Simple Summary
Xylophagous insects, such as cockroaches and their close relatives, feed on wood, a food source that is very difficult to digest because it is primarily composed of lignin and cellulose, contains plant defense chemicals, and provides very little nitrogen, an essential nutrient. To survive on such a poor diet, these insects rely on the help of their gut microbiota, which supply enzymes to break down plant fibers, detoxification and antioxidant functions to cope with harmful chemicals, and even include bacteria capable of adding nitrogen to their food supply. In our study, we compared the gut bacteria of different wood-feeding insects and found that, while each species possessed unique microbial communities, they shared similar functional traits essential for breaking down wood and conserving nitrogen. We also observed that all insect gut microbiota contained antibiotic resistance genes, with cockroaches—including C. punctulatus—harboring the highest levels. These findings enhance our understanding of how insects thrive on such extreme diets and underscore the role of gut microbes in recycling plant material in nature. This knowledge may also inform new strategies for converting plant waste and addressing the global challenge of antibiotic resistance.
Abstract
Gut microbiota enable wood-feeding insects to digest recalcitrant diets. Two DNA-based analyses were performed. Amplicon sequencing of gut microbiota samples from Cryptocercus punctulatus showed inter-individual heterogeneity with visually distinct ordination patterns; however, no statistically significant differences were detected. Shotgun metagenomics was used to compare the taxonomic and functional profiles of C. punctulatus gut microbiota with those of other xylophagous Dictyoptera. Despite taxonomic differences, C. punctulatus microbiota revealed functional convergence with termites (Mastotermes darwiniensis and Nasutitermes sp.). Carbohydrate metabolism was performed by different bacterial phyla across all insects. All insect species possessed metabolic potential for cellulose, hemicellulose, pectin, and starch digestion, but lignin degradation capabilities were not detected. Termites showed higher abundance of chitin and xylan degradation pathways and nitrogen fixation genes, though nitrogen fixation was also present in Cryptocercus cockroaches. Genes for oxidative stress tolerance were present across all species but were most abundant in cockroaches, particularly, Cryptocercus. All insects harbored antibiotic resistance genes, with highest levels found in cockroaches. These findings indicate that metabolic requirements for wood digestion shape gut microbial community assembly across xylophagous insects, with distinct microbial taxa contributing to cellulose and hemicellulose breakdown. Moreover, the widespread presence of antibiotic resistance genes raises concerns about the potential transmission of antibiotic resistance within insect-associated microbiomes.
1. Introduction
Xylophagous insects are specialized on lignocellulose-rich wood diets that present important challenges: lignin, crystalline cellulose, diverse phenolics and secondary metabolites (e.g., alkaloids, coumarins, flavonoids, stilbenes, tannins, terpenoids, tropolones), and extremely low nitrogen content. To overcome these difficulties, their gut microbiota includes specialized bacterial taxa such as Clostridia, Bacteroidia, and Gammaproteobacteria that produce a suite of cellulolytic and hemicellulolytic enzymes (e.g., endoglucanases, xylanases) critical for breaking down cellulose and hemicellulose. Additionally, genera like Pseudomonas and Burkholderiales contribute to oxidative modification of lignin and possess antioxidant enzymes that help detoxify harmful plant secondary metabolites [1,2,3,4]. Given the complexity of lignocellulose digestion and the specialized adaptations required, understanding the gut microbiota of specific xylophagous lineages provides crucial insights into evolutionary digestive strategies.
The chemical composition of wood and herbaceous plants is variable, but generally contains 50–65% carbohydrates such as hemicellulose and cellulose, 20–25% phenolic compounds (e.g., lignin, monolignols, and other phenolic compounds), and 20% other components such as secondary metabolites. Regarding nitrogen content, woody plant tissues usually contain only 0.03–0.10% nitrogen [5,6]. The C/N ratio of most woody species is between 350 and 500:1 and can be as high as 1250:1 in some conifer species [6]. Cellulose and hemicellulose polymers require specialized enzymes for their digestion [7]. Relevant enzymes (provided by the host and the symbiotic microbiota) involved in hydrolysis include endoglucanases, beta-glucosidases, xylanases, hemicellulases, esterases and other glycoside hydrolases. The lignin contains functional groups such as carbonyl, methoxyl, phenolic hydroxyl, and alcoholic hydroxyl groups that greatly impact its reactivity [6]. Degradation of lignin can result in the production of oxygen radicals that may increase oxidative stress, creating a hostile environment for microorganisms [7].
These challenging nutritional constraints have driven the evolution of specialized digestive strategies across different insect lineages, most notably within the superorder Dictyoptera, a well-established insect lineage that includes termites, cockroaches, and mantids. Six termite families and the wood-feeding cockroach genus Cryptocercus share the ability to digest lignocellulose with a complex consortium of gut bacteria and protists, whereas the higher-termite family Termitidae has lost its protists and relies solely on bacteria [8]. Despite their shared evolutionary history and lignocellulose diets, comprehensive comparative analyses of gut microbiota across xylophagous Dictyoptera remain scarce. Most research on wood-feeding Dictyoptera, especially termites, uses certain cockroach lineages as evolutionary reference points rather than conducting broad, systematic comparative analyses across all wood-feeding groups. Cryptocercus cockroaches are the primary model for termite evolution due to their close phylogenetic relationship and shared traits, while other wood-feeding Dictyoptera are less frequently included in comparative frameworks [9,10]. While termites mainly consume wood, many cockroaches exhibit diverse diets, including omnivorous scavenging, feeding on leaf litter, and decaying wood [11,12]. Notable lignocellulose-eating cockroaches are Cryptocercus spp. from East Asia and North America, Parasphaeria boleiriana from Brazil [13], and Panchlora nivea from the Caribbean. Cryptocercus and Parasphaeria inhabit rotting logs, while Panchlora lives in decomposing palm trunks, leaf litter, and refuse piles of fungus-farming ants [14]. This ecological diversity suggests corresponding variation in gut microbiome composition and function.
The basic digestive tract architecture (foregut, midgut, hindgut) is conserved across insects, yet its physicochemical heterogeneity (pH, redox potential, oxygen gradients) structures distinct microbial communities [4,15]. In cockroaches, the hindgut is typically anoxic, neutral to slightly alkaline and mildly reducing [11]. The microbiome substantially influences digestion and nutrient absorption: germ-free Periplaneta americana individuals exhibit underdeveloped digestive organs, which normalize after re-inoculation with conspecific gut microbes [16].
The aims of this study were to elucidate the bacterial gut normobiota of the wood-feeding cockroach C. punctulatus and to compare its microbiota composition and predicted functional capabilities with those of other xylophagous Dictyoptera insects, particularly termites (Mastotermes darwiniensis—“lower” termite and Nasutitermes sp.—“higher” termite). The significance of this work lies in advancing the understanding of evolutionary digestive strategies in insects that feed on lignocellulose-rich wood diets, which are difficult to digest due to the presence of complex polymers like lignin and cellulose, plant defense chemicals, and very low nitrogen content. The gut microbiota is crucial for these insects to digest wood by providing specialized enzymes for fiber breakdown, detoxification, nitrogen fixation, and antioxidant functions. Despite their close phylogenetic relatedness, comprehensive comparative analyses across xylophagous Dictyoptera—including cockroaches and termites—are scarce. Comparative analyses of termite and cockroach microbiomes could help the understanding of lignocellulose adaptation across Dictyoptera, and have applied implications for sustainable agriculture and biotechnology related to lignocellulose bioconversion.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
Cryptocercus punctulatus specimens were collected in Virginia, USA, by Dr. Michael Dolan (University of Massachusetts, Amherst, MA, USA) and maintained under laboratory conditions for several months, using the same type of wood from which the cockroaches had been collected in the forest. Three adult C. punctulatus cockroaches were transported to our laboratory in Barcelona, Catalonia, Spain, for analysis. During transport, the insects were kept in tubes at room temperature. Upon arrival, the specimens exhibited vitality, activity, and an apparent health status adequate for experimental procedures. The cockroaches were immediately dissected under sterile conditions to extract the intact gut. Briefly, the abdomens were opened using a sterile scalpel, and the entire gut was collected and placed into an Eppendorf tube for subsequent DNA extraction. Disinfection and dissection were performed in a laminar flow cabinet to avoid contamination.
