Simple Summary
The diamondback moth is a serious pest of crucifer crops. To control this pest, the use of intercropping (such as trap crops) is gaining attention since they are ecologically safe. However, such approaches require an understanding host plants which are most attractive to the pest. In this study, we quantified different volatiles released by Barbarea vulgaris in response to the diamondback moth larval feeding. We investigated olfactory responses of the adult diamondback moth to natural and simulated volatiles released by infested plants. We also investigated how volatile effects changed in response to larval feeding treatments. Overall, our findings indicated the relationship between key volatile compound, host plant cues emission and regulation of the diamondback moth adult female behavior due to key volatile triggered by the diamondback moth larvae feeding on B. vulgaris.
Abstract
The diamondback moth (DBM) is a destructive pest of crucifer crops. In this study, DBM larvae shown to herbivore induced plant volatiles (HIPVs) that were attractive to adult females exposed in a Y-tube olfactometer. Our results showed that olfactory responses of adult females to HIPVs induced by third instar larvae feeding on Barbarea vulgaris were significantly higher (20.40 ± 1.78; mean moths (%) ± SD) than those induced by first instar larvae (14.80 ± 1.86; mean moths (%) ± SD). Meanwhile, a significant concentration of Sulphur-containing isothiocyanate, 3-methylsulfinylpropyl isothiocyanate, and 4-methylsulfinyl-3-butenyl isothiocyanate were detected in HIPVs released by third instar larvae compared to those released by first instar larvae while feeding on B. vulgaris. When the DBM females were exposed to synthetic chemicals, singly and in blend form, a similar response was observed as to natural HIPVs. Our study demonstrated that the relationship between isothiocyanates acting as plant defense compounds, host plant cues emission and regulation of the DBM adult female behavior due to key volatile triggered by the DBM larvae feeding on B. vulgaris.
1. Introduction
Plants are continuously at risks of attack by herbivore insect. As a result, they have evolved many inducible defense mechanisms to avoid damage [1]. For example, herbivory may modify the reallocation of primary plant metabolites or trigger other resistance-related plant responses in undamaged neighbors which act directly or indirectly against herbivores [2,3]. Direct defenses include a diverse array of strategies, such as strengthening of plant cell walls by regulating secondary metabolites, induction of hypersensitive cell death, and production of toxic and deterrent substances, such as glucosinolates and saponins in crucifers [4,5,6,7]. Indirect defenses can involve the emission of volatile compounds in response to arthropod feeding, which can influence trophic guilds by attracting natural enemies [8,9].
The diamondback moth (DBM), Plutella xylostella L., is a serious pest of crucifer crops with a cosmopolitan distribution [10]. The DBM has developed resistance to many chemical insecticides, as well as Bt toxins [11] making it difficult to control [12]. Efforts to find alternative control approaches include cultural methods, such as the use of trap crops and intercropping [11]. Such approaches require an understanding of competing host plants which are most attractive to the pest.
Host plant volatiles play an important role in host finding, recognition and acceptance by herbivores [13,14,15,16,17,18]. The brassica family produces a wide range of compounds which are protective against polyphagous species [19]. However, some of these secondary metabolites, such as glucosinolates, are used for host recognition cues by specialist Lepidoptera, including the DBM [19,20,21]. In addition, some plant volatiles are induced HIPVs, which can mediate ecological functions, including directly inhibiting development of herbivores and pathogens [11,14,15].
In this study, we hypothesize that host attraction by the DBM may reflect upregulated levels of key volatile compounds. Thus, we quantified different volatiles released by brassica plants in response to the DBM larval feeding. We investigated olfactory responses of adult the DBM to natural and simulated volatiles released by infested plants. We also studied how volatile effects changed in response to different larval feeding treatments.
2. Materials and Methods
2.1. Plants and Insects
Seed of wintercress (Barbarea vulgaris) (G-type R. Br, glabrous type; Hedeland population) obtained from the Department of Plant and Environmental Sciences, University of Copenhagen, Denmark were stored at 4 °C until use. Seeds were sown in a greenhouse under long-day conditions, 25 °C (light) and 20 °C (dark) with 16:8-h photoperiod and 60–75% relative humidity (RH). Plants were watered weekly and fertilized every other week. After two weeks, 120 seedlings were transplanted individually into 1-L plastic pots and maintained in climate-controlled room at 25 °C (light), 20 °C (dark), 16:8 h photoperiod and 60–65% RH.
