Maximizing the Potential of Attractive Targeted Sugar Baits (ATSBs) for Integrated Vector Management
Abstract
Simple Summary
Abstract
1. Introduction and Historical Perspective of Insect Baiting
2. Active Ingredients Used in ATSBs
3. Attractants Incorporated into TSBs
4. Methods of ATSB Deployment
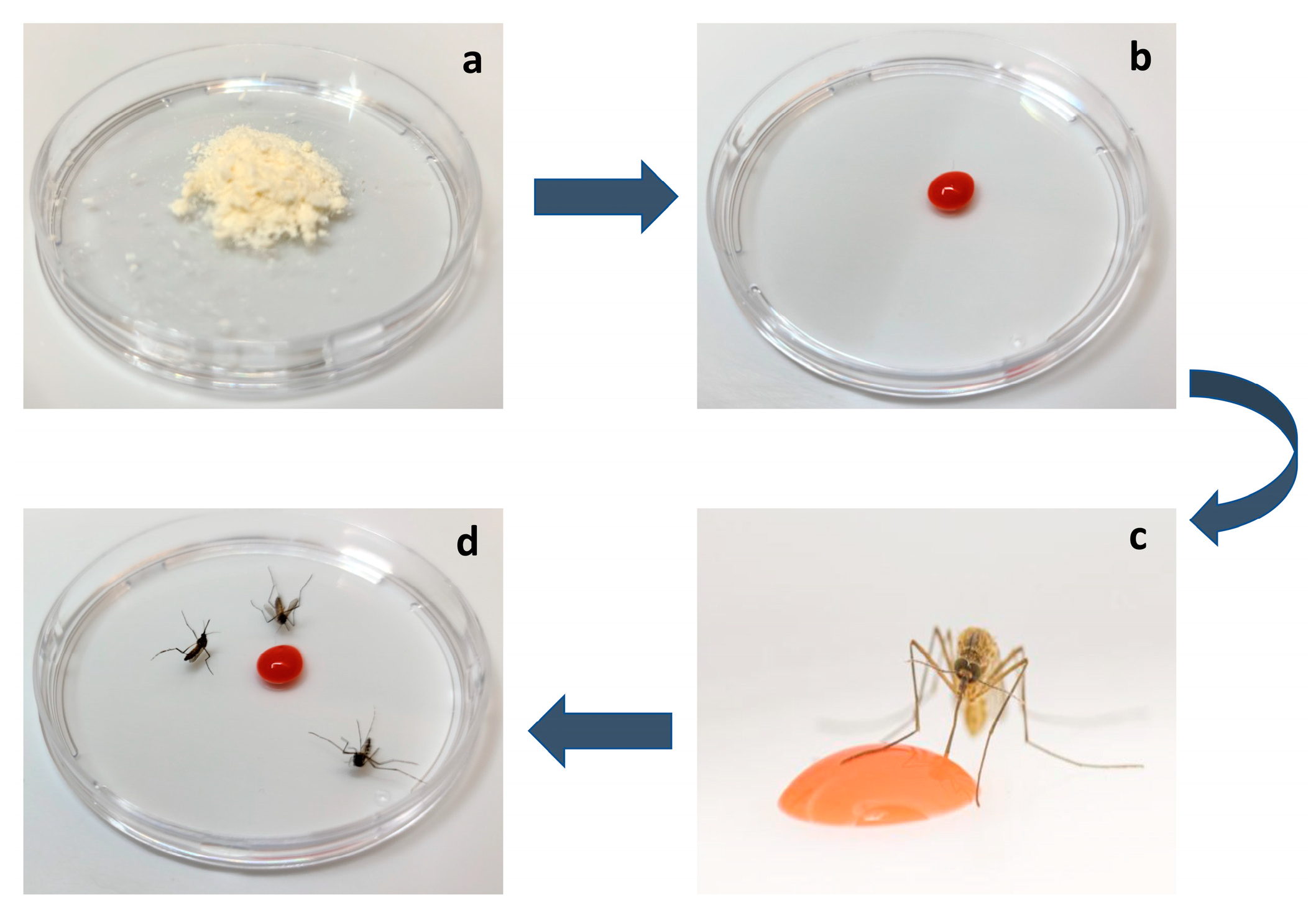
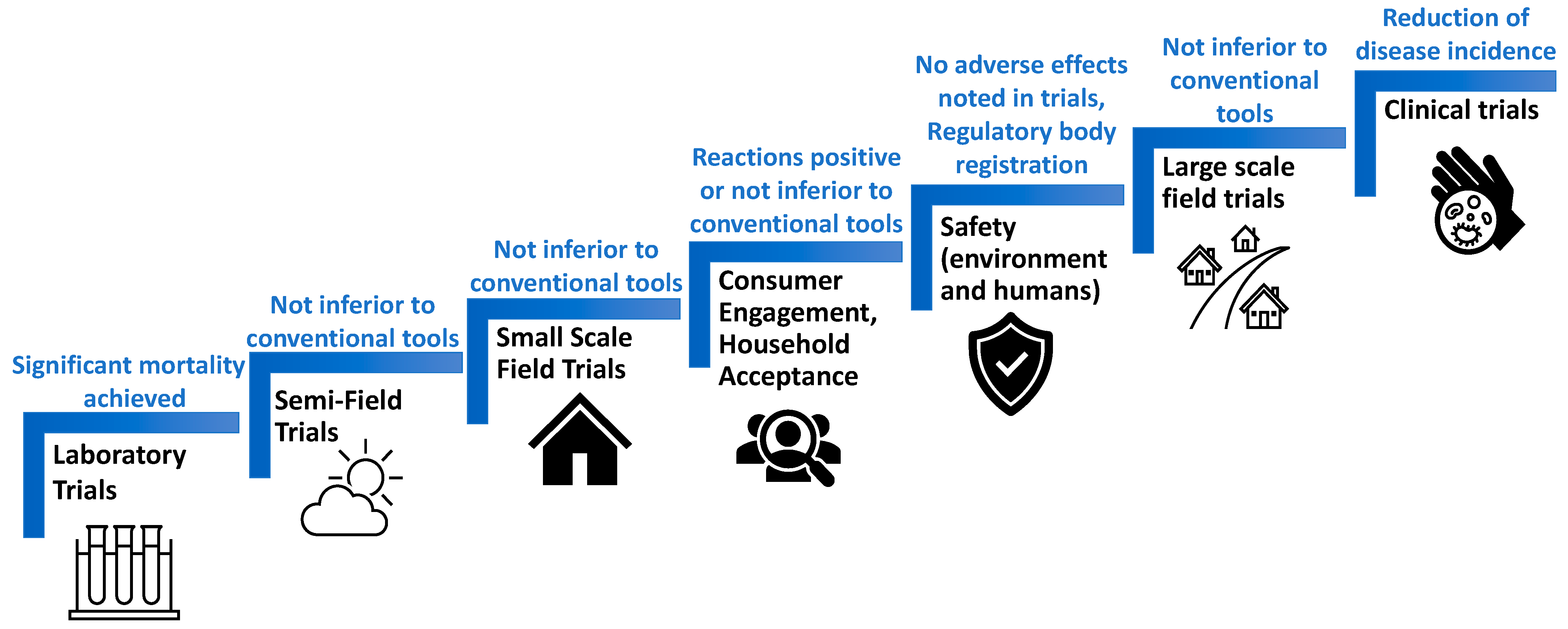
5. ATSB Efficacy Studies
6. Incorporating ATSBs in Integrated Vector Management (IVM)
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Vector-Borne Diseases: Key Facts; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 25 April 2023).