The gut tissue was homogenized using a FastPrep system with 0.1 mm glass beads. Bulk DNA was then extracted by repeated phenol–chloroform washing [13]. Following homogenization, an equal volume of phenol–chloroform–isoamyl alcohol solution (typically 25:24:1, v/v/v, Thermo Fisher Scientific, Madrid, Spain) was added to the homogenate to denature proteins and separate nucleic acids. The mixture was shaken and centrifuged at 12,000× g for 10 min at room temperature to separate phases. The upper aqueous phase containing DNA was carefully transferred to a new tube. This phenol–chloroform extraction step was repeated 2–3 times until the interphase was clear, indicating thorough removal of proteins and lipids. After the final extraction, DNA was precipitated by adding 0.1 volume of 3 M sodium acetate (pH 5.2) (Thermo Fisher Scientific) and 2 volumes of cold 100% ethanol (Thermo Fisher Scientific), followed by incubation at –20 °C for at least 1 h or overnight. The sample was then centrifuged at 12,000× g for 10 min at 4 °C to pellet the DNA. The pellet was washed with 70% ethanol to remove residual salts, air-dried briefly, and resuspended in an appropriate volume of TE buffer (Thermo Fisher Scientific).
2.2. Cryptocercus punctulatus Metagenome Sequencing and Data Processing
Samples were pooled, and shotgun metagenomic sequencing was performed using the Illumina MiSeq_RunMicro300 cycles (2 × 150) platform by the Genomic and Bioinformatic Service of the Autonomous University of Barcelona (sample Cp_shotgun).
Paired-end fastq file sequences generated by Illumina MiSeq were assembled using the MEGAHIT tool in Galaxy Version 1.2.9 [https://usegalaxy.eu/] [17]. The assembled sequences were then submitted to the DOE-JGI Metagenome Annotation Pipeline (MAP v.4) [https://gold.jgi.doe.gov/] [18]. For genomic assembly, the Velvet algorithm package was used. The pipeline runs against curated models, derived from full-length genes within IMG, while keeping the best-scoring models. The identification of protein-coding genes was performed using a consensus of four different ab initio gene prediction tools: prokaryotic GeneMark.hmm (v.2.8), MetaGeneAnnotator (v. Aug 2008), Prodigal (v. 2.6.2) and FragGeneScan. Protein-coding genes with translations shorter than 32 amino acids were deleted. Assignment was made at 90% of the KO gene sequence covered by the alignment [18]. KO genes refer to genes that have been assigned a KEGG Orthology (KO) identifier within the KEGG (Kyoto Encyclopedia of Genes and Genomes) database. The use of MAP for metagenomic analysis enabled the detection of partial genes associated with various functional activities. However, given these limitations, the functional annotations should be considered preliminary and interpreted as putative activity potential.
Data available in the JGI database Gold pj. Ga0134290 (C. punctulatus) (https://gold.jgi.doe.gov/analysis_project?id=Ga0134290 (accessed on 1 November 2025). General statistics from JGI: Reads were assembled into 177,102 scaffolds, reflecting a highly fragmented metagenome. Quality filtering by JGI indicated that over 80% of bases achieved Q30 or higher, suggesting reasonable base accuracy. Among all predicted coding sequences, 18.5% matched KEGG Orthology (KO) terms, indicating moderate annotation coverage common to fragmented gut metagenomes. Ribosomal RNA genes were rare (0.05% of 16S and 0.18% of 18S rRNA). BLAST (version 2.13.0)-based taxonomic classification at 60% identity cutoff yielded 20% bacterial hits, 2% eukaryotic, and roughly 78% unassigned, the latter representing short or chimeric contigs and poorly conserved sequences. The high proportion of unclassified scaffolds and low rRNA recovery indicate incomplete coverage of microbial diversity. These limitations restrict the precision of gene and pathway abundance estimates but still provide valuable community-level insights into the C. punctulatus gut microbiome.
2.3. Downloaded Data for Comparative Analysis
Several C. punctulatus 16S rRNA amplicon datasets were retrieved from the NCBI Bioproject Database. Sample Cp (Bioproject PRJDB7102) [19]; Cp_1, Cp_2 and Cp_3 (Bioproject PRJNA238270) [20]; Sample Cp_4 (Bioproject PRJNA217467) [21], and Sample Cp_5 (Bioproject PRJNA284583) [13] (Table 1).

Table 1.
C. punctulatus normobiota amplicon sequencing samples.
Several gut microbiome shotgun metagenomes of different xylophagous Dictyoptera species were retrieved from the JGI-GOLD database. Their associated information is summarized in Table 2.

Table 2.
Shotgun metagenomes of different xylophagous Dictyoptera species.
2.4. Bioinformatics and Statistical Analyses
Amplicon reads were processed by the amplicon analysis pipeline of the SILVA base data (http://www.arb-silva.de). The pipeline automatically performs stringent quality filtering steps: All reads are aligned to the SILVA reference database using the SINA aligner. Reads that fail to align properly (non-rDNA fragments, chimeras, poor similarity, or mismatched orientation) are discarded. Reads that pass alignment undergo checks for minimum length, ambiguous bases (N > 2%) and homopolymers (>8 bp). Taxonomic abundance tables were generated at multiple ranks, with a focus on the phylum level. Relative abundances were calculated by expressing the read counts assigned to each phylum as a percentage of the total classified reads per sample. Differences in microbiota composition between groups were assessed using the Kolmogorov–Smirnov test for non-parametric data. Statistical significance was determined at p < 0.05. Alpha diversity was measured by the Shannon index. Shannon’s index H is an estimator of taxa diversity (combining richness and evenness) and was computed in R using the vegan package [22]. For amplicon sequencing, principal coordinate analysis (PCoA) plots of normalized sample community distributions were generated using Bray–Curtis dissimilarity and analyzed within the MTP platform of EzBio-Cloud Apps (ChunLab Inc., Seoul, Republic of Korea) (https://www.EZbiocloud.net/).
For each insect host species, metagenomic datasets obtained from the JGI GOLD database and the present study were processed using the JGI Metagenome Annotation Pipeline, which includes sequence quality control, gene prediction, taxonomic classification, and functional annotation against the Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology (KO) database. Data analyzed by the JGI metagenome annotation pipeline resulted in very low resolution for eukaryotic organisms.
For shotgun metagenome samples, Bray–Curtis dissimilarity was used to measure beta diversity and produce PCoA plots via the web-based JGI Genomes Online Database (https://gold.jgi.doe.gov/).
Functional potential was assessed by compiling KO annotations from the JGI genomes online database. KO abundance values were normalized to relative abundance (% of total annotated genes) to account for differences in sequencing depth.
3. Results
3.1. Bacterial Gut Patterns in Cryptocercus punctulatus
The gut bacterial microbiota of multiple C. punctulatus samples collected from natural environments in Virginia and North Carolina, USA (Cp, Cp_1, Cp_2, Cp_3, Cp_4 and Cp_5) were compared with that of C. punctulatus, Cp_shotgun (this study). No significant differences were detected by the Kolmogorov–Smirnov test; however, three distinct gut bacterial patterns were identified: Pattern-1 in Cp, Cp_1, Cp_2, and Cp_3; Pattern-2 in Cp_4; and Pattern-3 in Cp_5 and Cp_shotgun. Pattern-1 was characterized by a high proportion of Bacteroidota; Pattern-2, by Bacillota and Pattern-3, by an increased proportion of Pseudomonadota (Figure 1, Supplementary Figure S1).
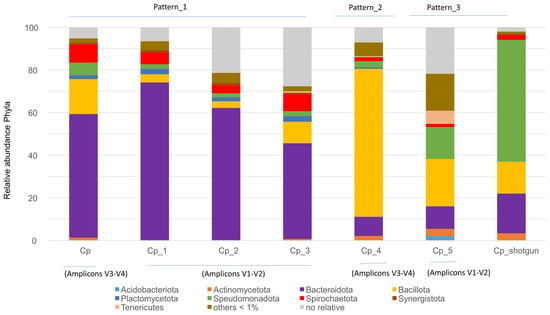
Figure 1.
