Simple Summary
The cotton aphid is a major global pest, damaging crops through its explosive reproduction. A tiny wasp, Binodoxys communis, shows promise as a natural control method but scientists do not fully understand how it manipulates the aphid’s reproduction across different host stages. Our research reveals that parasitism by the wasp impairs aphid development and severely suppresses reproduction, with the most pronounced effects on younger aphids. We discovered that silencing a single master regulator gene is sufficient to control young aphids, whereas suppressing reproduction in adults requires the coordinated downregulation of multiple genes. This explains why the wasp favors nymphs—an efficient “low-cost, high-reward” strategy honed by evolution. These insights deepen our grasp of nature’s pest battles and pinpoint genes for better eco-friendly farming tools, helping farmers protect crops without the use of harsh chemicals.
Abstract
The cotton aphid Aphis gossypii is a globally significant agricultural pest that threatens crop production through its prolific reproduction. While the parasitoid wasp Binodoxys communis offers promising potential for biological control, the molecular mechanisms underlying its reproductive manipulation of aphid hosts remain poorly understood. Here, we investigated the stage-specific parasitism strategies of B. communis on A. gossypii using integrated biological observations and transcriptomic analysis. Parasitism significantly prolonged aphid development and suppressed reproduction across all host stages, with severity inversely correlated with host age at parasitism. Transcriptomic analysis of ovaries of parasitized aphids revealed 1168 differentially expressed genes, with temporal progression from minimal changes in nymphs (7 DEGs at day 1) to extensive disruption in adults (549 DEGs at day 3). Notably, juvenile hormone acid methyltransferase (JHAMT), the rate-limiting enzyme in juvenile hormone biosynthesis, emerged as a master regulator that is differentially targeted across host stages. In 3rd instar nymphs, single-gene suppression of JHAMT (−3.23-fold change) achieved effective reproductive control, whereas adult parasitism required progressive manipulation of multiple genes including JHAMT, FOHSDR, ALDH, and JHEH. The vitellogenin-vitellogenin receptor system only showed coordinated downregulation in adults, whereas nymphs exhibited preemptive receptor suppression before vitellogenesis onset. These findings demonstrate that B. communis has evolved to exploit a developmental window where host manipulation is most efficient—3rd instar nymphs, which possess sufficient resources for parasitoid development and lack the complex compensatory mechanisms found in adults. This “low-cost, high-reward” strategy based on precision targeting of master regulators in nymphs compared to multi-gene assault in adults, revealing the evolutionary optimization of parasitoid manipulation strategies. Our results provide molecular insights into parasitoid-host coevolution and identified key regulatory targets for developing innovative biological control strategies against this important agricultural pest.
1. Introduction
Aphis gossypii Glover (Hemiptera: Aphididae) is a highly adaptable and prolific pest with a worldwide distribution that causes serious damage to cotton production [,,]. In recent years, large-scale outbreaks of this aphid have resulted in substantial economic losses []. The challenges of effective aphid management are further compounded by climate change, crop diversification, and agricultural intensification, necessitating the development of innovative control strategies. Binodoxys communis Gahan (Hymenoptera: Braconidae), a koinobiont endoparasitoid, is a promising biological control agent for various aphid species, including cotton, corn, and soybean aphids [,,]. Parasitoid wasps orchestrate host manipulation through four primary mechanisms mediated by venom proteins and peptides: suppression of host immunity [,,,,,], growth and development [,,,], nutrient metabolism [,,], and reproduction [,,,]. Among these, reproductive manipulation represents a particularly effective strategy, as it directly eliminates host population growth while preserving host resources for parasitoid development [].
Parasitoids exhibit selective host preferences influenced by developmental stage traits, reflecting an evolutionary optimization strategy in which they exploit developmental windows for efficient manipulation through minimal molecular intervention [,,,,]. Although some aphidiid parasitoids can oviposit in all host stages, including adults, they typically exhibit a preference for 2nd–3rd or 2nd–4th instar nymphs [,]. Despite extensive research on parasitoid-induced alterations in host immunity, development, and metabolism [,,,,], the molecular mechanisms governing stage-specific strategies, particularly how B. communis regulates the reproductive capacity of A. gossypii, remain largely unexplored. The ovary, as the primary reproductive organ, serves as an ideal system for investigating parasitoid-mediated reproductive manipulation at the molecular level. Understanding the transcriptomic changes in ovaries of parasitized aphids could reveal key regulatory targets and clarify the molecular basis for the efficiency of stage-specific manipulation strategies.
In this study, we investigated the impact of B. communis parasitism on the development and reproduction of A. gossypii across different host stages. Through comprehensive transcriptomic analysis of ovarian tissues from parasitized and non-parasitized aphids, we aimed to: (1) characterize the molecular mechanisms underlying reproductive suppression; (2) identify stage-specific gene expression patterns that explain parasitoid host preferences; and (3) elucidate the molecular basis of parasitoid preference for specific host stages. These findings will enhance the understanding of parasitoid-host coevolution and provide theoretical foundation for optimizing biological control strategies in agricultural systems.
2. Materials and Methods
2.1. Insects and Plants
The cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae), was reared parthenogenetically at the Cotton Research Institute of the Chinese Academy of Agricultural Sciences (36°5′34.8″ N, 114°31′47.19″ E) under controlled conditions of 26.0 ± 1 °C, 65 ± 5% relative humidity (RH), and a 16:8 h (light: dark) photoperiod. The parasitoid wasp Binodoxys communis Gahan (Hymenoptera: Braconidae) was maintained under identical conditions. Cotton plants (Gossypium hirsutum cv. Zhongmian49) from the Mid-term Database of Cotton Germplasm Resources at the same institute were used as host plants and were cultivated at 26.0 ± 1 °C, 70 ± 5% RH, and a 14:10 h (light: dark) photoperiod.