- Karunaratne, S.H.; Surendran, S.N. Mosquito control: A review on the past, present and future strategies. J. Natl. Sci. Found. Sri Lanka. 2022, 50, 277. [Google Scholar] [CrossRef]
- Karunaratne, S.H.; De Silva, W.A.; Weeraratne, T.C.; Surendran, S.N. Insecticide resistance in mosquitoes: Development, mechanisms and monitoring. Ceylon J. Sci. 2018, 47, 299–309. [Google Scholar] [CrossRef]
- Hodgson, E.; Levi, P.E. Pesticides: An important but underused model for the environmental health sciences. Environ. Health Perspect. 1996, 104 (Suppl. 1), 97–106. [Google Scholar] [PubMed]
- Wang, G.H.; Gamez, S.; Raban, R.R.; Marshall, J.M.; Alphey, L.; Li, M.; Rasgon, J.L.; Akbari, O.S. Combating. mosquito-borne diseases using genetic control technologies. Nat. Commun. 2021, 12, 4388. [Google Scholar] [CrossRef]
- Alphey, N.; Bonsall, M.B. RIDL: Modelling release of insects carrying a dominant lethal. In Transgenic Insects: Techniques and Applications; CABI: Wallingford, UK, 2014; pp. 263–282. [Google Scholar]
- Fiorenzano, J.M.; Koehler, P.G.; Xue, R.D. Attractive Toxic Sugar Bait (ATSB) for control of mosquitoes and its impact on non-target organisms: A review. Int. J. Environ. Res. Public Health 2017, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Dethier, V.J. Chemical insect attractants and repellents. Soil Sci. 1948, 65, 204. [Google Scholar] [CrossRef]
- Kofoid, C.A.; Light, S.F.; Horner, A.C.; Randall, M. Termites and their control. Nature 1934, 133, 929–930. [Google Scholar]
- Esenther, G.R.; Beal, R.H. Attractant-mirex bait suppresses activity of Reticulitermes spp. J. Econ. Entomol. 1974, 67, 85–88. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Simpson, S.J. Bugs in the system: Insects and their impact on human affairs. Nature 1995, 374, 842. [Google Scholar]
- Mallis, A.; Story, K. Handbook of Pest Control; MacNair-Dorland: New York, NY, USA, 1969. [Google Scholar]
- Lea, A.O. Sugar-baited insecticide residues against mosquitoes. Mosq. News. 1965, 25, 1. [Google Scholar]
- Dhang, P. Innovations in insect baiting and its role in reducing insecticide load in urban pest control. Int. Pest Control 2016, 58, 210. [Google Scholar]
- Shin, E.; Park, C.; Ahn, Y.J.; Lee, D.K.; Chang, K.S. Insecticidal and repellent activities of insecticide-sucrose solutions to Culex pipiens molestus (Diptera: Culicidae) under laboratory and field conditions. Pest Manag. Sci. 2011, 67, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.D.; Müller, G.C.; Kline, D.L.; Barnard, D.R. Effect of application rate and persistence of boric acid sugar baits applied to plants for control of Aedes albopictus. J. Am. Mosq. Control Assoc. 2011, 27, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Kravchenko, V.D.; Schlein, Y. Decline of Anopheles sergentii and Aedes caspius populations following presentation of attractive toxic (spinosad) sugar bait stations in an oasis. J. Am. Mosq. Control Assoc. 2008, 24, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef]
- Schlein, Y.; Muller, G.C. An approach to mosquito control: Using the dominant attraction of flowering Tamarix jordanis trees against Culex pipiens. J. Med. Entomol. 2008, 45, 384–390. [Google Scholar] [CrossRef]
- Müller, G.C.; Beier, J.C.; Traore, S.F.; Toure, M.B.; Traore, M.M.; Bah, S.; Doumbia, S.; Schlein, Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar. J. 2010, 9, 210. [Google Scholar] [CrossRef]
