Using UPLC–MS/MS to Evaluate the Dissemination of Pyriproxyfen by Aedes Mosquitoes to Combat Cryptic Larval Habitats after Source Reduction in Kaohsiung in Southern Taiwan
Abstract
1. Introduction
2. Materials and Methods
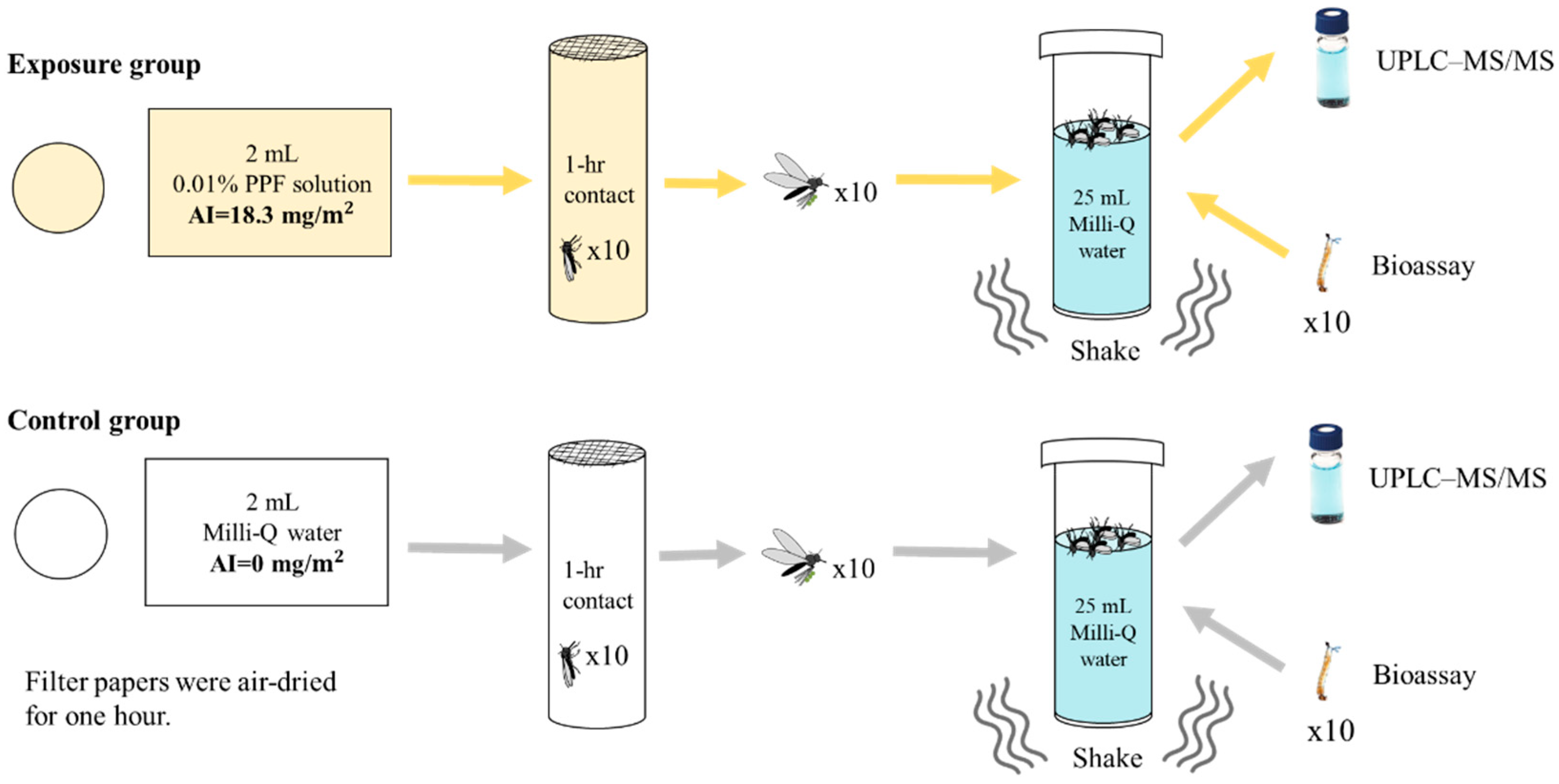
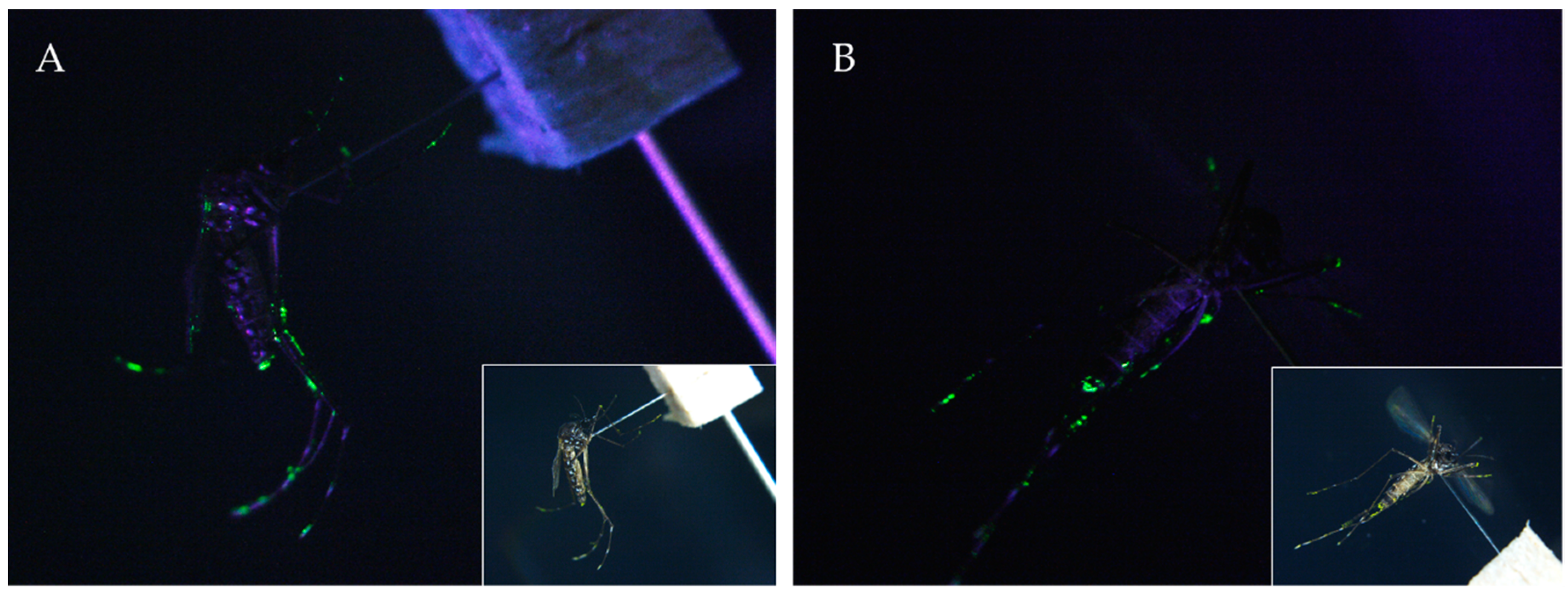
2.1. Laboratory Test of PPF Being Carried and Disseminated by Adult Mosquitoes
