Competing Vegetation Structure Indices for Estimating Spatial Constrains in Carabid Abundance Patterns in Chinese Grasslands Reveal Complex Scale and Habitat Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Carabid Abundances
2.2. Landscape Remotely Sensed Imagery
2.3. Selection of Vegetation Indices
2.4. Sampling Sites
2.5. Explaining Carabid Abundance
3. Results
3.1. Overall Results
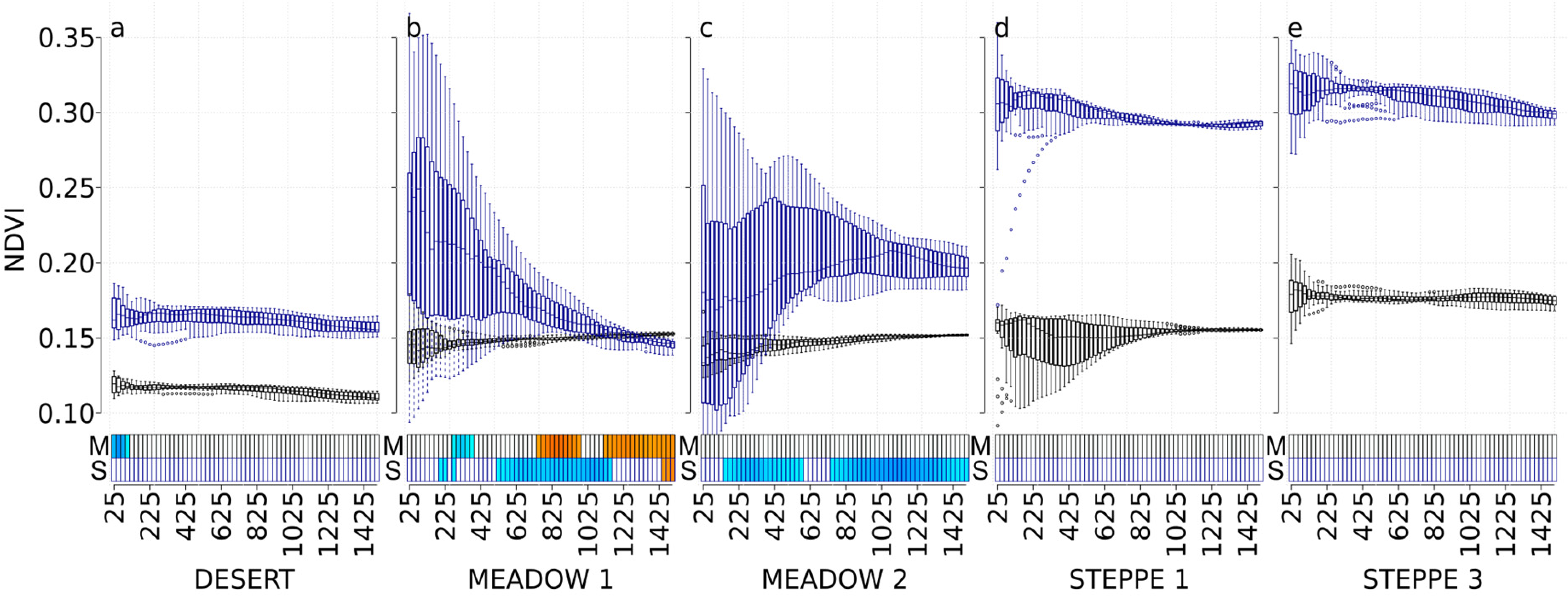
3.2. NDVI
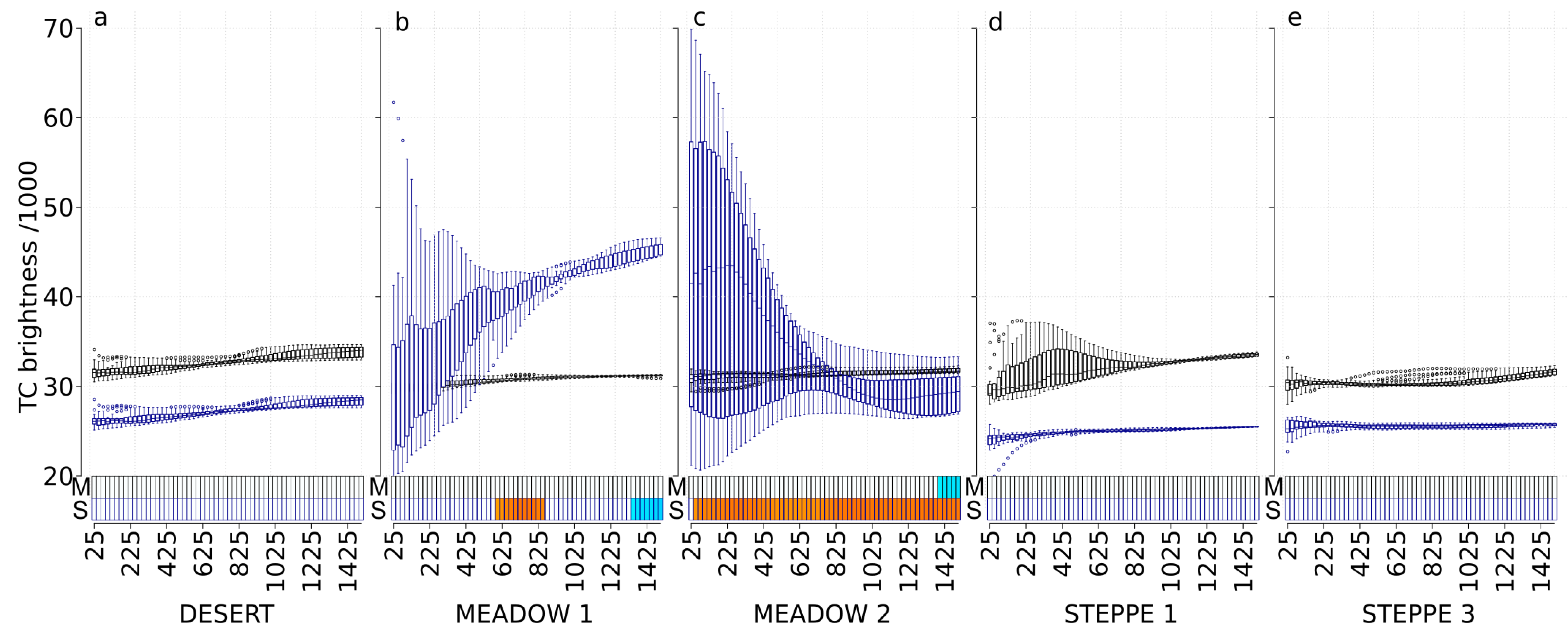
3.3. TC Brightness (TC-B)
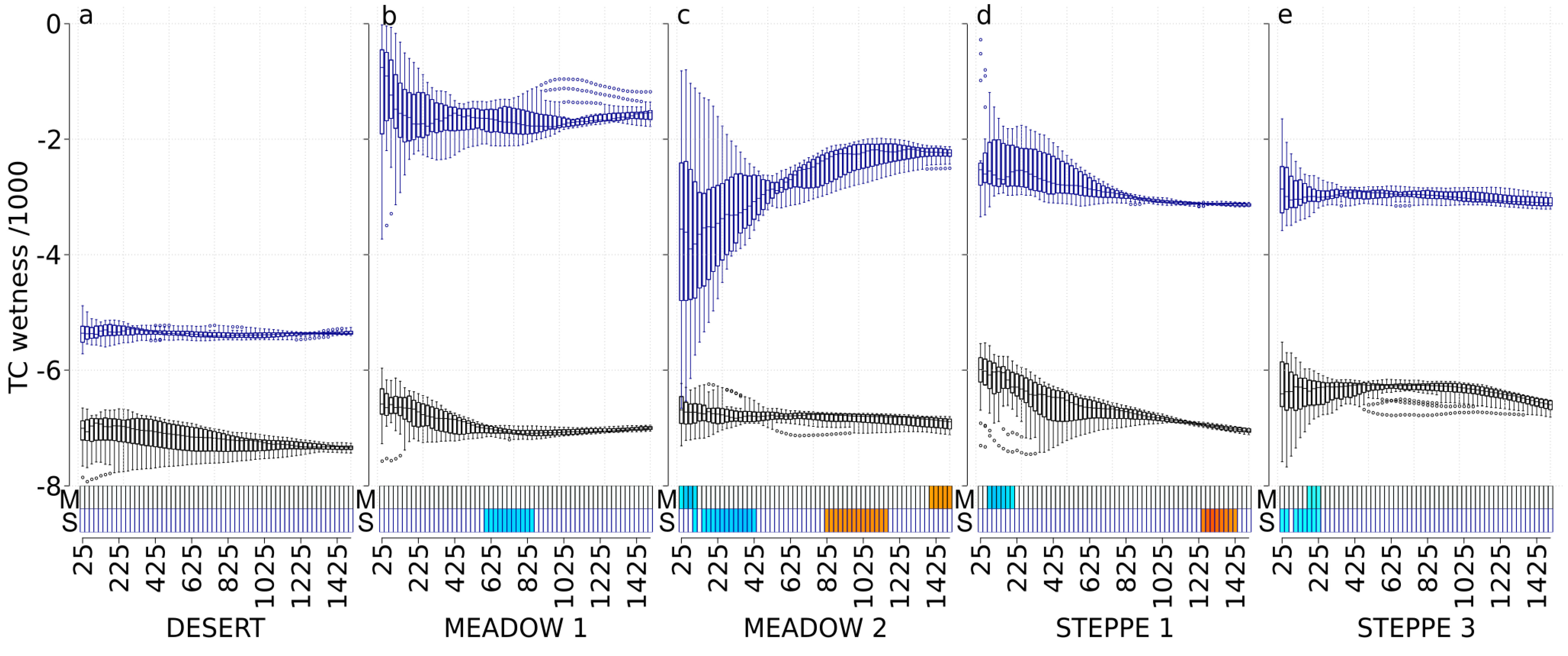
3.4. TC Wetness (TC-W)
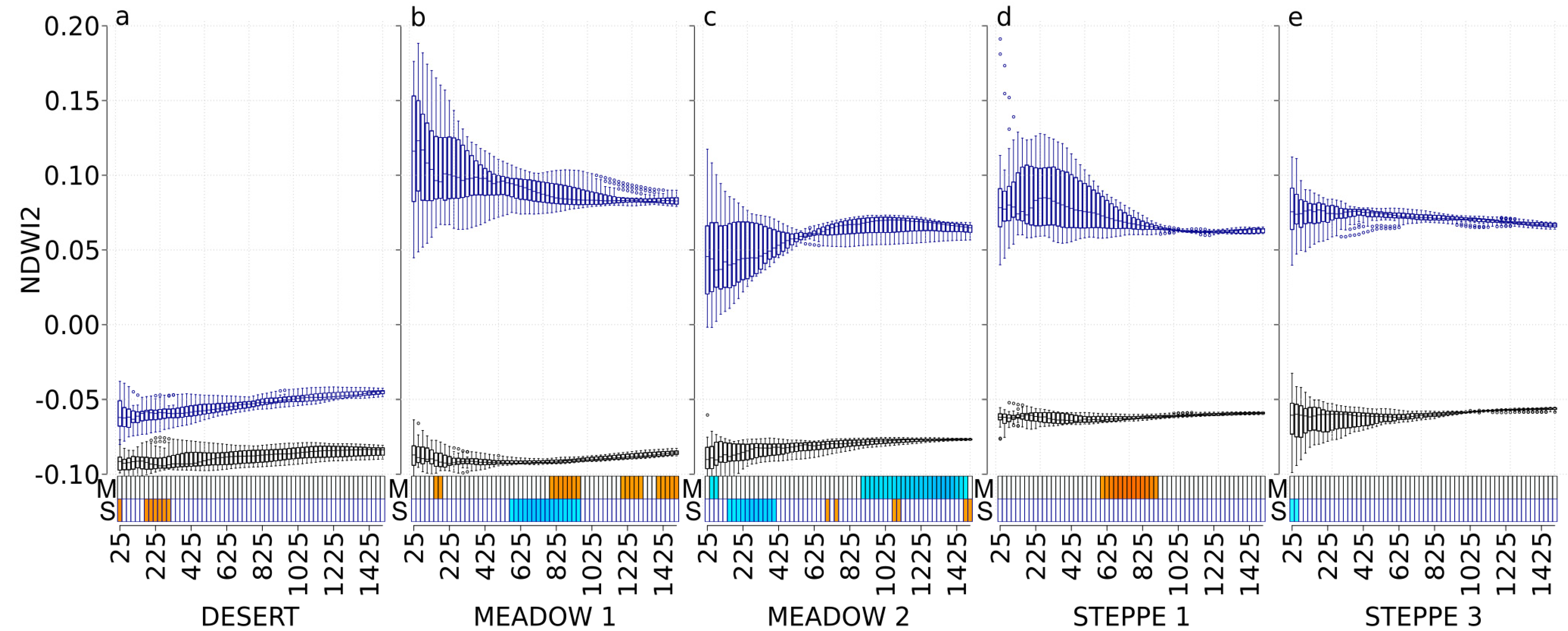
3.5. NDWI2
3.6. MNDWI
3.7. SATVI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holland, J.D.; Bert, D.G.; Fahrig, L. Determining the Spatial Scale of Species’ Response to Habitat. BioScience 2004, 54, 227–233. [Google Scholar] [CrossRef]
- Aviron, S.; Burel, F.; Baudry, J.; Schermann, N. Carabid assemblages in agricultural landscapes: Impacts of habitat features, landscape context at different spatial scales and farming intensity. Agri-Environ. Schemes Landsc. Exp. 2005, 108, 205–217. [Google Scholar] [CrossRef]
- Gaucherel, C.; Burel, F.; Baudry, J. Multiscale and surface pattern analysis of the effect of landscape pattern on carabid beetles distribution. Ecol. Indic. 2007, 7, 598–609. [Google Scholar] [CrossRef]
- Kotze, D.J.; Brandmayr, P.; Casale, A.; Dauffy-Richard, E.; Dekoninck, W.; Koivula, M.J.; Lövei, G.L.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty years of carabid beetle research in Europe—From taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. ZooKeys 2011, 100, 55–148. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M.; Albuquerque, S.; Bichier, P.; Cohen, H.; Egerer, M.H.; Kirk, C.; Will, K.W. Local and Landscape Drivers of Carabid Activity, Species Richness, and Traits in Urban Gardens in Coastal California. Insects 2019, 10, 112. [Google Scholar] [CrossRef]