- Mascari, T.M.; Foil, L.D. Laboratory evaluation of insecticide-treated sugar baits for control of phlebotomine sand flies (Diptera: Psychodidae). J. Am. Mosq. Control Assoc. 2010, 26, 398–402. [Google Scholar] [CrossRef]
- Schlein, Y.; Müller, G.C. Experimental control of Phlebotomus papatasi by spraying attractive toxic sugar bait (ATSB) on vegetation. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 766–771. [Google Scholar] [CrossRef]
- Lewis, D.J.; Domoney, C.R. Sugar meals in Phlebotominae and Simuliidae (Diptera). Proc. R. Entomol. Soc. Ser. A Gen. Entomol. 1966, 41, 175–179. [Google Scholar] [CrossRef]
- Foster, W.A.; Takken, W. Nectar-related vs. human-related volatiles: Behavioural response and choice by female and male Anopheles gambiae (Diptera: Culicidae) between emergence and first feeding. Bull. Entomol. Res. 2004, 94, 145–157. [Google Scholar] [CrossRef]
- Stone, C.M.; Hamilton, I.M.; Foster, W.A. A survival and reproduction trade-off is resolved in accordance with resource availability by virgin female mosquitoes. Anim. Behav. 2011, 81, 765–774. [Google Scholar] [CrossRef]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide resistance management and industry: The origins and evolution of the Insecticide Resistance Action Committee (IRAC) and the mode of action classification scheme. Pest Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef]
- Diarra, R.A.; Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; et al. Testing configurations of attractive toxic sugar bait (ATSB) stations in Mali, West Africa, for improving the control of malaria parasite transmission by vector mosquitoes and minimizing their effect on non-target insects. Malar. J. 2021, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.; Samal, R.R.; Kumar, M.; Verma, V.; Sagar, R.K.; Singh, S.P.; Raghavendra, K. Laboratory evaluation of the efficacy of deltamethrin-laced attractive toxic sugar bait formulation on Anopheles stephensi. Malar. J. 2023, 22, 92. [Google Scholar] [CrossRef] [PubMed]
- Stewart, Z.P.; Oxborough, R.M.; Tungu, P.K.; Kirby, M.J.; Rowland, M.W.; Irish, S.R. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS ONE 2013, 8, e84168. [Google Scholar] [CrossRef]
- Traore, M.M.; Junnila, A.; Traore, S.F.; Doumbia, S.; Revay, E.E.; Kravchenko, V.D.; Schlein, Y.; Arheart, K.L.; Gergely, P.; Xue, R.D.; et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 2020, 19, 72. [Google Scholar] [CrossRef]
- Barbosa, D.S.; Rodrigues, M.M.S.; Silva, A.A.E. Evaluation of attractive toxic sugar baits (ATSB) against Aedes aegypti (Diptera: Culicidae) in laboratory. Trop. Biomed. 2019, 36, 578–586. [Google Scholar]
- Kumar, G.; Sharma, A.; Dhiman, R. Laboratory evaluation of the efficacy of boric acid containing toxic sugar baits against Anopheles culicifacies, An. stephensi and Aedes aegypti mosquitoes. J. Vector Borne Dis. 2022, 59, 52–56. [Google Scholar]
- Qualls, W.A.; Müller, G.C.; Traore, S.F.; Traore, M.M.; Arheart, K.L.; Doumbia, S.; Schlein, Y.; Kravchenko, V.D.; Xue, R.D.; Beier, J.C. Indoor use of attractive toxic sugar bait (ATSB) to effectively control malaria vectors in Mali, West Africa. Malar. J. 2015, 14, 301. [Google Scholar] [CrossRef]
- Revay, E.E.; Müller, G.C.; Qualls, W.A.; Kline, D.L.; Naranjo, D.P.; Arheart, K.L.; Kravchenko, V.D.; Yefremova, Z.; Hausmann, A.; Beier, J.C.; et al. Control of Aedes albopictus with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in St. Augustine, Florida. Parasitol. Res. 2014, 113, 73–79. [Google Scholar] [CrossRef]