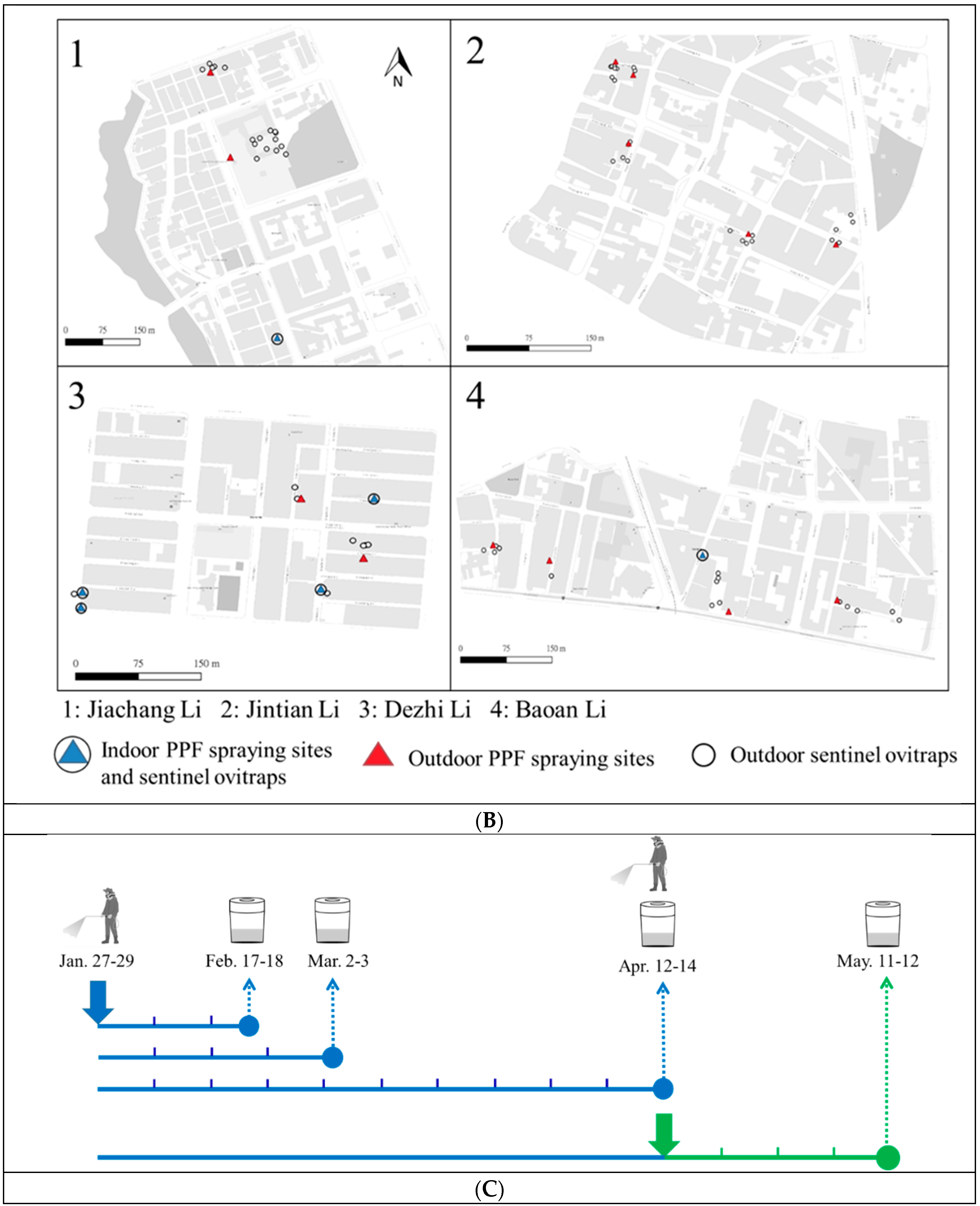
2.2. Field Trial in Kaohsiung City
2.3. Mosquito Bioassay
2.4. Detection and Quantitation of PPF in Water Samples Using UPLC–MS/MS
3. Results
3.1. Laboratory Simulation Test of PPF Being Carried and Disseminated into Small Water Containers
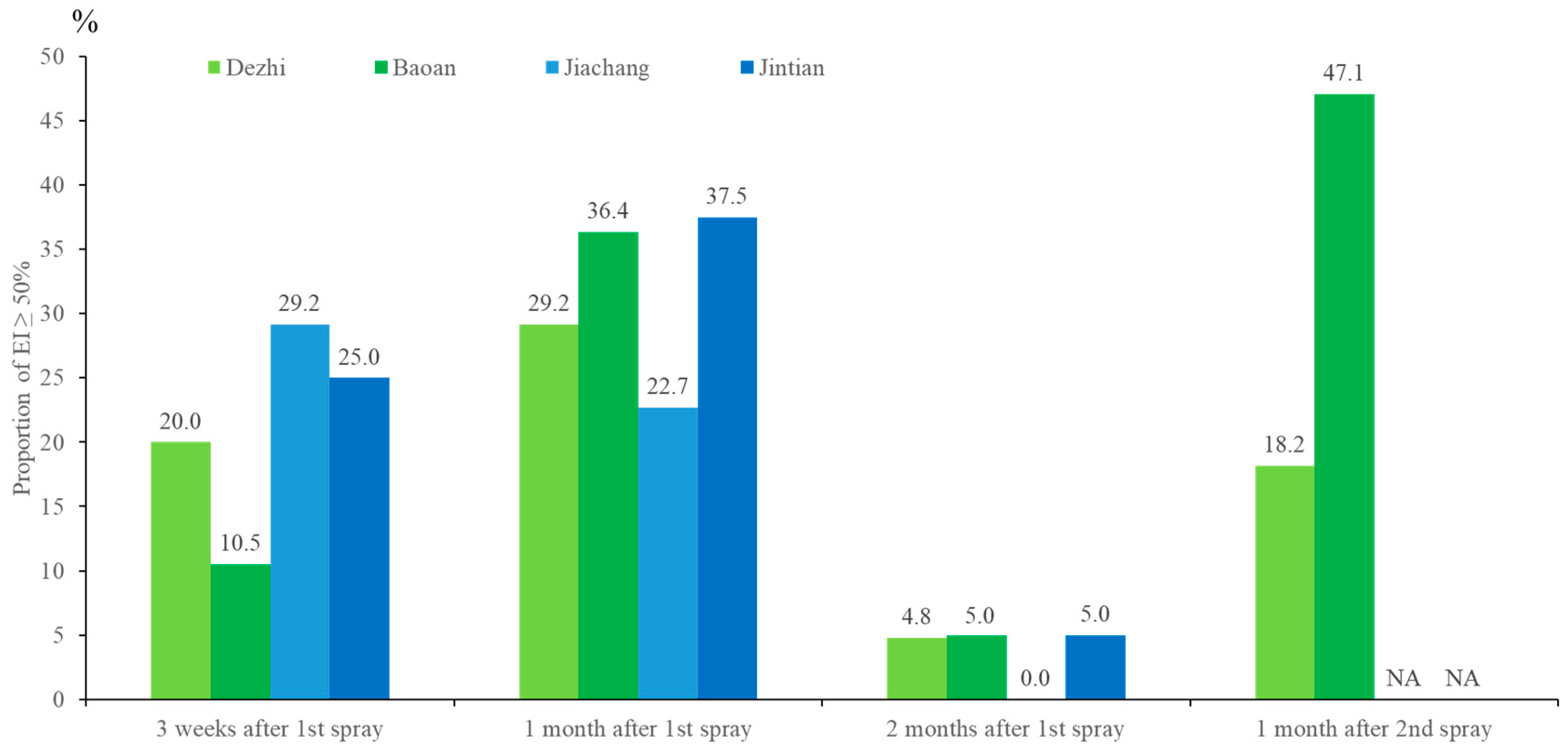