- Horváth, R.; Magura, T.; Tóthmérész, B.; Eichardt, J.; Szinetár, C. Both local and landscape-level factors are important drivers in shaping ground-dwelling spider assemblages of sandy grasslands. Biodivers. Conserv. 2019, 28, 297–313. [Google Scholar] [CrossRef]
- Meyer, S.T.; Heuss, L.; Feldhaar, H.; Weisser, W.W.; Gossner, M.M. Land-use components, abundance of predatory arthropods, and vegetation height affect predation rates in grasslands. Agric. Ecosyst. Environ. 2019, 270–271, 84–92. [Google Scholar] [CrossRef]
- Torma, A.; Császár, P.; Bozsó, M.; Balázs, D.; Valkó, O.; Kiss, O.; Gallé, R. Species and functional diversity of arthropod assemblages (Araneae, Carabidae, Heteroptera and Orthoptera) in grazed and mown salt grasslands. Agric. Ecosyst. Environ. 2019, 273, 70–79. [Google Scholar] [CrossRef]
- Jouveau, S.; Toïgo, M.; Giffard, B.; Castagneyrol, B.; van Halder, I.; Vétillard, F.; Jactel, H. Carabid activity-density increases with forest vegetation diversity at different spatial scales. Insect Conserv. Divers. 2019, 13, 36–46. [Google Scholar] [CrossRef]
- Meentemeyer, V.; Box, E.O. Scale Effects in Landscape Studies. In Landscape Heterogeneity and Disturbance; Turner, M.G., Ed.; Springer: New York, NY, USA, 1987; pp. 15–34. [Google Scholar]
- Turner, M.G.; O’Neill, R.V.; Gardner, R.H.; Milne, B.T. Effects of changing spatial scale on the analysis of landscape pattern. Landsc. Ecol. 1989, 3, 153–162. [Google Scholar] [CrossRef]
- Martin, A.E.; Fahrig, L. Measuring and selecting scales of effect for landscape predictors in species–habitat models. Ecol. Appl. 2012, 22, 2277–2292. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.Z.; Hu, Z.Z.; Zhao, J.; Zhang, D.G.; Hou, F.J.; Lin, H.L.; Mu, X.D. A grassland classification system and its application in China. Rangel. J. 2008, 30, 199–209. [Google Scholar] [CrossRef]
