Simple Summary
The moth Loxostege sticticalis (Lepidoptera: Pyralidae) is a typical migrant insect pest found in North America, Eastern Europe, and Asia. The natural pheromone compounds of adult moths are involved in courtship and mating behavior. In this article, we examine the peripheral mechanisms of sex communication, demonstrating that the pheromone receptor (PR) LstiPR2 responds specifically to the major sex pheromone compound E11-14:OAc, which results in the activation of the “a” neuron in sensilla trichodea. The other four LstiPRs showed no response to any sex pheromone compounds. This study will contribute to a new, environmentally friendly strategy for integrated pest management of L. sticticalis.
Abstract
Lepidopteran insects mainly rely on sex pheromones to complete sexual communications. Pheromone receptors (PRs) are expressed on the olfactory receptor neurons (ORNs) of the sensilla trichodea and play an essential role in sexual communication. Despite extensive investigations into the mechanisms of peripheral recognition of sex pheromones in Lepidoptera, knowledge about these mechanisms in L. sticticalis remains limited. In this study, five candidate LstiPRs were analyzed in a phylogenetic tree with those of other Lepidopteran insects. Electroantennography (EAG) assays showed that the major sex pheromone component E11-14:OAc elicited a stronger antennal response than other compounds in male moths. Moreover, two types of neurons in sensilla trichodea were classified by single sensillum recordings, of which the “a” neuron specifically responded to E11-14:OAc. Five candidate PRs were functionally assayed by the heterologous expression system of Xenopus oocytes, and LstiPR2 responded to the major sex pheromone E11-14:OAc. Our findings suggest that LstiPR2 is a PR sensitive to L. sticticalis’s major sex pheromone compound, E11-14:OAc. Furthermore, this study offers valuable insights into the sexual communication behavior of L. sticticalis, forming a foundation for further analysis of the species’ central nervous system.
1. Introduction
Insects have a sophisticated olfactory system to detect and distinguish a wide range of environmental odorants with high sensitivity and specificity [1,2]. Insects rely on the olfactory system to locate habitats and identify mating partners. Sex pheromones emitted from conspecifics are crucial for courtship and mating [3,4]. The sex pheromones of Lepidoptera species, composed of two or more components, are biosynthesized and released by the female sex gland to attract male moths [5]. Since the first insect sex pheromone ((Z,E)-10,12-hexadecadienol) was identified in Bombyx mori [6], increasing numbers of insect sex pheromone compounds have been identified and introduced into agriculture to replace conventional pest control agents [7,8,9,10,11,12].
In Lepidoptera, the primary olfactory organs for recognizing sex pheromones are the antennae. The surface of the antennae is covered with hair-like protrusions called sensilla. Many types of sensilla have been identified, such as trichodea, basiconica, coeloconica, chaetica, squamiformia, styloconica, and Böhm’s bristles [13,14]. The functions of different types of sensilla are diverse, including chemosensation, hygrosensation, theromosensation, and mechanosensation [15,16]. Among these sensilla types, the sensilla trichodea is associated with pheromone detection [17,18,19,20,21]. A typical feature of the sensilla trichodea is the presence of pores on the surface [20,22]. In male antennae, the hairs participating in pheromone sense are common in most moths, such as B. mori, Manduca sexta, Agrotis ipsilon, and Helicoverpa armigera [23].
Sexual communication involves several olfactory proteins that detect, recognize, and degrade peripheral sex pheromones. Odorant binding proteins (OBPs), chemosensory proteins (CSPs), odorant receptors (ORs), ionotropic receptors (IRs), sensory neuron membrane proteins (SNMPs), and odorant degrading enzymes (ODEs) play individual and cooperative roles in these processes [17,24]. Each OR is generally co-expressed with the OR co-receptor (Orco), facilitating chemical signal recognition. This process transforms the chemical signals into electrical signals, regulating chemical communication in insects [4,25]. Pheromone receptors (PRs) are the main ligand gated-ion channels used to bind to pheromones, which are members of the ORs superfamily [24].
Recent studies have reported the functions of insect PRs using Xenopus oocytes [26,27]. For instance, the AdisOR1 from Athetis dissimilis exhibited a strong response to the sex pheromone compounds (Z)-9-tetradecenol (Z9-14:OH) and (Z,E)-9,12-tetradecadien-1-ol (Z9, E12-14:OH) [28]. Similarly, AlepOR3 from Athetis lepigone demonstrated high sensitivity to the sex pheromone (Z)-7-dodecenyl acetate (Z7-12:OAc) [29]. DpunPR45 from Dendrolimus punctatus showed a broad response to sex pheromone compounds [30]. The response profile of olfactory receptor neurons (ORNs) housed in the sensilla, which is determined by expressed PRs, is an essential molecular element in the peripheral olfactory process in insects [27,31]. PRs mainly participate in the sexual communication process of moths [32]. Therefore, PRs play a crucial role in detecting pheromone compounds, and it is necessary to research the functions of PRs in depth.