P. xylostella L. (strain Fuzhou-S) larvae were collected from a brassica field in Fuzhou in southeastern China (26.08° N, 119.28° E), and reared on an artificial diet [22], in a climate-controlled room maintained at 24 ± 1 °C (light) and 24 ± 1 °C (dark) with a 12:12 h photoperiod and 60–70% RH. Emerging adult moths were released into mesh cages (60 × 60 × 60 cm) in groups of 400 (50:50 sex ratio) for mating. Adult female moths were used in the experiments
2.2. Behavioral Response Treatments
B. vulgaris plants (60–70 day old) were randomly assigned to three comparison groups with the DBM adult females for olfactory response, (1) healthy (HB) control versus mechanically damaged plants (MB), (2) plants exposed to first instar (FLB) versus third instars (TLB), and (3) first instars reared on artificial diet (FLA) versus first instar larvae (FL). Mechanical damage was induced by cutting across 30% of the B. vulgaris leaf veins with a sterilized knife. In each treatment, the DBM larvae were released on plants using a camel hair brush. There were four replicates for each treatment.
2.3. Olfactory Responses to Volatiles
Three treatment combinations were used to test the attraction of the chemicals to mated DBM adult female with an olfactometer: (1) healthy (HB) control versus mechanically damaged plants (MB), (2) plants exposed to first instar (FLB) versus third instar (TLB), and (3) first instars reared on artificial diet (FLA) versus first instar larvae (FL). The preference of the mated DBM adult female for HIPVs was studied in two-choice tests with a closed system Y-tube olfactometer, using the method described by Lin et al. [23]. The test chamber consisted of a Y-shaped glass tube (base tube: 10.5 cm; Y arms: 10 cm; tube internal diameter: 1.6 cm, and 90° angle between the two arms). Each arm was connected to a flow meter and an odor source container consisting of a glass jar (30 cm high, 28 cm diameter) large enough to hold a potted B. vulgaris plant. Parafilm was used to cover the soil of the pot, isolating it from the plant foliage. The airflow was purified through a charcoal filter and then passed through a humidifier bottle. The humidified airflow was split between two channels, and each channel was directed through an odor container. The two odor flows were sent through each arm of the olfactometer. The airflow through each arm was 300 mL/min as verified by a flow meter. The experiment was conducted at 20–25 °C with 60–70% RH, in a black box with an artificial light source from a single 35 W fluorescent tube placed above the arms of the Y-tube.
For olfactory responses, mated adult females were released individually at the open end of the Y-tube (joint arm). The time spent by adult females in each arm was recorded over 300 s, which began after an adult female touched the cotton placed at the end of the Y-tube arm. The olfactometer treatments were alternated between arms every five tests to prevent locational bias. Every 10 trials, the olfactometer tube was washed with alcohol and dried, and the two plants were replaced. Fifty adult females were tested per sets of plant treatments with a new unexposed adult female used for each trial replicate. In total, there were five replicates in this experiment.
2.4. Headspace Collection and Volatiles Analysis
We investigated the production of isothiocyanate (SIT) levels in response to larval feeding as potential host-attraction cues. The DBM larvae (3rd instar larvae) were infested on the surface of the plants with a camel hair brush. Volatiles were collected (after 24 h) from the olfactometer headspace as described by Pineda et al. [24]. The four treatments were: healthy B. vulgaris; B. vulgaris fed by first instar larvae (n = 50); B. vulgaris fed by third instar larvae (n = 50); and mechanically damaged B. vulgaris (n = 4).
For the assessment, each plant pot was wrapped with parafilm (Neenah, WI54956, USA) to prevent the soil odor from mixing with plant odor. Plants were placed in glass jars (10 L, cleaned with acetone, which was allowed to evaporate, and heated to 70 °C overnight before use). Pots containing soil alone were used as control treatments. Air was drawn from the glass jar through a glass tube (charcoal filter trap column, 3-mm ID, 65 mm long, 1.5 mg Prec charcoal, Klaüstrott, Chromatographie, Deerfield, MA, USA) using a mini vacuum pump (CD12/16NK, 1KL, Billerica, MA, USA), connected to the glass jar by a mini steel tube (2 mm ID) at 30 mL/min (negative pressure) for 24 h. Before use, the filter trap column was washed with dichloromethane-methanol (1:2 v/v), chloroform, acetone, dichloromethane, and n-pentene (in that order) and then heated to 100 °C for 12 h. All experiments were performed in a temperature-controlled room (24 ± 1 °C) with three replicates (n = 50) per treatment [23]. Collected volatiles were absorbed using Tenax® TA 200 mg (60/80 mesh; Grace-Alltech, Deerfield, IL, USA), eluted into 30% chloroform, and the mixture was subsequently stored at −20 °C until analysis.