Relative abundance of the major bacterial phyla in the gut microbiota of C. punctulatus across seven samples. Samples include Cp, Cp_1–Cp_5, and Cp_shotgun sample (this study). Three bacterial community patterns were observed, with no significant differences among groups (Kolmogorov–Smirnov test, p > 0.05).
Microbial diversity across the identified gut patterns, measured using the Shannon index, was 3.10 for Pattern-1 (Cp, Cp_1, Cp_2 and Cp_3), 4.515 for Pattern-2 (Cp_4), and 3.826 for Pattern-3 (Cp_5). Diversity analysis is commonly used to assess gut microbiota health. However, establishing a universally accepted definition remains challenging due to individual variation, diet, lifestyle, host genetics, and environmental factors [23]. In this study, the absence of significant differences among the patterns indicates that maintaining the samples Cp_5 and Cp_shotgun under laboratory conditions did not substantially alter their gut microbiota composition.
After establishing these bacterial gut patterns, we compared shotgun-derived taxa among different xylophagous insects.
3.2. Shotgun Taxa of Cryptocercus punctulatus and Other Xylophagous Dyctioptera
Shotgun metagenomics exhibited limited resolution for detecting protist-related sequences, most of which are unique to certain termite families and the related cockroach genus Cryptocercus [15]. Therefore, our analysis focused on bacterial microbiota diversity and functionality. Shotgun metagenomic profiling revealed that the most abundant bacterial phyla in C. punctulatus were Pseudomonadota (57.4%), Bacteroidota (18.8%), Bacillota (15%), Actinomycetota (3.2%) and Spirochaeota (2.2%). Archaea accounted for less than 1% of relative abundance, belonging primarily to Methanobacteriota (e.g., “Candidatus Methanoplasma”, Methanobacterium and Methanobrevibacter) (Figure 2a). Among Pseudomonadota Gamma- and Betaproteobacteria predominated. The dominant Gammaproteobacteria families were Enterobacteriaceae (e.g., Raoultella and Klebsiella) and Pseudomonadaceae (e.g., Pseudomonas). Rhodocyclaceae represented 63% of Betaproteobacteria. The four most abundant Bacteroidota families were Bacteroidaceae (23.3%), Tannerellaceae (23.4%), Dysgonomonadaceae (17.7%) and Prevotellaceae (9.6%). Within Bacillota, the Clostridia class constituted 64.4% primarily Lachnospiraceae and Clostridiaceae.
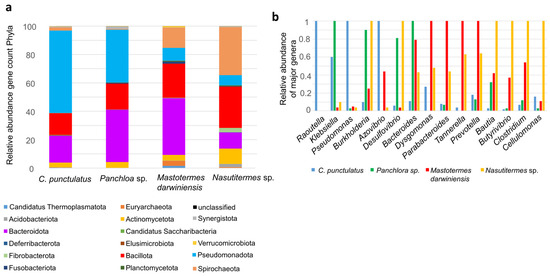
Figure 2.
Shotgun taxa of C. punctulatus and other xylophagous Dyctioptera. (a) The bar chart showed the relative abundance of bacterial phyla present in the gut microbiota of four insect species: C. punctulatus, Panchlora sp., M. darwiniensis, and Nasutitermes sp. The relative abundance is presented as gene count percentages for each phylum, as indicated by the color key below the chart. (b) Relative abundance of major genera identified in the four insect gut metagenomes. For each genus, the maximum number of detected sequences among all species was assigned a relative value of 1. Abundances in the other species are shown relative to this maximum value, facilitating cross-species comparisons of dominant genera.
Shotgun metagenomes from other Dictyoptera species exhibited distinct compositions. In the cockroach Panchlora sp., the predominant phyla were Pseudomonadota (36.7%), Bacteroidota (36.5%) and Bacillota (18.1%). The lower termite Mastotermes darwiniensis was dominated by Bacteroidota (38.9%), Bacillota (23.5%), Spirochaeota (13.8%) and Pseudomonadota (8.8%). The higher termite Nasutitermes sp. exhibited greater abundances of Spirochaeota (33.2%), Bacillota (28.6%), Bacteroidota (11.2%), Actinomycetota (10.6%) and Pseudomonadota (7%) (Figure 2a).
At >60% gene identity, genus-level distributions differed markedly between C. punctulatus and other Dictyoptera. In C. punctulatus, the most abundant genera were Raoultella, Klebsiella, Pseudomonas, Azovibrio and Dysgomonas. In Panchlora sp. Dominant genera included Klebisella, Burkholderia, Desulfovibrio and Bacteroides. The lower termite M. darwiniensis was enriched in Dysgomonas, Parabacteroides, Tannerella and Prevotella, whereas the higher termite Nasutitermes sp. was dominated by Burkholderia, Desulfovibrio, Blautia, Butyrivibrio, Clostridium and Cellulomonas. Spirochaete genera such as Treponema were also more prevalent in Nasutitermes sp. and M. darwiniensis (Figure 2b).
Principal coordinate analysis (PCoA) based on family-level taxa showed that C. punctulatus harbors a distinct microbiota compared with both termites and the more distantly related cockroach Panchlora sp. (Supplementary Figure S2a). However, PCoA clustering based on KEGG orthologs (KO) profiles showed that C. punctulatus grouped closely with termites, particularly the phylogenetically ancient lower termite M. darwiniensis (Supplementary Figure S2b).
3.3. Functional Nutritional Shotgun Metagenome of Cryptocercus punctulatus and Other Xylophagous Dyctioptera
Shotgun metagenomics was used to evaluate the functional potential of the gut microbiota in C. punctulatus. Predicted proteins were classified into KEGG orthologs (KOs) and mapped to the dominant bacterial phyla. Functional analysis revealed that the major gut bacteria in the host primarily participate in amino acid metabolism, carbohydrate metabolism, energy production, and the metabolism of vitamins and cofactors. To a lesser extent, they contribute to terpenoid and polyketide metabolism and to xenobiotic degradation (Figure 3). Although the overall metabolic profiles were broadly similar across Dictyoptera examined, the specific bacterial taxa performing these functions differed. For example, carbohydrate metabolism in C. punctulatus was primarily associated with Bacteroidota, whereas in M. darwiniensis and Panchlora sp. it was mainly linked to Bacillota, and in Nasutitermes sp. to Actinomycetota and Bacteroidota (Figure 3). Genes related to xenobiotic detoxification represented a minor fraction of the relative total KO abundance. These findings suggest that core gut bacterial roles involve nutrient provisioning, lignocellulose digestion, and, to a lesser degree, detoxification.
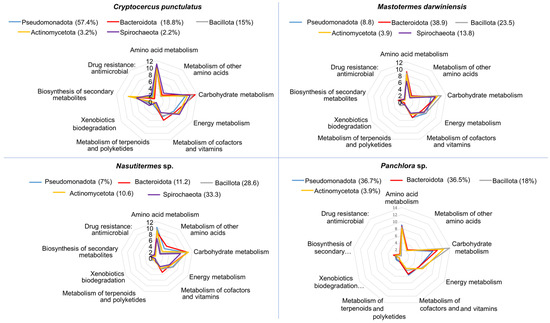
Figure 3.
Radial plots depicting the predicted functional potential of the major bacterial phyla within the gut microbiota of four insect species: C. punctulatus, M. darwiniensis, Nasutitermes sp., and Panchlora sp. For each host, the four most abundant phyla are displayed, with their relative abundance indicated in parentheses. Each axis in the radial graphs represents a major metabolic or functional category predicted for the bacterial community, including: amino acid metabolism, metabolism of other amino acids, carbohydrate metabolism, energy metabolism, metabolism of cofactors and vitamins, metabolism of terpenoids and polyketides, xenobiotic biodegradation, biosynthesis of secondary metabolites, and antimicrobial drug resistance. Values indicate the frequency of gene functions predicted for each pathway, based on metagenomic data.