Loxostege sticticalis (Lepidoptera: Pyralidae), commonly known as the beet webworm, is a devastating pest causing substantial ecological and economic damage to numerous crops across North America, Eastern Europe, and Asia [33,34,35]. L. sticticalis was added to the National Class I list of crop insect pests in 2020 by the Chinese Ministry of Agriculture and Rural Affairs. Like most moths, the male moth of L. sticticalis engages in courtship and mating by detecting the sex pheromone components emitted by female moths [36,37]. Sex pheromones in Lepidoptera are primarily classified into three types—Type 0, Type I, and Type II—based on their chemical structures [38]. The sex pheromone components of L. sticticalis belong to Type I pheromones, consisting of straight-chain alcohols, aldehydes, and acetates with 10–18 carbon atoms. The compounds 11€-tetradecenyl acetate (E11-14:OAc), 11(E)-tetradecenal (E11-14:Ald), E-11-tetradecenol (E11-14:OH), 1-tetradecanol (14:OH), tetradecyl acetate (14:OAc), and 1-dodecanol (12:OH) are the sex pheromone components of L. sticticalis [38,39,40]. A mixture of (E11-14:OAc):(E11-14:Ald) and (E11-14:OAc):(E11-14:Ald):(E11-14:OH) at a ratio of 1:1 and 5:3:12, respectively, was found to be optimal lure rations for L. sticticalis male moths in wind tunnel assays and field assays [39].
While the technique of RNA-Seq [34] has identified the five candidate LstiPRs involved in pheromone detection in L. sticticalis, the function of these genes remains unknown. In this study, we cloned the full-length sequences of five candidate LstiPRs and LstiORco from the antennae of male specimens. The functions of the candidate LstiPRs were characterized using heterologous expression in the Xenopus oocyte system with a two-electrode voltage clamp, and single sensillum recordings (SSRs) were performed to analyze the function of olfactory receptor neurons in response to sex pheromone compounds.
2. Materials and Methods
2.1. Insects
In June 2021, over a thousand larvae of L. sticticalis, ranging from two to five instars, were collected from Hohhot, Inner Mongolia, China (40°82′17″ N, 111°71′58″ E). The larvae were reared with fresh Chenopodium album at a constant temperature of 22 ± 1 °C, a photoperiod of 16 h of light and 8 h of darkness, and a relative humidity of 75 ± 5%. The last-instar larvae were transferred to a box containing clean, sandy soil at 15% humidity to facilitate pupation. Newly emerged moths were immediately separated by sex and kept in 500 mL beakers with a 5% honey solution.
2.2. RNA Extraction and cDNA Synthesis
The antennae were isolated from three-day-old male adults, immediately snap-frozen in liquid nitrogen, and stored at −80 °C for subsequent use. The total RNA of male antennae (30 pairs) was extracted using TRIZOL® Reagent (Invitrogen) according to the manufacturer’s instructions. The quality and quantity of the RNA were assessed by agarose gel electrophoresis (AGE) and NANODROP 2000 (ThermoFisher, Waltham, MA, USA), respectively. The first-strand cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (ThermoFisher, Waltham, MA, USA).
2.3. Phylogenetic Analysis
Phylogenetic trees were constructed using amino acid sequences of candidate genes from LstiPRs, LstiORco, from Lepidoptera species, including B. mori [41], Cnaphalocrocis medinalis [42], Ostrinia furnacalis [43], and L. sticticalis [34]. The amino acid sequences were aligned using Multiple Alignments using Fast Fourier Transform (MAFFT; https://www.ebi.ac.uk/Tools/msa/mafft/ (accessed on 20 April 2022)) with default parameters, and the phylogenetic tree was constructed using the Jones Taylor Thornton (JTT) method in RAxML version 8 with 1000 bootstrap tests [44].
2.4. Cloning Full-Length Sequence of LstiPRs
Following transcriptome analysis and polymerase chain reaction (PCR), the full-length cDNA sequences of five candidate LstiPRs were obtained. The LstiPR-specific primer pairs were designed using Primer Premier 5.0 software (PRIMER Biosoft, Palo Alto, CA, USA) (Table 1). The volumes of the amplification reaction were 50 μL, including 2 μL male antennal cDNA (1 μg μL−1), 2 μL (each) of the primer pairs (10 μM), 19 μL ddH2O, and 25 μL 2 × PrimerSTAR® HS DNA Polymerase (TaKaRa, Japan). PCR conditions were: pre-denaturation at 98 °C for 45 s, followed by 36 cycles of 98 °C for 10 s, 60 °C for 15 s, and 72 °C for 2 min. The final extension was at 72 °C for 10 min. Each LstiPR was initially ligated into the blunt cloning vector of the pEASY®-Blunt Simple Cloning Kit (TransGen Biotech, China) according to the manufacturer’s instructions. Subsequently, the LstiPRs were cloned into the pT7Ts-vector (Invitrogen, Waltham, MA, USA) by seamless cloning technology, according to the instructions of the ClonExpress® II One Step Cloning Kit (Vazyme, Nanjing, China).

Table 1.
Primers used in this study.
2.5. Pheromone Components
Six sex pheromone compounds have been identified from L. sticticalis previously, including (E)-11-tetradecenyl acetate (E11-14:OAc), (E)-11-tetradecenal (E11-14:Ald), (E)-11-tetradecen-1-ol (E11-14:OH), 1-tetradecanol (14:OH), tetradecyl acetate (14:OAc), and 1-dodecanol (12:OH). The pheromone compounds were purchased from Changzhou Nimrod Biotech Inc. (Changzhou, China) (Table 2). Stock solutions of the compounds were prepared in dimethyl sulfoxide at a concentration of 1 M. For the Xenopus oocyte system experiments, 1 × Ringer’s buffer (96 mM NaCl, 2 mM KCl, 5 mM MgCl2, 0.8 mM CaCl2, and 5 mM HEPES, pH 7.6) was used to dilute the stock solutions into working solutions.

Table 2.