- Tenywa, F.C.; Kambagha, A.; Saddler, A.; Maia, M.F. The development of an ivermectin-based attractive toxic sugar bait (ATSB) to target Anopheles arabiensis. Malar. J. 2017, 16, 338. [Google Scholar] [CrossRef]
- Alomar, A.A.; Eastmond, B.H.; Rapti, Z.; Walker, E.D.; Alto, B.W. Ingestion of spinosad-containing toxic sugar bait alters Aedes albopictus vector competence and vectorial capacity for dengue virus. Front. Microbiol. 2022, 13, 933482. [Google Scholar] [CrossRef] [PubMed]
- Kline, D.L.; Muller, G.C.; Junnila, A.; Xue, R.D. Attractive toxic sugar baits (ATSB): A novel vector management tool. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018. [Google Scholar]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef]
- Davis, J.; Bibbs, C.S.; Müller, G.C.; Xue, R.-D. Evaluation of Bacillus thuringiensis israelensis as toxic sugar bait against adult Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes. J. Vector Ecol. 2021, 46, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Schlein, Y.; Pener, H. Bait-fed adult Culex pipiens carry the larvicide Bacillus sphaericus to the larval habitat. Med. Vet. Entomol. 1990, 4, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Schlein, Y.; Müller, G.C. Decrease of larval and subsequent adult Anopheles sergentii populations following feeding of adult mosquitoes from Bacillus sphaericus-containing attractive sugar baits. Parasit. Vectors 2015, 8, 244. [Google Scholar] [CrossRef]
- Lindh, J.M.; Terenius, O.; Eriksson-Gonzales, K.; Knols, B.G.J.; Faye, I. Re-introducing bacteria in mosquitoes-A method for determination of mosquito feeding preferences based on coloured sugar solutions. Acta Trop. 2006, 99, 173–183. [Google Scholar] [CrossRef]
- Ondiaka, S.N.; Masinde, E.W.; Koenraadt, C.J.; Takken, W.; Mukabana, W.R. Effects of fungal infection on feeding and survival of Anopheles gambiae (Diptera: Culicidae) on plant sugars. Parasit. Vectors 2015, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Robert, L.L.; Perich, M.J.; Schlein, Y.; Jacobson, R.L.; Wirtz, R.A.; Lawyer, P.G.; Githure, J.I. Phlebotomine sand fly control using bait-fed adults to carry the larvicide Bacillus sphaericus to the larval habitat. J. Am. Mosq. Control Assoc. 1997, 13, 140–144. [Google Scholar]
- Scott, J.M.; Seeger, K.E.; Gibson-Corrado, J.; Muller, G.C.; Xue, R.D. Attractive toxic sugar bait (ATSB) mixed with pyriproxyfen for control of larval Aedes albopictus (Diptera: Culicidae) through fecal deposits of adult mosquitoes. J. Med. Entomol. 2017, 54, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, A.; Scott, J.M.; Qualls, W.A.; Müller, G.C.; Xue, R.D. Attractive toxic sugar baits mixed with pyriproxyfen sprayed on plants against adult and larval Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 896–899. [Google Scholar] [CrossRef]
- Junnila, A.; Revay, E.E.; Müller, G.C.; Kravchenko, V.; Qualls, W.A.; Xue, R.D.; Allen, S.A.; Beier, J.C.; Schlein, Y. Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in beta-cyclodextrin as the active ingredient. Acta Trop. 2015, 152, 195–200. [Google Scholar] [CrossRef] [PubMed]
