J.; Zhou, K. Descriptions of one new genus and six new species of Machilidae (Insecta: Archaeognatha) from China: Morphological and molecular data. J. Nat. Hist. 2011, 45, 1131–1164. [Google Scholar] [CrossRef]
- Song, Z.S. Machilontus (s. str.) medogensis Song & Huang, sp. nov. from Tibet, the northernmost record of the genus Machilontus Silvestri, 1912 and the first record of the family Meinertellidae (Insecta: Microcoryphia: Machiloidea) in China. Zootaxa 2011, 2822, 61–68. [Google Scholar] [CrossRef]
- Silvestri, F. Note sui Machilidae. III. Descrizione di un nuovo genere e di sei nuove specie. Redia 1906, 3, 325–335. [Google Scholar]
- Silvestri, F. Schwedisch-chinesiche wissenschaftliche Expedition nach den nordwestlichen Provinzen Chinas (38. Thysanura, Machilidae). Ark. Zool. 1934, 27, 1–7. [Google Scholar]
- Silvestri, F. Descripzione di alcuni Machilidae (Thysanura) della China. Notes Entomol. Chin. Du Mus. Heude Shanghai 1936, 3, 103–115. [Google Scholar]
- Silvestri, F. Contributto alla conoscenza dei Machilidae (Insecta, Thysanura) del Giappone. Bollettino del Laboratorio di Zoologia, generale e agraria della R. Sc. Super. D’agricoltura Portici 1943, 32, 283–306. [Google Scholar]
- Mendes, L.F.; Gaju-Ricart, M.; de Roca, C.B.; Molero-Baltanás, R. On some Silvestri species of Machilidae (Microcoryphia, Insecta) which types are in the Muséum National d’Histoire Naturelle, Paris. Pedobiologia 2000, 44, 268–284. [Google Scholar] [CrossRef]
- Li, P.; Yu, D.N.; Zhang, J.Y. One new species of the genus Pedetontinus (Microcoryphia, Machilidae) from China with morphological and molecular data. Acta Zootaxon Sin. 2012, 37, 740–746. [Google Scholar]
- Kaplin, V. Description of a new species of the bristletail genus Pedetontinus Silv. (Thysanura, Machilidae) from China with a review of species of this genus. Entomol. Rev. 2015, 95, 73–86. [Google Scholar] [CrossRef]
- Kaplin, V. A new species of the genus Allopsontus Silv. (Microcoryphia, Machilidae) from northwestern china. Entomol. Rev. 2016, 96, 126–131. [Google Scholar] [CrossRef]
- Kaplin, V. A new species of bristletails of the genus Silvestrichilis Wygodzinsky, 1950 (Archaeognatha: Machilidae) from South China. Far East. Entomol. 2019, 376, 23–28. [Google Scholar] [CrossRef]
- Huang, F.S.; Song, Z.S.; Liang, A.P. A new bristletail species of the genus Allopsontus Silvestri (Microcoryphia: Machilidae) from Shaanxi, China. Orient. Insects 2006, 40, 267–272. [Google Scholar] [CrossRef]
- Deng, K.Z.; Zhang, J.Y.; Yu, D.N. A new species of the genus Haslundichilis (Microcoryphia, Machilidae) from China and redescription of Haslundichilis hedini (Silvestri). Acta Zootaxonomica Sin. 2011, 36, 882–887. [Google Scholar]
- Shen, C.Y.; Wang, L.Y.; Wu, H.Y.; Zhang, J.Y. A new species of Silvestrichilis Wygodzinsky, 1950 (Insecta: Microcoryphia) from Wudang Mountain, Hubei, China, with the description of both sexes. Zootaxa 2025, 5621, 437–452. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yu, D.N.; Zhang, J.Y. One new species of the genus Pedetontinus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sin. 2011, 36, 36–39. [Google Scholar]
- Shen, C.Y.; Yang, T.; Ji, J.H.; Zhang, J.Y. Six New Species of Genus Pedetontus Silvestri, 1911 (Microcoryphia: Machilidae), from Southern China. Insects 2025, 16, 916. [Google Scholar] [CrossRef] [PubMed]
- Kaplin, V. On the classification and phylogeny of the Machilidae (Thysanura, Microcoryphia). Entomol. Rev. 1985, 64, 117–131. [Google Scholar]