The pheromone compounds used in this study.
2.6. Electroantennography Responses
Electroantennography (EAG) was used to evaluate the response of the antennae of three-day-old male adults to six pheromone compounds of females in L. sticticalis. Briefly, the pheromone compounds were dissolved in paraffin oil and diluted to 10 μg μL–1. A filter paper (0.5 cm × 5 cm) was loaded with 10 μL pheromone compound using a Pasteur pipette, and the duration of stimulation was set to 0.8 s with an interval of 3 min. The negative control was 10 μL paraffin oil only. Fifteen to twenty male antennae were stimulated with each pheromone compound, and the difference in electric signal values before and after stimulation was compared. The signals were amplified by a 10 × AC/DC preamplifier (Syntech) and recorded by Syntech EAG software (EAGPro 2.0). The values were normalized to the negative control.
2.7. Single Sensillum Recordings
Signal sensillum recordings (SSRs) revealed the peripheral neural coding of L. sticticalis males detecting pheromones. Three-day-old male antennae of sensilla trichodea were utilized to record responses to pheromone compounds. A single male moth was immobilized inside a 1 mL plastic pipette with the narrow end trimmed off. The moth was delicately pushed into the plastic tip, leaving one antenna and head outside, and secured by dental wax. The tungsten microelectrodes were sharpened with a solution of KNO2 (40%). The recording electrode was inserted into the antenna’s sensilla, while the reference electrode was inserted into the opposite eye. Each trial was performed to test the responses to 10 μL of pheromone compounds at a concentration of 10 μg μL−1 that was dripped onto a piece of filter paper (0.5 cm × 5 cm) and inserted into a Pasteur pipette. The continuous purified and humidified air flow stream was set at 1.2 L min−1 with a 0.3 s stimulus air pulse. A total of eight sensilla trichodea were recorded successfully. The recorded signal was amplified through a 10 × AC/DC preamplifier (Syntech) and recorded by a data acquisition controller (10 s, starting 1 s before stimulation) (IDAC-4, Syntech, Kirchzarten, Germany). The software Autospike (Syntech, Kirchzarten, Germany) was used for digitizing and displaying action potentials on a computer screen. The response of the sensilla trichodea was measured by the change in action potential frequency relative to the frequency of spontaneous action potentials (spikes s−1) before and after stimulation [45].
2.8. Xenopus Oocyte System and Two-Voltage Clamp Recordings
The cDNA sequences of candidate pheromone receptors were cloned, and cRNA was synthesized according to the mMESSAGE mMACHINETM T7 Kit (Thermo Fisher Scientific) instructions. A mixture of each candidate LstiPR and LstiORco cRNA (1:1) was injected into Xenopus laevis oocytes (stageV/VI), which were treated with 2 mg mL−1 collagenase I in Washing Buffer (96 mM NaCl, 2 mM KCl, 5 mM MgCl2, and 5 mM HEPES, pH 7.6) for about 1 h at room temperature and then incubated overnight in Barth solution (96 mM NaCl, 2 mM KCl, 0.8 mM CaCl2, 5 mM MgCl2, 5 mM HEPES, 50 mg/mL tetracycline, 5% horse serum, 100 mg ml−1 streptomycin, and 550 mg ml−1 sodium pyruvate, pH 7.6). After incubating for three days at 18 °C, the two-electrode voltage clamp system was employed to measure the cellular current elicited by stimulation with various pheromones at a concentration of 10−4 M. Dose-response curves were performed using a concentration range of 10−7 to 5 × 10−3 M for each pheromone compound, testing at least six oocyte replicates. The electric signal was amplified using an OC-725C oocyte clamp (Warner Instruments, Hamden, CT, USA) at a holding potential of −80 mV. The data were analyzed with Digidata 1440A and pCLAMP 10.0 software (Axon Instruments Inc., Union City, CA, USA).
2.9. Data Analysis
Data analysis were performed using SPSS 17.0 (IBM Corp., Chicago, IL, USA), and graphs were created with GraphPad Prism 7.0 (GraphPad Software Inc., Boston, CA, USA). The electrophysiological values (currents and spikes s−1) were presented as the mean ± SEM, and the responses of LstiORco/LstiPRx to tested pheromone compounds and single sensillum recording data were analyzed using a one-way nested analysis of variance, followed by a Tukey’s post hoc test (p < 0.05) (IBM, Endicott, NY, USA).
3. Results
3.1. Gene Cloning and Phylogenetic Analysis
The full-length coding regions of the five LstiPRs were amplified, and the complete open reading frames of LstiPR1, LstiPR2, LstiPR3, LstiPR4, and LstiPR5 were found to contain 978 bp, 1263 bp, 1086 bp, 1308 bp, and 1038 bp, encoding polypeptides of 326, 421, 362, 436, and 346 amino acids, respectively. The pairwise sequence identities among these five LstiPRs ranged from 29.91% (LstiPR1/LstiPR2) to 60.80% (LstiPR2/LstiPR5) (Table 3).

Table 3.
Pairwise sequence identities among five LstiPRs (%).
A phylogenetic tree was constructed from candidate LstiORs together with other ORs from B. mori [41], C. medinalis [42], and O. furnacalis [43] based on maximum likelihood (Figure 1). A clade composed of highly conserved ORco genes from L. sticticalis, and the three other species was evident. We observed that the LstiORs share high homology with those of O. furnacalis, and LstiORco is part of the widely expressed and highly conserved ORco family in insects. The five LstiPRs were clustered into the PR clade, which was easily distinguishable from other Lepidopteran species due to their high degree of similarity (purple shaded region in Figure 1).
