Silencing the Circadian Clock Genes Cycle and Clock Disrupts Reproductive–Metabolic Homeostasis but Does Not Induce Reproductive Diapause in Arma chinensis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Sample Preparation
2.2. Molecular Cloning and Sequence Analysis
2.3. Real-Time Fluorescence Quantitative PCR Analysis
2.4. Diel Expression of AcCyc and AcClk Under Non-Diapause or Diapause Conditions
2.5. RNA Interference Bioassays
2.6. The Assessement of Potential Off-Targets of dsAcCyc and dsAcClk
2.7. Statistical Analysis
3. Results
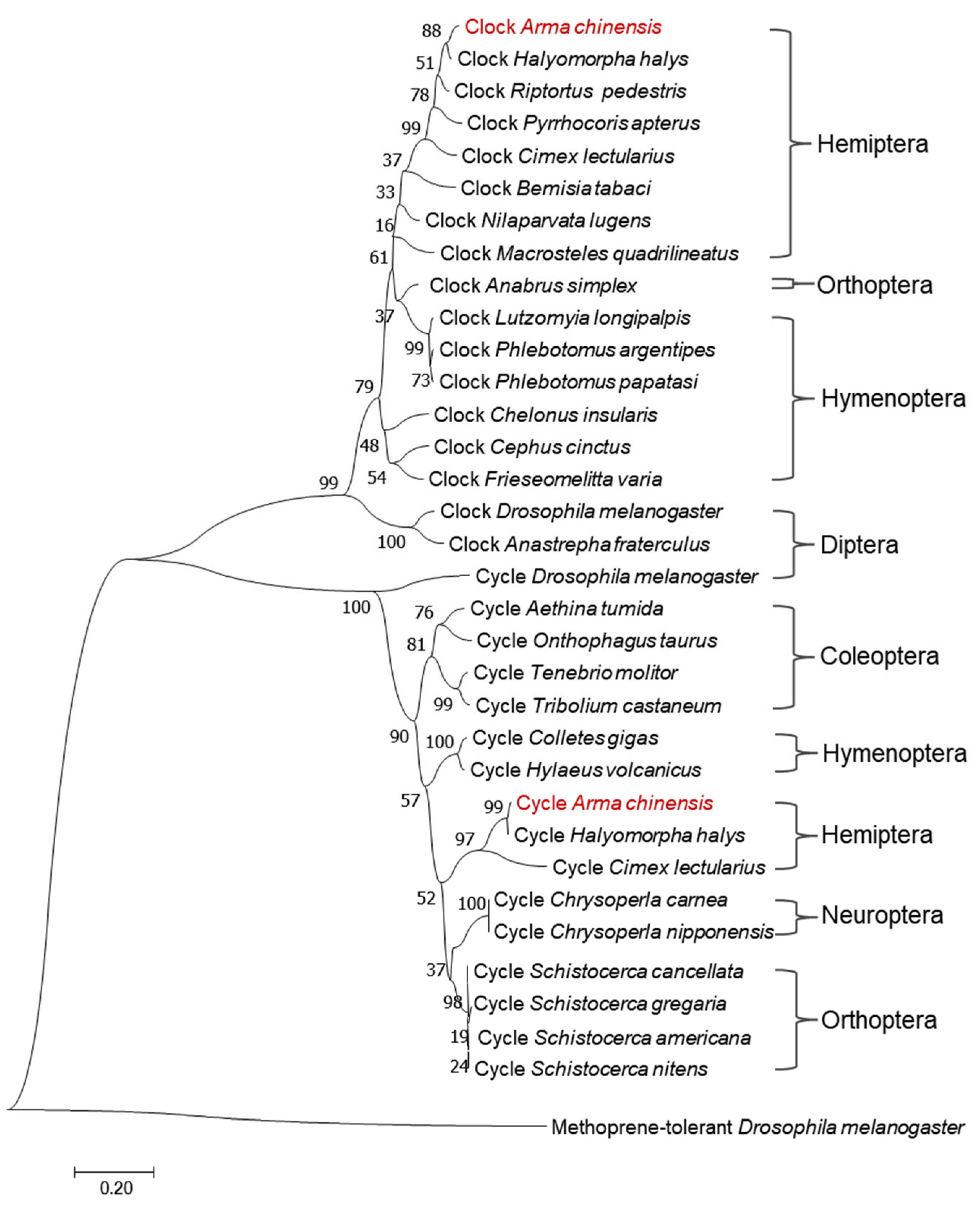
3.1. Identification and Characterization of the A. chinensis AcCyc and AcClk Gene
3.2. Spatiotemporal Expression Patterns of AcCyc and AcClk During Diapause and Non-Diapause Conditions
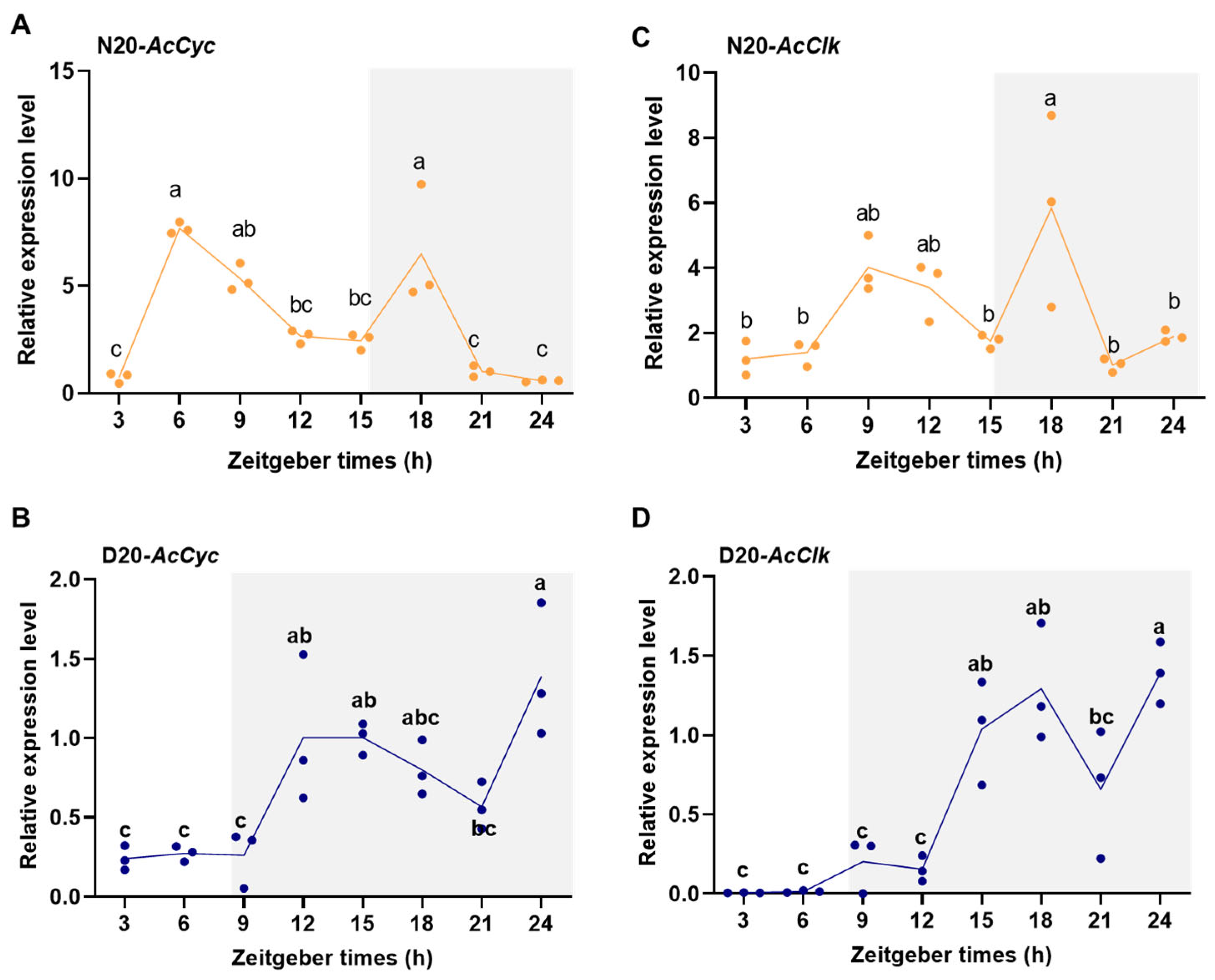
3.3. Rhythmic Expression of AcCyc and AcClk in Adults During Diapause and Non-Diapause Conditions
3.4. Silencing AcCyc and AcClk Genes Impeded Ovarian Development and Reduced Triglyceride (TAG) Content in Non-Diapausing Females
3.5. Silencing AcCyc and AcClk Genes Regulate Other Circadian Clock Genes
3.6. Silencing AcCyc and AcClk Genes Downregulate Juvenile Hormone Pathway Genes
3.7. Potential Off-Targets of dsAcCyc and dsAcClk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, Y.; Walker, A.A.; Meng, L.; Herzig, V.; Li, B. The predatory stink bug Arma custos (Hemiptera: Pentatomidae) produces a complex proteinaceous venom to overcome caterpillar prey. Biology 2023, 12, 691. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.Y.; Wu, H.H.; Coudron, T.A.; Zhang, L.S.; Wang, M.Q.; Liu, C.X.; Chen, H.Y. A meridic diet for continuous rearing of Arma chinensis (Hemiptera: Pentatomidae: Asopinae) on a zoophytogenous artificial diet and a secondary prey. Biol. Control 2013, 67, 491–497. [Google Scholar] [CrossRef]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Chen, J.J.; Liu, M.Y.; He, W.W.; Reynolds, J.A.; Wang, Y.N.; Wang, M.Q.; Zhang, L.S. Enhanced degradation of juvenile hormone promotes reproductive diapause in the predatory ladybeetle Coccinella septempunctata. Front. Physiol. 2022, 13, 877153. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Zhu, L.; Zhu, F.; Lei, C.L.; Wang, X.P. Juvenile hormone facilitates the antagonism between adult reproduction and diapause through the methoprene-tolerant gene in the female Colaphellus bowringi. Insect Biochem. Mol. Biol. 2016, 74, 50–60. [Google Scholar] [CrossRef]