- McDermott, E.G.; Morris, E.K.; Garver, L.S. Sodium ascorbate as a potential toxicant in attractive sugar baits for control of adult mosquitoes (Diptera: Culicidae) and Sand Flies (Diptera: Psychodidae). J. Med. Entomol. 2019, 56, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Revay, E.E.; Schlein, Y.; Tsabari, O.; Kravchenko, V.; Qualls, W.; De-Xue, R.; Beier, J.C.; Traore, S.F.; Doumbia, S.; Hausmann, A.; et al. Formulation of attractive toxic sugar bait (ATSB) with safe EPA-exempt substance significantly diminishes the Anopheles sergentii population in a desert oasis. Acta Trop. 2015, 150, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Limitation of Plant Biopesticides. In Advances in Plant Biopesticides; Singh, D., Ed.; Springer: New Delhi, India, 2014. [Google Scholar]
- Airs, P.M.; Bartholomay, L.C. RNA interference for mosquito and mosquito-borne disease control. Insects 2017, 8, 4. [Google Scholar] [CrossRef]
- Wiltshire, R.M.; Duman-Scheel, M. Advances in oral RNAi for disease vector mosquito research and control. Curr. Opin. Insect Sci. 2020, 40, 18–23. [Google Scholar] [CrossRef]
- Duman-Scheel, M. Saccharomyces cerevisiae (baker’s yeast) as an interfering RNA expression and delivery system. Curr. Drug Targets 2019, 20, 942–952. [Google Scholar] [CrossRef]
- Hapairai, L.K.; Mysore, K.; Sun, L.; Li, P.; Wang, C.W.; Scheel, N.D.; Lesnik, A.; Scheel, M.P.; Igiede, J.; Wei, N.; et al. Characterization of an adulticidal and larvicidal interfering RNA pesticide that targets a conserved sequence in mosquito G protein-coupled dopamine 1 receptor genes. Insect Biochem. Mol. Biol. 2020, 120, 103359. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Li, P.; Wang, C.W.; Scheel, N.D.; Lesnik, A.; Igiede, J.; Scheel, M.P.; Wei, N.; et al. Characterization of a dual-action adulticidal and larvicidal interfering RNA pesticide targeting the Shaker gene of multiple disease vector mosquitoes. PLoS Negl. Trop. Dis. 2020, 14, e0008479. [Google Scholar] [CrossRef]
- Mysore, K.; Sun, L.; Hapairai, L.K.; Wang, C.W.; Igiede, J.; Roethele, J.B.; Scheel, N.D.; Scheel, M.P.; Li, P.; Wei, N.; et al. A yeast RNA-interference pesticide targeting the Irx gene functions as a broad-based mosquito larvicide and adulticide. Insects 2021, 12, 986. [Google Scholar] [CrossRef]
- Mysore, K.; Sun, L.; Hapairai, L.K.; Wang, C.W.; Roethele, J.B.; Igiede, J.; Scheel, M.P.; Scheel, N.D.; Li, P.; Wei, N.; et al. A broad-based mosquito yeast interfering RNA pesticide targeting Rbfox1 represses notch signaling and kills both larvae and adult mosquitoes. Pathogens 2021, 10, 1251. [Google Scholar] [CrossRef] [PubMed]
- Taracena, M.; Hunt, C.; Pennington, P.; Andrew, D.; Jacobs-Lorena, M.; Dotson, E.; Wells, M. Effective oral RNA interference (RNAi) administration to adult Anopheles gambiae mosquitoes. J. Vis. Exp. 2022, 1, 181. [Google Scholar]
- Hapairai, L.K.; Mysore, K.; Chen, Y.; Harper, E.I.; Scheel, M.P.; Lesnik, A.M.; Sun, L.; Severson, D.W.; Wei, N.; Duman-Scheel, M. Lure-and-kill yeast interfering RNA larvicides targeting neural genes in the human disease vector mosquito Aedes aegypti. Sci. Rep. 2017, 7, 13223. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Harper, E.I.; Chen, Y.; Eggleson, K.K.; Realey, J.S.; Scheel, N.D.; Severson, D.W.; Wei, N.; et al. Yeast interfering RNA larvicides targeting neural genes induce high rates of Anopheles larval mortality. Malar. J. 2017, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Ojha, V.P.; Pasi, S. Applicability of attractive toxic sugar baits as a mosquito vector control tool in the context of India: A review. Pest Manag. Sci. 2021, 77, 2626–2634. [Google Scholar] [CrossRef]