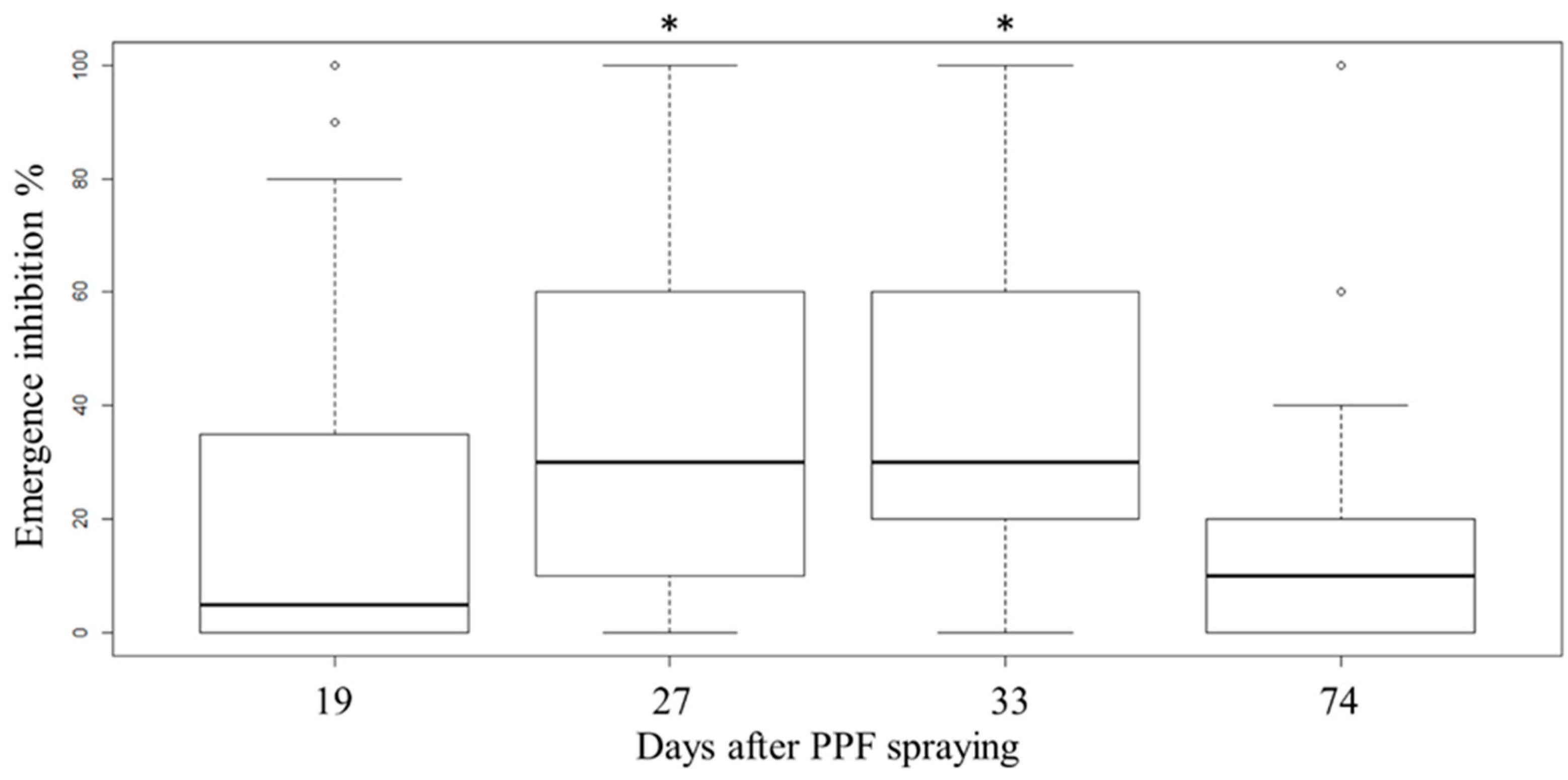
3.2. PPF Dissemination Efficacy in a Field Trial in Kaohsiung
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, C.F.; Hou, J.N.; Chen, T.H.; Chen, W.J. Discriminable roles of Aedes aegypti and Aedes albopictus in establishment of dengue outbreaks in Taiwan. Acta Trop. 2014, 130, 17–23. [Google Scholar] [CrossRef]
- Lin, C.; Wang, C.Y.; Teng, H.J. The study of dengue vector distribution in Taiwan from 2009 to 2011. Taiwan Epidemiol. Bull. 2014, 30, 304–310. [Google Scholar]
- Chen, W.J. Dengue outbreaks and the geographic distribution of dengue vectors in Taiwan: A 20-year epidemiological analysis. Biomed. J. 2018, 41, 283–289. [Google Scholar] [CrossRef]
- Wang, S.F.; Wang, W.H.; Chang, K.; Chen, Y.H.; Tseng, S.P.; Yen, C.H.; Wu, D.C.; Chen, Y.M.A. Severe Dengue Fever Outbreak in Taiwan. Am. J. Trop. Med. Hyg. 2016, 94, 193–197. [Google Scholar] [CrossRef]
- Taiwan Centers for Disease Control. Guidelines for Dengue/Chikungunya Control 2019; Taiwan Centers for Disease Control: Taipei, Taiwan, 2019.