The adsorbed samples were analyzed using a gas chromatograph (Agilent Technologies (Santa Clara, CA, USA) 7890B GC System) mass spectrometer (Agilent Technologies 5977A MSD) (GC-MS) with an HP-5 column (30 mm × 0.25 mm i.d., 1.0 μm film thickness, Agilent). The GC oven temperature was increased from 40 °C (5-min hold) to 280 °C at a rate of 10 °C per min. Column effluent was ionized by electron impact ionization at 70 eV. Mass spectra were acquired by scanning from 35 to 350 m/z at a scan rate of 5.38 scans/sec.
Compounds were identified using the deconvolution software AMDIS (version 2.64, NIST, USA) in combination with NIST 05 and Wiley 7th edition spectral libraries, and by comparing retention indices with those from the literature.
2.5. Adult Female Response to Synthetic HIPV Sources
The response of the adult female DBM was compared between natural and synthetic HIPVs sources. For testing synthetics of active chemicals (sulfur-containing isothiocyanate, 3-methylsulfinylpropyl isothiocyanate, and 4-methylsulfinyl-3-butenyl isothiocyanate volatile compounds from the headspace) were purchased from Macklin Biochemical Co. Ltd. (Shanghai, China) at 97–99% purity.
For testing single doses, 3-methylsulfinylpropyl isothiocyanate were dissolved with triethyl citrate (TEC) to 0.01, 0.5, 5, 10, 20, and 30 nmol·mL−1. In addition, 4-methylsulfinyl-3-butenyl isothiocyanate was dissolved with TEC to 0.1, 0.5, 1, 5, 10, and 25 nmol·mL−1 concentrations. The range of dilutions used for the bioassay varied from Quantities of chemicals emitted from cotton roll to Quantities detected in B. vulgaris by GC-MS (Supplementary Materials Tables S1 and S2). For testing blends, 3-methylsulfinylpropyl isothiocyanate and 4-methylsulfinyl-3-butenyl isothiocyanate were tested at the following ratios; blend HB (18.71 and 0.10 nmol·mL−1, respectively), blend MB (8.32 and 1.36 nmol·mL−1, respectively), blend FLB (22.91 and 1.89 nmol·mL−1, respectively) and blend TLB (18.78 and 1.43 nmol·mL−1, respectively (Table S1). Each suspension solution (1 mL) was applied to a small cotton roll and placed into the volatiles collecting chamber. The response of mated adult DBM to each blend were tested in the Y-tube olfactometer as described above. The selected adult females were starved for 24 h before the behavioral test, and the test was replicated five times.
2.6. Statistical Analysis
Responses of adult females to HIPVs in the five treatments were compared using chi-square tests for dependent samples with five replicates. We performed Pearson Correlation, the p-value for each test (not merely “significant” or “p < 0.05”), to determine the correlation between the olfactory responses of the adult DBM females to B. vulgaris volatile blends. We used SPSS 19.0 for overall statistical analyses.
3. Results
3.1. Quantities of Isothiocyanate Detected from the Headspace of Multiple Damaged Barbarea vulgaris Plants
The DBM larval feeding affected the levels of isothiocyanate volatiles produced by B. vulgaris plants. In the FLB and TLB treatments, means of 3-methylsulfinylpropyl isothiocyanate were significantly lower, while the MB treatments were not significantly different from the HB (control). (Table 1). These findings demonstrated that the FLB suppressed the emission of this sulphur containing isothiocyanates (SIT) volatile compound. The first instar feeding caused a decreased attraction of adult females to host plants (Table 2) (Mean quantity (ng) ± SE; 0.12 ± 0.03 c). In contrast, third instar larvae feeding on B. vulgaris did not affect the attraction of adult females to host plants when compared with first instars (Figure 1).

Table 1.
Mean quantities (ng) ± SE of isothiocyanate from the headspace of multiple damaged Barbarea vulgaris plants.

Table 2.
The peak area of the main volatile compounds emitted from multi-treated Barbarea vulgaris plants.
