Simple Summary
Nilaparvata lugens (Brown rice planthopper, BPH), a migratory rice pest with strong fecundity and insecticide resistance, severely threatens global rice production. This study aimed to explore how trehalose-6-phosphate synthase—a key gene in trehalose biosynthesis—affects N. lugens’s reproduction and related molecular mechanisms. We used RNA interference (RNAi) to silence three trehalose-6-phosphate synthase paralogs (TPS1, TPS2, TPS3) in female N. lugens. Results showed that TPS silencing significantly delayed ovarian development, prolonged the pre-oviposition period, reduced total egg production and offspring hatch rate, and downregulated the expression of vitellogenin (Vg) and its receptor (VgR)—critical for oocyte maturation. It also disrupted hormonal signaling (juvenile hormone and 20-hydroxyecdysone pathways) and nutrient-sensing cascades (insulin/IGF and TOR pathways), as well as altered lipid metabolism. This dual effect of impairing energy supply and hormonal regulation highlights TPS gene as a promising molecular target for developing eco-friendly strategies to control N. lugens, which is vital for safeguarding rice yield and food security.
Abstract
Nilaparvata lugens is a migratory pest with high fecundity and outstanding drug resistance, which poses a devastating danger to rice production. This study investigated the reproductive regulation mechanism of N. lugens, specifically silencing the trehalose-6-phosphate synthase gene (TPS) via RNAi to elucidate how TPS governs the trehalose metabolic network through modulation of trehalose biosynthesis. Insect fecundity hinges on the synchronized progression of oogenesis and the tightly controlled expression of vitellogenin (Vg). In N. lugens, this coordination is orchestrated by an integrated molecular network that converges juvenile hormone signaling (JH), 20-hydroxyecdysone pathways (20E), insulin/IGF signaling (IIS), and the target of rapamycin cascade (TOR), collectively dictating the reproductive output of the species. Using TPS knockdown as the entry point, this study dissects the lipid-metabolic circuitry of N. lugens and uncovers how hormonal signaling cascades orchestrate reproduction by precisely modulating vitellogenin (Vg) and its cognate receptor VgR. Synthesized double-stranded terpene synthase genes (dsTPSs) can degrade mRNA, inhibit protein translation, and ultimately lead to the silencing of TPS genes, simultaneously crippling energy provision and hormonal signaling to orchestrate a multi-pronged suppression of reproduction. This dual-action intervention offers a promising molecular target for environmentally friendly management of N. lugens.
1. Introduction
Oryza sativa (rice) underpins the food security and livelihoods of hundreds of millions worldwide, especially in impoverished regions. However, rice production is continually imperiled by a range of insect pests and diseases that erode both yield and grain quality [,]. Chief among these threats is Nilaparvata lugens (Brown rice planthopper, BPH), the most devastating migratory pest of rice, closely followed by its congener, Sogatella furcifera (White-backed planthopper, WBPH), a potent vector of microbial and viral pathogens. Together, they inflict staggering yield losses across rice-growing regions [,]. Specifically, adults and nymphs of N. lugens congregate at the rice root zone, inserting their piercing–sucking stylets into phloem vessels to draw sap at rates that can exceed three times their own body weight per day [,]. Concurrently, salivary phenolic toxins breach cellular membranes, inflicting cascading physiological injury throughout the plant []. Furthermore, N. lugens excretes copious honeydew that becomes a fertile substrate for saprophytic fungi; the resulting sooty film blankets leaf surfaces and sharply curtails rice photosynthetic efficiency []. In addition, N. lugens exhibits remarkable fecundity: during oviposition, female adults repeatedly pierce rice stems, generating wounds that accelerate water loss in the host plant and provide entry points for destructive pathogens, including sheath blight and rice blast pathogens []. In recent years, the pest has devastated millions of hectares of paddies and acquired resistance to multiple commercial insecticides []. Consequently, the development of next-generation, biologically based green pesticides has become an urgent imperative.
RNA interference (RNAi) has emerged as an indispensable tool for functional genomics and a transformative platform for species-specific pest management []. Microinjection remains the gold-standard method for double-stranded RNA (dsRNA), enabling direct introduction of these molecules into embryos or the hemocoel. This allows hemolymph circulation to rapidly disseminate the trigger throughout the insect body [,,], and consistent with this, extensive studies have demonstrated that silencing the TPS and TRE genes disrupts chitin metabolism in Acyrthosiphon pisum [], while suppression of HpNAG and HpNAGK reduces cuticle thickness and chitin content in the coleopteran Holotrichia parallela Motschulsky []. Similarly, silencing the Ago1 and Ago2 genes in S. furcifera disrupts ecdysis and leads to lethality []. Thus, suppressing the expression of key genes can prove lethal to insects, establishing RNAi as a promising, gene-based weapon for next-generation pest control.
Trehalose functions as the principal hemolymph sugar and primary energy reserve in insects, orchestrating growth during chronic cold stress and serving as a central regulator of development, stress tolerance, flight capacity, and reproductive output [,,]. Trehalose biosynthesis hinges on the trehalose-6-phosphate synthase gene (TPS). In adults, three TPS paralogs are selectively expressed in the head, legs, wings, and epidermis []. Silencing TPS expression arrests trehalose formation and drives a compensatory rise in free glucose []. The expression level of the TPS gene directly governs trehalose concentration; when insects face starvation or high energy expenditure, TPS activity is promptly up-regulated, accelerating trehalose synthesis to maintain hemolymph glucose homeostasis [,]. During overwintering, Helicoverpa armigera markedly up-regulates TPS expression and accumulates trehalose, a metabolic safeguard that underpins H. armigera’s cold-hardiness [,]. Additionally, starvation triggers a marked rise in hemolymph trehalose concentration in Spodoptera exigua []. Similarly, during the pupal stage, Bombyx mori accumulates trehalose to fuel adult eclosion; silencing TPS disrupts ecdysone signaling and arrests pupation altogether []. In contrast, feeding decisions in A. pisum are dictated by the dynamic balance of intracellular trehalose and glucose levels []. Collectively, these examples all illustrate the importance of trehalose for insects. Silencing TPS genes in insects, exemplified by Heortia vitessoides and Tribolium castaneum, precipitates lethality and severe malformations, underscoring the gene’s pivotal role in coordinating energy and chitin metabolism [,]. Inhibiting trehalase reduces the synthesis of trehalose. Trehalose can be hydrolyzed into glucose, which provides energy for these physiological activities of insects, thereby ensuring the normal progress of the reproductive process and playing a crucial role in reproduction [,].
Behavioral assays show that N. lugens and S. furcifera exhibit a strong preference for rice seedlings as oviposition sites. However, as the crop matures, the oviposition propensity of S. furcifera decreases precipitously, while N. lugens maintains continuous egg-laying activity []. Vitellogenin (Vg) and its receptor (VgR) are key regulators of insect reproduction, as they control oocyte maturation, embryogenesis, and ultimately mediate population dynamics [,]. Studies in Periplaneta americana have shown that during yolk deposition, juvenile hormone (JH) and 20-hydroxyecdysone (20E) operate downstream of indoleamine- and peptide-mediated cues to orchestrate vitellogenesis []. More broadly, insect vitellogenesis is regulated by an integrated network of hormonal and nutrient-sensing pathways, including JH, 20E, the insulin/insulin-like growth factor signaling (IIS) pathway, and the target of rapamycin (TOR) pathway. These pathways collectively govern yolk synthesis and deposition [,].
This study had two interrelated objectives. First, to clarify the role of the TPS gene in N. lugens reproduction, we used RNAi to silence the TPS gene, then quantified changes in the insect’s reproductive output and delineated transcriptional reprogramming of key reproductive signaling pathways. Second, to propose a TPS-targeted control strategy for this pest: by disrupting N. lugens’ trehalose metabolism via TPS silencing, we aimed to validate the TPS gene as a viable molecular target and lay the foundation for developing a potent, species-specific control measure against this devastating agricultural pest.