- French, B.W.; Elliott, N.C. Temporal and spatial distribution of ground beetle (Coleoptera:Carabidae) assemblages in grasslands and adjacent wheat fields. Pedobiologia 1999, 43, 73–84. [Google Scholar]
- Liu, J.-L.; Li, F.-R.; Sun, T.-S.; Ma, L.-F.; Liu, L.-L.; Yang, K. Interactive effects of vegetation and soil determine the composition and diversity of carabid and tenebrionid functional groups in an arid ecosystem. J. Arid Environ. 2016, 128, 80–90. [Google Scholar] [CrossRef]
- Lü, Y.; Fu, B.; Wei, W.; Yu, X.; Sun, R. Major Ecosystems in China: Dynamics and Challenges for Sustainable Management. Environ. Manag. 2011, 48, 13–27. [Google Scholar] [CrossRef]
- Han, Z.; Song, W.; Deng, X.; Xu, X. Grassland ecosystem responses to climate change and human activities within the Three-River Headwaters region of China. Sci. Rep. 2018, 8, 9079. [Google Scholar] [CrossRef]
- Wu, N.; Liu, A.; Wang, Y.; Li, L.; Chao, L.; Liu, G. An Assessment Framework for Grassland Ecosystem Health with Consideration of Natural Succession: A Case Study in Bayinxile, China. Sustainability 2019, 11, 1096. [Google Scholar] [CrossRef]
- Zheng, K.; Wei, J.Z.; Pei, J.Y.; Cheng, H.; Zhang, X.L.; Huang, F.Q.; Li, F.M.; Ye, J.S. Impacts of climate change and human activities on grassland vegetation variation in the Chinese Loess Plateau. Sci. Total Environ. 2019, 660, 236–244. [Google Scholar] [CrossRef]
- Li, X.R.; Jia, X.H.; Dong, G.R. Influence of desertification on vegetation pattern variations in the cold semi-arid grasslands of Qinghai-Tibet Plateau, North-west China. J. Arid Environ. 2006, 64, 505–522. [Google Scholar] [CrossRef]