Individual KO genes detected were grouped into functional categories (e.g., carbohydrate and nitrogen pathways). Because each category comprises a different number of putative KOs, comparisons are valid only within categories. Putative genes for carbohydrate hydrolysis were identified across all insect microbiomes examined, supporting the digestion of cellulose, starch, pectin, xylan, chitin, and butanoate (Figure 4a). The inferred capacities for chitin and xylan degradation were higher in Nasutitermes sp. than in the cockroaches and M. darwiniensis (Figure 4a). Inositol phosphate metabolism was less represented in cockroaches than in termites, with similar trends observed for glyoxylate and dicarboxylate metabolism (Figure 4a). Short-chain fatty acids such as propionate and butyrate produced from dietary carbohydrates by gut microbes may be utilized by the host. Notably, propionate metabolism was particularly well represented in C. punctulatus.
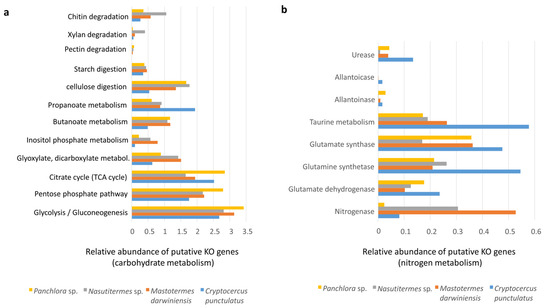
Figure 4.
Putative KO genes related to carbohydrate metabolism and nitrogen metabolism. (a) Bar graph showing the relative abundance of putative KO genes related to diverse carbohydrate metabolic pathways analyzed by shotgun metagenomics in the gut microbiota of Panchlora sp. (yellow), Nasutitermes sp. (grey), Mastotermes darwiniensis (orange), and C. punctulatus (blue). Metabolic pathways evaluated include chitin, xylan, pectin, starch, and cellulose degradation, etc. (b) Bar graph displaying the relative abundance of putative KO genes involved in nitrogen metabolism for the same four insect species.
Nitrogen acquisition strategies, including nitrogen fixation and nitrogenous waste recycling, were also examined (Figure 4a). Putative nitrogen fixation via the nitrogenase complex was higher in termites; however, in C. punctulatus, it may also represent an important nitrogen source (Figure 4b), partly due to nitrogen-fixing ectosymbionts (Treponema spirochaetes and members of the Bacteroidales) associated with Barbulanympha and Trichonympha protists, as well as other diazotrophic gut microorganisms [15,24]. Genes encoding urease, allantoicase, and allantoainase were detected in C. punctulatus and Panchlora sp. and M. darwiniensis. C. punctulatus exhibited higher urease activity, likely reflecting the role of the endosymbiont Blattabacterium. In this study, Blattabacterium accounted for approximately 3.3% of the total community, a subset of the 18% assigned to Bacteroidota, possibly reflecting contamination from the fat body, which houses Blattabacterium during intestinal dissection. Because the fat body closely envelops the intestine in cockroaches, inadvertent disruption during dissection can release Blattabacterium into gut samples [25] (Figure 4b).
3.4. Functional Stress and Antibiotic Shotgun Metagenome of Cryptocercus punctulatus and Other Xylophagous Dyctioptera
The insect gut is a major site of detoxification and stress response. Cytochrome P450 monooxygenases (P450s) catalyze key redox reactions and detoxify a wide range of xenobiotics [26]. P450 genes were detected in all insects examined, indicating potential for xenobiotic degradation; however, they represented less than 1% of total KO annotations, suggesting that detoxification plays a relatively minor role compared with pathways such as carbohydrate metabolism. Other oxidative stress–related enzymes—including superoxide dismutases, catalases, peroxidases, and components of the glutathione system—were present in all insects, with particularly high representation in cockroaches, especially C. punctulatus (Figure 5a) [27,28]. Ferroptosis, an iron-dependent, lipid peroxidation–associated form of regulated cell death [29], was slightly higher in Panchlora sp.
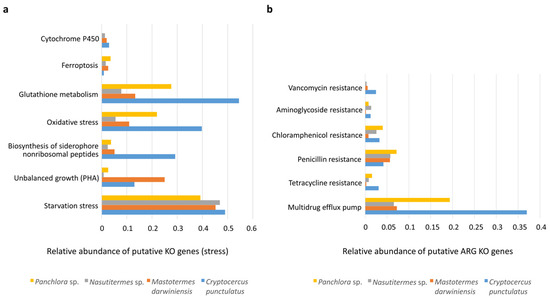
Figure 5.
Putative KO genes related to stress and antibiotic resistance. (a) Bar graph depicting the relative abundance of putative KO genes involved in various cellular stress response mechanisms across the gut microbiomes of Panchlora sp. (yellow), Nasutitermes sp. (grey), M. darwiniensis (orange), and C. punctulatus (blue). The relative abundance of these KO genes could reflect the adaptive capacity of microbial communities to cope with environmental stressors and maintain cellular homeostasis within the insect gut environment. (b) Bar graph showing the relative abundance of putative ARG (antibiotic resistant genes) KO genes conferring resistance to different classes of antibiotics in the same four insect species. Resistance mechanisms evaluated include genes for vancomycin, aminoglycosides, chloramphenicol, penicillin, and tetracycline, as well as genes encoding multidrug efflux pumps (panel (b)).
Despite access to dietary carbon sources, nutrient starvation stress markers (e.g., K03087, K03088) were detected in both cockroaches and termites. In addition, genes involved in the synthesis of the reserve polymer polyhydroxyalkanoate (PHA) (e.g., K03821, K00023, K05973) were observed in M. darwiniensis and C. punctulatus, indicative of unbalanced growth (Figure 5a). PHA accumulation is typically stimulated under nutrient limitation (e.g., P, N, Fe) combined with excess carbon availability. The co-occurrence of starvation response and PHA synthesis pathways suggests that gut microbiota experience intermittent nutrient deprivation, likely due to fluctuations in dietary intake or competition within the densely populated gut environment.
Antibiotic resistance KO genes associated with vancomycin, aminoglycoside, chloramphenicol, penicillin, tetracycline, and multidrug efflux pumps were detected in all insects examined (see Figure 5b). Among these, multidrug resistance pumps and penicillin resistance genes were most abundant. Cockroaches exhibited a greater number of antibiotic resistance-related KO genes than termites (Figure 5b).
4. Discussion
4.1. Bacterial Gut Patterns from Cryptocercus punctulatus
The symbiotic gut microbiota is integral to insect physiology, particularly in wood-feeding termites and the xylophagous cockroach Cryptocercus, which rely on microbial partners to process nutrient-poor, lignocellulosic diets. These gut microorganisms facilitate the digestion of recalcitrant substrates, synthesize essential vitamins and nutrients, and provide additional functions fundamental to host fitness [4,30,31,32].
The methodologies employed—amplicon sequencing targeting the V1–V2 and V3–V4 regions of the 16S rRNA gene and whole metagenome shotgun sequencing—are known to introduce inherent biases that can influence the detection and quantification of microbial taxa. Amplicon sequencing depends on PCR primer specificity, which may preferentially amplify certain taxa while underrepresenting or missing others, particularly those with mismatches in primer-binding regions. Conversely, shotgun metagenomics sequences all DNA fragments present in a sample without primer bias, but its taxonomic resolution and sensitivity can be affected by sequencing depth, host DNA contamination, and bioinformatic limitations [33,34]. The V1–V2 region generally exhibits higher species-level taxonomic richness and diversity than the V4–V5 region. Certain bacterial genera, such as some Bacillota and Actinobacteriota, are more abundantly detected with V1–V2 primers, whereas V4–V5 may underrepresent these taxa or show different relative abundances [35]. Across the C. punctulatus samples analyzed in this study, the relative abundance of dominant bacterial taxa Bacteroidota, Bacillota, and Pseudomonadota varied among samples. Despite methodological biases, Pattern-1 encompassed samples from both the V1–V2 and V4–V5 regions (Cp, Cp_1, Cp_2, and Cp_3) (Figure 1). However, no statistically significant differences at the phylum level were detected between pattern-1 and pattern-2 or between Pattern-1 and Pattern-3 (Kolmogorov–Smirnov test, p > 0.05) (Figure 1). This suggests a degree of compositional flexibility within the gut community, with multiple stable bacterial configurations persisting without compromising functional integrity.