2. Materials and Methods
2.1. Source and Breeding of N. lugens
Rice cultivar Taichung Native 1 (TN1) served as the host plant N. lugens was originally field-collected from paddies at the China National Rice Research Institute and maintained on TN1 seedlings for more than 60 consecutive generations under controlled conditions. TN1 seeds were surface-sterilized and imbibed in sterile water at warm water for 24–48 h. During the warm season, germinated seeds were grown on autoclaved soil for a week; in the cool season, perform hydroponic cultivation in an artificial climate chamber. Virgin males and females were paired in mesh cages containing fresh TN1 seedlings. After mating, females oviposited into the leaf sheaths, and eggs hatched 6–7 days later. The environmental conditions of the artificial climate chamber are set as follows: temperature 27 °C ± 1 °C, relative humidity 65 ± 5%, and photoperiod 18 L:6 D (Light:Dark). Replace fresh rice seedlings in a timely manner during the breeding process to ensure sufficient food supply for the population.
2.2. Total RNA Isolation and cDNA Synthesis
Total RNA was extracted from adult N. lugens using Trizol reagent []. Each treatment consisted of three biological replicates, each comprising five adults. The purity and concentration of RNA were detected by Nanodrop 2000 (Thermo Fisher, Waltham, MA, USA), and the integrity of RNA was detected by 1% agarose gel electrophoresis. If the RNA quality is qualified, it will be stored at −80 °C for subsequent experiments. The final concentration of each RNA was adjusted to 1000 ng/μL for subsequent cDNA synthesis. RNA was used as template, and the prime script RT reagent Kit with gDNA Eraser Kit (Takara, Japan) was used for cDNA transcription reaction.
2.3. Synthesis and Purification of dsRNA
The specific primers for TPS1, TPS2 and TPS3 of N. lugens were designed using Primer Premier 5 software (Premier Biosoft, Canada) in Table 1, in which the T7 promoter sequence was GGATCCTAATACGACTCACTATAGG. Double-stranded RNA was synthesized according to the method of T7 Ribo MAXTM Express RNAi System Kit (Promega, America). The same method was used to synthesize the dsRNA of green fluorescent protein gene (GFP) as the control group.

Table 1.
Primers for dsRNA synthesis.
2.4. Microinjection and Post-Injection Rearing of N. lugens
Prepare a capillary glass tube with an inner diameter of 0.5 mm, and use a P-1000 needle puller (Sutter instrument, America) to pull the glass tube into a micro injection needle with a thin tip. Newly emerged N. lugens females were briefly anesthetized with CO2 and microinjected under a stereomicroscope (Leica, Germany) using a calibrated microinjector. Injections were delivered into the soft membranous region between the meso- and metathoracic coxae of freshly anesthetized N. lugens females. A fine-tipped glass needle (tapered with forceps to prevent bending and minimize mechanical damage) was used for each microinjection. Each experiment was performed with three replicates, and five adult N. lugens were injected for each replicate. Two injection treatments were established: (i) dsGFP (negative control) and (ii) an equimolar cocktail (1:1:1) of the three synthesized dsRNAs—dsTPS1, dsTPS2, and dsTPS3—designated dsTPSs [,].
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Gene-specific qRT-PCR primers were designed with Primer Premier 5, and the Actin gene served as the endogenous reference. Primer sequences are listed in Table 2 (all primer efficiencies are greater than 90%). The qRT-PCR reaction system was as follows: TB Green Premix Ex Taq 5 μL, ddH2O 3.6 μL, forward primer F 0.2 μL, reverse primer R 0.2 μL, cDNA 1 μL. The qRT-PCR reaction was performed on a Bio-Rad CFX system using the following thermal profile: pre-denaturation at 95 °C for 30 s, denaturation at 95 °C for 5 s, extension at 60 °C for 30 s, and 35 cycles. Amplicon specificity was verified via amplification and melt-curve analyses, and relative gene expression was quantified by the 2−ΔΔCT method [].

Table 2.
Primers for qRT-PCR.
2.6. Integrated Ovarian Transcriptomic and Metabolomic Profiling
Transcriptome sampling: Females microinjected with dsRNA and untreated males were paired 1:1 on fresh rice seedlings; 3 days later, ovaries were dissected under a stereomicroscope. Three biological replicates were prepared, each pooling 5–10 ovaries. Total RNA was isolated as described in Section 2.2, then the RNA sample was sent to the Beijing Genomics Institute (Shenzhen, China) for transcriptome sequencing (sequencing platform: DNBSEQ, sequencing depth ≥ 60×).
Metabolome sampling: Microinjected females and untreated males were paired 1:1 on fresh rice seedlings. After 3 days, ovaries were dissected under a Leica EZ4 HD stereomicroscope, yielding six biological replicates of 15–25 ovaries each. The ovaries were frozen in liquid nitrogen, and the samples were sent to the Beijing Genomics Institute (Shenzhen, China) for non-targeted metabolomics sequencing.
Single-stranded circular DNA library (a transcriptome library constructed from mRNA), using a polyA enrichment strategy which binds to the polyA tail of mRNA via OligodT magnetic beads to enrich mRNA with a polyA structure and exclude rRNA interference.
Total RNA-seq raw reads were first filtered using SOAPnuke software (BGI Shenzhen) to remove reads with low quality, adapter contamination, and excessively high content of unknown base N, resulting in clean reads. Differentially expressed genes (DEGs) were analyzed using DESeq2 software with the parameter setting of q-value (adjusted p-value) ≤ 0.05 []. The screened DEGs were subjected to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis. In addition, this experiment employed Principal Component Analysis (PCA) to examine the overall distribution of individual samples within each group and the degree of dispersion between groups.
The following experimental steps were completed by the company: Using a liquid extracted from a mixture of methanol, ethanol and water, extract a sample of brown-flush ovaries, add two sterilized small steel beads to grind, and then grind the grinding liquid in an ultrasound bath at 4 °C. Mix the methanol and water according to the volume ratio of 9:1, and prepare a solvent; take the grinding liquid, put it in a frozen vacuum enrichment, then add 200 μL of the solvent to dissolve, then concentrate for 15 min, take the liquid to transfer to the above sample bottle. In addition, 20 μL of liquid is extracted from each sample separately and mixed together to form a QC sample, and LC-MS analysis is carried out in sync with each treatment group sample to assess the repeatability and stability of the analysis process. The extracted metabolites were separated and tested using the Q Exactive HF High Resolution Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) in both positive and negative ions.
2.7. Detection of the Fecundity of N. lugens
Ovarian dissection and imaging: Injected females were paired 1:1 with untreated males. On days 3, 5 and 7 post-mating, females were briefly anesthetized with CO2 and ovaries were dissected intact under a stereomicroscope (Leica, Germany).
Female N. lugens after injection were paired and reared with untreated male N. lugens at a 1:1 ratio. The oviposition status was observed daily, and the number of days from pairing to the start of oviposition is defined as the pre-oviposition period. On day 3 post-mating, ovaries were dissected, staged, and the number of mature oocytes was quantified []; more than 15 females were examined per treatment. Based on oocyte maturity, color, and number within the ovarioles, the adult ovaries were classified into five distinct stages: milky transparent (Grade I), vitellogenic (Grade II), mature (Grade III), egg-laying (Grade IV), and late egg-laying (Grade V).
Offspring hatch-rate assessment: Injected females were paired 1:1 with untreated males. Three days later, each mated female was transferred to a feeding device containing fresh TN1 seedlings immersed in clean water, at a density of 1–3 females per device. Hatch rates of the resulting eggs were recorded. Each trial comprised ≥ 9 replicates. Collect the eggs laid by the female of N. lugens from the 3rd day to the 6th day after eclosion, and then remove the adults. Count the egg hatching of N. lugens every 24 h under the condition of artificial climate chamber until no further nymphs emerge. Unhatched eggs were examined and categorized under a stereomicroscope.
2.8. Determination of Triglyceride Content
Injected females were paired 1:1 with untreated males. After 72 h, fat bodies and ovaries were dissected. Each treatment comprised three biological replicates, each pooling 5–8 tissues (fat bodies or ovaries) from N. lugens.