- Xu, D.; You, X.; Xia, C. Assessing the spatial-temporal pattern and evolution of areas sensitive to land desertification in North China. Ecol. Indic. 2019, 97, 150–158. [Google Scholar] [CrossRef]
- Sun, J.; Hou, G.; Miao, L.; Fu, G.; Zhan, T.-Y.; Zhou, H.; Tsunekawa, A.; Haregeweyn, N. Effects of climatic and grazing changes on desertification of alpine grasslands, Northern Tibet. Ecol. Indic. 2019, 107, 105647. [Google Scholar] [CrossRef]
- Pizzolotto, R. Ground Beetles (Coleoptera, Carabidae) as a Tool for Environmental Management: A Geographical Information System Based on Carabids and Vegetation for the Karst Near Trieste (Italy). In Carabid Beetles: Ecology and Evolution; Desender, K., Dufrêne, M., Loreau, M., Luff, M.L., Maelfait, J.-P., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 343–351. [Google Scholar]
- French, B.W.; Elliott, N.C.; Berberet, R.C.; Burd, J.D. Effects of Riparian and Grassland Habitats on Ground Beetle (Coleoptera: Carabidae) Assemblages in Adjacent Wheat Fields. Environ. Entomol. 2001, 30, 225–234. [Google Scholar] [CrossRef]
- Ewuim, S.C. The effect of land use on the community structure distribution and abundance of ground beetles (Insecta: Coleoptera) in a guinea savanna in Nigeria. Anim. Res. 2008, 5, 913–916. [Google Scholar] [CrossRef]
- Fattorini, S.; Vigna Taglianti, A. Use of taxonomic and chorological diversity to highlight the conservation value of insect communities in a Mediterranean coastal area: The carabid beetles (Coleoptera, Carabidae) of Castelporziano (Central Italy). Rendiconti Lincei 2015, 26, 625–641. [Google Scholar] [CrossRef]
- Yu, X.-D.; Lü, L.; Wang, F.-Y.; Luo, T.-H.; Zou, S.-S.; Wang, C.-B.; Song, T.-T.; Zhou, H.-Z. The Relative Importance of Spatial and Local Environmental Factors in Determining Beetle Assemblages in the Inner Mongolia Grassland. PLoS ONE 2016, 11, e0154659. [Google Scholar] [CrossRef]