- Guo, S.; Tian, Z.; Wu, Q.W.; King-Jones, K.; Liu, W.; Zhu, F.; Wang, X.P. Steroid hormone ecdysone deficiency stimulates preparation for photoperiodic reproductive diapause. PLoS Genet. 2021, 17, e1009352. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Lounibos, L.P. Evolution of dormancy and its photoperiodic control in pitcher-plant mosquitoes. Evolution 1977, 31, 546–567. [Google Scholar] [CrossRef]
- Chen, J.J.; Liu, X.X.; Guo, P.H.; Teets, N.M.; Zhou, J.C.; Chen, W.B.; Luo, Q.Z.; Kanjana, N.; Li, Y.Y.; Zhang, L.S. Regulation of forkhead box O transcription factor by insulin signaling pathway controls the reproductive diapause of the lady beetle, Coccinella septempunctata. Int. J. Biol. Macromol. 2024, 258 Pt 1, 128104. [Google Scholar] [CrossRef]
- Kostál, V. Eco-physiological phases of insect diapause. J. Insect Physiol. 2006, 52, 113–127. [Google Scholar] [CrossRef]
- Denlinger, D.L. Encyclopedia of Insects, 2nd ed.; Academic Press: Salt Lake City, UT, USA, 2009; pp. 267–271. [Google Scholar]
- Meuti, M.E.; Denlinger, D.L. Evolutionary links between circadian clocks and photoperiodic diapause in insects. Integr. Comp. Biol. 2013, 53, 131–143. [Google Scholar] [CrossRef]
- Dolezel, D. Photoperiodic time measurement in insects. Curr. Opin. Insect Sci. 2015, 7, 98–103. [Google Scholar] [CrossRef]
- Saunders, D.S. Dormancy, diapause, and the role of the circadian system in insect photoperiodism. Annu. Rev. Entomol. 2020, 65, 373–389. [Google Scholar] [CrossRef]
- Tataroglu, O.; Emery, P. The molecular ticks of the Drosophila circadian clock. Curr. Opin. Insect Sci. 2015, 7, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Stehlík, J.; Závodská, R.; Shimada, K.; Sauman, I.; Kostál, V. Photoperiodic induction of diapause requires regulated transcription of timeless in the larval brain of Chymomyza costata. J. Biol. Rhythms 2008, 23, 129–139. [Google Scholar] [CrossRef]
- Tobita, H.; Kiuchi, T. Knockouts of positive and negative elements of the circadian clock disrupt photoperiodic diapause induction in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2022, 149, 103842. [Google Scholar] [CrossRef]