Determination of triglycerides in fat body: Triglyceride (TG) content in fat bodies was quantified using Triglyceride assay kit (Nanjing Jiancheng, China). The quantity of quinones generated in the reaction correlates directly with triglyceride content, with higher levels yielding darker colorimetric signals. The calculation formula of triglyceride content is as follows:
Triglyceride content (mmol/L) = (Sample OD value − Blank OD value)/(Calibration OD value blank OD value) × Concentration of calibrator (mmol/L)
Triglyceride (TG) quantification in ovaries: The TG assay procedure was identical to that described above. However, given that ovaries are not high-fat tissues, protein content was also measured in the pre-treated samples to normalize TG levels. Protein concentration was determined using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Absorbance was measured at 562 nm using a microplate reader. TG content was then calculated based on the protein concentration of the sample as follows:
Triglyceride content (mmol/L) = [(Sample OD value − Blank OD value)/(Calibration OD value blank OD value) × Concentration of calibrator (mmol/L)]/Protein concentration of the sample (gprot/L)
2.9. Data Analysis
Statistical significance of the data was assessed using IBM SPSS Statistics 20 software, with normality and homogeneity of variance evaluated. Differences between control and treatment groups were compared using one-way analysis of variance (ANOVA) or independent sample t-test. Post hoc tests were performed using the Tukey method for one-way ANOVA, with different letters indicating significant differences between groups (p < 0.05). For the independent sample t-test, “*” denoted a significant difference when p < 0.05, “**” indicated an extremely significant difference when p < 0.01, “***” represented an even more extremely significant difference when p < 0.001, and “ns” indicated no significant difference. Data are presented as mean ± SD. GraphPad Prism version 9.0 software was used for data visualization (all data conformed to a normal distribution).
3. Results
3.1. RNAi Efficacy and Transcriptomic-Metabolomic Analysis
On day 3 post-injection, females were sampled for RNAi validation. Quantitative real-time PCR (qRT-PCR) revealed significant downregulation of the three trehalose-6-phosphate synthase genes—TPS1, TPS2, and TPS3—indicating effective dsRNA-mediated silencing (Figure 1A). Through the analysis of ovarian transcriptome and metabolome, we found that the correlation coefficients within the dsGFP and dsTPSs groups exceeded 99% and 96%, respectively, confirming robust sample reproducibility across both treatments (Figure 1B). Moreover, analysis of inter-group transcriptomic correlation showed that the correlation coefficient between the dsGFP and dsTPSs groups exceeded 94%, confirming high transcriptomic similarity between the two groups. Consistent with this result, principal component analysis (PCA) demonstrated that all samples from the two treatment groups were tightly clustered in the PCA space (Figure 1C), which further verified the high degree of similarity in their transcriptomic profiles. The results of KEGG analysis are pivotal for elucidating the biological pathways and molecular mechanisms associated with gene functions. The results indicated significant enrichment in pathways related to Fatty acid elongation, Fatty acid metabolism, Proximal tubule bicarbonate reabsorption, and Fatty acid degradation (Figure 1D). Integrating KEGG pathway enrichment analysis from both positive and negative ion modes, the differentially abundant metabolites between the dsTPSs and dsGFP groups were primarily enriched in pathways related to the biosynthesis of Glutathione metabolism, Ascorbate and aldehyde metabolism, etc. (Figure 1E,F). In summary, the transcriptomic and metabolomic data are highly consistent, providing crucial insights into the role of glucose metabolism in regulating the fecundity of N. lugens (The transcriptomic-metabolomic analysis and metabolomics run metrics are provided in the Supplementary Files).
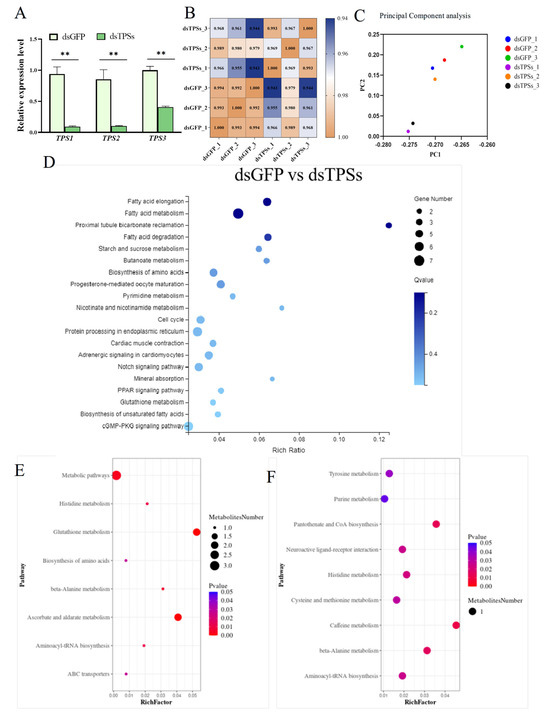
Figure 1.
The relative expression levels of TPS1, TPS2, and TPS3 in N. lugens on the third day after dsTPSs injection (A). Correlation analysis and principal component analysis among the transcriptome samples (B,C). KEGG metabolic pathway analysis of DEGs (D). KEGG pathway enrichment analysis of differential metabolites and analysis of crucial metabolites (E,F) ((E,F) represent the KEGG enrichment pathway between dsGFP and dsTPSs groups in the negative ion and positive ion mode). For the independent sample t-test, “**” indicated an extremely significant difference when p < 0.01.
3.2. Impact of Trehalose-6-Phosphate Synthase Gene on Ovarian Development in Female N. lugens
Examining the ovarian morphology of N. lugens, we observed that compared to the control group injected with dsGFP, the ovarian area of N. lugens injected with dsTPSs was significantly reduced on days 3 and 5. Additionally, the number of mature eggs in development was lower, and ovarian development was insufficient. However, by day 7, ovarian development appeared sufficient (Figure 2A). On day 3 post-injection, ovaries were dissected from females injected with dsGFP, staged, and mature oocyte counts were recorded. Most ovaries from dsGFP-injected females reached Grade III, with only a few remaining at Grade II. In contrast, ovaries from females injected with dsTPSs exhibited a significant increase in Grade I and II (Figure 2B) and a marked reduction in mature eggs (Figure 2C), indicating that dsTPSs treatment impeded ovarian growth and significantly inhibited early-stage ovarian development in N. lugens.
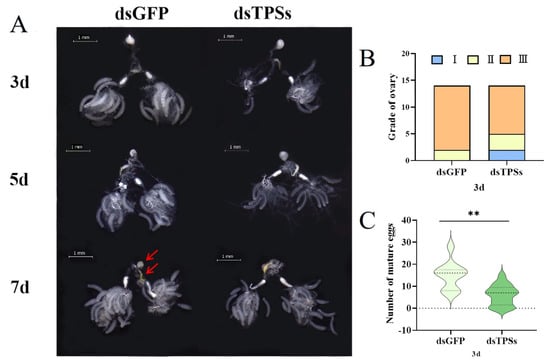
Figure 2.
The ovarian development of N. lugens on the third, fifth, and seventh days after dsTPSs injection, magnifications of 16× were used (Scale bar = 1 mm). The white part indicated by the arrow is the bursa copulatrix, and the yellow part indicated by the arrow is the spermatheca (A), as well as the ovarian grade and the number of mature oocytes on the third day, N = 15 (B,C). For the independent sample t-test, “**” indicated an extremely significant difference when p < 0.01.
3.3. Impact of Trehalose-6-Phosphate Synthase Gene Silencing on Egg Production and Hatch Rate in Female N. lugens
Following injection with dsGFP and dsTPSs, the mean pre-oviposition periods of N. lugens were 2.65 d and 3.53 d, respectively. The pre-oviposition period of the dsTPSs-injected group was significantly longer than that of the dsGFP control group (Figure 3A). Figure 2A shows that the ovarian development of brown planthoppers injected with dsTPSs is inhibited. Correspondingly, the results showed that compared with the control group injected with dsGFP, the total egg production of N. lugens injected with dsTPSs was significantly reduced (Figure 3B). In addition, the hatching rate of N. lugens’ offspring was significantly reduced in the treatment group injected with dsTPSs (Figure 3C). It can be seen from the figure that the hatching failure of N. lugens may be related to the development error of the anterior–posterior axis of the egg or the eggshell (Figure 3D). Three days after the dsTPSs injection, the relative expression levels of Vg and VgR were significantly downregulated (Figure 3E,F), which was associated with the observed insufficient development of egg granules (Figure 3D) and ovarian hypoplasia (Figure 2A).