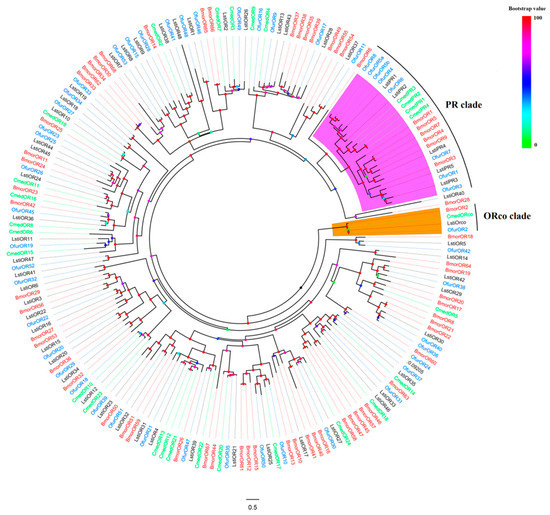
Figure 1.
Phylogenetic relationship between odorant receptors (ORs) of L. sticticalis and other species. The sequences of various ORs were aligned using MAFFT with default parameters, and the tree was constructed by RAxML 8.0 with a bootstrap of 1000 replicates. The clade of the purple region indicates pheromone receptors (PRs), and the clade of the orange region indicates OR co-receptors (ORco).
3.2. Electroantennogram Assays of Pheromone Compounds in L. sticticalis Males
The antennal EAG response of L. sticticalis males to six pheromone compounds was evaluated. The results indicate that E11-14:OAc, at 10 μg μL–1, elicited the strongest response, which was significantly higher than responses to the other pheromones (p < 0.05, Figure 2). The pheromone component, 14:OH, elicited a moderate EAG response (Figure 2). However, the other four pheromone components (12:OH, 14:OAc, E11-14:OH, and E11-14:Ald) elicited weak EAG responses (Figure 2).
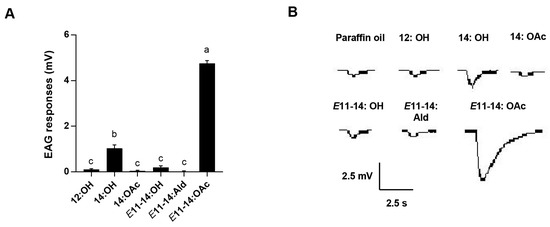
Figure 2.
Electroantennogram (EAG) of responses of L. sticticalis males to pheromone compounds. (A) Responses of males to six pheromone compounds. Error bars represent ± SEM (n = 15–20). Lowercase letters indicate significant differences among tested pheromone compounds (p < 0.05). (B) Tracings of the response of male L. sticticalis to six different pheromone compounds.
3.3. The Responses of the Sensilla Trichodea ORNs of L. sticticalis Males to Pheromones
We recorded responses of sensilla trichodea to pheromones from various positions on the antennae of male L. sticticalis. The results revealed that each sensillum housed two olfactory neurons: one large-spiking and one small-spiking ORN. These were differentiated by their spike amplitudes in single sensillum recordings (Figure 3A). Among the six pheromone components, E11-14:OAc elicited a specific response at a concentration of 10 µg μL–1 to the “a” ORN (Figure 3B). However, the remaining five pheromone components did not induce any changes in the number of spikes (Figure 3B,C).
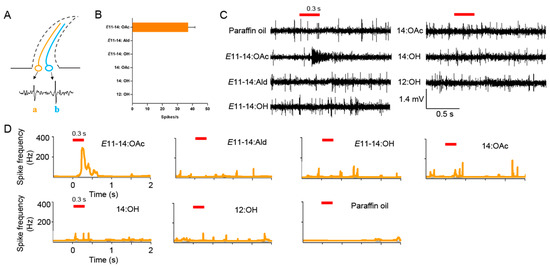
Figure 3.
The responses of sensilla trichodea in the antennae of L. sticticalis males to pheromone components. (A) Distinct ORNs housed in the sensilla trichodea. The order and color of neurons are randomly chosen. “a” and “b” represent the large- and small-spiking ORN, respectively. (B) The response profile of sensilla trichoidea. The error bar represents ± SEM (n = 8). (C) The response traces of signal sensilla recordings of the sensilla trichodea of L. sticticalis males to pheromone components. The red stub line represents stimulus air pulse. (D) Peristimulus time histogram (PSTH) showing the responses of ORN “a” (orange) to six odor stimuli and control. The red stub line represents stimulus air pulse.
3.4. Functional Reconstitution of LstiORco and LstiPRx in Heterologous Cells
The Xenopus oocyte system recorded the responses of the five candidate LstiPRs to six pheromone compounds. The results show that LstiPR2 was mainly responsive to E11-14:OAc, the main sex pheromone of L. sticticalis, with a current value of 702.93 ± 100.86 nA (Figure 4A). In dose-response experiments, LstiPR2/LstiORco was sensitive to E11-14:OAc at a low threshold of the response ranging from 310.57 ± 26.84 nA (10−7 M) to 561.167 ± 77.56 nA (5 × 10−4 M), with an EC50 value of 1.249 × 10−5 M (Figure 4B). The other four receptors (LstiPR1, LstiPR3, LstiPR4, and LstiPR5) showed no response to the pheromone compounds of L. sticticalis (Figure 4C).