Beyond methodological effects, another factor potentially influencing the observed profiles the type of wood from which specimens were collected. The forests of North Carolina and Virginia, where C. punctulatus specimens were collected (Mount Collins, South Mountains, Haywood County, and Mountain Lake), belong to the Southern and Central Appalachian mixed hardwood ecoregion, characterized by similar dominant tree taxa. Although tree species differ slightly among sites, the dominant vegetation across these forests consists primarily of hardwood taxa (oak, maple, birch, hickory, and beech) with conifers such as fir and spruce present only at higher elevations. These tree species share broadly similar lignocellulosic compositions, characterized by high cellulose and hemicellulose content and secondary compounds typical of temperate hardwoods. Therefore, substrate quality and nutrient availability for C. punctulatus may be comparable across sites [36], suggesting that diet may not be the primary driver of gut bacterial community structure.
Cryptocercus punctulatus is currently divided into four known karyotype groups (2n = 37, 39, 43 and 45). Despite genetic differences among these groups, no ecological or habitat use differences have been reported within their examined range [37]. The specimens used in this study were Cp_1 (2n = 43), Cp_2 (2n = 43), and Cp_3 (2n = 45) [20]. Although they had different karyotypes, they exhibited similar bacterial community patterns, supporting the conclusion that neither host genetic background nor local diet strongly influences phylum-level microbiota structure in these populations. Instead, ecological and functional stability—likely reflecting evolutionary pressures for efficient lignocellulose degradation appears to conserve core gut community features across host genetic variants and localities.
Such flexibility is likely rooted in the early establishment of the microbiota during Cryptocercus development. In these subsocial cockroaches, proctodeal trophallaxis from adults to juveniles ensures vertical transmission of symbionts and the formation of core microbial communities [37]. The complete symbiotic microbiota assembles sequentially and is not finalized until the third instar, when individuals attain nutritional independence, although they continue to maintain contact with adults [38].
The observed interindividual variation likely reflects the inherent ecological plasticity of the gut microbiome. These findings reinforce the view that the C. punctulatus gut microbiota achieves a balance between stability and compositional flexibility, fitting within a host–microbe ecological framework where multiple taxonomic assemblages can sustain similar functional outcomes. This resilience likely represents an adaptive feature of xylophagous symbioses, ensuring consistent host nutrition despite environmental fluctuations or experimental variation.
Samples maintained under laboratory conditions (Cp_5, Cp_shotgun) did not differ significantly from those collected in nature, suggesting that laboratory maintenance does not substantially disrupt the Cryptocercus gut community. Previous studies have reported that insects kept in laboratory environments over extended periods often develop gut communities differing in both diversity and composition from those of wild-caught individuals, largely due to dietary and environmental changes [39,40]. This observation is particularly relevant for comparative analyses of Cp_shotgun samples for taxonomic and functional features relative to metagenomic datasets from wild-caught insect species, consistent with previous findings on cockroach gut microbiomes [41].
4.2. Shotgun Taxa Metagenome of Cryptocercus punctulatus and Other Xylophagous Dyctioptera
The Cp_shotgun metagenome from C. punctulatus was compared with metagenomic datasets from other xylophagous Dictyoptera. The specialized bacterial genera observed in each insect appear to be characteristic features enabling them to thrive on lignocellulosic diets [42,43]. At the genus level, the C. punctulatus gut is dominated by Raoultella, Klebsiella, Pseudomonas, Azovibrio and Dysgonomonas. These genera are functionally relevant to lignocellulose-based diets, as they are implicated in complex polysaccharide degradation, short-chain fatty acid production, vitamin biosynthesis, and nitrogen fixation. Such capabilities are consistent with the nutritional demands of a wood-feeding lifestyle and align with previous reports linking these taxa to cellulose and hemicellulose breakdown in other arthropod hosts [44,45,46].
Principal coordinate analysis at the family-level revealed that C. punctulatus harbors a distinct bacterial assemblage relative to both termites and the distantly related cockroach Panchlora sp. This divergence likely reflects host-specific gut environments, including differences in redox gradients, metabolic niches and nutrient availability, together with the unique evolutionary history of symbiosis in each lineage. Distinct microbial community organizations were observed among the insects studied. “Lower” termites, such as M. darwiniensis, maintain a mixed protist–bacteria symbiosis, while higher termites (e.g., Nasutitermes sp). exhibit bacteria-dominated communities enriched in taxa specializing in hemicellulose and chitin degradation. C. punctulatus preserves a protist–bacteria consortium similar to that of lower termites, but with distinctive bacterial contributions reflecting its cockroach ancestry. Wood digestion in Dictyoptera may, therefore, have arisen through both shared symbiotic legacies and independent innovations in host–microbe associations [3,15].
4.3. Functional Nutritional Shotgun Metagenome of Cryptocercus punctulatus and Other Xylophagous Dyctioptera
Across all xylophagous Dictyoptera, gut microbiomes encode extensive repertoires of carbohydrate-active enzymes (CAZymes) for cellulose, hemicellulose, pectin, and starch hydrolysis, whereas canonical lignin-degrading enzymes (laccases, manganese peroxidases, and lignin peroxidases) were essentially absent, with only low-abundance katG and ligD homologs detected. This supports the notion that enhancing polysaccharide accessibility rather than lignin mineralization suffices for wood digestion [47]. Nasutitermes sp. displayed the highest xylanolytic and chitinolytic potential, predominantly mediated by Spirochaetota and Fibrobacteriota CAZymes [48], likely reflecting fungal biomass degradation within decaying wood [49,50]. In nature, the main chitin degraders are bacteria from the phyla Bacteroidota and Bacillota, which produce CAZymes capable of lysing fungal cell walls and insect cuticles, composed of protein and chitin [50,51]. Specifically, these bacteria putatively generate mycolytic enzymes that break down chitin in these structures [50] (Figure 4a).
Inositol phosphate metabolism encompasses biochemical pathways involved in the synthesis, breakdown, and signaling of inositol phosphates, which play critical roles in cell signaling, membrane trafficking, and gene regulation [52]. Inositol phosphate metabolic pathways were less represented in cockroaches compared with termites (Figure 4a). Similar results were observed for glyoxylate and dicarboxylate metabolism, which enable organisms to utilize acetate as an energy source when glucose availability is limited. The glyoxylate cycle converts acetyl-CoA to malate while bypassing carbon dioxide loss, allowing greater carbon incorporation into biomass. Oxaloacetate and malate provide carbon skeletons for amino acid synthesis. Thus, the glyoxylate cycle supplies simple sugars, biosynthetic precursors, and amino acid substrates when glucose is scarce [53]. The reduced representation of these pathways in cockroaches compared with termites suggests differences in acetate and glucose metabolism between these groups, implying that cockroaches may depend more heavily on glucose metabolism.
Insects have evolved various strategies to cope with low dietary nitrogen, including symbiotic nitrogen fixation, efficient nitrogen recycling, and optimization of nitrogen intake. Key nitrogen-fixing symbionts include diazotrophic bacteria such as Spirochaeota and Bacteroidota, which provide fixed nitrogen to their hosts [13]. Insects also recycle nitrogenous wastes such as uric acid, urea, and ammonia via glutamine synthetase and glutamate synthase pathways. They excrete nitrogenous waste in forms such as uric acid, ammonia, allantoin and allantoate via the Malpighian tubules and hindgut. Other nitrogen acquisition mechanisms include coprophagy (fecal consumption), proctodeal trophallaxis (anus-to-mouth feeding), predation or cannibalism, and ingestion of shed cuticles [25,51]. Termites relied chiefly on diazotrophic bacteria for nitrogen fixation, whereas Cryptocercus exhibited elevated gene counts for uric-acid and urea recycling and non-protein amino acid (e.g., taurine) metabolism, indicating a shift toward waste-derived nitrogen salvage [54,55,56] (Figure 4b).
4.4. Functional Stress and Antibiotic Shotgun Metagenome of Cryptocercus punctulatus and Other Xylophagous Dyctioptera
Changes in the gut microbial communities of xylophagous insects can occur in response to environmental stressors. Factors such as diet, temperature extremes, or exposure to insecticides can alter the diversity and composition of gut bacteria [57,58,59]. The microbiota must also tolerate intrinsic gut conditions, including toxic plant compounds (e.g., alkaloids, terpenoids, phenols, etc.), oxygen exposure, variable pH, nutrient limitation, and competitive exclusion conditions that impose substantial stress on microorganisms.