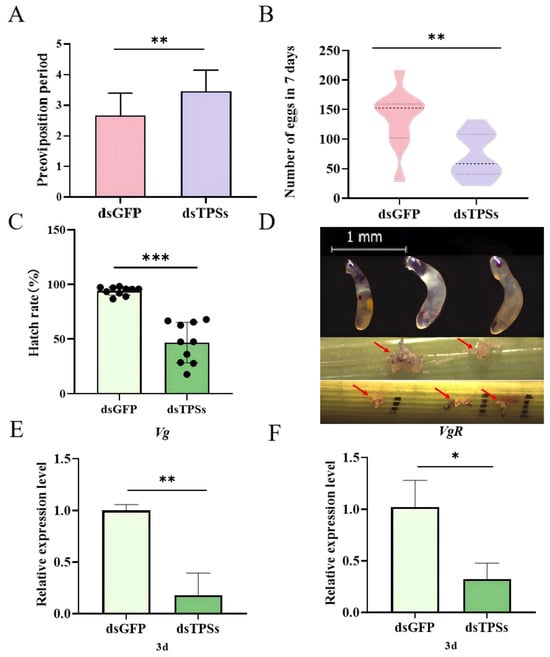
Figure 3.
Preoviposition and total number of eggs in 7 days of N. lugens after dsTPSs injection (A,B). Hatching rate (C) and non-hatching phenotypes of N. lugens offspring, magnifications of 32× were used (The arrow indicates the unhatched egg grains in the rice) (D) and the relative expression of Vg and VgR (E,F) after dsTPSs injection. For the independent sample t-test, “*” denoted a significant difference when p < 0.05, “**” indicated an extremely significant difference when p < 0.01, “***”represented an even more extremely significant difference when p < 0.001.
3.4. Impact of Trehalose-6-Phosphate Synthase Gene on JH and 20E Signaling Pathways
Three days post-injection of dsTPSs, the juvenile hormone biosynthetic gene JHAMT showed significantly downregulated expression compared to controls (0.01 < p ≤ 0.05; Figure 4A), while the JH intracellular receptor-encoding gene Met was profoundly reduced (p ≤ 0.001; Figure 4B). Additionally, the 20-hydroxyecdysone receptor genes ECR and USP were both downregulated (p ≤ 0.001; Figure 4C,D). These findings indicate that dsTPSs injection attenuates both JH and 20E signaling in N. lugens.
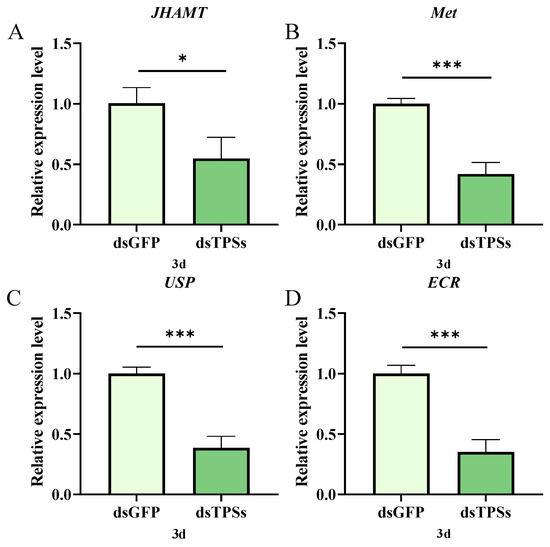
Figure 4.
The relative expression levels of the juvenile hormone gene JHAMT and its receptor gene Met (A,B), the Ecdysone receptor gene ECR and the supervalve protein gene USP in N. lugens on the third day after dsTPS injection (C,D). For the independent sample t-test, “*” denoted a significant difference when p < 0.05, “***” represented an even more extremely significant difference when p < 0.001.
3.5. Impact of Trehalose-6-Phosphate Synthase Gene on Nutrient Signaling Pathways
On the third day post-dsTPSs injection, the expression level of the insulin receptor gene InR1 in N. lugens exhibited a highly significant downward trend compared to the control group (0.001 < p ≤ 0.01) (Figure 5A). Similarly, the expression level of another insulin receptor gene, InR2, also decreased significantly (0.01 < p ≤ 0.05) (Figure 5B). As a core molecule in the regulatory pathway of cell growth and metabolism, the mRNA level of the TOR gene was significantly reduced compared to the control group (0.01 < p ≤ 0.05) (Figure 5C). S6K, a downstream effector protein of mTOR, showed no significant difference in gene expression compared to the control group (p > 0.05) (Figure 5D).
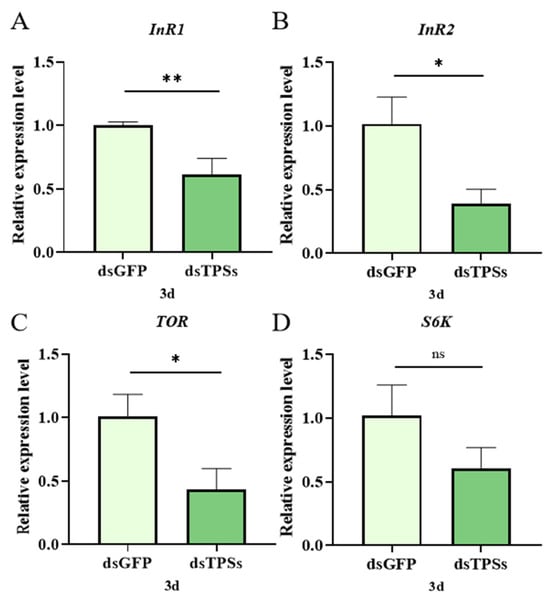
Figure 5.
The relative expression of insulin-like peptide signaling pathway-related genes (InR1 and InR2) (A,B) and target genes of rapamycin signaling pathway (TOR and S6K) (C,D) in N. lugens on the third day after dsTPSs injection. For the independent sample t-test, “*” denoted a significant difference when p < 0.05, “**” indicated an extremely significant difference when p < 0.01, and “ns” indicated no significant difference.
3.6. Impact of Trehalose-6-Phosphate Synthase Gene on Lipid Metabolism
The experimental results indicated that, on day 3 post-dsTPSs injection, triglyceride (TG) content in fat bodies did not significantly differ from controls (Figure 6A). However, TG levels in ovaries were significantly elevated (Figure 6B). The impact of TPS silencing on lipid metabolism in N. lugens was further examined via qRT-PCR. Results indicated that fatty acid synthase gene (Fas) mRNA levels were significantly reduced (0.01 < p ≤ 0.05) on day 3 post-dsTPSs injection (Figure 6C). Conversely, adipokinetic hormone gene (AKH), which regulates lipid mobilization, exhibited a downward trend but no significant difference compared to controls (Figure 6D).
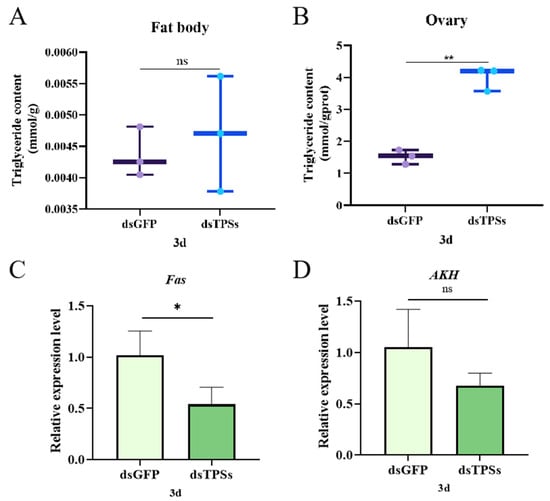
Figure 6.
The relative expression levels of triglycerides in the adiposomes and ovaries of N. lugens on the third day after the injection of dsTPSs (A,B), as well as the relative expression of the fatty acid synthase gene Fas (C) and the lipid-activating hormone gene AKH in N. lugens (D). For the independent sample t-test, “*” denoted a significant difference when p < 0.05, “**” indicated an extremely significant difference when p < 0.01, and “ns” indicated no significant difference.
4. Discussion
Characterized by its migratory behavior, prolific reproductive capacity, propensity for outbreaks, and robust resistance to insecticides, N. lugens poses a significant threat to rice production. Effective management of this pest must adhere to the principle of “prevention first, integrated control”. In recent years, research has increasingly focused on the development of novel, eco-friendly pesticides [,,,]. This study, commencing with the reproductive biology of N. lugens, employs RNAi technology to silence the TPS gene, thereby disrupting trehalose synthesis in vivo. This intervention results in diminished reproductive capacity in adult insects, laying a foundation for the potential development of RNAi-based biopesticides in the future.