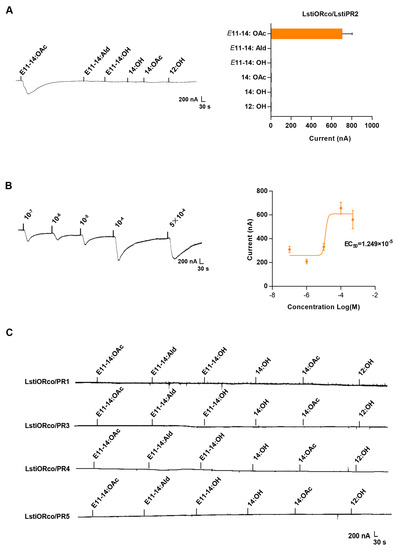
Figure 4.
Stimulation of LstiORco/LstiPRx with pheromone compounds in Xenopus oocytes. All LstiPRs were co-expressed with LstiORco. The concentration of pheromone compounds was 10−4 M, and stimulation was for 15 s. (A) Response profile of LstiORco/LstiPR2 in the Xenopus oocyte system stimulated with pheromone compounds. Error bars indicate ± SEM (n = 6–10). (B) Dose-response curve of LstiORco/LstiPR2 in Xenopus oocytes stimulated with E11-14:OAc. (C) Lack of response of LstiPR1, LstiPR3, LstiPR4, and LstiPR5 to different pheromone compounds.
4. Discussion
Many studies have shown that PRs are vital in insects’ sexual communication [32]. Previous studies identified the pheromone compounds and the candidate PRs of L. sticticalis [34,39], but they provided limited characterization and functional analysis of these LstiPRs. To further investigate their roles in sexual communication in L. sticticalis, we identified five candidate LstiPRs based on sequence homology and phylogenetic analysis [26]. According to the phylogenetic tree, five candidate LstiPRs clustered into the PRs clade due to their high homology. Therefore, we hypothesized that these five candidate LstiPRs could potentially detect sex pheromones in L. sticticalis based on the features of PRs in other moths [46,47].
Sexual communication is essential for courtship and mating behaviors in insects, especially in Lepidoptera [4,37,48]. In general, antennae function as the primary olfactory organs involved in courtship rituals [1,49]. Thus, the antennal responses of male moths to these pheromones were measured by EAG assays to identify the active sex pheromone compounds. Among the six pheromone compounds tested, E11-14:OAc elicited the highest EAG responses. Consistent with previous studies, E11-14:OAc had an induction effect on the entire wind tunnel process, including flight, upwind, approach, and landing [39,40]. However, the other five pheromone components showed weak EAG responses in males, which may be related to the low numbers of sensilla on the antennae of males [13,15,50]. Many studies have shown that PRs are expressed on the ORNs of dendrites of the sensilla trichodea, which primarily sense pheromones [51,52]. Thus, sensilla trichodea were classified using single sensillum recordings to further characterize the functional types, interpreting the recognition of olfactory sensory neurons as pheromone compounds in L. sticticalis. The results show that two neurons, “a” and “b,” were identified in the sensilla trichodea of L. sticticalis. ORN “a” in sensilla trichodea was activated by E11-14:OAc. Usually, two or three neurons are housed in the sensilla trichodea in Lepidoptera [53]. For instance, in Spodoptera frugiperda male’s antennae, two neurons are housed in Type I and III sensilla trichodea, and three are housed in the Type II sensilla trichodea. In addition, E11-14:OAc was the main pheromone compound in some moths, such as O. furnacalis and Mythimna loreyi [46,53]. Our results are consistent with previous studies, namely that the sensilla primarily detected the pheromone compounds [53]. Interestingly, the large-spiking ORN in the male L. sticticalis trichoid sensilla was activated by E11-14:OAc, also found in S. frugiperda and M. loreyi [10,53]. However, the remaining pheromone compounds did not induce any response in the sensilla trichodea ORNs, leading us to speculate that other types of antennal sensilla might be responsible for detecting these compounds. Previous studies have shown that intra-specific pheromone compounds can also be sensed by the ORNs of sensilla auricillicum [54]. From the above two experimental results, some discrepancies are clear. EAG results showed that all tested pheromones could activate the male’s antennae response, whereas SSR results indicated that only E11-14:OAc could elicit the male’s antennae response. The reason for this discrepancy is that EAG tested the response of the entire moth antennae, whereas SSR assays only tested the sensilla trichodea type. Thus, we speculated that the 14:OH might be recognized by other types of sensilla [14].
To further advance our understanding of the sexual communication process, it is imperative to elucidate the function of PRs, as they play a crucial role in detecting pheromone components predominantly by olfactory genes [11,36]. Currently, Xenopus oocytes, as a heterologous expression system in vitro, have been used for studying many species, such as Epiphyas postvittana [11], Heliothis virescens [55], and O. furnacalis [46]. Therefore, we assayed the response of five candidate LstiPRs from L. sticticalis to known pheromone compounds by using the Xenopus oocyte system and found that LstiPR2 was elicited explicitly by the pheromone component E11-14:OAc, while the other four candidate LstiPRs (LstiPR1, LstiPR3, LstiPR4, and LstiPR5) were not activated by any test pheromone compounds. The results show that LstiPRs exhibited a narrow tuning to the sex pheromone chemical signal from female L. sticticalis, in contrast to the broadly tuned PRs identified in O. furnacalis [46]. These results differ from most studies conducted on other moth species. The possible reason may be that when the PRs are expressed in vivo, other olfactory elements must complete the whole process, such as OBPS, CSPs, and SNMPs [56]. Furthermore, the non-responding candidate LstiPRs (LstiPR1, LstiPR3, LstiPR4, and LstiPR5) might be able to detect host volatiles or other types of odors, as previously reported for Cydia pomonella, where CpomOR3 was elicited by a volatile pear ester [57].