- Serrano, A.R.M.; Capela, R.A.; Santos, C.V.-D.N. Biodiversity and notes on carabid beetles from Angola with description of new taxa (Coleoptera: Carabidae). Zootaxa 2017, 4353, 201–256. [Google Scholar] [CrossRef]
- Kang, L.; Han, X.; Zhang, Z.; Sun, O.J. Grassland ecosystems in China: Review of current knowledge and research advancement. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 997–1008. [Google Scholar] [CrossRef]
- Purtauf, T.; Dauber, J.; Wolters, V. The response of carabids to landscape simplification differs between trophic groups. Oecologia 2005, 142, 458–464. [Google Scholar] [CrossRef]
- Lassau, S.A.; Hochuli, D.F. Testing predictions of beetle community patterns derived empirically using remote sensing. Divers. Distrib. 2008, 14, 138–147. [Google Scholar] [CrossRef]
- Lafage, D.; Secondi, J.; Georges, A.; Bouzillé, J.-B.; Pétillon, J. Satellite-derived vegetation indices as surrogate of species richness and abundance of ground beetles in temperate floodplains. Insect Conserv. Divers. 2014, 7, 327–333. [Google Scholar] [CrossRef]
- Tsafack, N.; Rebaudo, F.; Wang, H.; Nagy, D.D.; Xie, Y.; Wang, X.; Fattorini, S. Carabid community structure in northern China grassland ecosystems: Effects of local habitat on species richness, species composition and functional diversity. PeerJ 2019, 6, e6197. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J.; van Etteb, J. Raster: Geographic Data Analysis and Modeling; R Package Version 2.8-19. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 10 September 2018).
- Leutner, B.; Horning, N.; Schwalb-Willmann, J. RStoolbox: Tools for Remote Sensing Data Analysis in R. Available online: https://cran.r-project.org/web/packages/RStoolbox/index.html (accessed on 10 September 2018).