The experimental results indicated that the differentially abundant metabolites between the dsTPSs and dsGFP groups were primarily enriched in metabolic pathways, including glutathione metabolism and ascorbate/aldarate metabolism (Figure 1E,F). Glutathione (GSH), an essential tripeptide, is a cornerstone in enzymatic detoxification, crucial for modulating reactive oxygen species (ROS) levels and implicated in myriad key physiological functions [,,]. Glutathione is indispensable for embryonic development and modulates reproductive efficiency by regulating oxidative stress, energy metabolism, and signal transduction [,,,,]. Similarly, ascorbic acid has long been linked to fertility: its interplay with aldose acid metabolism fine-tunes germ cell quality, hormonal balance, and embryonic development, thereby influencing reproductive outcomes []. During oocyte maturation, these metabolites further support reproductive processes by shielding oocytes from ROS-mediated damage and ensuring adequate nutrient supply via their antioxidant properties [,,]. In the present study, KEGG analysis revealed that differentially expressed genes (DEGs) in the dsTPSs group were significantly enriched in pathways related to Fatty acid metabolism and Fatty acid degradation (Figure 1D), consistent with the role of TPS genes in metabolic regulation. To validate TPS gene silencing efficiency, we detected the relative expression of three trehalose-6-phosphate synthase genes (TPS1, TPS2, and TPS3) in N. lugens on day 3 post-RNAi: all three genes showed significant downregulation (Figure 1A). This result differs from a related study where TPS1 expression in N. lugens increased on day 3 following dsTPSs injection []; we propose that this discrepancy may stem from differences in the injected dsTPSs concentrations, a factor known to affect RNAi efficiency. We further investigated key genes and metabolites in fatty acid metabolism. Fas, a highly conserved key gene in fatty acid biosynthesis that promotes lipid accumulation during the lipid storage phase and maintains lipid homeostasis by upregulating lipid metabolism [,], showed significant downregulation in the dsTPSs group (Figure 6C). Consistent with this, triglyceride (TG) levels— a core indicator of lipid metabolism in insects []—exhibited tissue-specific changes: fat body TG content showed no significant difference (Figure 6A), while ovarian TG levels increased markedly (Figure 6B). We hypothesize that this elevation in ovarian TG may reflect aberrant lipid metabolism, a phenomenon often associated with reproductive dysfunction. Notably, triglycerides stored in the fat body are progressively hydrolyzed into ATP by lipase [,]. Combined with our findings of TPS gene silencing, Fas downregulation, and altered TG distribution, these results collectively suggest that silencing TPS genes disrupts fatty acid metabolism pathways. This disruption may reduce ATP production, thereby modulating the reproductive processes of N. lugens.
Trehalose, known as the “blood glucose” of insect hemolymph, acts as a central energy source for ovarian development and a key protector against oocyte stress []. Its synthesis in insect ovaries follows a well-defined pathway: trehalose-6-phosphate synthase catalyzes the formation of trehalose-6-phosphate from UDP-glucose and glucose-6-phosphate, and this intermediate is subsequently converted to trehalose by trehalose-6-phosphate phosphatase (TPP) [,,]. This metabolic pathway is particularly active during vitellogenesis, as it fuels the accumulation of lipids and proteins in developing oocytes []—a role supported by studies in Helicoverpa armigera, where TPS/TPP transcription is upregulated during the pre-pupal and pupal stages to meet reproductive energy demands []. Vitellogenesis, another core process for insect reproduction, relies on the synthesis, transport, and deposition of Vg. Specifically, Vg is synthesized in the fat body, transported to the ovary via hemolymph, and taken up by mature oocytes through mediation by VgR; once inside oocytes, Vg precipitates and accumulates to form vitellin, an essential nutrient reserve for embryonic development [,,]. In the present study, we observed that on day 3 post-injection of dsTPSs, the expression levels of both Vg and VgR in N. lugens were significantly downregulated compared to the control group (Figure 3E,F)—a result that links TPS silencing to impaired vitellogenesis. To further contextualize this finding, we referenced studies on the AKH signaling pathway, which interacts with trehalose metabolism to regulate insect reproduction. Previous work has shown that silencing the AKH receptor gene (AKHR) reduces Vg expression in the fat body and inhibits Vg deposition in oocytes, while concurrently decreasing trehalose levels in both fat bodies and hemolymph []; this reduction in hemolymph trehalose ultimately leads to decreased egg-laying and fecundity in N. lugens []. These results collectively suggest that inhibiting trehalose metabolism (either via TPS silencing or AKHR knockdown) curtails Vg deposition. In our study, AKH expression did not show a statistically significant difference between the dsTPSs group and the control (Figure 6D). This trend, combined with our direct expression of Vg/VgR downregulation (Figure 3E,F), may indicate a potential crosstalk between TPS-mediated trehalose metabolism and AKH signaling in regulating N. lugens reproduction. Additionally, relevant studies have shown that trehalase inhibitors block trehalase activity in eggs, preventing oocytes from absorbing required vitellogenin and thus reducing ovarian vitellogenin accumulation; this aligns with our finding that disrupting trehalose synthesis (via TPS silencing) impairs Vg/VgR expression, reinforcing the link between trehalose metabolism and vitellogenesis. This ultimately lowers egg hatch rates and curtails the reproductive capacity of Spodoptera frugiperda []. RNAi-mediated knockdown of G-protein coupled receptor genes (AAEL003647 and AAEL019988) in Aedes aegypti reduced egg production by approximately 30% and induced abnormal ovarian development []. Vg synthesis occurs not only in the ovaries but also in the fat body. Notably, the TAG content in the ovaries was significantly decreased (Figure 6B). The insufficient energy supply derived from TAG in the ovaries directly inhibited either Vg synthesis or receptor-mediated endocytosis of Vg, ultimately leading to impaired yolk deposition []. In this study, injection of dsTPSs in N. lugens led to a reduction in egg-laying numbers and a significantly lower hatch rate compared to the control group (Figure 3B,C). This was accompanied by delayed ovarian development (Figure 2A) and egg abnormalities such as eye-spot inversion and eye-spot absence (Figure 3D). It is speculated that the silencing of the TPS gene leads to a decrease in Vg expression, which prevents oocytes from accumulating sufficient yolk. This causes oocyte development to arrest or degenerate, thereby resulting in reduced egg volume, thinner eggshells, decreased egg-laying quantity, and lower hatching rate. This also indicates that the inhibitory effect of the TPS gene on the reproduction of N. lugens is exerted by acting on egg grains.
The synthesis of Vg requires energy. Insufficient trehalose content leads to energy deficiency, which inhibits Vg synthesis. Meanwhile, Vg expression is also affected by a variety of signaling pathways. JH and 20E are two of the most critical insect hormones, orchestrating the entire reproductive process through intricate synergistic or antagonistic interactions [,,]. JH activates the transcription of the Vg gene in adipocytes via the receptor Met, thereby initiating and sustaining vitellogenesis and regulating ovarian development during insect reproduction. Meanwhile, 20E not only governs insect molting but also plays a pivotal role in oocyte maturation and oviposition [,]. In this study, injection of dsTPSs in N. lugens significantly reduced ovarian area on days 3 and 5 post-injection (Figure 2A), increased the number of Grade I and II ovaries (Figure 2B), and decreased the number of mature eggs (Figure 2C). Concurrently, expression levels of JHAMT and Met were downregulated (Figure 4A,B), while key 20E signaling pathway receptors—USP and ECR—were also significantly reduced (Figure 4C,D). These results suggest that ovarian TPS expression is tightly regulated by JH: TPS silencing disrupts the intricate coordination and regulation between JH and 20E, arresting vitellogenesis and oocyte maturation and delaying ovarian development in female N. lugens.