- Wang, C.H.; Chang, N.T.; Wu, H.H.; Ho, C.M. Integrated control of the dengue vector Aedes aegypti in Liu-Chiu village, Ping-Tung County, Taiwan. J. Am. Mosq. Control Assoc. 2000, 16, 93–99. [Google Scholar]
- World Heath Organization. Handbook for Integrated Vector Management; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Wang, S.F.; Chang, K.; Loh, E.W.; Wang, W.H.; Tseng, S.P.; Lu, P.L.; Chen, Y.H.; Chen, Y.M.A. Consecutive large dengue outbreaks in Taiwan in 2014–2015. Emerg. Microbes Infect. 2016, 5, e123. [Google Scholar] [CrossRef]
- Caputo, B.; Ienco, A.; Cianci, D.; Pombi, M.; Petrarca, V.; Baseggio, A.; Devine, G.J.; della Torre, A. The “auto-dissemination” approach: A novel concept to fight Aedes albopictus in urban areas. PLoS Negl. Trop. Dis. 2012, 6, e1793. [Google Scholar] [CrossRef]
- Itoh, T.; Kawada, H.; Abe, A.; Eshita, Y.; Rongsriyam, Y.; Igarashi, A. Utilization of bloodfed females of Aedes aegypti as a vehicle for the transfer of the insect growth regulator pyriproxyfen to larval habitats. J. Am. Mosq. Control Assoc. 1994, 10, 344–347. [Google Scholar]
- Devine, G.J.; Perea, E.Z.; Killeen, G.F.; Stancil, J.D.; Clark, S.J.; Morrison, A.C. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc. Natl. Acad. Sci. USA 2009, 106, 11530–11534. [Google Scholar] [CrossRef]
- Invest, J.; Lucas, J. Pyriproxyfen as a mosquito larvicide. In Proceedings of the Sixth International Conference on Urban Pests, Veszprem, Hungary, 13–16 July 2008; pp. 239–245. [Google Scholar]
- Sihuincha, M.; Zamora-Perea, E.; Orellana-Rios, W.; Stancil, J.D.; Lopez-Sifuentes, V.; Vidal-Ore, C.; Devine, G.J. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J. Med. Entomol. 2005, 42, 620–630. [Google Scholar] [CrossRef]
- Gomez, A.; Seccacini, E.; Zerba, E.; Licastro, S. Comparison of the insecticide susceptibilities of laboratory strains of Aedes aegypti and Aedes albopictus. Mem. Inst. Oswaldo Cruz 2011, 106, 993–996. [Google Scholar] [CrossRef]
- Snetselaar, J.; Andriessen, R.; Suer, R.A.; Osinga, A.J.; Knols, B.G.; Farenhorst, M. Development and evaluation of a novel contamination device that targets multiple life-stages of Aedes aegypti. Parasites Vectors 2014, 7, 200. [Google Scholar] [CrossRef]
- Seixas, G.; Paul, R.E.L.; Pires, B.; Alves, G.; de Jesus, A.; Silva, A.C.; Devine, G.J.; Sousa, C.A. An evaluation of efficacy of the auto-dissemination technique as a tool for Aedes aegypti control in Madeira, Portugal. Parasites Vectors 2014, 7, 200. [Google Scholar] [CrossRef]
- World Health Organization. Monitoring and Managing Insecticide Resistance in Aedes Mosquito populations. Interim Guidance for Entomologists; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Wang, Y.; Suman, D.S.; Bertrand, J.; Dong, L.; Gaugler, R. Dual-treatment autodissemination station with enhanced transfer of an insect growth regulator to mosquito oviposition sites. Pest Manag. Sci. 2014, 70, 1299–1304. [Google Scholar] [CrossRef]
- Unlu, I.; Suman, D.S.; Wang, Y.; Klingler, K.; Faraji, A.; Gaugler, R. Effectiveness of autodissemination stations containing pyriproxyfen in reducing immature Aedes albopictus populations. Parasites Vectors 2017, 10, 139. [Google Scholar] [CrossRef]
- Chandel, K.; Suman, D.S.; Wang, Y.; Unlu, I.; Williges, E.; Williams, G.M.; Gaugler, R. Targeting a hidden enemy: Pyriproxyfen autodissemination strategy for the control of the container mosquito Aedes albopictus in cryptic habitats. PLoS Negl. Trop. Dis. 2016, 10, e0005235. [Google Scholar] [CrossRef]