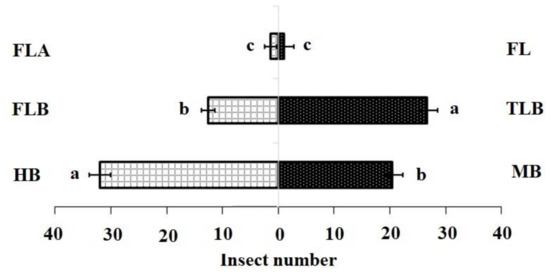
Figure 1.
Olfactory responses of Plutella xylostella adults to natural volatiles. Bars represent the mean (±SE) response of adult female (compared using the χ2 test) exposed to natural volatiles from Barbarea vulgaris plants. Different letters indicate differences between two treatments at p < 0.05. HB, healthy B. vulgaris; FLB, first instar larvae feeding on B. vulgaris; TLB, third instar larvae feeding on B. vulgaris; MB, mechanically damaged B. vulgaris; FLA, first instar larvae feeding on artificial diet; FL, first instar larvae only (FL).
3.2. Y-Tube Olfactometer Bioassays
Our Y-tube olfactometer bioassay responses indicated that HIPVs affected the behavior of adult female moths. Firstly, healthy plants were more attractive to the DBM adult female compared with mechanically damaged plants (Table 3). Moreover, plants infested with first instars were less attractive to the DBM adult females than plants infested with third instars or healthy plants (Table 3).

Table 3.
Olfactory response of the DBM adult females to volatile blends emitted from multi-treated Barbarea vulgaris plants.
3.3. Adult Female Responses to Synthetic Compounds
The responses of the adult female DBM to single dosages and blends of 3-methylsulfinylpropyl isothiocyanate and 4-methylsulfinyl-3-butenyl as odor sources are shown in Figure 2 and Figure 3. In the single dosage treatment, 3-methylsulfinylpropyl isothiocyanate concentrations of 30 nmol·mL−1 (χ2 = 32.22, df = 4, p = 0.001), 20 nmol·mL−1 (χ2 = 29.20, df = 4, p = 0.015), 10 nmol·mL−1 (χ2 = 25.4, df = 4, p = 0.001), and 5 nmol·mL−1 (χ2 = 17.80, df = 4, p = 0.002) were significantly more attractive to adult females than HB (control). However, no significant differences were observed between the control and concentrations of 0.50 nmol·mL−1 (χ2 = 1.80, df = 4, p = 0.205) and 0.01 nmol·mL−1 (χ2 = 1.40, df = 4, p = 0.213) (Figure 2).
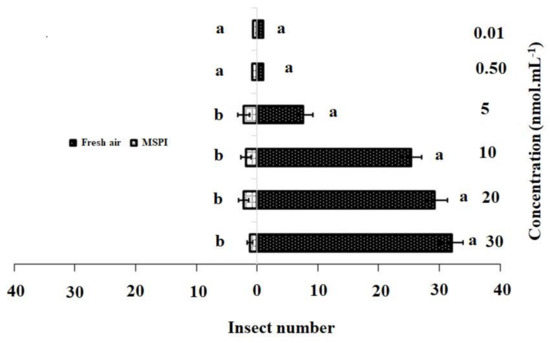
Figure 2.
Responses of P. xylostella adult females to synthetic 3-methylsulfinylpropyl isothiocyanate (MSPI) compared with control. Bars represent the mean (± SE) response of adult female exposed to synthetic active volatile from Barbarea vulgaris plants. Different letters indicate differences between two treatments at p < 0.05.
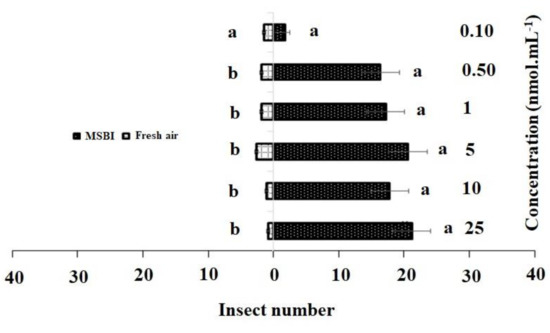
Figure 3.
Responses of P. xylostella adult females to synthetic 4-methylsulfinyl-3-butenyl isothiocyanate (MSBI) compared with controls. Bars represent the mean (± SE) response of adult female exposed to synthetic active volatile from Barbarea vulgaris plants. Different letters indicate differences between two treatments at p < 0.05.