2.2. The Growth and Development of A. gossypii in Response to the Parasitism by B. communis
To assess the impact of parasitism by B. communis on the growth and development of A. gossypii, wingless aphids at various developmental stages (1st, 2nd, 3rd, and 4th instars) were placed on glass dishes lined with cotton leaves. Mated female B. communis were introduced for parasitization, and a cotton ball soaked in honey water was provided as a food source. Non-parasitized aphids of the same age served as controls. Parasitism was confirmed by observing the stinging behavior. Subsequently, both parasitized and control aphids were transferred to an agar-based culture system with fresh cotton leaves for further observation. Only aphids that successfully developed into mummies were recorded as parasitized and used for subsequent data analysis. Three replicates were established, each containing 20 aphids. The developmental stages of both groups were recorded daily.
2.3. The Reproduction of A. gossypii in Response to the Parasitism by B. communis
The effect of parasitism on the reproductive capacity of A. gossypii was evaluated using a similar experimental setup as described in Section 2.2. Parasitized and non-parasitized wingless aphids were monitored daily for offspring production. The experiment was conducted in 3 replicates, each with 20 aphids.
2.4. Ovary Dissection and RNA Extraction
Wingless 3rd instar nymphs and adults A. gossypii, both parasitized and non-parasitized by B. communis, were utilized for ovary dissection. Parasitism was confirmed by observing the stinging behavior, and successfully parasitized aphids were promptly removed. Both the parasitized aphids and the non-parasitized aphids were maintained on a cotton leaf under the same incubator conditions. Ovary dissection was performed under a microscope after 1 and 3 days of parasitism for 3rd instar nymphs and adults. Only aphid samples in which parasitoid larvae were confirmed present during dissection were processed for RNA extraction and sequencing. Ovaries from non-parasitized aphids of the same age was dissected as controls. The collected samples were frozen in liquid nitrogen and stored at −80 °C until RNA extraction for transcriptome sequencing. Three replicates were prepared, each containing 60 ovaries. Total RNA was extracted from the ovaries of both parasitized and non-parasitized A. gossypii using the TRIzol method. RNA concentration and purity were assessed using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA), and RNA integrity was verified by 1.5% agarose gel electrophoresis. Only samples with A260/A280 ratios between 1.8–2.0 and clear 28S/18S rRNA bands were used for sequencing.
2.5. Library Preparation and Transcriptome Sequencing
Poly(A)+ mRNA was isolated from total RNA using oligo(dT) magnetic beads. Purified mRNA was fragmented to ~300 bp using fragmentation buffer. First-strand cDNA was synthesized using random hexamer primers and reverse transcriptase, followed by second-strand synthesis to generate double-stranded cDNA. Libraries were prepared by end-repair, A-tailing, and adapter ligation. Sequencing was performed on an Illumina NovaSeq 6000 platform (Majorbio, Shanghai, China), generating 150 bp paired-end reads.
2.6. Data Processing and Analysis
Raw reads were processed using fastp v0.19.5 (https://github.com/OpenGene/fastp, accessed on 13 December 2024) to remove adapters, low-quality bases (Q < 30), ambiguous nucleotides (>10% N), and short reads (<50 bp). Clean reads were mapped to the A. gossypii reference genome using HISAT2 v2.1.0 (http://ccb.jhu.edu/software/hisat2/index.shtml, accessed on 18 December 2021) with default parameters. Transcript assembly and abundance quantification were performed using StringTie v2.1.2 (https://ccb.jhu.edu/software/stringtie/, accessed on 21 December 2021) and RSEM v1.3.3, respectively. Gene expression was normalized as FPKM (Fragments Per Kilobase per Million mapped reads). Genes with FPKM > 1 were retained for analysis. Differential expression analysis was conducted using DESeq2 with thresholds of |log2(fold change)| ≥ 1 and adjusted p-value < 0.05. Functional enrichment analyses were performed using GOatools v0.6.5 for Gene Ontology and custom R scripts for KEGG pathways. Enrichment significance was determined using Fisher’s exact test with Benjamini–Hochberg correction (P-adjust < 0.05).
2.7. RT-qPCR to Assess Gene Expression Change
Seven reproduction-related differentially expressed genes (DEGs) were selected for validation. Gene-specific primers were designed using Primer 6 and synthesized by Sangon Biotech (Shanghai, China) (Table 1). RT-qPCR was performed using SYBR Green chemistry with β-actin serving as the reference gene for normalization. Relative expression was calculated using the 2−ΔΔCT method []. Three biological replicates with three technical replicates each were analyzed.

Table 1.
Primer sequences for reproduction-related genes and reference gene.
2.8. Statistical Analysis
Data were analyzed using SPSS version 20 (IBM Corp., Armonk, NY, USA). Differences between parasitized and control groups were assessed using the appropriate statistical tests (e.g., Student’s t-test or ANOVA), with the significance threshold set at p < 0.05. Confidence intervals (CIs) were calculated at 95%. Figures were generated using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA) and the Majorbio cloud platform (https://www.majorbio.com, accessed on 20 May 2025).