- Gaugler, R.; Suman, D.; Wang, Y. An autodissemination station for the transfer of an insect growth regulator to mosquito oviposition sites. Med. Vet. Entomol. 2012, 26, 37–45. [Google Scholar] [CrossRef]
- Ohashi, K.; Nakada, K.; Ishiwatari, T.; Miyaguchi, J.; Shono, Y.; Lucas, J.R.; Mito, N. Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 1052–1058. [Google Scholar] [CrossRef]
- Ohba, S.Y.; Ohashi, K.; Pujiyati, E.; Higa, Y.; Kawada, H.; Mito, N.; Takagi, M. The effect of pyriproxyfen as a “population growth regulator” against Aedes albopictus under semi-field conditions. PLoS ONE 2013, 8, e67045. [Google Scholar] [CrossRef]
- Suman, D.S.; Farajollahi, A.; Healy, S.; Williams, G.M.; Wang, Y.; Schoeler, G.; Gaugler, R. Point-source and area-wide field studies of pyriproxyfen autodissemination against urban container-inhabiting mosquitoes. Acta Trop. 2014, 135, 96–103. [Google Scholar] [CrossRef]
- Doud, C.W.; Hanley, A.M.; Chalaire, K.C.; Richardson, A.G.; Britch, S.C.; Xue, R.D. Truck-mounted area-wide application of pyriproxyfen targeting Aedes aegypti and Aedes albopictus in Northeast Florida. J. Am. Mosq. Control Assoc. 2014, 30, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.M.; Farooq, M.; Estep, A.S.; Xue, R.D.; Kline, D.L. Evaluation of pyriproxyfen dissemination via Aedes albopictus from a point-source larvicide application in Northeast Florida. J. Am. Mosq. Control Assoc. 2017, 33, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Khemrattrakool, P.; Yanola, J.; Lumjuan, N.; Somboon, P. Pyriproxyfen-treated polypropylene sheets and resting boxes for controlling mosquitoes in livestock operations. Insects 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Lwetoijera, D.W.; Dongus, S.; Matowo, N.S.; Lorenz, L.M.; Devine, G.J.; Majambere, S. Sterilising effects of pyriproxyfen on Anopheles arabiensis and its potential use in malaria control. Parasites Vectors 2013, 6, 144. [Google Scholar] [CrossRef]
- Mbare, O.; Lindsay, S.W.; Fillinger, U. Pyriproxyfen for mosquito control: Female sterilization or horizontal transfer to oviposition substrates by Anopheles gambiae sensu stricto and Culex quinquefasciatus. Parasites Vectors 2014, 7, 280. [Google Scholar] [CrossRef]
- Maoz, D.; Ward, T.; Samuel, M.; Muller, P.; Runge-Ranzinger, S.; Toledo, J.; Boyce, R.; Velayudhan, R.; Horstick, O. Community effectiveness of pyriproxyfen as a dengue vector control method: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005651. [Google Scholar] [CrossRef]
- Mbare, O.; Lindsay, S.W.; Fillinger, U. Testing a pyriproxyfen auto-dissemination station attractive to gravid Anopheles gambiae sensu stricto for the development of a novel attract-release -and-kill strategy for malaria vector control. BMC Infect. Dis. 2019, 19, 800. [Google Scholar] [CrossRef]
- Suman, D.S.; Wang, Y.; Faraji, A.; Williams, G.M.; Williges, E.; Gaugler, R. Seasonal field efficacy of pyriproxyfen autodissemination stations against container-inhabiting mosquito Aedes albopictus under different habitat conditions. Pest Manag. Sci. 2018, 74, 885–895. [Google Scholar] [CrossRef]