Similarly, in the single treatment of 4-methylsulfinyl-3-butenyl, concentrations of 25 nmol·mL−1 (χ2 = 21.20, df = 4, p = 0.013), 10 nmol·mL−1 (χ2 = 17.20, df = 4, p = 0.007), 5 nmol·mL−1(χ2 = 20.16, df = 4, p = 0.012), and 1 nmol·mL−1 (χ2 = 17.81, df = 4, p = 0.005) had significant effects on adult females as compared to the control, while no significant differences were observed between the control and 0.50 nmol·mL−1 (χ2 = 5.00, df = 4, p = 0.288) and 0.10 nmol·mL−1 (χ2 = 1.80, df = 4, p = 0.205) concentrations (Figure 3).
For the blended treatments, the results showed that the HB blend (χ2 = 21.20, df = 4, p = 0.001) was more attractive to adult females than the FLB blends (χ2 = 4.20, df = 4, p = 0.12) or TLB blends (χ2 = 9.01, df = 4, p = 0.20), while the HB blend (χ2 = 21.20, df = 4, p = 0.10) and MB blend (χ2 = 17.60, df = 4, p = 0.15) showed no significant difference (Figure 4).
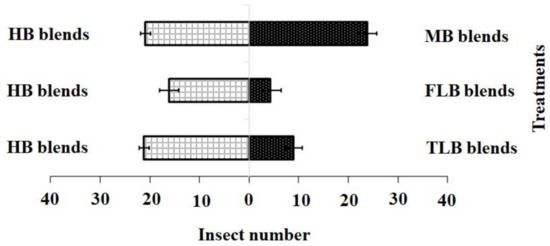
Figure 4.
Responses of P. xylostella adult females to synthetic blends. HB, healthy B. vulgaris; FLB, first instar larvae feeding on B. vulgaris; TLB, third instar larvae feeding on B. vulgaris; MB, mechanically damaged B. vulgaris. Bars represent the mean (± SE) response of adult female exposed to synthetic active volatile from Barbarea vulgaris plants. Different letters indicate differences between two treatments at p < 0.05.
Sulfur-containing isothiocyanate; 3-methylsulfinylpropyl isothiocyanate, and 4-methylsulfinyl-3-butenyl isothiocyanate volatile compounds from the headspace were significantly correlated with the olfactory response of adult females. Therefore were considered for single and blended bioassays, while the others were not (Table 4).

Table 4.
Correlation between olfactory response of adult females to the multi-treated Barbarea vulgaris plant volatiles and peak area of the main volatile compounds emitted from multi-treated B. vulgaris.
Adult female responses to the two active chemical compound blends in a Y-olfactometer were compared using the χ2 test. (1) blend HB: 3-methylsulfinylpropyl isothiocyanate 18.71 nmol·mL−1; (2) blend FLB: 3-methylsulfinylpropyl isothiocyanate 8.32 nmol·mL−1 and 4-methylsulfinyl-3-butenyl isothiocyanate 1.36 nmol·mL−1; (3) blend TLB: 3-methylsulfinylpropyl isothiocyanate 22.91 nmol·mL−1 and 4-methylsulfinyl-3-butenyl isothiocyanate 1.89 nmol·mL−1; (4) blend MB: 3-methylsulfinylpropyl isothiocyanate 18.78 nmol·mL−1 and 4-methylsulfinyl-3-butenyl isothiocyanate 1.43 nmol·mL−1. Bars indicate mean ± SE; means followed by different letters indicate significant differences at p < 0.05.
4. Discussion
Crucifer plants effectively use their secondary metabolites to protect themselves against herbivores. HIPVs are an evolved plants response that stimulates behavioral response in herbivores [25,26,27,28,29,30]. However, host plant recognition by herbivores is dependent on the perception of a specific substance, or combination of substances, at or near the surface of leaves [31]. Therefore, our study results show that volatile constituents (3-methylsulfinylpropyl isothiocyanate and 4-methylsulfinyl-3-butenyl isothiocyanate) may be just as effective as non-volatile constituents (glucosinolates) to oviposit [20,32,33,34,35,36,37]. These results suggested that DBM may be aware of host plants while in flight.