3. Results
3.1. The Growth and Development of A. gossypii in Response to the Parasitism by B. communis
As shown in Figure 1, parasitism by B. communis significantly delayed the growth and development of A. gossypii compared to non-parasitized controls. The duration of the 4th instar stage was extended by 19.1 h when aphids were parasitized as 2nd instars, and by 4 h when parasitized as 3rd instars. Parasitism consistently prolonged developmental times from 1st to 2nd, 3rd and 4th instars (from 16.9, 31.7, and 29.2 h to 27.7, 39.5, and 37.8 h, respectively), with no molting observed beyond the 4th instar. Regardless of the initial instar (1st, 2nd, 3rd, or 4th), parasitized A. gossypii nymphs consistently exhibited prolonged developmental durations across all instars (2nd to adult), with all stages demonstrating similar extension trends. These results indicated that parasitism by B. communis significantly impaired the growth and development of A. gossypii.

Figure 1.
Developmental durations at different host stages [mean ± standard error (number of individuals that completed the stage)]. Letters indicate the significance of the same parasitic days at the same age (T tests with CIs = 95%).
3.2. Reproduction of A. gossypii in Response to the Parasitism by B. communis
The fecundity of A. gossypii was significantly reduced by B. communis compared to the non-parasitized individuals, with reduction severity dependent on host developmental stage. The maximum reduction occurred in 1st instar, while minimal reduction occurred in 4th instar. After parasitization, the average number of offspring numbers of 1st, 2nd, 3rd, and 4th aphids were 1.34, 2.12, 5.46, and 7.20, respectively, while the average number of offspring of non-parasitized aphids were 33.4, 35.6, 35.7, and 31.2 (Figure 2).

Figure 2.
The offspring production of Aphis gossypii in Response to Parasitism by Binodoxys communis [mean ± standard error means reproductive number (±SD) per generation]. Different letters indicate statistically significant differences between treatments within the different generations (Tukey’s HSD test with CIs = 95%).
3.3. Transcriptome Sequencing and Differential Expression Analysis
A total of 155.53 Gb of clean data were generated after quality filtering, (≥6.03 Gb/sample), with a GC content ranging from 32.57% to 36.29%. Data reliability was confirmed by Q20 (>97%) and Q30 (>91.6%) scores. A total of 1168 differentially expressed genes (DEGs) were identified across all comparisons between parasitized and non-parasitized samples. Specifically, after the 3rd instar nymphs were parasitized for 1 day (Figure 3A,B), 7 DEGs were found (2 upregulated, 5 downregulated). After the 3rd instar nymphs were parasitized for 3 days (Figure 3A,C), 324 DEGs were identified (217 upregulated, 107 downregulated). After the adults were parasitized for 1 day (Figure 4A,B), 288 DEGs were detected (108 upregulated, 180 downregulated). After the adults were parasitized for 3 days (Figure 4A,C), 549 DEGs were observed (320 upregulated, 229 downregulated).
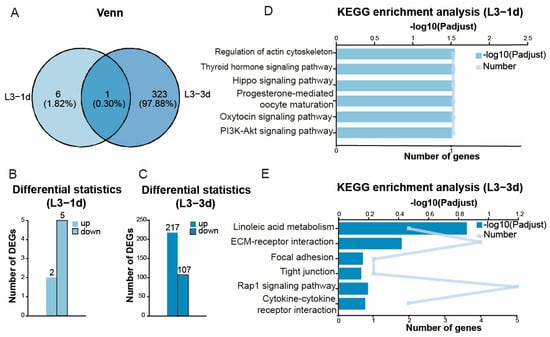
Figure 3.
Differential gene expression and KEGG enrichment analysis of ovaries of 3rd instar Aphis gossypii nymphs following parasitism by Binodoxys communis. (A) Venn diagram of differentially expressed genes (DEGs) in 3rd instar A. gossypii nymphs parasitized by B. communis on day 1 (L3-1d) vs. day 3 (L3-3d). Data annotations indicate gene counts and proportions. (B) Number of DEGs after the nymphs were parasitized for 1 day. (C) Number of DEGs after the nymphs were parasitized for 3 days. (D) KEGG enrichment analysis after the nymphs were parasitized for 1 day. (E) KEGG enrichment analysis after the nymphs were parasitized for 3 days.

Figure 4.
Differential gene expression and KEGG enrichment analysis of ovaries of Aphis gossypii adults following parasitism by Binodoxys communis. (A) Venn diagram of differentially expressed genes (DEGs) in A. gossypii adults parasitized by B. communis on day 1 (Ad-1d) vs. day 3 (Ad-3d). Data annotations indicate gene counts and proportions. (B) Number of DEGs after the adults were parasitized for 1 day. (C) Number of DEGs after the adults were parasitized for 3 days. (D) KEGG enrichment analysis after the adults were parasitized for 1 day. (E) KEGG enrichment analysis after the adults were parasitized for 3 days.
3.4. KEGG Pathway Analysis
KEGG pathway analysis revealed key pathways implicated in the reproductive regulation affected by parasitism. Most notably, the insect hormone biosynthesis pathway was significantly enriched after the adults were parasitized for 1 and 3 days, representing a primary target through which parasitoids manipulate host reproduction (Figure 4D,E), but this pathway was not enriched after the 3rd instar nymphs were parasitized for 1 day (Figure 3D) and showed no significant enrichment (p = 0.80) after the 3rd instar nymphs were parasitized for 3 days (Figure 3E). The pathway controls the synthesis of juvenile hormone and ecdysone, master regulators of insect reproduction and development. Additionally, the activation of apoptosis pathways after parasitizing for 1 and 3 days in adults provided direct molecular evidence for programmed cell death in ovarian tissues, which is likely responsible for the reproductive failure (Figure 4D,E). Furthermore, after the adults were parasitized for 3 days, activation of antigen-processing and presentation pathways (Figure 4E) suggested a reallocation of host resources from reproduction to immune responses.