- World Health Organization. Pyriproxyfen in Drinking-Water: Use for Vector Control in Drinking-Water Sources and Containers—Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Marcombe, S.; Darriet, F.; Agnew, P.; Etienne, M.; Yp-Tcha, M.M.; Yebakima, A.; Corbel, V. Field efficacy of new larvicide products for control of multi-resistant Aedes aegypti populations in Martinique (French West Indies). Am. J. Trop. Med. Hyg. 2011, 84, 118–126. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A. Economic and disease burden of dengue in Southeast Asia. PLoS Negl. Trop. Dis. 2013, 7, e2055. [Google Scholar] [CrossRef]
- Cafferata, M.L.; Bardach, A.; Rey-Ares, L.; Alcaraz, A.; Cormick, G.; Gibbons, L.; Romano, M.; Cesaroni, S.; Ruvinsky, S. Dengue epidemiology and burden of disease in Latin America and the Caribbean: A systematic review of the literature and meta-analysis. Value Health Reg. Issues 2013, 2, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Huang, Y.H.; Shu, P.Y.; Wu, H.S.; Lin, Y.S.; Yeh, T.M.; Liu, H.S.; Liu, C.C.; Lei, H.Y. Characteristic of dengue disease in Taiwan: 2002–2007. Am. J. Trop. Med. Hyg. 2010, 82, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S.; Farajollahi, A.; Healy, S.P.; Clark, G.G.; Fonseca, D.M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 2014, 9, e101992. [Google Scholar] [CrossRef] [PubMed]
- Macoris, M.; Cerone, F.; Rigueti, M.; Galvani, K.C.; Macoris, M.L. Effect of pyriproxyfen in Aedes aegypti populations with different levels of susceptibility to the organophosphate temephos. Dengue Bull. 2008, 32, 186–198. [Google Scholar]
- Su, X.; Guo, Y.; Deng, J.; Xu, J.; Zhou, G.; Zhou, T.; Li, Y.; Zhong, D.; Kong, L.; Wang, X.; et al. Fast emerging insecticide resistance in Aedes albopictus in Guangzhou, China: Alarm to the dengue epidemic. PLoS Negl. Trop. Dis. 2019, 13, e0007665. [Google Scholar] [CrossRef]


| Group | Emergence Inhibition of Ae. aegypti (%) | PPF Concentration in Water (ng/L) * | Average PPF (ng) Per Mosquito Disseminated into 25 mL of Water † |
|---|---|---|---|
| Control (Water) | 0 | ND | ND |
| PPF exposure group 1 | 100 | 17.71 ± 0.38 | 0.044 ± 0.001 |
| PPF exposure group 2 | 100 | 33.63 ± 2.63 | 0.084 ± 0.007 |
| PPF exposure group 3 | 100 | 16.08 ± 1.45 | 0.040 ± 0.004 |
| Average in exposure groups | 100 | 22.47 ± 1.49 | 0.056 ± 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-A.; Lai, Y.-T.; Wu, K.-C.; Yen, T.-Y.; Chen, C.-Y.; Tsai, K.-H. Using UPLC–MS/MS to Evaluate the Dissemination of Pyriproxyfen by Aedes Mosquitoes to Combat Cryptic Larval Habitats after Source Reduction in Kaohsiung in Southern Taiwan. Insects 2020, 11, 251. https://doi.org/10.3390/insects11040251
Chen Y-A, Lai Y-T, Wu K-C, Yen T-Y, Chen C-Y, Tsai K-H. Using UPLC–MS/MS to Evaluate the Dissemination of Pyriproxyfen by Aedes Mosquitoes to Combat Cryptic Larval Habitats after Source Reduction in Kaohsiung in Southern Taiwan. Insects. 2020; 11(4):251. https://doi.org/10.3390/insects11040251
Chicago/Turabian StyleChen, Ying-An, Yi-Ting Lai, Kuo-Chih Wu, Tsai-Ying Yen, Chia-Yang Chen, and Kun-Hsien Tsai. 2020. "Using UPLC–MS/MS to Evaluate the Dissemination of Pyriproxyfen by Aedes Mosquitoes to Combat Cryptic Larval Habitats after Source Reduction in Kaohsiung in Southern Taiwan" Insects 11, no. 4: 251. https://doi.org/10.3390/insects11040251
APA StyleChen, Y.-A., Lai, Y.-T., Wu, K.-C., Yen, T.-Y., Chen, C.-Y., & Tsai, K.-H. (2020). Using UPLC–MS/MS to Evaluate the Dissemination of Pyriproxyfen by Aedes Mosquitoes to Combat Cryptic Larval Habitats after Source Reduction in Kaohsiung in Southern Taiwan. Insects, 11(4), 251. https://doi.org/10.3390/insects11040251