- Cui, W.Z.; Qiu, J.F.; Dai, T.M.; Chen, Z.; Li, J.L.; Liu, K.; Wang, Y.J.; Sima, Y.H.; Xu, S.Q. Circadian clock gene period contributes to diapause via GABAeric-diapause hormone pathway in Bombyx mori. Biology 2021, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Daimon, T.; Shiomi, K.; Udaka, H.; Numata, H. Involvement of the clock gene period in the photoperiodism of the Silkmoth Bombyx mori. Zoolog Sci. 2021, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, L.; Zhu, L.; Tian, Z.; Shen, Z.; Cheng, J.; Zhang, S.; Li, Z.; Liu, X. Mutation of the clock gene timeless disturbs diapause induction and adult emergence rhythm in Helicoverpa armigera. Pest. Manag. Sci. 2023, 79, 1876–1884. [Google Scholar] [CrossRef]
- Kotwica-Rolinska, J.; Damulewicz, M.; Chodakova, L.; Kristofova, L.; Dolezel, D. Pigment dispersing factor is a circadian clock output and regulates photoperiodic response in the linden bug, Pyrrhocoris apterus. Front. Physiol. 2022, 13, 884909. [Google Scholar] [CrossRef]
- Kotwica-Rolinska, J.; Pivarciova, L.; Vaneckova, H.; Dolezel, D. The role of circadian clock genes in the photoperiodic timer of the Linden bug Pyrrhocoris apterus during the nymphal stage. Physiol. Entomol. 2017, 42, 266–273. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. Feeding mistiming decreases reproductive fitness in flies. Cell Metab. 2011, 13, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, W.; Li, Y.; Chen, J.; Teets, N.M.; Zhang, L. Metabolic and transcriptional regulation of reproductive diapause in Arma chinensis. iScience 2025, 28, 111761. [Google Scholar] [CrossRef]
- Shen, Z.; Luo, Q.; Mao, J.; Li, Y.; Wang, M.; Zhang, L. Molecular identification of two thioredoxin genes and their function in antioxidant defense in Arma chinensis diapause. Front. Physiol. 2024, 15, 1440531. [Google Scholar] [CrossRef]
- Luo, Q.; Mao, J.; Li, Y.; Wang, M.; Zhang, L.; Shen, Z. Molecular characterization of a novel thioredoxin-related transmembrane protein gene AcTMX3 that plays important roles in antioxidant defence in Arma chinensis diapause. Insect Mol. Biol. 2025, 34, 218–227. [Google Scholar] [CrossRef]
- Wang, S.L.; Zhang, M.S.; Zhou, L.; Jing, X.Y.; Zhang, H.Z.; Li, Y.Y.; Zhang, L.S. Gene expression pattern and function analysis of juvenile hormone epoxy-hydrolase during diapause of Arma chinensis. Jiangsu Agric. Sci. 2024, 52, 43–51. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, Z.; Chen, J.; Li, Y.; Mao, J.; Wang, M.; Zhang, L. Functional analysis of adipokinetic hormone signaling in reproductive diapause of Coccinella septempunctata. Pest. Manag. Sci. 2024, 80, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Cao, Z.C.; Wu, Y.H.; Tang, R.; Sun, S.L.; Yang, Z.; Liu, T.X.; Jing, X.F. Functional analysis of DGAT1 in the cotton bollworm, Helicoverpa armigera. Entomol. Gen. 2022, 42, 891–899. [Google Scholar] [CrossRef]