- Ji, J.H.; Wu, H.Y.; Gao, Y.X.; Shen, C.Y.; Yang, Z.W.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. Unusual genetic diversity within Thereuopoda clunifera (Wood, 1862) (Chilopoda: Scutigeromorpha) revealed by phylogeny and divergence times using mitochondrial genomes. Insects 2025, 16, 486. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, L.; Ayivi, S.P.G.; Storey, K.B.; Ma, Y.; Yu, D.N.; Zhang, J.Y. Cryptic species exist in Vietnamella sinensis Hsu, 1936 (Insecta: Ephemeroptera) from studies of complete mitochondrial genomes. Insects 2022, 13, 412. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Shen, C.Y.; Cheng, H.Y.; Chen, Y.X.; Wu, H.Y.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. Mitogenome-Based phylogeny with divergence time estimates revealed the presence of cryptic species within Heptageniidae (Insecta, Ephemeroptera). Insects 2024, 15, 745. [Google Scholar] [CrossRef]
- Ye, F.; Lan, X.E.; Zhu, W.B.; You, P. Mitochondrial genomes of praying mantises (Dictyoptera, Mantodea): Rearrangement, duplication, and reassignment of tRNA genes. Sci. Rep. 2016, 6, 25634. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.Y.; Shen, S.Q.; Zhang, Z.Y.; Xu, X.D.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. Comparative mitogenomes of two Coreamachilis species (Microcoryphia: Machilidae) along with phylogenetic analyses of Microcoryphia. Insects 2021, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Lee, E.M.; Jo, Y.H.; Park, H.C.; Kim, S.R.; Hwang, J.S.; Jin, B.R.; Kang, P.D.; Kim, K.-G.; Han, Y.S. Complete nucleotide sequence and organization of the mitogenome of the silk moth Caligula boisduvalii (Lepidoptera: Saturniidae) and comparison with other lepidopteran insects. Gene 2008, 413, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.; Cameron, S.; Whiting, M. The complete mitochondrial genome sequence of the Mormon cricket (Anabrus simplex: Tettigoniidae: Orthoptera) and an analysis of control region variability. Insect Mol. Biol. 2007, 16, 239–252. [Google Scholar] [CrossRef]
- Clary, D.O.; Wolstenholme, D.R. The mitochondrial DNA molecule of Drosophila yakuba: Nucleotide sequence, gene organization, and genetic code. J. Mol. Evol. 1985, 22, 252–271. [Google Scholar] [CrossRef]
- Regier, J.C.; Shultz, J.W.; Ganley, A.R.; Hussey, A.; Shi, D.; Ball, B.; Zwick, A.; Stajich, J.E.; Cummings, M.P.; Martin, J.W.; et al. Resolving arthropod phylogeny: Exploring phylogenetic signal within 41 kb of protein-coding nuclear gene sequence. Syst. Biol. 2008, 57, 920–938. [Google Scholar] [CrossRef]
- Regier, J.C.; Shultz, J.W.; Zwick, A.; Hussey, A.; Ball, B.; Wetzer, R.; Martin, J.W.; Cunningham, C.W. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 2010, 463, 1079–1083. [Google Scholar] [CrossRef]
- Meusemann, K.; von Reumont, B.M.; Simon, S.; Roeding, F.; Strauss, S.; Kück, P.; Ebersberger, I.; Walzl, M.; Pass, G.; Breuers, S.; et al. A phylogenomic approach to resolve the arthropod tree of life. Mol. Biol. Evol. 2010, 27, 2451–2464. [Google Scholar] [CrossRef] [PubMed]
- Von Reumont, B.M.; Meusemann, K.; Szucsich, N.U.; Dell’Ampio, E.; Gowri-Shankar, V.; Bartel, D.; Simon, S.; Letsch, H.O.; Stocsits, R.R.; Luan, Y.X.; et al. Can comprehensive background knowledge be incorporated into substitution models to improve phylogenetic analyses? A case study on major arthropod relationships. BMC Evol. Biol. 2009, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Von Reumont, B.M.; Jenner, R.A.; Wills, M.A.; Dell’Ampio, E.; Pass, G.; Ebersberger, I.; Meyer, B.; Koenemann, S.; Iliffe, T.M.; Stamatakis, A.; et al. Pancrustacean phylogeny in the light of new phylogenomic data: Support for Remipedia as the possible sister group of Hexapoda. Mol. Biol. Evol. 2012, 29, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M. Comment on phylogenetic analyses with four new Cretaceous bristletails reveal inter-relationships of Archaeognatha and Gondwana origin of Meinertellidae. Cladistics 2020, 36, 227–231. [Google Scholar] [CrossRef]