Herbivorous insects have evolved strategies to detoxify compounds encountered in their habitats, including enzymes such as carboxylesterase, glutathione-S-transferases (GSTs), and cytochrome P450 monooxygenases (CYP450s Cytochrome P450 enzymes, a large family of heme-containing oxidases, occur across bacteria, fungi, plants, and animals. After ingestion, plant tissues enter the insect digestive tract, where the gut bacterial community aids in digestion, nutrient absorption, and detoxification of harmful phytochemicals [60]. For example, Pseudomonas species can degrade monoterpenes and diterpene acids [61]. Cytochrome P450 KO genes had lower relative abundance in the metagenomes of both cockroaches and termites, suggesting that the microbiota did not play a fundamental role in detoxification, which may instead be mediated by the insects’ own enzymes—a pattern observed in several insect taxa [28,62].
Oxygen-rich tissues surround insect guts, as air is delivered through the tracheal system. Oxygen permeates into the hindgut contents up to 150–200 mm below the epithelium, where the gut microbiota depletes through respiration, generating a microoxic periphery surrounding an anoxic center [62,63]. A stable, physiological state in the host may support high microbial diversity, likely maintaining a sharp O2–H2 gradient within the gut [63,64]. Enzymes that combat oxidative stress such as superoxide dismutases, catalases, peroxidases, and glutathione, as well as the oxidative process of ferroptosis, were detected across all insects examined. Cockroaches, particularly C. punctulatus, exhibited notably high levels of oxidative stress-related enzymes, with catalase genes showing greater relative abundance than those for superoxide dismutase (SOD) (Figure 5a). A similar pattern has been reported in the American cockroach Periplaneta americana [28]. The abundance of these redox-regulating components in cockroaches suggests adaptability to shifts in gut redox states, which may fluctuate depending on lignocellulose consumption [63,65].
Antibiotic resistance genes (ARGs) are widespread in diverse environments—soil, freshwater, marine systems, wastewater treatment plants, and even pristine habitats—reflecting their ancient evolutionary origins [66,67]. However, the extensive use of antibiotics has greatly increased ARG prevalence. In this context, wildlife can serve as indicators of environmental pollution by antibiotic resistance genes, especially in densely populated human areas [68,69,70].
Genes conferring resistance to vancomycin, aminoglycosides, chloramphenicol, β-lactams, tetracyclines, and multidrug efflux pumps were detected across all samples, with cockroach metagenomes harboring the greatest resistome diversity (Figure 5b). Because the analyzed insects originated from pristine environments with minimal anthropogenic influence, their resistomes were likely shaped mainly by intrinsic microbial interactions and natural selective pressures rather than direct human activity. These results provide a valuable baseline for assessing naturally occurring ARG levels in environmental microbial communities.
Termites analyzed in this study exhibited lower relative ARG abundance than cockroaches, likely due to ecological and biological differences, including their eusocial lifestyle. The specialized feeding behaviors of termites actively maintain microbiome stability and limit ARG acquisition [71]. Cockroaches, in contrast, exhibit more opportunistic ecological strategies, exposing them to broader environmental microbial reservoirs. While Cryptocercus cockroaches also engage in proctodeal feeding, their social structures are less rigid, and their environmental interactions are more variable. This lifestyle promotes a dynamic gut microbiome capable of incorporating environmental bacteria, including those carrying ARGs. The detection of ARGs in C. punctulatus and Panchlora sp. may indicate environmental dissemination from distant human sources, though their role in ARG transmission is likely limited compared to urban insects. Urban environments—with high antibiotic use and contamination—promote ARG proliferation, as seen in synanthropic cockroaches such as Blattella germanica [72]. Studies in gregarious cockroaches, such as Pycnoscelus surinamensis, have shown increased ARG abundance following antibiotic exposure, with resistance subsequently transmitted to untreated populations through social interaction [73].
The gut microbiota of cockroaches—from both forest and urban environments—thus represents a potential sentinel for anthropogenic antibiotic pollution and mediator of gene flow between ecological reservoirs [74].
5. Conclusions
This study provides valuable insights into the gut microbiota of the wood-feeding cockroach C. punctulatus and its functional comparison with other xylophagous Dictyoptera. Several limitations should be considered. Methodological differences, such as the use of amplicon versus shotgun metagenomics, may introduce biases in microbial detection and quantification, potentially affecting the precision of taxonomic and functional comparisons. Additionally, the study focused primarily on bacterial communities, as protist detection had low resolution in metagenomic analyses, leaving some symbiotic roles unexplored. Future research should aim to refine metagenomic methods to improve eukaryotic microbiota resolution and expand comparative analyses across broader ecological settings. Despite contrasting community compositions, xylophagous cockroaches and termites exhibit remarkable functional convergence in carbon and nitrogen metabolism, while cockroaches appear to serve as hotspots for environmentally acquired antibiotic resistance genes. The high antibiotic resistance observed in cockroaches compared with termites suggests environmental acquisition, highlighting the need for comparative studies between urban and pristine habitats to better understand resistance gene sources and dissemination dynamics. Understanding the composition and functional capabilities of xylophagous insect gut microbiomes not only advances our knowledge of host–microbe coevolution, but also carries significant implications for sustainable agriculture—informing biotechnological strategies for lignocellulose bioconversion and nutrient cycling—and for public health, given that the spread of antibiotic resistance genes in environmental reservoirs may influence the emergence of resistant pathogens in agricultural and urban ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16111128/s1, Figure S1: Principal coordinates analysis of the community distribution of several C. punctulatus samples based on phyla relative abundance obtained by amplicon sequencing made by statgraphics centurion v18.0 program. Cp (V3–V4 region); Cp_1–Cp_3 (V1-V2 region); Cp_4 (V3-V4 region); and Cp_5 (V1-V2 region) (see Table 1). Figure S2: Principal coordinates analysis of the community distribution. (a) Based on taxonomy annotation of shotgun metagenomes processed in the JGI database. (b) Based on functional annotation of shotgun metagenomes processed in the JGI database (panel b) [http://www.jgi.doe.gov/].
Author Contributions
Conceptualization, M.B., R.G. and D.M.-G.; methodology, M.B.; formal analysis, M.B.; data curation, M.B. and D.M.-G.; writing—original draft preparation, M.B. and R.G.; writing—review and editing, M.B., R.G. and D.M.-G.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by MCIN/AEI/10.13039/501100011033 (project PID2021-123735OB-C22).
Data Availability Statement
Data availability in the JGI database Gold pj. Ga0134290 (C. punctulatus) (https://gold.jgi.doe.gov/analysis_project?id=Ga0134290 (accessed on 1 November 2025).