- Chust, G.; Pretus, J.L.; Ducrot, D.; Ventura, D. Scale dependency of insect assemblages in response to landscape pattern. Landsc. Ecol. 2004, 19, 41–57. [Google Scholar] [CrossRef]
- Crist, E.P.; Kauth, R.J. The Tasseled Cap de-mystified. Photogramm. Eng. Remote Sens. 1986, 52, 81–86. [Google Scholar]
- McFeeters, S.K. The use of the Normalized Difference Water Index (NDWI) in the delineation of open water features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Xu, H. Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. Int. J. Remote Sens. 2006, 27, 3025–3033. [Google Scholar] [CrossRef]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Marsett, R.C.; Qi, J.; Heilman, P.; Biedenbender, S.H.; Watson, M.C.; Amer, S.; Weltz, M.; Goodrich, D.; Marsett, R. Remote Sensing for Grassland Management in the Arid Southwest. Rangel. Ecol. Manag. 2006, 59, 530–540. [Google Scholar] [CrossRef]
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R; Chapman & Hall/CRC Interdisciplinary Statistics: Noca Raton, FL, USA, 2015; pp. 1–810. [Google Scholar]
- Pettorelli, N.; Ryan, S.J.; Mueller, T.; Bunnefeld, N. The Normalized Difference Vegetation Index (NDVI): Unforeseen successes in animal ecology. Clim. Res. 2011, 46, 15–27. [Google Scholar] [CrossRef]
- Dubinin, V.; Svoray, T.; Dorman, M.; Perevolotsky, A. Detecting biodiversity refugia using remotely sensed data. Landsc. Ecol. 2018, 33, 1815–1830. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: 2018. Available online: http://www.r-project.org/ (accessed on 10 September 2018).
- Imdadullah, M.; Aslam, M.; Altaf, S. mctest: An R Package for Detection of Collinearity among Regressors. R J. 2016, 8, 495–505. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modeling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Batáry, P.; Kovács, A.; Báldi, A. Management effects on carabid beetles and spiders in Central Hungarian grasslands and cereal fields. Comm. Ecol. 2008, 9, 247–254. [Google Scholar] [CrossRef]
- Gallé, R.; Happe, A.-K.; Baillod, A.B.; Tscharntke, T.; Batáry, P. Landscape configuration, organic management, and within-field position drive functional diversity of spiders and carabids. J. Appl. Ecol. 2019, 56, 63–72. [Google Scholar] [CrossRef]