- Cen, W.; Li, J.W.; He, J.T.; Chen, X.Y.; Li, L.Y.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. Morpho-Molecular discordance and cryptic diversity in jumping Bristletails: A mitogenomic analysis of Pedetontus silvestrii (Insecta: Archaeognatha: Machilidae). Insects 2025, 16, 452. [Google Scholar] [CrossRef]
- Marshall, D.C.; Hill, K.B.; Moulds, M.; Vanderpool, D.; Cooley, J.R.; Mohagan, A.B.; Simon, C. Inflation of molecular clock rates and dates: Molecular phylogenetics, biogeography, and diversification of a global cicada radiation from Australasia (Hemiptera: Cicadidae: Cicadettini). Syst. Biol. 2016, 65, 16–34. [Google Scholar] [CrossRef]
- Shear, W.A.; Bonamo, P.M.; Grierson, J.D.; Rolfe, W.I.; Smith, E.L.; Norton, R.A. Early land animals in North America: Evidence from Devonian age arthropods from Gilboa, New York. Science 1984, 224, 492–494. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Beall, B.S.; Hueber, F.M. Early insect diversification: Evidence from a Lower Devonian bristletail from Québec. Science 1988, 242, 913–916. [Google Scholar] [CrossRef]
- Rasnitsyn, A.P.; Aristov, D.S.; Gorochov, A.V.; Rowland, J.M.; Sinitshenkova, N.D. Important new insect fossils from Carrizo Arroyo and the Permo-Carboniferous faunal boundary. N. M. Mus. Nat. Hist. Sci. Bull. 2004, 25, 215–246. [Google Scholar]
- Rasnitsyn, A.P. Taxonomy and morphology of Dasyleptus Brongniart, 1885, with description of a new species (Insecta: Machilida: Dasyleptidae). Russ. Entomol. J. 1999, 8, 145–154. [Google Scholar]
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef]
- Ballard, J.W.O.; Kreitman, M. Is mitochondrial DNA a strictly neutral marker? Trends. Ecol. Evol. 1995, 10, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.T.; Li, Q.; Zhang, J.Y.; Storey, K.B.; Yu, D.N. Characterization of the mitochondrial genomes of two toads, Anaxyrus americanus (Anura: Bufonidae) and Bufotes pewzowi (Anura: Bufonidae), with phylogenetic and selection pressure analyses. PeerJ 2020, 8, e8901. [Google Scholar] [CrossRef]
- Li, X.D.; Jiang, G.F.; Yan, L.Y.; Li, R.; Mu, Y.; Deng, W.A. Positive selection drove the adaptation of mitochondrial genes to the demands of flight and high-altitude environments in grasshoppers. Front. Genet. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Guan, J.Y.; Zhang, Z.Y.; Cao, Y.R.; Cai, Y.Y.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. Insight into the phylogenetic relationships among three subfamilies within Heptageniidae (Insecta: Ephemeroptera) along with low-temperature selection pressure analyses using mitogenomes. Insects 2021, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.F.; Cen, W.; Storey, K.B.; Liu, L.J.; Yu, D.N.; Zhang, J.Y. Comparative mitogenomic analysis of three Chionea species (Tipulomorpha: Limoniidae): Insights into phylogenetic relationships and selection pressure. Insects 2025, 16, 720. [Google Scholar] [CrossRef]