5. Conclusions
In conclusion, our results provide robust molecular and electrophysiological evidence that E11-14:OAc is the major pheromone component in L. sticticalis. Interestingly, the pheromone component E11-14:OAc exists in the sex pheromone components of many other species, such as Ostrina spp. [46], Proeulia auraria [58], and Spodoptera litura [12]. Therefore, further investigation into the molecular mechanisms that differentiate between inter- and intra-specific pheromone components in L. sticticalis is required, which is of great significance for optimizing the current pheromone lure.
Author Contributions
Conceptualization, K.-J.L. and S.-J.G.; methodology, Y.Z.; software, Y.Z.; validation, Y.-Y.L.; formal analysis, Y.Z. and H.-B.H.; investigation, H.-B.H.; resources, L.-B.X. and W.-H.W.; data curation, Y.-Y.L., K.-J.L. and H.W.; writing-original draft reparation, Y.Z. and H.-B.H.; writing-review and editing, Y.Z. and H.-B.H.; visualization, L.-F.H. and W.-H.W.; supervision, S.-J.G. and L.-B.X.; project administration, Y.Z.; funding acquisition, Y.Z. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Central Government to Guide Local Science and Technology Development Fund Projects of China (Grant No. 2021ZY0041 to Y.Z.), Natural Science Foundation of Inner Mongolia (Grant No. 2022MS03012 to Y.Z.), the special fund for basic scientific research business of central public welfare scientific research institutes (Grant No. 1610332022010 to Y.Z.), and the National Key Research and Development Program of China (Grant No. 2022YFD1401400 to H.W.).
Data Availability Statement
All the data and resources generated for this study are included in the article.
Acknowledgments
We thank Chen-xi Dong from Lan Kao Vocational College of San Nong for helping to raise the beet webworm. All authors are very thankful to anonymous reviewers for helping to improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Auer, T.O.; Khallaf, M.A.; Silbering, A.F.; Zappia, G.; Ellis, K.; Álvarez-Ocaña, R.; Arguello, J.R.; Hansson, B.S.; Jefferis, G.S.X.E.; Caron, S.J.C.; et al. Olfactory receptor and circuit evolution promote host specialization. Nature 2020, 579, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.G. A review of chemosensation and related behavior in aquatic insects. J. Insect Sci. 2011, 11, 62. [Google Scholar] [CrossRef]
- Walgenbach, J.F.; Schoof, S.C.; Bosch, D.; Escudero-Colomar, L.-A.; Lingren, B.; Krawczyk, G. Comparison of sex pheromone and kairomone-enhanced pheromone lures for monitoring oriental fruit moth (Lepidoptera: Tortricidae) in mating disruption and non-disruption tree fruit orchards. Environ. Entomol. 2021, 50, 1063–1074. [Google Scholar] [CrossRef]
- Zanolli, P.; Annoscia, D.; Zanni, V.; Nazzi, F.; Pavan, F. Behavioural evidence and chemical identification of a female sex pheromone in Anagrus atomus (Hymenoptera: Mymaridae). J. Chem. Ecol. 2021, 47, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpa, F.A.; Jacquin-Joly, E. Sex in the night: Fatty acid-derived sex pheromones and corresponding membrane pheromone receptors in insects. Biochimie 2014, 107, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Butenandt, V.A.; Beckmann, R.; Stamm, D.; Hecker, E. Über den sexual-lockstoff des seidenspinners Bombyx mori. reindarstellung und konstitution. Z. Naturforschg 1959, 14, 283–284. [Google Scholar]
- Fleischer, J.; Rausch, A.; Dietze, K.; Erler, S.; Cassau, S.; Krieger, J. A small number of male-biased candidate pheromone receptors are expressed in large subsets of the olfactory sensory neurons in the antennae of drones from the European honey bee Apis mellifera. Insect Sci. 2022, 29, 749–766. [Google Scholar] [CrossRef]
- Grant, G.G.; Katovich, S.A.; Hall, D.J.; Lombardo, D.A.; Lessor, K.N.S.A. Sex pheromone identification and trapping of Dioryctria resinosella (Lepidoptera, Pyralidae). Environ. Entomol. 1993, 22, 154–161. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, J.W.; Lee, D.H.; Huh, M.J.; Nam, I.; Park, J.H.; Jung, M.; Park, I.K. Identification of sex pheromone components of Korean Dioryctria abietella (Lepidoptera: Pyralidae) population and synergism of pheromone and pine cone volatile blends. J. Econ. Entomol. 2022, 115, 178–186. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Guo, M.B.; Hou, X.Q.; Yang, B.; Wang, G.R. Optimization of a pheromone lure by analyzing the peripheral coding of sex pheromones of Spodoptera frugiperda in China. Pest Manag. Sci. 2022, 78, 2995–3004. [Google Scholar] [CrossRef]