Acknowledgments
JGI staff members who contributed to obtaining the analysis of metagenome data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.D.; Geib, S.M.; Carlson, J.E.; Tien, M.; McKenna, D.; Hoover, K. Functional genomics and microbiome profiling of the Asian long horned beetle (Anoplophora glabripennis) reveal insights into the digestive physiology and nutritional ecology of wood feeding beetles. BMC Genom. 2014, 15, 1096. [Google Scholar] [CrossRef]
- Berlanga, M.; Guerrero, R. The holobiont concept: The case of xylophagous termites and cockroaches. Symbiosis 2016, 68, 49–60. [Google Scholar] [CrossRef]
- Scharf, M.E. Challenges and physiological implications of wood feeding in termites. Curr. Opin. Insect Sci. 2020, 41, 79–85. [Google Scholar] [CrossRef]
- Rowell, R.M.; Pettersen, R.; Tshabalada, M.A. Cell wall chemistry. In Handbook of Wood Chemistry and Wood Composites, 2nd ed.; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2013; Volume 3, pp. 34–72. [Google Scholar]
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2014, 2, 12. [Google Scholar] [CrossRef]
- Montella, S.; Ventorino, V.; Lombard, V.; Henrissat, B.; Pepe, O.; Faraco, V. Discovery of genes coding for carbohydrate-active enzyme by metagenomic analysis of lignocellulosic biomasses. Sci. Rep. 2017, 7, 42623. [Google Scholar] [CrossRef]
- Brune, A.; Dietrich, C. The gut microbiota of termites: Digesting the diversity in the light of ecology and evolution. Annu. Rev. Microbiol. 2015, 69, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.; Tokuda, G.; Watanabe, H.; Rose, H.; Slaytor, M.; Maekawa, K.; Bandi, C.; Noda, H. Evidence from multiple gene sequences indicates that termites evolved from wood-feeding cockroaches. Curr. Biol. 2000, 10, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Klass, K.; Nalepa, C.; Lo, N. Wood-feeding cockroaches as models for termite evolution (Insecta: Dictyoptera): Cryptocercus vs. Parasphaeria boleiriana. Mol. Phyl. Evol. 2008, 46, 809–817. [Google Scholar] [CrossRef]
- Lampert, N.; Mikaelyan, A.; Brune, A. Diet is not the primary driver of bacterial community structure in the gut of litter-feeding cockroaches. BMC Microbiol. 2019, 30, 238. [Google Scholar] [CrossRef]
- Jahnes, B.C.; Sabree, Z.L. Nutritional symbiosis and ecology of host-gut microbe systems in the Blattodea. Curr. Opin. Insect Sci. 2020, 39, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Llorens, C.; Comas, J.; Guerrero, R. Gut bacterial community of the xylophagous cockroaches Cryptocercus punctulatus and Parasphaeria boleiriana. PLoS ONE 2016, 11, e0152400. [Google Scholar] [CrossRef] [PubMed]
- Gontang, E.A.; Aylward, F.O.; Carlos, C.; del Río, T.G.; Chovatia, M.; Fern, A.; Lo, C.-C.; Malfatti, S.A.; Tringe, S.G.; Currie, C.R.; et al. Major changes in microbial diversity and community composition across gut sections of a juvenile Panchlora cockroach. PLoS ONE 2017, 12, e0177189. [Google Scholar] [CrossRef]
- Berlanga, M. Functional symbiosis and communication in microbial ecosystems. The case of wood-eating termites and cockroaches. Int. Microbiol. 2015, 18, 159–169. [Google Scholar] [CrossRef]
- Jahnes, B.C.; Poudel, K.; Staats, A.M.; Sabree, Z.L. Microbial colonization promotes model cockroach gut tissue growth and development. J. Insect Physiol. 2021, 133, 104274. [Google Scholar] [CrossRef]
- Hiltemann, S.; Rasche, H.; Gladman, S.; Hotz, H.-R.; Larivière, D.; Blankenberg, D.; Jagtap, P.D.; Wollmann, T.; Bretaudeau, A.; Goué, N.; et al. Galaxy Training: A powerful framework for teaching! PLoS Comput. Biol. 2023, 19, e1010752. [Google Scholar] [CrossRef]
- Huntemann, M.; Ivanova, N.N.; Mavromatis, K.; Tripp, H.J.; Paez-Espino, D.; Tennessen, K.; Palaniappan, K.; Szeto, E.; Pillay, M.; Chen, I.-M.A.; et al. The standard operation procedure of the DOE-JGI metagenome annotation pipeline (MAP v.4). Stand. Genom. Sci. 2016, 11, 17. [Google Scholar] [CrossRef]
- Utami, Y.D.; Kuwahara, H.; Igai, K.; Murakami, T.; Sugaya, K.; Morikawa, T.; Nagura, Y.; Yuki, M.; Deevong, P.; Inoue, T.; et al. Genome analyses of uncultured TG2/ZB3 bacteria in “margulisbacteria” specifically attached to ectosymbiotic spirochetes of protists in the termite gut. ISME J. 2019, 13, 455–467. [Google Scholar] [CrossRef]
- Tai, V.; James, E.R.; Nalepa, C.A.; Scheffranhn, R.H.; Perlman, S.J.; Keeling, P.J. The role of host phylogeny varies in shaping microbial diversity in the hindguts of lower termites. Appl. Environ. Microbiol. 2015, 81, 1059–1070. [Google Scholar] [CrossRef]
- Dietrich, C.; Brune, A. The cockroach origin of the termite gut microbiota: Patterns in bacterial community structure reflect major evolutionary events. Appl. Environ. Microbiol. 2014, 80, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.G.; Saccenti, E. The power of microbiome studies: Some consideration on which alpha and beta metrics to use and how to report results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.; Petitfils, C.; De Vos, W.; Tilg, H.; El-Omar, E. What defines a healthy gut microbiome? Gut 2024, 73, 333378. [Google Scholar] [CrossRef]
- Tai, V.; Carpenter, L.J.; Weber, P.K.; Nalepa, C.A.; Perlman, S.J.; Keeling, P.J. Genome evolution and nitrogen fixation in bacterial ectosymbionts of a protist inhabiting wood-feeding cockroaches. Appl. Environ. Microbiol. 2016, 82, 4682–4695. [Google Scholar] [CrossRef]
- Hansen, A.K.; Pers, D.; Russell, J.A. Symbiotic solutions to nitrogen limitation and amino acid imbalance in insect diets. Adv. Insect Physiol. 2020, 58, 161–205. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The central role of cytochrome P450 in xenobiotic metabolism—A brief review on a fascinating enzyme family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Xu, J.; Strange, J.P.; Welker, D.L.; James, R.R. Detoxification and stress response genes expressed in a western North American bumble bee, Bombus huntii (Hymenoptera: Apidae). BMC Genom. 2013, 14, 5075–5082. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, J.; Liu, M.; Liu, Z. Midgut transcriptome of the cockroach Periplaneta americana and its microbiota: Digestion, detoxification and oxidative stress response. PLoS ONE 2016, 11, e0155254. [Google Scholar] [CrossRef]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Develop. 2018, 32, 602–619. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Bauer, E.; Strauss, A.S.; Vogel, H.; Marz, M.; Kaltenpoth, M. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. Proc. Royal Soc. B Biol. Sci. 2014, 281, 20141838. [Google Scholar] [CrossRef]
- Gupta, A.; Nair, S. Dynamics of insect-microbiome interactions influence host and microbial symbiont. Front. Microbiol. 2020, 11, 1357. [Google Scholar] [CrossRef]
- Bukin, Y.; Galachyants, Y.; Morozov, I.; Bukin, S.; Zakharenko, A.; Zemskaya, T. The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci. Data 2019, 6, 190007. [Google Scholar] [CrossRef] [PubMed]
- Durazzi, F.; Sala, C.; Castellani, G.; Manfreda, G.; Remondini, D.; De Cesare, A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 2021, 11, 3030. [Google Scholar] [CrossRef]
- Deissova, T.; Zapletalová, M.; Kunovský, L.; Kroupa, R.; Grolich, T.; Kala, Z.; Linhartová, P.B.; Lochman, J. 16S rRNA gene primer choice impacts off-target amplification in human gastrointestinal tract biopsies and microbiome profiling. Sci. Rep. 2023, 13, 12577. [Google Scholar] [CrossRef]
- Addison, B.; Winange, M.C.D.; Pu, Y.; Ragauskas, A.J.; Harman-Ware, A.E. Solid-satate NMR at natural isotopic abundance for bioenergy application. Biotechnol. Biofuels Bioprod. 2025, 18, 46. [Google Scholar] [CrossRef]
- Nalepa, C.A. Origin of termite eusociality: Trophallaxis integrates the social, nutricional, and microbial environments. Ecol. Èntomol. 2015, 40, 323–335. [Google Scholar] [CrossRef]