- Lu, K.; Zhou, J.M.; Chen, X.; Li, W.R.; Li, Y.; Cheng, Y.B.; Yan, J.; You, K.K.; Yuan, Z.N.; Zhou, Q. Deficiency of Brummer impaires lipid mobilization and jh-mediated vitellogenesis in the brown planthopper, Nilaparvata lugens. Front. Physiol. 2018, 9, 1535. [Google Scholar] [CrossRef]
- Lye, P.Y.; Chika, S.; Fukushima, Y.; Takaki, K.; Liew, M.W.O.; Yamamoto, M.; Wakabayashi, K.; Mori, H.; Kotani, E. Cytotoxin-mediated silk gland organ dysfunction diverts resources to enhance silkworm fecundity by potentiating nutrient-sensing IIS/TOR pathways. iScience 2024, 27, 108853. [Google Scholar] [CrossRef]
- Tamai, T.; Shiga, S.; Goto, S.G. Roles of the circadian clock and endocrine regulator in the photoperiodic response of the brown-winged green bug Plautia stali. Physiol. Entomol. 2019, 44, 43–52. [Google Scholar] [CrossRef]
- Bajgar, A.; Jindra, M.; Doleze, D. Autonomous regulation of the insect gut by circadian genes acting downstream of juvenile hormone signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 4416–4421. [Google Scholar] [CrossRef]
- Smykal, V.; Chodakova, L.; Hejnikova, M.; Briedikova, K.; Wu, B.C.-H.; Vaneckova, H.; Chen, P.; Janovska, A.; Kyjakova, P.; Vacha, M.; et al. Steroid receptor coactivator TAIMAN is a new modulator of insect circadian clock. PLoS Genet. 2023, 19, e1010924. [Google Scholar] [CrossRef]
- Mukai, A.; Mano, G.; Marteaux, L.D.; Shinada, T.; Goto, S.G. Juvenile hormone as a causal factor for maternal regulation of diapause in a wasp. Insect Biochem. Mol. Biol. 2022, 144, 103758. [Google Scholar] [CrossRef]
- Ma, H.Y.; Li, Y.Y.; Li, L.; Tan, Y.; Pang, B.P. Juvenile hormone regulates the reproductive diapause through Methoprene-tolerant gene in Galeruca daurica. Insect Mol. Biol. 2021, 30, 12710. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Guo, S.; Li, J.X.; Zhu, F.; Liu, W.; Wang, X.P. Juvenile hormone biosynthetic genes are critical for regulating reproductive diapause in the cabbage beetle. Insect Biochem. Mol. Biol. 2021, 139, 103654. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, B.; Tian, Z.; Loof, A.D.; Wang, J.L.; Wang, X.P.; Liu, W. Key role of juvenile hormone in controlling reproductive diapause in females of the Asian lady beetle Harmonia axyridis. Pest Manag. Sci. 2021, 78, 6619. [Google Scholar] [CrossRef] [PubMed]
- Smykal, V.; Dolezel, D. Evolution of proteins involved in the final steps of juvenile hormone synthesis. J. Insect Physiol. 2023, 145, 104487. [Google Scholar] [CrossRef]