- Yuvaraj, J.K.; Jordan, M.D.; Zhang, D.-D.; Andersson, M.N.; Löfstedt, C.; Newcomb, R.D.; Corcoran, J.A. Sex pheromone receptors of the light brown apple moth, Epiphyas postvittana, support a second major pheromone receptor clade within the Lepidoptera. Insect Biochem. Mol. Biol. 2022, 141, 103708. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Liu, Y.; Jacquin-Joly, E.; Dong, S.; Wang, G. Identification and functional characterization of sex pheromone receptors in the common cutworm (Spodoptera litura). Chem. Senses 2015, 40, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Bawin, T.; Collard, F.; De Backer, L.; Yarou, B.B.; Compère, P.; Francis, F.; Verheggen, F.J. Structure and distribution of the sensilla on the antennae of Tuta absoluta (Lepidoptera: Gelechiidae). Micron 2017, 96, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Zacharuk, R.Y.; Shields, V.D. Sensilla of immature insects. Annu. Rev. Enotomol. 1991, 36, 331–354. [Google Scholar] [CrossRef]
- da Silva, K.B.; da Silva, C.B.; Júnior, K.A.L.R.; de Freitas, J.M.D.; de Freitas, J.D.; Chia, G.S.; Tinôco, R.S.; da Costa, J.G.; Goulart, H.F.; Santana, A.E.G. Morphology and distribution of antennal sensilla of Automeris liberia (Lepidoptera: Saturniidae). Micron 2019, 123, 102682. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.A.; Ignell, R.; Carlson, J.R. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 2005, 25, 8359–8367. [Google Scholar] [CrossRef]
- Cloonan, K.; Rizzato, A.R.; Ferguson, L.; Hillier, N.K. Detection of heliothine sex pheromone components in the Australian budworm moth, Helicoverpa punctigera: Electrophysiology, neuroanatomy, and behavior. J. Comp. Physiol. A 2020, 206, 939–950. [Google Scholar] [CrossRef]
- Kohl, J.; Huoviala, P.; Jefferis, G.S. Pheromone processing in Drosophila. Curr. Opin. Neurobiol. 2015, 34, 149–157. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Cheng, P.; Huang, X.; Gong, M. An odorant receptor from Anopheles sinensis in China is sensitive to oviposition attractants. Malar. J. 2018, 17, 348. [Google Scholar] [CrossRef]
- Riesgo-Escovar, J.R.; Piekos, W.B.; Carlson, J.R. The Drosophila antenna: Ultrastructural and physiological studies in wild-type and lozenge mutants. J. Comp. Physiol. A 1997, 180, 151–160. [Google Scholar] [CrossRef]
- Wang, G.; Vásquez, G.M.; Schal, C.; Zwiebel, L.J.; Gould, F. Functional characterization of pheromone receptors in the tobacco budworm Heliothis virescens. Insect Mol. Biol. 2011, 20, 125–133. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Terrado, M.; Pinnelli, G.R.; Sanes, J.; Plettner, E. Binding interactions, structure-activity relationships and blend effects in pheromone and host olfactory detection of herbivorous Lepidoptera. In Olfactory Concepts of Insect Control-Alternative to Insecticides, 2nd ed.; Picimbon, J.F., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; Volume 2, pp. 265–310. [Google Scholar]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Larsson, M.C.; Domingos, A.I.; Jones, W.D.; Chiappe, M.E.; Amrein, H.; Vosshall, L.B. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 2004, 43, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Grosse-Wilde, E.; Gohl, T.; Dewer, Y.M.; Raming, K.; Breer, H. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). Proc. Natl. Acad. Sci. USA 2004, 101, 11845–11850. [Google Scholar] [CrossRef]
- Nakagawa, T.; Sakurai, T.; Nishioka, T.; Touhara, K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 2005, 307, 1638–1642. [Google Scholar] [CrossRef]
- Liu, X.L.; Sun, S.J.; Khuhro, S.A.; Elzaki, M.E.A.; Yan, Q.; Dong, S.L. Functional characterization of pheromone receptors in the moth Athetis dissimilis (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2019, 158, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Du, L.X.; Xu, J.W.; Wang, B.; Zhang, X.Q.; Yan, Q.; Wang, G. Functional characterization of four sex pheromone receptors in the newly discovered maize pest Athetis lepigone. J. Insect Physiol. 2018, 113, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Cao, S.; Zhang, Z.; Kong, X.; Liu, F.; Wang, G.; Zhang, S. Evolution of sex pheromone receptors in Dendrolimus punctatus Walker (lepidoptera: Lasiocampidae) is divergent from other moth species. Insect Biochem. Mol. Biol. 2020, 122, 103375. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell Mol. Life Sci. 2018, 75, 485–508. [Google Scholar]
- Fleischer, J.; Krieger, J. Insect pheromone receptors-key elements in sensing intraspecific chemical signals. Front. Cell. Neurosci. 2018, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Lv, M.; Mao, N.; Wang, C.; Cheng, Y.; Zhang, L.; Jiang, X.; Luo, L. Molecular characterization of a lysozyme gene and its altered expression profile in crowded beet webworm (Loxostege sticticalis). PLoS ONE 2016, 11, e0161384. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.S.; Li, K.B.; Zhang, S.; Cao, Y.Z.; Yin, J. Identification of candidate chemosensory genes by transcriptome analysis in Loxostege sticticalis Linnaeus. PLoS ONE 2017, 12, e0174036. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Li, E.; Chen, Q.; Kang, H.; Zhang, S.; Li, K.; Wang, Y.; Jiao, Y.; Ren, B. A herbivore-induced plant volatile of the host plant acts as a collective foraging signal to the larvae of the meadow moth, Loxostege sticticalis (Lepidoptera: Pyralidae). J. Insect. Physiol. 2019, 118, 103941. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Sakurai, T.; Namiki, S.; Kanzaki, R. Molecular and neural mechanisms of sex pheromone reception and processing in the silkmoth Bombyx Mori. Front. Physiol. 2014, 5, 125. [Google Scholar] [CrossRef]