- Faddeeva, A.; Studer, R.; Kraaijeveld, K.; Sie, D.; Ylstra, B.; Mariën, J.; op den Camp, H.; Datema, E.; Den Dunnen, J.T.; Van Straalen, N.; et al. Collembolan transcriptomes highlight molecular evolution of hexapods and provide clues on the adaptation to terrestrial life. PLoS ONE 2015, 10, e0130600. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Zhang, J.Y.; Zhou, C.F.; Gai, Y.H.; Song, D.X.; Zhou, K.Y. The complete mitochondrial genome of Parafronurus youi (Insecta: Ephemeroptera) and phylogenetic position of the Ephemeroptera. Gene 2008, 424, 18–24. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Bioinform. Methods Protoc. 2000, 132, 71–91. [Google Scholar]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Donath, A.; Jühling, F.; Al-Arab, M.; Bernhart, S.H.; Reinhardt, F.; Stadler, P.F.; Middendorf, M.; Bernt, M. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 2019, 47, 10543–10552. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.L.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Grant, J.R.; Stothard, P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36, W181–W184. [Google Scholar] [CrossRef]
- Illustrator, A. Adobe Illustrator. 2021. Available online: https://www.adobe.com/products/illustrator.html#modal-hash (accessed on 2 July 2025).
- Lorenz, R.; Bernhart, S.H.; Höner zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA package 2.0. algorithms. Mol. Biol. 2011, 6, 26. [Google Scholar]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- He, K.; Zhang, J.Y.; Deng, K.Z.; Chen, Z. The complete mitochondrial genome of the bristletail Songmachilis xinxiangensis (Archaeognatha: Machilidae). Mitochondr. DNA 2013, 24, 99–101. [Google Scholar] [CrossRef]
- Cameron, S.L.; Miller, K.B.; D’Haese, C.A.; Whiting, M.F.; Barker, S.C. Mitochondrial genome data alone are not enough to unambiguously resolve the relationships of Entognatha, Insecta and Crustacea sensu lato (Arthropoda). Cladistics 2004, 20, 534–557. [Google Scholar] [CrossRef]
- Carapelli, A.; Liò, P.; Nardi, F.; Van der Wath, E.; Frati, F. Phylogenetic analysis of mitochondrial protein coding genes confirms the reciprocal paraphyly of Hexapoda and Crustacea. BMC Evol. Biol. 2007, 7 (Suppl. S2), S8. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlowski, L. The mitochondrial genome of the bristletail Petrobius brevistylis (Archaeognatha: Machilidae). Insect Mol. Biol. 2006, 15, 253–258. [Google Scholar] [CrossRef]
- Shen, S.Q.; Cai, Y.Y.; Xu, K.K.; Chen, Q.P.; Cao, S.S.; Yu, D.N.; Zhang, J.Y. The complete mitochondrial genome of Pedetontus zhejiangensis (Microcoryphia: Machilidae) and its phylogeny. Mitochondr. DNA B 2020, 5, 3143–3145. [Google Scholar] [CrossRef]
- Cook, C.E.; Yue, Q.; Akam, M. Mitochondrial genomes suggest that hexapods and crustaceans are mutually paraphyletic. Proc. R. Soc. B Biol. Sci. 2005, 272, 1295–1304. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Xia, X.; Xie, Z. DAMBE: Software package for data analysis in molecular biology and evolution. J. Hered. 2001, 92, 371–373. [Google Scholar] [CrossRef]
- Schrago, C.G.; Aguiar, B.O.; Mello, B. Comparative evaluation of maximum parsimony and Bayesian phylogenetic reconstruction using empirical morphological data. J. Evol. Biol. 2018, 31, 1477–1484. [Google Scholar] [CrossRef]
- Reyes-Hernández, J.L.; Hansen, A.K.; Jenkins Shaw, J.; Solodovnikov, A. Phylogeny-based taxonomic revision and niche modelling of the rove beetle genus Loncovilius Germain, 1903 (Coleoptera: Staphylinidae: Staphylininae). Zool. J. Linn. Soc. 2024, 202, zlad143. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Vrieze, S.I. Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol. Methods 2012, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree Version 1.4.4. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 July 2025).