- Batáry, P.; Báldi, A.; Szél, G.; Podlussány, A.; Rozner, I.; Erdős, S. Responses of grassland specialist and generalist beetles to management and landscape complexity. Divers. Distrib. 2007, 13, 196–202. [Google Scholar] [CrossRef]
- Huang, W.; Luo, J.; Zhao, J.; Zhang, J.; Ma, Z. Predicting Wheat Aphid Using 2-Dimensional Feature Space Based on Multi-Temporal Landsat TM. In Proceedings of the 2011 IEEE International Geoscience and Remote Sensing Symposium, Vancouver, BC, Canada, 24–29 July 2011; pp. 1830–1833. [Google Scholar]
- White, J.C.; Coops, N.C.; Hilker, T.; Wulder, M.A.; Carroll, A.L. Detecting mountain pine beetle red attack damage with EO-1 Hyperion moisture indices. Int. J. Remote Sens. 2007, 28, 2111–2121. [Google Scholar] [CrossRef]
- Hiramatsu, S.; Usio, N. Assemblage Characteristics and Habitat Specificity of Carabid Beetles in a Japanese Alpine-Subalpine Zone. Psyche 2018, 2018, 9754376. [Google Scholar] [CrossRef]
- Leasure, D.R. Landsat to monitor an endangered beetle population and its habitat: Addressing annual life history and imperfect detection. Insect Conserv. Divers. 2017, 10, 385–398. [Google Scholar] [CrossRef]
- Prins, E. Landsat approaches to map agro-pastoral farming in the wetlands of southern Sudan. Int. J. Remote Sens. 2018, 39, 854–878. [Google Scholar] [CrossRef]
- Maynard, C.L.; Lawrence, R.L.; Nielsen, G.A.; Decker, G. Ecological site descriptions and remotely sensed imagery as a tool for rangeland evaluation. Can. J. Remote Sens. 2007, 33, 109–115. [Google Scholar] [CrossRef]

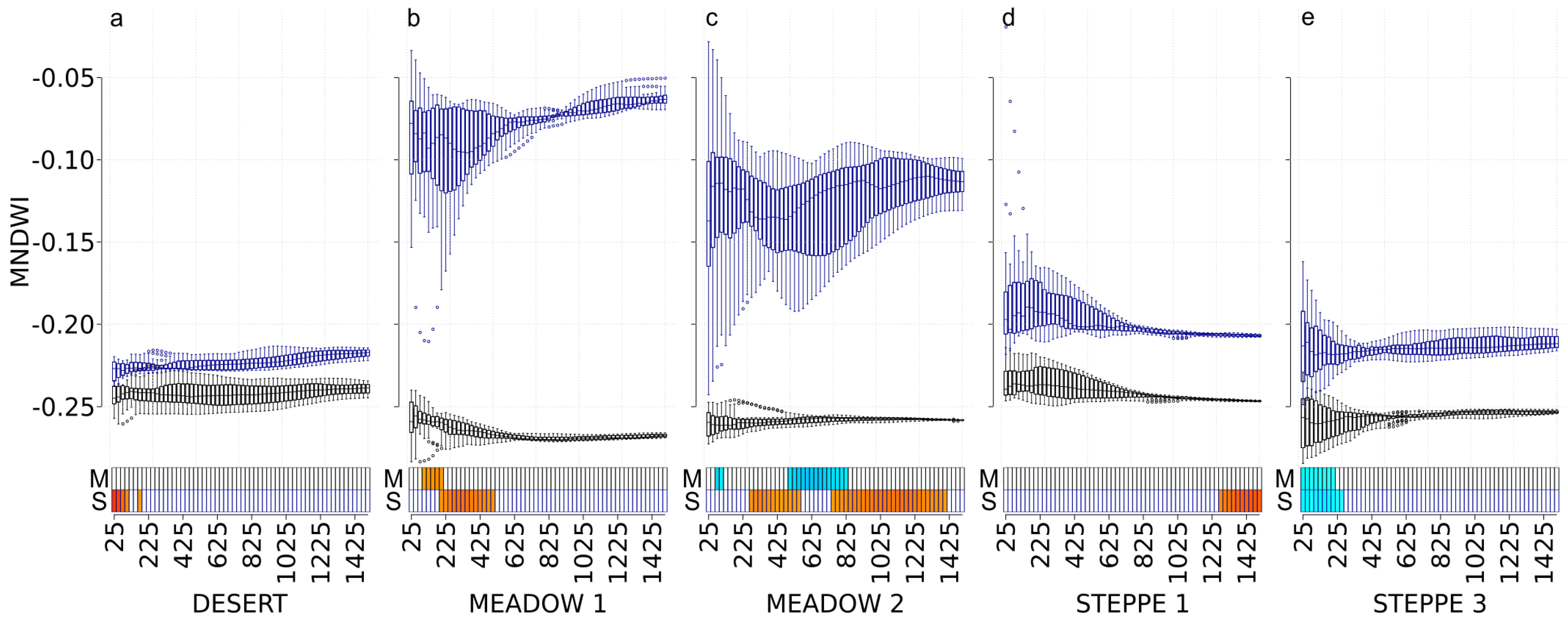
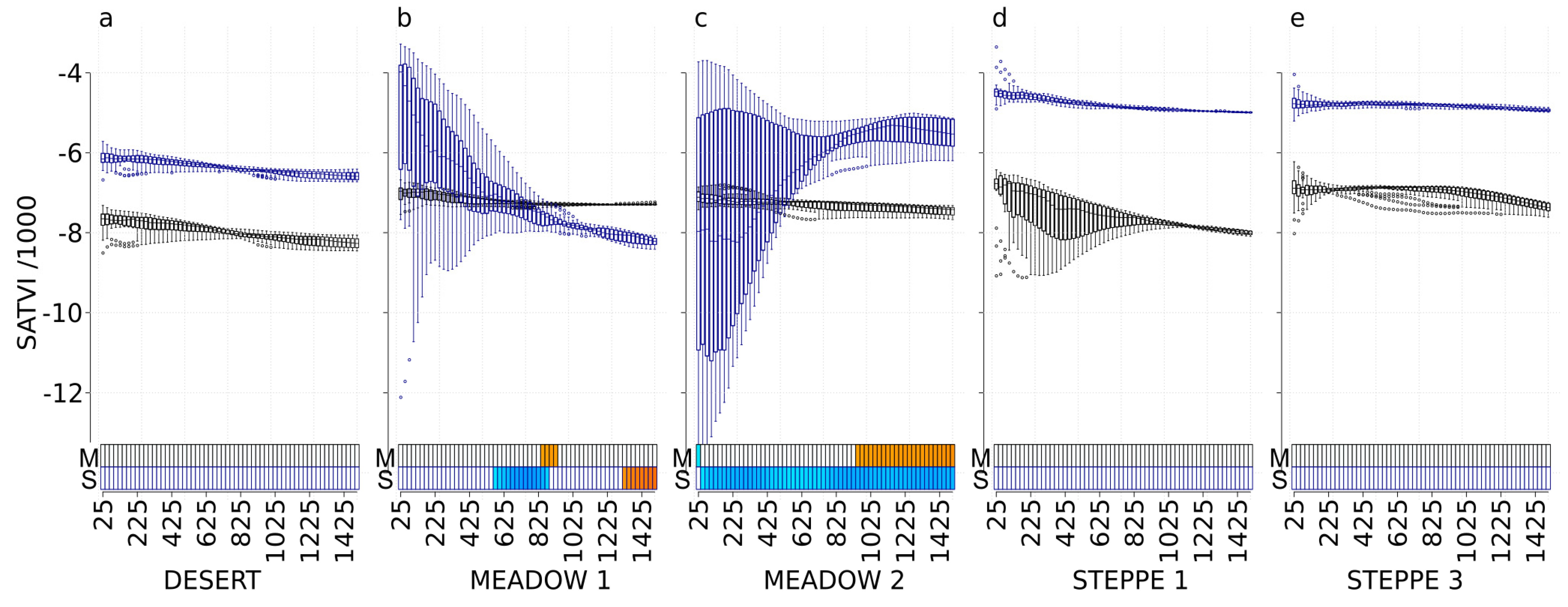
| Site | χ2 | df | p-Value |
|---|---|---|---|
| Desert steppe | 16.667 | 24 | 0.2749 |
| Meadow steppe 1 | 26.667 | 24 | 0.6405 |
| Meadow steppe 2 | 20 | 24 | 0.6064 |
| Typical steppe 1 | 16.667 | 24 | 0.2749 |
| Typical steppe 2 | 53.333 | 24 | 0.0011 |
| Typical steppe 3 (Typical steppe 2 with 4 points excluded) | 24 | 24 | 0.9232 |
| May/September | Brightness | Wetness | MNDWI | NDVI | NDWI2 | SATVI |
|---|---|---|---|---|---|---|
| Brightness | − | 0.53 ∙ | 0.88 *** | −0.70 ** | 0.26 | −0.91 *** |
| Wetness | −0.72 ** | − | 0.74 * | 0.09 | 0.93 * | −0.13 |
| MNDWI | 0.70 ** | −0.07 | − | −0.60 * | 0.47 | −0.70 ** |
| NDVI | −0.80 ** | 0.90 *** | −0.36 * | − | 0.42 | 0.88 *** |