Ovarian development is also influenced by nutrient signaling pathways. N. lugens has two insulin receptor genes, InR1 and InR2 []. RNAi results showed that InR1 and InR2 expression significantly decreased on day 3 post-treatment (Figure 5A,B). InR1 typically activates the PI3K/Akt pathway, while InR2 acts as a negative regulator of this pathway [,]. Studies have demonstrated that amino acids activate S6K via the TOR pathway, enhancing the translation efficiency of Vg and driving ovarian cell proliferation. Given the significant reduction in TOR expression observed in this study (Figure 5C), a corresponding decrease in S6K activity would be expected. Although S6K levels did not significantly change in this experiment (Figure 5D), a downward trend was noted. Silencing the TPS gene results in insufficient TOR expression, which in turn reduces Vg content, aligning with the observed trends in this study. The interplay between the insulin-TOR signaling pathways modulates lipid metabolism, thereby influencing genetic regulatory mechanisms []. After knocking down the expression of PvVg in Polyrhachis vicina Roger, the expression levels of genes related to other pathways all decreased significantly. PvVg regulates PvERR expression by crosstalk with the JH and IIS-TOR signaling pathways []. This is consistent with the findings of this study, and the decreased expression of the relevant genes is a phenomenon caused by specific regulation. Our study found that TAG content in the fat body remained unchanged, while the TAG content in the ovaries decreased significantly (Figure 6A,B). We hypothesize that when trehalose is insufficient, the energy supply in the fat body is deficient, which directly inhibits the expression or activity of lipolytic enzymes. This prevents TAG from being effectively decomposed and released from the fat body, thus keeping the TAG content in the fat body unchanged. The lack of energy provided by TAG in the ovaries directly inhibits the synthesis of Vg or the receptor-mediated endocytosis process, resulting in insufficient yolk deposition.
5. Conclusions
Collectively, our results demonstrate that trehalose-6-phosphate synthase (TPS1, TPS2, TPS3) acts as a central node integrating energy metabolism, hormonal signaling, and nutrient sensing to regulate N. lugens reproduction. Targeting TPS via RNAi-based strategies can effectively suppress N. lugens’s reproductive capacity without relying on traditional insecticides, thereby providing a novel, eco-friendly molecular target for N. lugens management. This not only offers a promising approach to mitigate N. lugens-induced rice yield losses but also aligns with the goal of sustainable agriculture by reducing environmental risks associated with chemical pesticides.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16121195/s1, Text S1: Details for transcriptomics and metabolites; Table S1: Number of eggs in 7 days and hatching rate.
Author Contributions
Conceptualization, Y.H. and F.Z.; methodology, Y.H.; software, X.Z. (Xinyu Zhang) and Y.Z. (Yuya Zhang); validation, Y.H. and F.Z.; formal analysis, X.Z. (Xinyu Zhang); investigation, Y.Z. (Yanfei Zhou); resources, L.G.; data curation, Y.Z. (Yuya Zhang); writing—original draft preparation, Y.H.; writing—review and editing, F.Z. and M.Z.; visualization, Y.Z. (Yi Zhang); supervision, X.Z. (Xinyi Zhang); project administration, Y.L.; funding acquisition, M.Z. and B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 32272608).
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TPS | Trehalose-6-phosphate synthase |
| TRE | Trehalose-6-phosphate phosphatase |
| AKH | Adipokinetic hormone |
| JH | Juvenile hormone signaling |
| 20E | 20-hydroxyecdysone pathways |
| IIS | Insulin/IGF signaling |
| TOR | Target of rapamycin cascade |
References
- Haliru, B.S.; Rafii, M.Y.; Mazlan, N.; Ramlee, S.I.; Muhammad, I.; Akos, I.S.; Halidu, J.; Swaray, S.; Bashir, Y.R. Recent strategies for detection and improvement of brown planthopper resistance genes in rice: A Review. Plants 2020, 9, 1202. [Google Scholar] [CrossRef]
- Wang, S.L.; Cheng, R.L.; Lu, J.B.; Yu, X.P.; Zhang, C.X. A Cripavirus in the brown planthopper, Nilaparvata lugens. J. Gen. Virol. 2016, 97, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xiong, S.; Guan, X.; Tang, T.; Zhu, Z.; Zhu, X.; Hu, J.; Wu, J.; Zhang, S. Insight into rice resistance to the brown planthopper: Gene cloning, functional analysis, and breeding applications. Int. J. Mol. Sci. 2024, 25, 13397. [Google Scholar] [CrossRef]
- Anand, R.; Divya, D.; Mazumdar-Leighton, S.; Bentur, J.S.; Nair, S. Expression analysis reveals differentially expressed genes in BPH and WBPH associated with resistance in rice RILs derived from a Cross between RP2068 and TN1. Int. J. Mol. Sci. 2023, 24, 13982. [Google Scholar] [CrossRef]
- Guo, J.P.; Xu, C.X.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306. [Google Scholar] [CrossRef]
- Xu, H.X.; He, X.C.; Zheng, X.S.; Yang, Y.J.; Lu, Z.X. Influence of rice black streaked dwarf virus on the ecological fitness of non-vector planthopper Nilaparvata lugens (Hemiptera: Delphacidae). Insect Sci. 2014, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Lu, J.B.; Li, Q.; Bao, Y.Y.; Zhang, C.X. Combined transcriptomic/proteomic analysis of salivary gland and secreted saliva in three planthopper species. J. Proteom. 2018, 172, 25–35. [Google Scholar] [CrossRef]
- Peñalver-Cruz, A.; Horgan, F.G. Interactions between rice resistance to planthoppers and honeydew-related egg parasitism under varying levels of nitrogenous fertilizer. Insects 2022, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Qian, N.; Zheng, P.; Wang, Y.; Pan, S.; Li, Y.; Zhang, C.; Chen, J.; Teng, J. Characterization of actin and tubulin promoters from two sap-sucking pests, Nilaparvata lugens (Stål) and Nephotettix cincticeps (Uhler). Biochem. Biophys. Res. Commun. 2016, 470, 831–837. [Google Scholar] [CrossRef]
- Garrood, W.T.; Zimmer, C.T.; Gorman, K.J.; Nauen, R.; Bass, C.; Davies, T.G. Field-evolved resistance to imidacloprid and ethiprole in populations of brown planthopper Nilaparvata lugens collected from across South and East Asia. Pest. Manag. Sci. 2016, 72, 140–149. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Kunte, N.; McGraw, E.; Bell, S.; Held, D.; Avila, L.A. Prospects, challenges and current status of RNAi through insect feeding. Pest. Manag. Sci. 2020, 76, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Christiaens, O.; Liu, J.S.; Niu, J.Z.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef]
- Wang, G.; Gou, Y.; Guo, S.; Zhou, J.J.; Liu, C. RNA interference of trehalose-6-phosphate synthase and trehalase genes regulates chitin metabolism in two color morphs of Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 948. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Liu, Z.; Han, W.; Lu, X.; Guo, W. Identification and functional analysis of two potential RNAi targets for chitin degradation in Holotrichia parallela Motschulsky (Insecta Coleoptera). Pestic. Biochem. Physiol. 2022, 188, 105257. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.P.; Zhao, X.Y.; Smagghe, G.; Yang, X.B.; Yang, H.; Zeng, Q.H.; Jia, Z.Y.; Jiang, Z.C. RNAi of ago1 and ago2 disrupts molting in the white-backed planthopper (Sogatella furcifera). Arch. Insect Biochem. Physiol. 2025, 119, e70069. [Google Scholar]
- Tang, B.; Wang, S.; Wang, S.G.; Wang, H.J.; Zhang, J.Y.; Cui, S.Y. Invertebrate trehalose-6-phosphate synthase gene: Genetic architecture, biochemistry, physiological function, and potential applications. Front. Physiol. 2018, 9, 30. [Google Scholar] [CrossRef]