3.5. JHAMT-Centered Gene Regulation Underlies Parasitoid Host Preference
Among the 10 genes identified in the insect hormone biosynthesis pathway (Figure 5A,B), JHAMT (juvenile hormone acid methyltransferase) emerged as the most consistently affected target across all developmental stages and time points, although its manipulation differed dramatically between host stages. In 3rd instar nymphs, JHAMT was the sole hormone synthesis gene that was significantly downregulated (Log2FC = −3.23, p = 1.37 × 10−3) after parasitization for 3 days, achieving reproductive disruption through minimal molecular intervention. This contrasts sharply with adult parasitism, where JHAMT downregulation intensified progressively from −1.49 (p = 2.53 × 10−5) on day 1 to −4.47 (p = 2.19 × 10−4) on day 3 (Figure 5C), accompanied by the concurrent suppression of multiple other pathway components including FOHSDR, ALDH, and JHEH (Figure 5B). In contrast to the multi-gene suppression required for adults, precise targeting of the master regulator JHAMT in nymphs efficiently disrupts reproduction, which accounts for the parasitoid’s host preference.

Figure 5.
Expression patterns of key reproductive regulatory genes in Aphis gossypii ovaries following Binodoxys communis parasitism. (A) Heatmap showing expression profiles of genes related in the insect hormone biosynthesis pathway in nymphs. (B) Heatmap showing expression profiles of genes enriched in the insect hormone biosynthesis pathway in adults. (C). Log2FC of JHAMT expression at different parasitism stages. (D) Log2FC of vitellogenin (Vg) and vitellogenin receptor (VgR) expression. Annotation and Explanation: P and CK represent parasitized and control samples; L3-1d and L3-3d represent 3rd instar nymphs parasitized for 1 and 3 days; Ad-1d and Ad-3d represent adults parasitized for 1 and 3 days.
The stage-specific regulation of the vitellogenin system further supported the adaptive advantage of parasitizing nymphs (Figure 5D). The 3rd instar nymphs only showed vitellogenin receptor (VgR) downregulation on day 1 (Log2FC = −0.92, though p = 0.199), suggesting a subtle preparatory suppression prior to the onset of vitellogenesis. This preemptive strategy in nymphs contrasts with the delayed but severe suppression observed in adults, where both vitellogenin (Vg; Log2FC = −0.94, p = 6.05 × 10−5) and its receptor (VgR; Log2FC = −1.68, p = 1.34 × 10−2) required coordinated downregulation by day 3 to halt active yolk protein synthesis and uptake.
Collectively, our results reveal distinct profiles for regulating the juvenile hormone and vitellogenin systems in parasitized 3rd instar nymphs versus adults, thereby providing a molecular basis for stage-specific outcomes of parasitism.
3.6. RT-qPCR Validation
A total of 7 differentially expressed genes (DEGs) were selected for RT-qPCR validation, including those from the insect hormone biosynthesis pathway (EVM0006879, EVM0008033, EVM0008396), the insulin signaling pathway (EVM0003641, EVM0002548), and the vitellogenin and receptor pathway (EVM0011149, EVM0005650). The relative expression levels of these genes, as determined by RT-qPCR, were consistent with the transcriptomic data from RNA-Seq (Figure 6). This consistency validated the reliability of the transcriptome sequencing data.
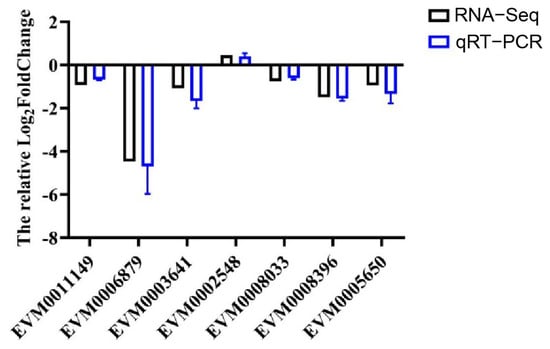
Figure 6.
Validation of RNA-Seq data by RT-qPCR analysis.
4. Discussion
The study provides comprehensive insights into the molecular mechanisms underlying B. communis parasitism of A. gossypii, revealing stage-specific strategies that optimize parasitoid fitness through differential host manipulation. Our integrated approach, which combined biological observations with transcriptomic analysis, demonstrates that B. communis employs distinct molecular tactics when parasitizing nymphs versus adults, with nymphs representing the optimal target for efficient reproductive suppression. Our results not only advance the understanding of parasitoid-host interactions but also identify potential molecular targets for novel pest management strategies.
The biological impacts of parasitism in our study manifested as significant developmental delays and reproductive suppression across all host stages, with severity inversely correlated with host age at parasitization. These observations have been documented in previous studies on Lysiphlebia japonica parasitizing Aphis gossypii, which prolonged development and severely suppressed reproduction []. Similarly, Cotesia ruficrus parasitization inhibited molting and pupation in Cnaphalocrocis medinalis [] while Microplitis similis extended 3rd instar duration in Spodoptera litura and S. exigua []. Furthermore, the venom of Aphidius ervi induces ovarian apoptosis in Acyrthosiphon pisum, inducing apoptosis of aphid ovarian cells and causing host sterilization []. These findings corroborate our data showing that parasitized aphids exhibited significantly reduced offspring production. Although these results confirm parasitoids’ regulatory role in aphid development and reproduction, the underlying molecular mechanisms necessitate further exploration.