- Xue, R.D.; Ali, A.; Kline, D.L.; Barnard, D.R. Field evaluation of boric acid- and fipronil-based bait stations against adult mosquitoes. J. Am. Mosq. Control Assoc. 2008, 24, 415–418. [Google Scholar] [CrossRef]
- Scott-Fiorenzano, J.M.; Fulcher, A.P.; Seeger, K.E.; Allan, S.A.; Kline, D.L.; Koehler, P.G.; Müller, G.C.; Xue, R.-D. Evaluations of dual attractant toxic sugar baits for surveillance and control of Aedes aegypti and Aedes albopictus in Florida. Parasit. Vectors 2017, 10, 9. [Google Scholar] [CrossRef]
- Qualls, W.A.; Müller, G.C.; Revay, E.E.; Allan, S.A.; Arheart, K.L.; Beier, J.C.; Smith, M.L.; Scott, J.M.; Kravchenko, V.D.; Hausmann, A.; et al. Evaluation of attractive toxic sugar bait (ATSB)-barrier for control of vector and nuisance mosquitoes and its effect on non-target organisms in sub-tropical environments in Florida. Acta Trop. 2014, 131, 104–110. [Google Scholar] [CrossRef]
- Khallaayoune, K.; Qualls, W.A.; Revay, E.E.; Allan, S.A.; Arheart, K.L.; Kravchenko, V.D.; Xue, R.D.; Schlein, Y.; Beier, J.C.; Müller, G.C. Attractive toxic sugar baits: Control of mosquitoes with the low-risk active ingredient dinotefuran and potential impacts on nontarget organisms in Morocco. Environ. Entomol. 2013, 42, 1040–1045. [Google Scholar] [CrossRef]
- Chanda, J.; Wagman, J.; Chanda, B.; Kaniki, T.; Ng‘andu, M.; Muyabe, R.; Mwenya, M.; Sakala, J.; Miller, J.; Mwaanga, G.; et al. Feeding rates of malaria vectors from a prototype attractive sugar bait station in Western Province, Zambia: Results of an entomological validation study. Malar. J. 2023, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Eisele, T.P.; Kleinschmidt, I.; Sarrassat, S.; terKuile, F.; Miller, J.; Chanda, J.; Silumbe, K.; Samuels, A.; Janssen, J.; Ogwang, C.; et al. Attractive targeted sugar bait phase III trials in Kenya, Mali, and Zambia. Trials 2022, 23, 640. [Google Scholar]
- Furnival-Adams, J.E.C.; Camara, S.; Rowland, M.; Koffi, A.A.; Ahoua Alou, L.P.; Oumbouke, W.A.; N’Guessan, R. Indoor use of attractive toxic sugar bait in combination with long-lasting insecticidal net against pyrethroid-resistant Anopheles gambiae: An experimental hut trial in Mbé, central Côte d’Ivoire. Malar. J. 2020, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Schlein, Y. Efficacy of toxic sugar baits against adult cistern-dwelling Anopheles claviger. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 480–484. [Google Scholar] [CrossRef]
- Qualls, W.A.; Xue, R.; Revay, E.E.; Allan, S.A.; Müller, G.C. Implications for operational control of adult mosquito production in cisterns and wells in St. Augustine, FL using attractive sugar baits. Acta Trop. 2012, 124, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, D.P.; Qualls, W.A.; Müller, G.C.; Samson, D.M.; Roque, D.; Alimi, T.; Arheart, K.; Beier, J.C.; Xue, R.D. Evaluation of boric acid sugar baits against Aedes albopictus (Diptera: Culicidae) in tropical environments. Parasitol. Res. 2013, 112, 1583–1587. [Google Scholar] [CrossRef]
- WHO. Strategy Development and Monitoring for Parasitic Diseases and Vector Control Team. Global Strategic Framework for Integrated Vector Management; World Health Organization: Geneva, Switzerland, 2004. Available online: https://apps.who.int/iris/handle/10665/68624 (accessed on 28 April 2023).
- WHO. Handbook for Integrated Vector Management; World Health Organization: Geneva, Switzerland, 2012. Available online: https://apps.who.int/iris/handle/10665/44768 (accessed on 28 April 2023).