- Nalepa, C.A. Early development of nymphs and establishment of hindgut symbiosis in Cryptocercus punctulatus (Dictyoptera: Cryptocercidae). Anna. Entomol. Soc. Am. 1990, 83, 786–789. [Google Scholar] [CrossRef]
- Waltmann, A.; Willcox, A.C.; Balasubramanian, S.; Mayorí, K.B.; Guerrero, S.M.; Sanchez, R.S.S.; Roach, J.; Pino, C.C.; Gilman, R.H.; Bern, C.; et al. Hindgut microbiota in laboratory-reared and wild Triatoma infestans. PLoS Neglected Trop. Dis. 2019, 13, e0007383. [Google Scholar] [CrossRef]
- Baltar, J.; Pavan, M.; Correa-Antonio, J.; Couto-Lima, D.; Maciel-De-Freitas, R.; David, M. Gut Bacterial Diversity of Field and Laboratory-Reared Aedes albopictus Populations of Rio de Janeiro, Brazil. Viruses 2023, 15, 1309. [Google Scholar] [CrossRef] [PubMed]
- Tinker, K.; Ottesen, E. Differences in Gut Microbiome Composition Between Sympatric Wild and Allopatric Laboratory Populations of Omnivorous Cockroaches. Front. Microbiol. 2021, 12, 703785. [Google Scholar] [CrossRef]
- Kundu, P.; Manna, B.; Majumder, S.; Ghosh, A. Species-wide metabolic interaction network for understanding natural lignocellulose digestion in termite gut microbiota. Sci. Rep. 2019, 9, 16329. [Google Scholar] [CrossRef]
- Marynowska, M.; Goux, X.; Sillam-Dussès, D.; Rouland-Lefèvre, C.; Halder, R.; Wilmes, P.; Gawron, P.; Roisin, Y.; Delfosse, P.; Calusinska, M. Compositional and functional characterization of biomass-degrading microbial communities in guts and plant fiber- and soil-feeding higher termites. Microbiome 2020, 8, 96. [Google Scholar] [CrossRef]
- Vera-Ponce de Leon, A.; Jahnes, B.C.; Duan, J.; Camuy-Vélez, L.A.; Sabree, Z.L. Cultivable, host-specific Bacteroidetes symbionts exhibit diverse polysaccharolytic strategies. Appl. Environ. Microbiol. 2020, 86, e00091-20. [Google Scholar] [CrossRef]
- Saati-Santamaria, Z.; Rivas, R.; Kolarik, M.; Garcia-Fraile, P. A new perspective of Pseudomonas—Host interactions: Distribution and potential ecological functions of the genus Pseudomonas within the Bark Beetle holobiont. Biology 2021, 10, 164. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Lu, X. Diversity and functional roles of the gut microbiota in Lepidopteran insects. Microorganisms 2022, 10, 1234. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef]
- Tokuda, G.; Mikaelyan, A.; Fukui, C.; Brune, A. Fiber-associated spirochetes are major agents of hemicellulose degradation in the hindgut of wood-feeding higher termites. Proc. Natl. Acad. Sci. USA 2018, 155, E11996–E12004. [Google Scholar] [CrossRef]
- Gazal, V.; Bailez, O.; Viana-Bailez, A.M.; Aguilar Menenzes, E.; Barsanulfo Menendez, E. Decayed wood affecting the attraction of the pest arboretum termite Nasutitermes corniger (Isoptera: Termitidae) to resource foods. Sociobiology 2012, 59, 287–295. [Google Scholar] [CrossRef][Green Version]
- Hu, H.; Rodrigues da Costa, R.; Pilgaard, B.; Schiott, M.; Lange, L.; Poulsen, M. Fungiculture in termites is associated with mycolytic gut bacterial community. mSphere 2019, 4, e00165-19. [Google Scholar] [CrossRef]
- Tong, R.L.; Aguilera-Olivares, D.; Chouvenc, T.; Su, N.-Y. Nitrogen content of the exuviae of Coptotermes gestroi (Wasmann) (Blattodea: Rhinotermitidae). Heliyon 2021, 7, e06697. [Google Scholar] [CrossRef]
- Stentz, R.; Osborne, S.; Horn, N.; Li, A.W.H.; Hautefort, I.; Bongaerts, R.; Rouyer, M.; Bailey, P.; Shears, S.B.; Hemmings, A.M.; et al. A bacterial homolog of eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 2014, 6, 646–656. [Google Scholar] [CrossRef]
- Yang, P.; Liu, W.; Chen, Y.; Gong, A.-D. Engineering the glyoxylate cycle for chemical bioproduction. Front. Bioeng. Biotechnol. 2022, 10, 1066651. [Google Scholar] [CrossRef]
- Fisher, S.H. Regulation of nitrogen metabolism in Bacillus subtilis: Vive la difference. Mol. Microbiol. 1999, 32, 223–232. [Google Scholar] [CrossRef]
- Reitzer, L. Nitrogen assimilation and global regulation in Escherichia coli. Ann. Rev. Microbiol. 2003, 57, 155–176. [Google Scholar] [CrossRef]
- Rohwerder, T. New structural insights into bacterial sulfoacetaldehyde and taurine metabolism. Biochem. J. 2020, 477, 1367–1371. [Google Scholar] [CrossRef]
- Ge, S.-X.; Li, T.-F.; Ren, L.-L.; Zong, S.-X. Host-plant adaptation in xylophagous insect-microbiome systems: Contributions of longicorns and gut symbionts revealed by parallel metatranscriptome. iScience 2023, 26, 106680. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.; Moeller, A.H. The effects of temperature on animal gut microbiomes. Front. Microbiol. 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Palau, M.; Guerrero, R. Gut microbiota dynamics and functionality in Reticulitermes grassei after a 7-day dietary shift and ciprofloxacin treatment. PLoS ONE 2018, 13, e0209789. [Google Scholar] [CrossRef]
- Zhao, M.; Lin, X.; Guo, X. The role of insect symbiotic bacteria in metabolizing phytochemicals and agrochemicals. Insects 2022, 13, 583. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.S.; Aylward, F.O.; Adams, S.M.; Erbilgin, N.; Aukema, B.H.; Currie, C.R.; Suen, G.; Raffa, K.F. Mountain pine beetles colonizing historical and naïve host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl. Environ. Microbiol. 2013, 79, 3468–3475. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Zhu, F.; Shen, Z.; Jiang, H.; Li, Z.; Liu, X.; Xu, H. Function of cytochrome P450s and gut microbiome in biopesticide adaptation of Grapholita molesta on different host diets. Int. J. Mol. Sci. 2023, 24, 15435. [Google Scholar] [CrossRef]
- Brune, A.; Emerson, D.; Breznak, J.A. The termite gut microflora as an oxygen sink: Microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl. Environ. Microbiol. 1995, 61, 2681–2687. [Google Scholar] [CrossRef]
- Berlanga, M.; Paster, B.J.; Guerrero, R. The taxophysiological paradox: Changes in the intestinal microbiota of the xylophagous cockroach Cryptocercus punctulatus depending on the physiological state of the host. Int. Microbiol. 2009, 12, 227–236. [Google Scholar] [CrossRef]
- Pester, M.; Brune, A. Hydrogen is the central free intermediate during lignocellulose degradation by termite gut symbionts. ISME J. 2007, 1, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.-A.T.; Chen, Q.-L.; He, J.-Z.; Hu, H.-W. Microbial regulation of natural antibiotic resistance: Understanding the protist-bacteria interactions for evolution of soil resistome. Sci. Total Environ. 2020, 705, 135882. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B.M. Honeybees and tetracycline resistance. mBio 2013, 4, e00045-13. [Google Scholar] [CrossRef]
- Liu, C.C.; Yao, H.Y.; Wang, C.W. Black soldier fly larvae can effectively degrade oxytetracycline bacterial residue by means of the gut bacterial community. Front. Microbiol. 2021, 12, 663972. [Google Scholar] [CrossRef]
- Maestre-Carballa, L.; Navarro-López, V.; Martinez-Garcia, M. A Resistome roadmap: From the human body to pristine environments. Front. Microbiol. 2022, 13, 858831. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Greening, C. Termite-engineered microbial communities of termite nest structures: A new dimension to the extended phenotype. FEMS Microbiol. Rev. 2022, 46, fuac034. [Google Scholar] [CrossRef] [PubMed]
- Llop, P.; Latorre, A.; Moya, A. Experimental epidemiology of antibiotic resistance: Looking for an appropriate animal model system. Microbiol. Spectrum. 2018, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bogri, A.; Jensen, E.E.B.; Borchert, A.V.; Brinch, C.; Otani, S.; Aarestrup, F.M. Transmission of antimicrobial resistance in the gut microbiome of gregarious cockroaches: The importance of interaction between antibiotic exposed and non-exposed populations. mSystems 2024, 9, e0101823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).