- Near, T.J.; Sanderson, M.J. Assessing the quality of molecular divergence time estimates by fossil calibrations and fossil–based model selection. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2004, 359, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Rannala, B. Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Mol. Biol. Evol. 2006, 23, 212–226. [Google Scholar] [CrossRef]
- Püschel, H.P.; O’Reilly, J.E.; Pisani, D.; Donoghue, P.C. The impact of fossil stratigraphic ranges on tip-calibration, and the accuracy and precision of divergence time estimates. Palaeontology 2020, 63, 67–83. [Google Scholar] [CrossRef]
- Keilbach, R. Bibliographie und Liste der Arten tierischer Einschlüsse in fossilen Harzen sowie ihrer Aufbewahrungsorte. Deut. Entomol. Z. 1982, 29, 129–286. [Google Scholar] [CrossRef]
- Sturm, H.; Poinar Jr, G.O. Cretaceomachilis libanensis, the oldest known bristle-tail of the family Meinertellidae (Machiloidea, Archaeognatha, Insecta) from the Lebanese Amber. Deut. Entomol. Z. 1998, 45, 43–48. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef]
- Yang, Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol. Biol. Evol. 1998, 15, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nielsen, R.; Yang, Z. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol. Biol. Evol. 2005, 22, 2472–2479. [Google Scholar] [CrossRef]
- Bielawski, J.P.; Yang, Z. A maximum likelihood method for detecting functional divergence at individual codon sites, with application to gene family evolution. J. Mol. Evol. 2004, 59, 121–132. [Google Scholar] [CrossRef]
- Bielawski, J.P.; Yang, Z. Maximum likelihood methods for detecting adaptive evolution after gene duplication. J. Struct. Funct. Genom. 2003, 3, 201–212. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Rannala, B. Phylogenetic methods come of age: Testing hypotheses in an evolutionary context. Science 1997, 276, 227–232. [Google Scholar] [CrossRef]
- Yang, Z.; Wong, W.S.; Nielsen, R. Bayes empirical Bayes inference of amino acid sites under positive selection. Mol. Biol. Evol. 2005, 22, 1107–1118. [Google Scholar] [CrossRef]
- Kaplin, V. A new species of bristletails of the genus Petrobiellus (Microcoryphia: Machilidae) from Sakhalin. Zoosystematica Ross. 2020, 29, 17–22. [Google Scholar] [CrossRef]
- Mtow, S.; Machida, R. What are Halomachilis akkesiensis and Halomachilis kojimai described from Hokkaido, Japan? (Insecta: Archaeognatha: Machilidae). Zootaxa 2024, 5543, 445–450. [Google Scholar] [CrossRef]
- Olfers, E.V. Die «Ur-Insekten» (Thysanura und Collembola im Bernstein). Schr. K. Phys.-ökon. Ges. Königsb. 1907, 48, 1–40. [Google Scholar]
- Wang, B.; Xu, C.; Jarzembowski, E.A. Ecological radiations of insects in the Mesozoic. Trends. Ecol. Evol. 2022, 37, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, C.; Sun, B.; Quan, C.; Wu, J.; Lin, Z. Paleo-CO2 variation trends and the Cretaceous greenhouse climate. Earth-Sci. Rev. 2014, 129, 136–147. [Google Scholar] [CrossRef]
- McLoughlin, S. The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Aust. J. Bot. 2001, 49, 271–300. [Google Scholar] [CrossRef]
- Laenen, B.; Shaw, B.; Schneider, H.; Goffinet, B.; Paradis, E.; Désamoré, A.; Heinrichs, J.; Villarreal, J.; Gradstein, S.; McDaniel, S. Extant diversity of bryophytes emerged from successive post-Mesozoic diversification bursts. Nat. Commun. 2014, 5, 5134. [Google Scholar] [CrossRef]