Transcriptomic analysis revealed the molecular underpinnings of these phenotypic changes, with 1168 DEGs identified across different stages and time points. The temporal progression of DEG numbers went from minimal changes after the nymphs were parasitized for 1 day (7 DEGs) to maximum changes after the adults were parasitized for 3 days (549 DEGs), which reflected the escalating molecular interplay between the parasitoid manipulation and host resistance. KEGG pathway analysis highlighted the central role of disrupted insect hormone biosynthesis. Concurrently, the activation of immune-related pathways revealed a “reproduction-immunity trade-off,” wherein hosts appear to redirect resources from reproduction to immune defense. This trade-off aligns with predictions of life-history evolution, where parasitism imposes selective pressures that compel hosts to sacrifice reproduction for survival assurance [].
Among the differentially expressed genes, 2 genes emerged as particularly significant for understanding parasitoid reproductive manipulation strategies. JHAMT showed stage-specific expression patterns that illuminated why parasitoids prefer nymphs. In nymphs, targeting JHAMT alone (−3.23-fold change at day 3) was sufficient for efficient reproductive disruption, whereas adult parasitism required both progressive JHAMT suppression (fold change from −1.49 to −4.47) and the alteration of multiple other genes. Juvenile hormone (JH) can be involved in the regulation of a series of physiological processes such as growth and development, metamorphosis, reproduction, embryogenesis and caste differentiation in insects [,,,,,]. JHAMT has been reported as a key regulatory enzyme in the synthesis of JH in Drosophila melanogaster [], Aedes aegypti [], and other insects. A previous study demonstrated that JHAMT knockdown dramatically reduced body length, body weight, survival rate, and pupation rate in Agrotis ipsilon [], which is consistent with our observations that a decrease in JHAMT leads to development delayed and suppressed fecundity in aphids. However, reduced JHAMT activity does not uniformly prolong developmental duration across insect species. In Leptinotarsa decemlineata, dsRNA-mediated JHAMT knockdown shortened developmental duration, inducing precocious metamorphosis and complete oviposition failure in females [].
The vitellogenin (Vg) system is indispensable for egg maturation and embryogenesis, thus playing a pivotal role in insect fecundity [,]. In our study, the vitellogenin-vitellogenin receptor system only showed coordinated suppression in adults (both Vg and VgR downregulated by day 3), whereas nymphs exhibited subtle VgR downregulation before vitellogenesis onset. Since vitellogenin synthesis is primarily regulated by juvenile hormone and ecdysone [], we propose that the suppression of the Vg system is a downstream consequence of the upstream disruption of hormone biosynthesis, particularly through JHAMT downregulation. This is supported by studies showing JH is a primary inducer of Vg in non-social insects [] and that JH analogs upregulate Vg expression [,]. Therefore, the suppression of Vg/VgR in parasitized aphids likely results from upstream hormonal manipulation rather than direct targeting. This indirect regulation through hormonal manipulation represents a more efficient parasitoid strategy than targeting individual reproductive proteins, as hormones act as master regulators controlling entire gene networks. Collectively, these molecular insights demonstrated that B. communis has evolved to exploit a developmental window where host manipulation is most efficient—3rd instar nymphs, which possess sufficient resources for parasitoid development and lack the complex compensatory mechanisms of adults, enabling “low-cost, high-reward” parasitism through precision targeting of master regulators rather than the multi-gene assault required in adults.
In conclusion, our findings elucidate the sophisticated molecular strategies employed by B. communis to manipulate host reproduction. The distinct stage-specific parasitism strategies reveal a remarkable evolutionary optimization: minimal intervention in nymphs versus a comprehensive molecular assault in adults, achieving an optimal balance between parasitoid fitness and host survival. While our study focused on the B. communis-A. gossypii system, the principles uncovered here likely extend to other parasitoid-aphid interactions, suggesting convergent evolution of reproductive manipulation mechanisms. Understanding these molecular intricacies not only advances fundamental knowledge of parasitoid biology but also opens new avenues for developing biocontrol strategies that specifically target the reproductive systems of pests.
5. Conclusions
This study provides the first comprehensive molecular characterization of how B. communis parasitism affects A. gossypii reproduction across different developmental stages. Through integrated biological and transcriptomic analyses, we demonstrated that parasitoids employ distinct molecular strategies when exploiting nymph versus adult hosts, identifying the 3rd instar nymph stage as the optimal developmental window for efficient reproductive manipulation. We identified JHAMT as a master regulator that can be precisely targeted in nymphs, contrasting with the multi-gene suppression required in adults, and revealed the evolutionary refinement of parasitoid strategies that maximize reproductive control while minimizing energetic investment. These findings not only advance our understanding of the molecular basis of parasitoid-host interactions but also provide valuable insights for developing innovative pest management strategies. By revealing the natural mechanisms through which parasitoids achieve “low-cost, high-reward” host manipulation, the findings reflect how parasitoids balance energy investment and parasitic benefits during long-term evolutionary processes. They also show how natural selection shapes optimal parasitic strategies and provides crucial insights into the broader evolutionary patterns of parasitoid-host interactions.