- Bayoh, M.N.; Walker, E.D.; Kosgei, J.; Ombok, M.; Olang, G.B.; Githeko, A.K.; Killeen, G.F.; Otieno, P.; Desai, M.; Lobo, N.F.; et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit. Vectors 2014, 20, 380. [Google Scholar] [CrossRef]
- Choi, L.; Wilson, A. Larviciding to control malaria. Cochrane Database Syst Rev. 2017, 2017, CD012736. [Google Scholar] [CrossRef]
- Kiware, S.S.; Chitnis, N.; Tatarsky, A.; Wu, S.; Castellanos, H.M.S.; Gosling, R.; Smith, D.; Marshall, J.M. Attacking the mosquito on multiple fronts: Insights from the vector control optimization model (VCOM) for malaria elimination. PLoS ONE 2017, 12, e0187680. [Google Scholar] [CrossRef]
- Killeen, G.F. Characterizing, controlling and eliminating residual malaria transmission. Malar. J. 2014, 23, 330. [Google Scholar] [CrossRef]
- Chaccour, C.; Killeen, G.F. Mind the gap: Residual malaria transmission, veterinary endectocides and livestock as targets for malaria vector control. Malar. J. 2016, 12, 24. [Google Scholar] [CrossRef]
- Omondi, S.; Kosgei, J.; Agumba, S.; Polo, B.; Yalla, N.; Moshi, V.; Abong’o, B.; Ombok, M.; McDermott, D.P.; Entwistle, J.; et al. Natural sugar feeding rates of Anopheles mosquitoes collected by different methods in western Kenya. Sci. Rep. 2022, 12, 20596. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.M.; White, M.T.; Ghani, A.C.; Schlein, Y.; Muller, G.C.; Beier, J.C. Quantifying the mosquito’s sweet tooth: Modelling the effectiveness of attractive toxic sugar baits (ATSB) for malaria vector control. Malar. J. 2013, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Marshall, J.M.; Qualls, W.A.; Schlein, Y.; McManus, J.W.; Arheart, K.L.; Hlaing, W.M.; Traore, S.F.; Doumbia, S.; Müller, G.C.; et al. Modelling optimum use of attractive toxic sugar bait stations for effective malaria vector control in Africa. Malar. J. 2015, 14, 492. [Google Scholar] [CrossRef]
- Beier, J.C.; Müller, G.C.; Gu, W.; Arheart, K.L.; Schlein, Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar. J. 2012, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Sissoko, F.; Junnila, A.; Traore, M.M.; Traore, S.F.; Doumbia, S.; Dembele, S.M.; Schlein, Y.; Traore, A.S.; Gergely, P.; Xue, R.D.; et al. Frequent sugar feeding behavior by Aedes aegypti in Bamako, Mali makes them ideal candidates for control with attractive toxic sugar baits (ATSB). PLoS ONE 2019, 14, e0214170. [Google Scholar] [CrossRef]
- Scott, T.W.; Amerasinghe, P.H.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Edman, J.D. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J. Med. Entomol. 2000, 37, 89–101. [Google Scholar] [CrossRef]
- WHO. Vector Control Advisory Group (VCAG) on New Tools, Technologies and Approaches: Terms of Reference; World Health Organization: Geneva, Switzerland, 2020. Available online: https://apps.who.int/iris/handle/10665/276401 (accessed on 30 April 2023).
- Duman Scheel, M.; Severson, D.W.; Eggleston, K.; Wei, N. RNAi Insecticide Materials and Methods. International Patent Application No. 62/361,704, 13 July 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njoroge, T.M.; Hamid-Adiamoh, M.; Duman-Scheel, M. Maximizing the Potential of Attractive Targeted Sugar Baits (ATSBs) for Integrated Vector Management. Insects 2023, 14, 585. https://doi.org/10.3390/insects14070585
Njoroge TM, Hamid-Adiamoh M, Duman-Scheel M. Maximizing the Potential of Attractive Targeted Sugar Baits (ATSBs) for Integrated Vector Management. Insects. 2023; 14(7):585. https://doi.org/10.3390/insects14070585
Chicago/Turabian StyleNjoroge, Teresia Muthoni, Majidah Hamid-Adiamoh, and Molly Duman-Scheel. 2023. "Maximizing the Potential of Attractive Targeted Sugar Baits (ATSBs) for Integrated Vector Management" Insects 14, no. 7: 585. https://doi.org/10.3390/insects14070585
APA StyleNjoroge, T. M., Hamid-Adiamoh, M., & Duman-Scheel, M. (2023). Maximizing the Potential of Attractive Targeted Sugar Baits (ATSBs) for Integrated Vector Management. Insects, 14(7), 585. https://doi.org/10.3390/insects14070585