- Benton, M.J. The origins of modern biodiversity on land. Philos. Trans. R. Soc. B. Biol. Sci. 2010, 365, 3667–3679. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Yuan, P.B.; Tsao, S.-J. Temporal and spatial records of active arc-continent collision in Taiwan: A synthesis. Geol. Soc. Am. Bull. 2006, 118, 274–288. [Google Scholar] [CrossRef]
- Li, C.F.; Xu, X.; Lin, J.; Sun, Z.; Zhu, J.; Yao, Y.; Zhao, X.; Liu, Q.; Kulhanek, D.K.; Wang, J. Ages and magnetic structures of the South China Sea constrained by deep tow magnetic surveys and IODP Expedition 349. Geochem. Geophys. Geosyst. 2014, 15, 4958–4983. [Google Scholar] [CrossRef]
- Ashman, L.G.; Shin, S.; Zwick, A.; Ślipiński, A.; McKenna, D.D. The first phylogeny of Australasian Lamiinae longhorn beetles (Coleoptera: Cerambycidae) reveals poor tribal classification and a complex biogeographic history. Syst. Entomol. 2022, 47, 213–230. [Google Scholar] [CrossRef]
- Decru, E.; Moelants, T.; De Gelas, K.; Vreven, E.; Verheyen, E.; Snoeks, J. Taxonomic challenges in freshwater fishes: A mismatch between morphology and DNA barcoding in fish of the north-eastern part of the Congo basin. Mol. Ecol. Resour. 2016, 16, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Kameda, Y.; Kimura, K.; Chiba, S. Substantial incongruence among the morphology, taxonomy, and molecular phylogeny of the land snails Aegista, Landouria, Trishoplita, and Pseudobuliminus (Pulmonata: Bradybaenidae) occurring in East Asia. Mol. Phylogenet. Evol. 2014, 70, 171–181. [Google Scholar] [CrossRef]
- Andreu, A.L.; Hanna, M.G.; Reichmann, H.; Bruno, C.; Penn, A.S.; Tanji, K.; Pallotti, F.; Iwata, S.; Bonilla, E.; Lach, B. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N. Engl. J. Med. 1999, 341, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Wilson, D.; Dutton, P.; Erecińska, M. Energy-coupling mechanisms in mitochondria: Kinetic, spectroscopic, and thermodynamic properties of an energy-transducing form of cytochrome b. Proc. Natl. Acad. Sci. USA 1970, 66, 1175–1182. [Google Scholar] [CrossRef]
- Davies, K.M.; Strauss, M.; Daum, B.; Kief, J.H.; Osiewacz, H.D.; Rycovska, A.; Zickermann, V.; Kühlbrandt, W. Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, 14121–14126. [Google Scholar] [CrossRef]
- Del Dotto, V.; Musiani, F.; Baracca, A.; Solaini, G. Variants in human ATP synthase mitochondrial genes: Biochemical dysfunctions, associated diseases, and therapies. Int. J. Mol. Sci. 2024, 25, 2239. [Google Scholar] [CrossRef]
- Lazarou, M.; Smith, S.M.; Thorburn, D.R.; Ryan, M.T.; McKenzie, M. Assembly of nuclear DNA-encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. FEBS J. 2009, 276, 6701–6713. [Google Scholar] [CrossRef]
- Bourens, M.; Barrientos, A. A CMC 1-knockout reveals translation-independent control of human mitochondrial complex IV biogenesis. EMBO Rep. 2017, 18, 477–494. [Google Scholar] [CrossRef]
- Hong, Y.H.; Huang, H.M.; Wu, L.; Storey, K.B.; Zhang, J.Y.; Zhang, Y.P.; Yu, D.N. Characterization of two mitogenomes of Hyla sanchiangensis (Anura: Hylidae), with phylogenetic relationships and selection pressure analyses of Hylidae. Animals 2023, 13, 1593. [Google Scholar] [CrossRef]
- Ngatia, J.N.; Lan, T.M.; Dinh, T.D.; Zhang, L.; Ahmed, A.K.; Xu, Y.C. Signals of positive selection in mitochondrial protein-coding genes of woolly mammoth: Adaptation to extreme environments? Ecol. Evol. 2019, 9, 6821–6832. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).