- Rebholz, Z.; Shewade, L.; Kaler, K.; Larose, H.; Schubot, F.; Tholl, D.; Morozov, A.V.; O’Maille, P.E. Emergence of terpene chemical communication in insects: Evolutionary recruitment of isoprenoid metabolism. Protein Sci. 2023, 32, e4634. [Google Scholar] [CrossRef]
- Lü, X.; Han, S.C.; Li, Z.G.; Li, L.Y.; Li, J. Gene characterization and enzymatic activities related to trehalose metabolism of in vitro reared Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) under sustained cold stress. Insects 2020, 11, 767. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, J.J.; Li, Y.; Gou, Y.; Quandahor, P.; Liu, C. Trehalose and glucose levels regulate feeding behavior of the phloem-feeding insect, the pea aphid Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 15864. [Google Scholar] [CrossRef]
- Ekta, S.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2014, 25, 357–367. [Google Scholar] [CrossRef]
- Elbein, A.D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; North, H.L.; Peng, Y.; Liu, H.; Liu, B.; Pan, R.; Zhou, Y.; Zheng, W.; Liu, K.; Yang, B.; et al. Adaptive evolution to the natural and anthropogenic environment in a global invasive crop pest, the cotton bollworm. Innovation 2023, 4, 100454. [Google Scholar] [CrossRef]
- Tellis, M.B.; Chaudhari, B.Y.; Deshpande, S.V.; Nikam, S.V.; Barvkar, V.T.; Kotkar, H.M.; Joshi, R.S. Trehalose transporter-like gene diversity and dynamics enhances stress response and recovery in Helicoverpa armigera. Gene 2023, 862, 147259. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hong, Y. Regulation of hemolymph trehalose level by an insulin-like peptide through diel feeding rhythm of the beet armyworm, Spodoptera exigua. Peptides 2015, 68, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.D.; Wang, Y.X.; Guo, Z.X.; Liu, Y.X.; Huang, Z.H.; Zhu, L.B.; Liu, M.H.; Liu, S.H.; Xu, J.P. Trehalose hydrolysis and transport-related genes promote Bombyx mori nucleopolyhedrovirus proliferation through the phosphoinositide 3-kinase-Akt signalling pathway in BmN cell. Dev. Comp. Immunol. 2023, 140, 104625. [Google Scholar] [CrossRef]
- Chen, J.X.; Lyu, Z.H.; Wang, C.Y.; Cheng, J.; Lin, T. RNA interference of a trehalose-6-phosphate synthase gene reveals its roles in the biosynthesis of chitin and lipids in Heortia vitessoides (Lepidoptera: Crambidae). Insect Sci. 2020, 27, 212–223. [Google Scholar] [CrossRef]
- Chen, Q.W.; Jin, S.; Zhang, L.; Shen, Q.D.; Wei, P.; Wei, Z.M.; Wang, S.G.; Tang, B. Regulatory functions of trehalose-6-phosphate synthase in the chitin biosynthesis pathway in Tribolium castaneum (Coleoptera: Tenebrionidae) revealed by RNA interference. Bull. Entomol. Res. 2018, 108, 388–399. [Google Scholar] [CrossRef]
- Wang, S.S.; Li, G.Y.; Liu, Y.K.; Luo, Y.J.; Xu, C.D.; Li, C.; Tang, B. Regulation of Carbohydrate Metabolism by Trehalose-6-Phosphate Synthase 3 in the Brown Planthopper, Nilaparvata lugens. Front. Physiol. 2020, 11, 575485. [Google Scholar] [CrossRef]
- Liu, Y.K.; Zhu, Y.; Wan, S.J.; Wang, X.Z.; Guan, L.W.; Xu, C.D.; Xie, B.H.; Wang, S.G.; Sun, S.S.; Tang, B. Trehalase regulates ovarian maturation and egg hatchability of Nilaparvata lugens. J. Pest. Sci. 2025, 98, 1645–1654. [Google Scholar] [CrossRef]
- Li, H.; Zaihui, Z.; Hongxia, H.; Weihua, M. Comparative transcriptome analysis of defense response of rice to Nilaparvata lugens and Chilo suppressalis infestation. Int. J. Biol. Macromol. 2020, 163, 2270–2285. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Niu, J.Z.; Ding, B.Y.; Zhang, Q.; Ye, C.; Zhang, W.; Smagghe, G.; Wang, J.J. Vitellogenin and its receptor play essential roles in the development and reproduction of the brown citrus aphid, Aphis (Toxoptera) citricidus. Insect Mol. Biol. 2018, 27, 221–233. [Google Scholar] [CrossRef]
- Lu, K.; Shu, Y.; Zhou, J.; Zhang, X.; Zhang, X.; Chen, M.; Yao, Q.; Zhou, Q.; Zhang, Q. Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stål). J. Insect Physiol. 2015, 73, 20–29. [Google Scholar] [CrossRef]
- Kamruzzaman, A.S.M.; Mikani, A.; Mohamed, A.A.; Elgendy, A.M.; Takeda, M. Crosstalk among indoleamines, neuropeptides and JH/20E in regulation of reproduction in the American cockroach, Periplaneta americana. Insects 2020, 11, 155. [Google Scholar] [CrossRef]
- Song, J.; Zhou, S. Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell Mol. Life Sci. 2020, 77, 1893–1909. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Nagaba, Y.; Elgendy, A.M.; Takeda, M. Regulation of vitellogenin genes in insects. Entomol. Sci. 2014, 17, 269–282. [Google Scholar] [CrossRef]
- Yang, M.M.; Zhao, L.N.; Shen, Q.D.; Xie, G.Q.; Wang, S.G.; Tang, B. Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the brown planthopper Nilaparvata lugens. Pest. Manag. Sci. 2017, 73, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, M.; Shen, Q.; Liu, X.; Shi, Z.; Wang, S.; Tang, B. Functional characterization of three trehalase genes regulating the chitin metabolism pathway in rice brown planthopper using RNA interference. Sci. Rep. 2016, 6, 27841. [Google Scholar] [CrossRef]
- Tang, B.; Yang, M.M.; Shen, Q.D.; Xu, Y.X.; Wang, H.J.; Wang, S.G. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, 10. [Google Scholar] [CrossRef]
- Lu, F.; Qi, J.G.; Qin, R.R. The processes of morphological change and grading criteria for ovarian development in the brown planthopper. Chin. Bull. Entomol. 2011, 48, 1394–1400. [Google Scholar]
- Yang, X.; Liu, S.; Lu, W.; Du, M.; Qiao, Z.; Liang, Z.; An, Y.; Gao, J.; Li, X. Delta and jagged are candidate target genes of RNAi biopesticides for the control of Nilaparvata lugens. Front. Bioeng. Biotechnol. 2022, 10, 1023729. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Biswas, A.; Sarkar, S.; Chakraborty, G.; Gaber, A.; Kobeasy, M.I.; Hossain, A. Evaluation and characterization of indigenous rice (Oryza sativa L.) landraces resistant to brown planthopper Nilaparvata lugens (Stål.) biotype 4. PeerJ 2022, 10, e14360. [Google Scholar] [CrossRef]
- Kang, K.; Cai, Y.; Yue, L.; Zhang, W. Effects of Different Nutritional Conditions on the Growth and Reproduction of Nilaparvata lugens (Stål). Front. Physiol. 2022, 12, 794721. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Albiter, H.; Mitford, R.; Genta, F.A.; Sant’Anna, M.R.; Dillon, R.J. Reactive oxygen species scavenging by catalase is important for female Lutzomyia longipalpis fecundity and mortality. PLoS ONE 2011, 9, e17486. [Google Scholar]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Rad. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Bashandy, T.; Guilleminot, J.; Vernoux, T.; Caparros-Ruiz, D.; Ljung, K.; Meyer, Y.; Reichheld, J. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 2010, 22, 376–391. [Google Scholar] [CrossRef]
- Xie, M.; Zhong, Y.; Lin, L.; Zhang, G.; Su, W.; Ni, W.; Qu, M.; Chen, H. Transcriptome analysis of Holotrichia oblita reveals differentially expressed unigenes related to reproduction and development under different photoperiods. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 42, 100959. [Google Scholar] [CrossRef]
- Abdelfattah, E.A.; Augustyniak, M.; Yousef, H.A. Stage-, sex- and tissue-related changes in H2O2, glutathione concentration, and glutathione-dependent enzymes activity in Aiolopus thalassinus (Orthoptera: Acrididae) from heavy metal polluted areas. Ecotoxicology 2021, 30, 478–491. [Google Scholar] [CrossRef]