| NDWI2 | −0.29 | 0.83 * | 0.34 | 0.75 * | − | 0.16 |
| SATVI | −0.98 *** | 0.81 ** | −0.63 * | 0.87 *** | 0.40 ∙ | − |
| Desert | Meadow 1 | Meadow 2 | Typ. Steppe 1 | Typ. Steppe 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| May | Sep | May | Sep | May | Sep | May | Sep | May | Sep | |
| NDVI | (−)25 * | (−)1250 ** | ||||||||
| Brightness | ||||||||||
| Wetness | (−)200 * | |||||||||
| NDWI2 | (+)300 * | (+)900 * | (−)25 ∙ | |||||||
| MNDWI | (+)25 *** | (+)1500 * | (+)1450 * | (+)1500 ** | (−)25 ** | (−)250 ∙ | ||||
| SATVI | (−)750 ** | |||||||||
| F | 8.406 | 20.92 | 5.874 | 11.59 | 5.663 | 9.57 | 6.945 | 12.95 | 7.845 | 4.747 |
| df | 1/13 | 2/12 | 1/13 | 1/13 | 1/13 | 1/13 | 2/12 | 1/13 | 1/24 | 2/23 |
| p-value | 0.012 | <0.001 | 0.031 | 0.005 | 0.033 | 0.009 | 0.010 | 0.003 | 0.010 | 0.019 |
| r-squared | 0.393 | 0.777 | 0.311 | 0.471 | 0.303 | 0.424 | 0.537 | 0.499 | 0.246 | 0.292 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsafack, N.; Fattorini, S.; Benavides Frias, C.; Xie, Y.; Wang, X.; Rebaudo, F. Competing Vegetation Structure Indices for Estimating Spatial Constrains in Carabid Abundance Patterns in Chinese Grasslands Reveal Complex Scale and Habitat Patterns. Insects 2020, 11, 249. https://doi.org/10.3390/insects11040249
Tsafack N, Fattorini S, Benavides Frias C, Xie Y, Wang X, Rebaudo F. Competing Vegetation Structure Indices for Estimating Spatial Constrains in Carabid Abundance Patterns in Chinese Grasslands Reveal Complex Scale and Habitat Patterns. Insects. 2020; 11(4):249. https://doi.org/10.3390/insects11040249
Chicago/Turabian StyleTsafack, Noelline, Simone Fattorini, Camila Benavides Frias, Yingzhong Xie, Xinpu Wang, and François Rebaudo. 2020. "Competing Vegetation Structure Indices for Estimating Spatial Constrains in Carabid Abundance Patterns in Chinese Grasslands Reveal Complex Scale and Habitat Patterns" Insects 11, no. 4: 249. https://doi.org/10.3390/insects11040249
APA StyleTsafack, N., Fattorini, S., Benavides Frias, C., Xie, Y., Wang, X., & Rebaudo, F. (2020). Competing Vegetation Structure Indices for Estimating Spatial Constrains in Carabid Abundance Patterns in Chinese Grasslands Reveal Complex Scale and Habitat Patterns. Insects, 11(4), 249. https://doi.org/10.3390/insects11040249