- Minakuchi, C.; Namiki, T.; Yoshiyama, M.; Shinoda, T. RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J. 2008, 275, 2919–2931. [Google Scholar] [CrossRef]
- Zheng, S.R.; Wang, Y.H.; Li, G.Y.; Qin, S.; Dong, Z.; Yang, X.; Xu, X.M.; Fang, G.Q.; Li, M.W.; Zhan, S. Functional polymorphism of CYCLE underlies the diapause variation in moths. Science 2025, 388, 2129. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Dai, Y.F.; Zhao, Y.L.; Li, X.; An, H.M.; Jones, K.K.; Wang, J.L.; Wanga, X.P.; Liu, W. Noncanonical action of circadian clock genes controls winter diapause entry via the NuA4/TIP60 complex in Harmonia axyridis. Proc. Natl. Acad. Sci. USA 2025, 122, e2510550122. [Google Scholar] [CrossRef] [PubMed]
- Suri, V.; Lanjuin, A.; Rosbash, M. TIMELESS-dependent positive and negative autoregulation in the Drosophila circadian clock. EMBO J. 1999, 18, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Peng, Y.; Peng, J.; Yu, S.; Wu, C.; Yang, X.; Xiao, Y. A supergene controls facultative diapause in the crop pest Helicoverpa armigera. Cell Rep. 2024, 43, 114939. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Luo, Q.; Zhang, M.; Lv, Z.; Liu, M.; Huang, X.; Li, Y.; Zhang, L. Silencing the Circadian Clock Genes Cycle and Clock Disrupts Reproductive–Metabolic Homeostasis but Does Not Induce Reproductive Diapause in Arma chinensis. Insects 2025, 16, 1192. https://doi.org/10.3390/insects16121192
Chen J, Luo Q, Zhang M, Lv Z, Liu M, Huang X, Li Y, Zhang L. Silencing the Circadian Clock Genes Cycle and Clock Disrupts Reproductive–Metabolic Homeostasis but Does Not Induce Reproductive Diapause in Arma chinensis. Insects. 2025; 16(12):1192. https://doi.org/10.3390/insects16121192
Chicago/Turabian StyleChen, Junjie, Qiaozhi Luo, Maosen Zhang, Zhuoling Lv, Meng Liu, Xiangchao Huang, Yuyan Li, and Lisheng Zhang. 2025. "Silencing the Circadian Clock Genes Cycle and Clock Disrupts Reproductive–Metabolic Homeostasis but Does Not Induce Reproductive Diapause in Arma chinensis" Insects 16, no. 12: 1192. https://doi.org/10.3390/insects16121192
APA StyleChen, J., Luo, Q., Zhang, M., Lv, Z., Liu, M., Huang, X., Li, Y., & Zhang, L. (2025). Silencing the Circadian Clock Genes Cycle and Clock Disrupts Reproductive–Metabolic Homeostasis but Does Not Induce Reproductive Diapause in Arma chinensis. Insects, 16(12), 1192. https://doi.org/10.3390/insects16121192