Author Contributions
T.Z. performed the data analysis and drafted the manuscript. C.B. drafted the manuscript. J.L. conducted the experiments and performed the data analysis. L.W., K.Z., D.L., J.J. and X.Z. assisted with the bioinformatics analysis, contributed to data interpretation, and participated in the manuscript revisions. X.G. designed the experiments, supervised the work, and secured the funding. W.M. contributed to the article preparation and data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from China Agriculture Research System and Agricultural Science (CARS-15-21) and Technology Innovation Program of the Chinese Academy of Agricultural Sciences, Biological Breeding Major Projects (2023ZD04062), Young Elite Scientists Sponsorship Program by CAST (2022QNRC001), and Youth Innovation Program of Chinese Academy of Agricultural Sciences (Y2023QC23).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All authors read and agreed to the publication of the study.
Data Availability Statement
The original data presented in the study are openly available in NCBI at PRJNA1354908.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lee, Y.; Thieme, T.; Kim, H. Complex evolution in Aphis gossypii group (Hemiptera: Aphididae), evidence of primary host shift and hybridization between sympatric species. PLoS ONE 2021, 16, e0245604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Wu, Y.K.; Zhang, Q.; Li, H.Q.; Pan, H.S.; Lu, W.; Wang, D.M.; Zhang, J.P.; Lu, Y.H. Aphid parasitism and parasitoid diversity in cotton fields in Xinjiang, China. PLoS ONE 2018, 13, e0207034. [Google Scholar] [CrossRef]
- Rebijith, K.B.; Asokan, R.; Kumar, N.K.K.; Krishna, V.; Chaitanya, B.N.; Ramamurthy, V.V. DNA barcoding and elucidation of cryptic aphid species (Hemiptera: Aphididae) in India. Bull. Entomol. Res. 2013, 103, 601–610. [Google Scholar] [CrossRef]
- Im, Y.; Park, S.E.; Lee, S.Y.; Kim, J.C.; Kim, J.S. Early-Stage Defense Mechanism of the Cotton Aphid Aphis gossypii Against Infection with the Insect-Killing Fungus Beauveria bassiana JEF-544. Front. Immunol. 2022, 13, 907088. [Google Scholar] [CrossRef] [PubMed]
- Du, L.G.; Zhao, L.K.; Elumalai, P.; Zhu, X.Z.; Wang, L.; Zhang, K.X.; Li, D.Y.; Ji, J.C.; Luo, J.Y.; Cui, J.J.; et al. Effects of sublethal fipronil exposure on cross-generational functional responses and gene expression in Binodoxys communis. Environ. Sci. Pollut. Res. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wyckhuys, K.A.G.; Stone, L.; Desneux, N.; Hoelmer, K.A.; Hopper, K.R.; Heimpel, G.E. Parasitism of the soybean aphid, Aphis glycines by Binodoxys communis: The role of aphid defensive behaviour and parasitoid reproductive performance. Bull. Entomol. Res. 2008, 98, 361–370. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.C.; Cheng, J.; Liu, S.; Yuan, H.B. Population dynamics and species composition of maize field parasitoids attacking aphids in northeastern China. PLoS ONE 2020, 15, e0241530. [Google Scholar] [CrossRef]
- Dorémus, T.; Cousserans, F.; Gyapay, G.; Jouan, V.; Milano, P.; Wajnberg, E.; Darboux, I.; Cônsoli, F.L.; Volkoff, A.N. Extensive Transcription Analysis of the Hyposoter didymator Ichnovirus Genome in Permissive and Non-Permissive Lepidopteran Host Species. PLoS ONE 2014, 9, e104072. [Google Scholar] [CrossRef]
- Gao, F.; Gu, Q.J.; Pan, J.; Wang, Z.H.; Yin, C.L.; Li, F.; Song, Q.S.; Strand, M.R.; Chen, X.X.; Shi, M. Cotesia vestalis teratocytes express a diversity of genes and exhibit novel immune functions in parasitism. Sci. Rep. 2016, 6, 26967. [Google Scholar] [CrossRef]
- Han, L.B.; Yin, L.H.; Huang, L.Q.; Wang, C.Z. Differential immunosuppression by Campoletis chlorideae eggs and ichnovirus in larvae of Helicoverpa armigera and Spodoptera exigua. J. Invertebr. Pathol. 2015, 130, 88–96. [Google Scholar] [CrossRef]
- Teng, Z.W.; Xu, G.; Gan, S.Y.; Chen, X.; Fang, Q.; Ye, G.Y. Effects of the endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae) parasitism, venom, and calyx fluid on cellular and humoral immunity of its host Chilo suppressalis (Lepidoptera: Crambidae) larvae. J. Insect Physiol. 2016, 85, 46–56. [Google Scholar] [CrossRef]
- Zhou, G.F.; Chen, C.X.; Cai, Q.C.; Yan, X.; Peng, N.N.; Li, X.C.; Cui, J.H.; Han, Y.F.; Zhang, Q.; Meng, J.H.; et al. Bracovirus Sneaks Into Apoptotic Bodies Transmitting Immunosuppressive Signaling Driven by Integration-Mediated eIF5A Hypusination. Front. Immunol. 2022, 13, 901593. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Ye, G.Y.; Fang, Q.; Hu, C. Alkaline phosphatase from venom of the endoparasitoid wasp, Pteromalus puparum. J. Insect Sci. 2010, 10, 14. [Google Scholar] [CrossRef]