- Zhao, L.; Xue, H.; Elumalai, P.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Luo, J.; Cui, J.; et al. Sublethal acetamiprid affects reproduction, development and disrupts gene expression in Binodoxys communis. Environ. Sci. Pollut. Res. Int. 2024. [Google Scholar] [CrossRef]
- Saranya, M.; Kennedy, J.S.; Anandham, R. Functional characterization of cultivable gut bacterial communities associated with rugose spiralling whitefly, Aleurodicus rugioperculatus Martin. 3 Biotech 2022, 12, 14. [Google Scholar] [CrossRef]
- Enya, S.; Yamamoto, C.; Mizuno, H.; Esaki, T.; Lin, H.K.; Iga, M.; Morohashi, K.; Hirano, Y.; Kataoka, H.; Masujima, T.; et al. Dual Roles of glutathione in ecdysone biosynthesis and antioxidant function during larval development in Drosophila. Genetics 2017, 207, 1519–1532. [Google Scholar] [CrossRef]
- Luck, M.R.; Jeyaseelan, I.; Scholes, R.A. Ascorbic acid and fertility. Biol. Reprod. 1995, 52, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Homma, T.; Lee, J.; Mitsuhashi, H.; Yamada, K.I.; Kimura, N.; Yamamoto, Y.; Fujii, A.J. Ascorbic acid and CoQ10 ameliorate the reproductive ability of Superoxide dismutase 1-deficient female mice. Biol. Reprod. 2020, 102, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Dutta, R.K.; Muralidhar, K.; Gupta, R.D. Decreased ascorbic acid biosynthesis in response to PMSG in the pre-pubertal female rat ovary. Res. Vet. Sci. 2020, 131, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, L.W.; Xing, X.R.; Xie, Y.Q.; Li, Y.J.; Liu, Z.X.; Wang, J.; Wu, F.A.; Sheng, S. Lipid dynamics, identification, and expression patterns of fatty acid synthase genes in an endoparasitoid, Meteorus pulchricornis (Hymenoptera: Braconidae). Int. J. Mol. Sci. 2020, 21, 6228. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, W.; Liu, C.; Chen, L.; Xu, Y.; Xiao, H.; Liang, G. Methoprene-Tolerant (Met) Is Indispensable for larval metamorphosis and female reproduction in the cotton bollworm Helicoverpa armigera. Front. Physiol. 2018, 9, 1601. [Google Scholar] [CrossRef] [PubMed]
- Gondim, K.C.; Atella, G.C.; Pontes, E.G.; Majerowicz, D. Lipid metabolism in insect disease vectors. Insect Biochem. Mol. Biol. 2018, 101, 108–123. [Google Scholar] [CrossRef]
- Arêdes, D.S.; Rios, T.; Carvalho-Kelly, L.F.; Braz, V.; Araripe, L.O.; Bruno, R.V.; Meyer-Fernandes, J.R.; Ramos, I.; Gondim, K.C. Deficiency of Brummer lipase disturbs lipid mobilization and locomotion, and impairs reproduction due to defects in the eggshell ultrastructure in the insect vector Rhodnius prolixus. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159442. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, H.; Li, Y.; Zhang, T.F.; Liu, Y.H. Trehalose-6-phosphate phosphatases are involved in trehalose synthesis and metamorphosis in Bactrocera minax. Insect Sci. 2022, 29, 1643–1658. [Google Scholar] [CrossRef]
- Magalhães, R.S.S.; De Lima, K.C.; De Almeida, D.S.G.; De Mesquita, J.F.; Eleutherio, E.C.A. Trehalose-6-phosphate as a potential lead candidate for the development of TPS1 inhibitors: Insights from the trehalose biosynthesis pathway in diverse yeast species. Appl. Biochem. Biotechnol. 2017, 181, 914–924. [Google Scholar] [CrossRef]
- Yang, H.J.; Cui, M.Y.; Zhao, X.H.; Zhang, C.Y.; Hu, Y.S.; Fan, D. Trehalose-6-phosphate synthase regulates chitin synthesis in Mythimna separata. Front. Physiol. 2023, 14, 1109661. [Google Scholar] [CrossRef]
- Santos, R.; Alves-Bezerra, M.; Rosas-Oliveira, R.; Majerowicz, D.; Meyer-Fernandes, J.R.; Gondim, K.C. Gene identification and enzymatic properties of a membrane-bound trehalase from the ovary of Rhodnius prolixus. Arch. Insect Biochem. Physiol. 2012, 81, 199–213. [Google Scholar] [CrossRef]
- Tellis, M.B.; Mohite, S.D.; Nair, V.S.; Chaudhari, B.Y.; Ahmed, S.; Kotkar, H.M.; Joshi, R.S. Inhibition of trehalose synthesis in lepidoptera reduces larval fitness. Adv. Biol. 2024, 8, e2300404. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, W.; Yang, L.; He, Q.; Zhou, S. Juvenile hormone promotes locust fat body cell polyploidization and vitellogenesis by activating the transcription of Cdk6 and E2f1. Insect Biochem. Mol. Biol. 2018, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 87–103. [Google Scholar] [CrossRef]
- Benrabaa, S.A.M.; Orchard, I.; Lange, A.B. The role of ecdysteroid in the regulation of ovarian growth and oocyte maturation in Rhodnius prolixus, a vector of Chagas disease. J. Exp. Biol. 2022, 1, jeb244830. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C.; Liu, C.; Song, Q.; Zhou, S. Rhythmic change of adipokinetic hormones diurnally regulates locust vitellogenesis and egg development. Insect Mol. Biol. 2020, 29, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wang, Y.; Chen, X.; Zhang, X.; Li, W.; Cheng, Y.; Li, Y.; Zhou, J.; You, K.; Song, Q.; et al. Adipokinetic hormone receptor mediates trehalose homeostasis to promote vitellogenin uptake by oocytes in Nilaparvata lugens. Front. Physiol. 2018, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Han, Y.; Mao, Q.; Fu, H.; Luo, Y.; Hua, L.; Liu, B.; Hu, G.; Wang, S.; Desneux, N.; et al. Regulation of three novel pepper thiothiazolidinones on the fecundity of Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 204, 106033. [Google Scholar] [CrossRef] [PubMed]
- Keyes-Scott, N.I.; Swade, K.R.; Allen, L.R.; Vogel, K.J. RNAi-mediated knockdown of two orphan G protein-coupled receptors reduces fecundity in the yellow fever mosquito Aedes aegypti. Front. Insect Sci. 2023, 3, 1197945. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.H.; Bomfim, L.; Atella, G.C.; Masuda, H.; Ramos, I. Silencing of RpATG6 impaired the yolk accumulation and the biogenesis of the yolk organelles in the insect vector R. prolixus. PLoS Negl. Trop. Dis. 2018, 12, e0006507. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, S.; Bamu, A.; Dai, H.; Lin, X. Nilaparvata lugens ERR2 regulates moulting and ovary development is related to hormone signalling. Insect Mol. Biol. 2023, 32, 376–386. [Google Scholar] [CrossRef]
- Ruan, Y.; Wong, N.K.; Zhang, X.; Zhu, C.; Wu, X.; Ren, C.; Luo, P.; Jiang, X.; Ji, J.; Wu, X.; et al. Vitellogenin receptor (VgR) mediates oocyte maturation and ovarian development in the pacific white shrimp (Litopenaeus vannamei). Front. Physiol. 2020, 11, 485. [Google Scholar] [CrossRef]
- Gruntenko, N.E.; Rauschenbach, I.Y. Interplay of JH, 20E and biogenic amines under normal and stress conditions and its effect on reproduction. J. Insect Physiol. 2008, 54, 902–908. [Google Scholar] [CrossRef]
- Huangfu, N.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Chen, L.; Gao, X.; Niu, L.; Gao, M.; Ji, J.; et al. Insulin Receptor substrate-1 (IRS1) regulates oogenesis and vitellogenesis in Propylea japonica by mediating the FOXO transcription factor expression, independent of JH and 20E signaling pathways. J. Agric. Food Chem. 2023, 71, 300–310. [Google Scholar] [CrossRef]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef]
- Xue, W.H.; Xu, N.; Chen, S.J.; Liu, X.Y.; Zhang, J.L. Neofunctionalization of a second insulin receptor gene in the wing-dimorphic planthopper, Nilaparvata lugens. PLoS Genet. 2021, 17, e1009653. [Google Scholar] [CrossRef] [PubMed]
- Oldham, S. Obesity and nutrient sensing TOR pathway in flies and vertebrates: Functional conservation of genetic mechanisms. Trends Endocrinol. Metab. 2011, 22, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Xi, G.S.; Zhao, J. Vitellogenin regulates estrogen-related receptor expression by crosstalk with the JH and IIS-TOR signaling pathway in Polyrhachis vicina Roger (Hymenoptera, Formicidae). Gen. Comp. Endocrinol. 2021, 310, 113836. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).