- Ando, T.; Inomata, S.; Yamamoto, M. Lepidopteran sex pheromones. Top Curr. Chem. 2004, 239, 51–96. [Google Scholar]
- Liu, A.P.; Cao, Y.X.; Xu, L.B.; Gao, S.J.; Gao, X.L.; Fan, G.M. The preliminary screening of synthetic pheromone of Loxostege sticticaiis. Chin. J. Appl. Enotomol. 2011, 48, 790–795. [Google Scholar]
- Shakhmaev, R.N.; Ishbaeva, A.U.; Shayakhmetova, I.S. Stereoselective synthesis of 11(E)-tetradecen-1-yl acetate- sex pheromone of sod webworm (Loxostege sticticalis). Russ. J. Gen. Chem. 2009, 79, 1171–1174. [Google Scholar] [CrossRef]
- Croset, V.; Rytz, R.; Cummins, S.F.; Budd, A.; Brawand, D.; Kaessmann, H.; Gibson, T.J.; Benton, R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010, 6, e1001064. [Google Scholar] [CrossRef]
- Zeng, F.-F.; Zhao, Z.-F.; Yan, M.-J.; Zhou, W.; Zhang, Z.; Zhang, A.; Lü, Z.-X.; Wang, M.-Q. Identification and comparative expression profiles of chemoreception genes revealed from major chemoreception organs of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). PLoS ONE 2015, 10, e0144267. [Google Scholar] [CrossRef]
- Yang, B.; Ozaki, K.; Ishikawa, Y.; Matsuo, T. Identification of candidate odorant receptors in Asian corn borer Ostrinia furnacalis. PLoS ONE 2015, 10, e0121261. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Leal, W.S. Electrophysiological measurements from a moth olfactory system. J. Vis. Exp. 2011, 49, e2489. [Google Scholar]
- Liu, W.; Jiang, X.C.; Cao, S.; Yang, B.; Wang, G.R. Functional studies of sex pheromone receptors in Asian corn borer Ostrinia furnacalis. Front. Physiol. 2018, 9, 591. [Google Scholar] [CrossRef]
- Wanner, K.W.; Nichols, A.S.; Allen, J.E.; Bunger, P.L.; Garczynski, S.F.; Linn, C.E.; Robertson, H.M.; Luetje, C.W. Sex pheromone receptor specificity in the European corn borer moth, Ostrinia nubilalis. PLoS ONE 2010, 5, e8685. [Google Scholar] [CrossRef]
- Vogel, H.; Heidel, A.J.; Heckel, D.G.; Groot, A.T. Transcriptome analysis of the sex pheromone gland of the noctuid moth Heliothis virescens. BMC Genom. 2010, 11, 29. [Google Scholar] [CrossRef]
- Renou, M.; Anton, S. Insect olfactory communication in a complex and changing world. Curr. Opin. Insect Sci. 2020, 42, 1–7. [Google Scholar] [CrossRef]
- Leal, W.S. Pheromone Reception. In The Chemistry of Pheromones and Other Semiochemicals, 2nd ed.; Schulz, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 341–360. [Google Scholar]
- Benton, R.; Sachse, S.; Michnick, S.W.; Vosshall, L.B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006, 4, e20. [Google Scholar] [CrossRef]
- Jiang, N.J.; Mo, B.T.; Guo, H.; Yang, J.; Tang, R.; Wang, C.Z. Revisiting the sex pheromone of the fall armyworm Spodoptera frugiperda, a new invasive pest in South China. Insect Sci. 2022, 29, 865–878. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.H.; Wang, L.; Yang, B.; Wang, G.R. Development of a new sex attractant via the peripheral coding of pheromones in Mythimna loreyi. J. Agric. Food Chem. 2023, 71, 2795–2803. [Google Scholar] [CrossRef]
- Ansebo, L.; Ignell, R.; Löfqvist, J.; Hansson, B.S. Responses to sex pheromone and plant odours by olfactory receptor neurons housed in sensilla auricillica of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2005, 51, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, Y.; Wang, B.; Wang, G.R. A single point mutation causes one-way alteration of pheromone receptor function in two Heliothis species. IScience 2021, 24, 102981. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Liu, Y.; Yang, T.; Pelosi, P.; Dong, S.L.; Wang, G.R. Pheromone binding proteins enhance the sensitivity of olfactory receptors to sex pheromones in Chilo suppressalis. Sci. Rep. 2015, 5, 13090. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.M.; Gonzalez, F.; Cattaneo, A.M.; Montagné, N.; Walker, W.B.; Bengtsson, M.; Anfora, G.; Ignell, R.; Jacquin-Joly, E.; Witzgall, P. A predicted sex pheromone receptor of codling moth Cydia pomonella detects the plant volatile pear ester. Front. Ecol. Evol. 2014, 2, 33. [Google Scholar] [CrossRef]
- Reyes-Garcia, L.; Cuevas, Y.; Ballesteros, C.; Curkovic, T.; Löfstedt, C.; Bergmann, J. A 4-component sex pheromone of the Chilean fruit leaf roller Proeulia auraria (Lepidoptera: Tortricidae). Cien. Inv. Agr. 2014, 41, 9–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).