- Edwards, J.P.; Bell, H.A.; Audsley, N.; Marris, G.C.; Kirkbride-Smith, A.; Bryning, G.; Frisco, C.; Cusson, M. The ectoparasitic wasp Eulophus pennicornis (Hymenoptera: Eulophidae) uses instar-specific endocrine disruption strategies to suppress the development of its host Lacanobia oleracea (Lepidoptera: Noctuidae). J. Insect Physiol. 2006, 52, 1153–1162. [Google Scholar] [CrossRef]
- Harvey, J.A. Dynamic effects of parasitism by an endoparasitoid wasp on the development of two host species: Implications for host quality and parasitoid fitness. Ecol. Entomol. 2000, 25, 267–278. [Google Scholar] [CrossRef]
- Kamiyama, T.; Shimada-Niwa, Y.; Mori, H.; Tani, N.; Takemata-Kawabata, H.; Fujii, M.; Takasu, A.; Katayama, M.; Kuwabara, T.; Seike, K.; et al. Parasitoid wasp venoms degrade Drosophila imaginal discs for successful parasitism. Sci. Adv. 2025, 11, eadq8771. [Google Scholar] [CrossRef]
- Kati, A.; Hardie, J. Regulation of wing formation and adult development in an aphid host, Aphis fabae, by the parasitoid Aphidius colemani. J. Insect Physiol. 2010, 56, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Nakamatsu, Y.; Tanaka, T. Venom of Euplectrus separatae causes hyperlipidemia by lysis of host fat body cells. J. Insect Physiol. 2004, 50, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.; Ellers, J. Lack of lipogenesis in parasitoids: A review of physiological mechanisms and evolutionary implications. J. Insect Physiol. 2008, 54, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Luo, J.Y.; Lv, L.M.; Wang, C.Y.; Li, C.H.; Zhu, X.Z.; Cui, J.J. Effects of Lysiphlebia japonica (Ashmead) on cotton-melon aphid Aphis gossypii Glover lipid synthesis. Insect Mol. Biol. 2015, 24, 348–357. [Google Scholar] [CrossRef]
- Ali, M.R.; Seo, J.; Lee, D.; Kim, Y. Teratocyte-secreting proteins of an endoparasitoid wasp, Cotesia plutellae, prevent host metamorphosis by altering endocrine signals. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2013, 166, 251–262. [Google Scholar] [CrossRef]
- Falabella, P.; Riviello, L.; Caccialupi, P.; Rossodivita, T.; Valente, M.T.; De Stradis, M.L.; Tranfaglia, A.; Varricchio, P.; Gigliotti, S.; Graziani, F.; et al. A γ-glutamyl transpeptidase of Aphidius ervi venom induces apoptosis in the ovaries of host aphids. Insect Biochem. Mol. Biol. 2007, 37, 453–465. [Google Scholar] [CrossRef]
- Tanaka, T.; Tagashira, E.; Sakurai, S. Reduction of Testis Growth of Pseudaletia separata Larvae after Parasitization by Cotesia kariyai. Arch. Insect Biochem. Physiol. 1994, 26, 111–122. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Ye, G.Y.; Dong, S.Z.; Fang, Q.; Hu, C. Venom of Pteromalus puparum (Hymenoptera: Pteromalidae) induced endocrine changes in the hemolymph of its host, Pieris rapae (Lepidoptera: Pieridae). Arch. Insect Biochem. Physiol. 2009, 71, 45–53. [Google Scholar] [CrossRef]
- Asgari, S.; Rivers, D.B. Venom Proteins from Endoparasitoid Wasps and Their Role in Host-Parasite Interactions. Annu. Rev. Entomol. 2011, 56, 313–335. [Google Scholar] [CrossRef]
- Badshah, H.; Ullah, F.; Calatayud, P.A.; Crickmore, N. Host stage preference and parasitism behaviour of Aenasius bambawalei an encyrtid parasitoid of Phenacoccus solenopsis. Biocontrol Sci. Technol. 2016, 26, 1605–1616. [Google Scholar] [CrossRef]
- Cheng, C.H.; Hwang, S.Y. Similar host instar preferences by three sympatric parasitoids of Chielomenes sexmaculata (Coleoptera: Coccinellidae): Potential host niche overlapping. Bull. Entomol. Res. 2025, 115, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Liu, T.X. Dynamic host-feeding and oviposition behavior of an aphid parasitoid Aphelinus asychis. Biocontrol 2018, 63, 533–542. [Google Scholar] [CrossRef]
- Luo, S.P.; Xia, S.K.; Lu, Y.H.; Wu, K.M. Parasitism efficiency and progeny fitness of Peristenus spretus Chen et van Achterberg vary with nymphal instar of host, Apolygus lucorum (Meyer-Dur). Biol. Control 2022, 167, 104839. [Google Scholar] [CrossRef]
- Schoellerl, E.N.; Redak, R.A. Host Stage Preferences of Encarsia noyesi, Idioporus affinis, and Entedononecremnus krauteri: Parasitoids of the Giant Whitefly Aleurodicus dugesii (Hemiptera: Aleyrodidae). Environ. Entomol. 2018, 47, 1493–1500. [Google Scholar] [CrossRef]
- Hågvar, E.B.; Hofsvang, T. Aphid parasitoids (Hymenoptera, Aphidiidae): Biology, host selection and use in biological control. Biocontrol News Inf. 1991, 12, 13–42. [Google Scholar]
- Rohne, O. Effect of temperature and host stage on performance of Aphelinus varipes Forster (Hym., Aphelinidae) parasitizing the cotton aphid, Aphis gossypii Glover (Hom., Aphididae). Appl. Entomol. 2002, 126, 572–576. [Google Scholar] [CrossRef]
- Beckage, N.E.; Gelman, D.B. Wasp parasitoid disruption of host development: Implications for new biologically based strategies for insect control. Annu. Rev. Entomol. 2004, 49, 299–330. [Google Scholar] [CrossRef] [PubMed]
- Kim-Jo, C.; Gatti, J.L.; Poirié, M. Drosophila Cellular Immunity Against Parasitoid Wasps: A Complex and Time-Dependent Process. Front. Physiol. 2019, 10, 603. [Google Scholar] [CrossRef]
- Wang, L.Z.; Wang, Z.H.; Xie, S.; Wang, P.Z.; Ye, X.Q.; Huang, J.H.; Wang, Z.Z.; Chen, X.X. Integration of transcriptomic and proteomic data uncovers the nutritional requirement of the immature development of an endoparasitoid wasp. Sci. Rep. 2025, 15, 17744. [Google Scholar] [CrossRef]
- Ye, G.-Y.; Hu, J.; Zhu, J.-Y.; Fang, Q.; Yan, Z.-C.; Wang, L. Recent advances in research on the mechanisms through which parasitoid wasps regulate host immunity and development. Chin. J. Appl. Entomol. 2019, 56, 382–400. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Gao, X.; Xue, H.; Luo, J.; Ji, J.; Zhang, L.; Niu, L.; Zhu, X.; Wang, L.; Zhang, S.; Cui, J. Molecular Evidence that Lysiphlebia japonica Regulates the Development and Physiological Metabolism of Aphis gossypii. Int. J. Mol. Sci. 2020, 21, 4610. [Google Scholar] [CrossRef]
- Li, S.J.; Huang, J.P.; Chang, Y.Y.; Quan, S.Y.; Yi, W.T.; Chen, Z.S.; Liu, S.Q.; Cheng, X.W.; Huang, G.H. Development of Microplitis similis (Hymenoptera: Braconidae) on two candidate host species, Spodoptera litura and Spodoptera exigua (Lepidoptera: Noctuidae). Fla. Entomol. 2015, 98, 736–741. [Google Scholar] [CrossRef]
- Digilio, M.C.; Isidoro, N.; Tremblay, E.; Pennacchio, F. Host castration by Aphidius ervi venom proteins. J. Insect Physiol. 2000, 46, 1041–1050. [Google Scholar] [CrossRef]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction–immunity trade-offs in insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef]
- Barton, L.J.; Sanny, J.; Dawson, E.P.; Nouzova, M.; Noriega, F.G.; Stadtfeld, M.; Lehmann, R. Juvenile hormones direct primordial germ cell migration to the embryonic gonad. Curr. Biol. 2024, 34, 505–518.e6. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Cardoso-Jaime, V.; Nouzova, M.; Michalkova, V.; Ramirez, C.E.; Fernandez-Lima, F.; Noriega, F.G. Juvenile hormone controls ovarian development in female Anopheles albimanus mosquitoes. Sci. Rep. 2019, 9, 2127. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The Juvenile Hormone Signaling Pathway in Insect Development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Truman, J.W.; Riddiford, L.M.; Konopova, B.; Nouzova, M.; Noriega, F.G.; Herko, M. The embryonic role of juvenile hormone in the firebrat, Thermobia domestica, reveals its function before its involvement in metamorphosis. eLife 2024, 12, RP92643. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, X.Q.; Feng, Q.L. Fat Body Biology in the Last Decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Bendena, W.G.; Zhang, J.; Burtenshaw, S.M.; Tobe, S.S. Evidence for differential biosynthesis of juvenile hormone (and related) sesquiterpenoids in Drosophila melanogaster. Gen. Comp. Endocrinol. 2011, 172, 56–61. [Google Scholar] [CrossRef]
- Mayoral, J.G.; Nouzova, M.; Yoshiyama, M.; Shinod, T.; Hernandez-Martinez, S.; Dolghih, E.; Turjanski, A.G.; Roitberg, A.E.; Priestap, H.; Perez, M.; et al. Molecular and functional characterization of a juvenile hormone acid methyltransferase expressed in the corpora allata of mosquitoes. Insect Biochem. Mol. Biol. 2009, 39, 31–37. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.M.; Shi, X.X.; Liu, D.Y.; Deng, Q.; Peng, J.N.; Li, M.Y.; Liu, S. Knockdown of the juvenile hormone acid O-methyltransferase gene impairs development of Agrotis ipsilon (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2025, 28, 102410. [Google Scholar] [CrossRef]
- Fu, K.Y.; Li, Q.; Zhou, L.T.; Meng, Q.W.; Lü, F.G.; Guo, W.C.; Li, G.Q. Knockdown of juvenile hormone acid methyl transferase severely affects the performance of Leptinotarsa decemlineata (Say) larvae and adults. Pest Manag. Sci. 2016, 72, 1231–1241. [Google Scholar] [CrossRef]
- Sappington, T.W.; Raikhel, A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 1998, 28, 277–300. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef]
- Tufail, M.; Nagaba, Y.; Elgendy, A.M.; Takeda, M. Regulation of vitellogenin genes in insects. Entomol. Sci. 2014, 17, 269–282. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sun, Z.Y.; Bai, H.; Palli, S.R. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guo, S.; Sun, D.; Zhu, L.; Zhu, F.; Lei, C.L.; Sheng, L.; Phelps, B.; Wang, X.P. Molecular characterization and juvenile hormone-regulated transcription of the vitellogenin receptor in the cabbage beetle Colaphellus bowringi. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2019, 229, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Shu, Y.H.; Zhou, J.L.; Zhang, X.Y.; Zhang, X.Y.; Chen, M.X.; Yao, Q.; Zhou, Q.; Zhang, W.Q. Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stal). J. Insect Physiol. 2015, 73, 20–29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).