In our study we used B. vulgaris which is considered as a trap crop against the DBM [4,5,34,35,38]. Our Y-tube olfactometer bioassay results show that HIPVs emitted from B. vulgaris affect the behavior of adult DBM females. We observed that mechanically damaged plants were less attractive to the DBM adult female compared with healthy plants (Table 3). Moreover, it is surprising that plants infested with third instars or healthy plants were more attractive to the DBM adult females than plants infested with first instars (Table 3). Our results also showed that the DBM responded positively to 3-methylsulfinylpropyl isothiocyanate and 4-methylsulfinyl-3-butenyl isothiocyanate, volatile compounds found on the leaf surfaces of crucifer plants [21]. This suggests that the up or down regulation of plant volatiles (isothiocyanate), significantly affect the DBM female attraction to oviposit on B. vulgaris plants.
Volatile compounds such as isothiocyanates are also involved in the behavioral response to hydrolysis output of glucosinolates in crucifers [38,39]. However, hydrolysis is expected to occur only when the leaf is infested with insects which can activate isothicyanates [40]. Isothiocyanates in crucifer plants [41,42,43,44,45] have been shown to occur in abundance in the airspace above undamaged plants [46]. Some studies also showed that HIPVs function as host-attraction cues for a range of specialist herbivores [28,29,47]. The host attraction phenomena was observed in by DBM and another crucifer feeders, the cabbage root fly [1,48].These finding suggested that the feeding by early instars decreased attraction of adult DBM females to host plant, which in result may regulates volatiles emission.
Additionally, responses to the synthetic active chemical compounds (Figure 2 and Figure 3) showed that volatile compounds (host-attraction cues) were perceived by olfactory behavior. Previously, it was reported that volatiles from various fertilized crucifer plants play a crucial role in herbivore host preferences [49], although isothiocyanates were not detected in damaged plants (within the threshold limits of the GC-MS system), these findings are echoed in our result that feeding by early instars suppressed emissions.
This study concurs with a previous study on the attractiveness of B. vulgaris to ovipositing the DBM [21], where adult females preferentially oviposited on B. vulgaris despite the fact that P. xylostella larvae do not survive on this dead-end trap crop [50,51,52,53,54,55,56,57,58]. Because, the manipulation of trap crops and their natural enemies has potential applications for pest control in agricultural settings. Thus, the manipulation of B. vulgaris could help in controlling DBM.
5. Conclusions
Our results provide evidence that the DBM adults can recognize hosts (food odor) in part by using larvae which activates volatile compounds from B. vulgaris plants. Our results also showed that host attraction cues (HIPVs) released from B. vulgaris damaged by larvae or mechanical means were involved in host attraction. Because when B. vulgaris is damaged by larvae it activates secondary metabolites, such as glucosinolates (non-volatile) and isothiocyanates (volatile), which increased the attraction among adult of the DBM females to oviposit. Thus, the results of this study suggested that B. vulgaris infested with early instar larvae with insect secretions may alter the amounts and balance of volatiles emitted by the infested plants, thus reducing host attraction. Additional detailed studies on the effect of the DBM-larvae secretions on damaged B. vulgaris are needed to explore structure-activity relationship and the sequence of events leading to host attraction.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/11/725/s1, Table S1: Quantities of the active synthetic compounds emitted from the cotton roll in different treatments over 12 h, Table S2: Predicted concentrations of synthetic compounds applied in the experiment.
Author Contributions
Conceptualization, M.H., Y.L. and L.W.; formal analysis, M.H., Y.L., S.A., S.B., J.G. and M.Q.; funding acquisition, M.H., L.W., J.G. and R.M.; investigation, M.H., Y.L., S.B. and M.Q.; methodology, M.H., Y.L., S.A., J.G. and M.Q.; project administration, M.H. and R.M.; supervision, R.M.; writing—original draft, M.H., Y.L., S.B., L.W., J.G. and M.Q.; writing—review and editing, M.H., S.A. and R.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Key Research and Development Program of China (2017YFF0210204); the GDAS Special Project of Science and Technology Development (2019GDASY-0501006, 2021GDASYL-20210103051, 2019GDASYL-0103055, 2018GDASCX-0107); Science and Technology Planning Project of Guangdong Province (No. 2017B020202005, No.2016B090923005); National Key Projects of R & D of China (2018YFD0201502), and Research Fund for the International Collaborative Program (KXGH17004), grant (CXZX2017211, CXZX2018101, CXZX2019008S, KF2015068) from FAFU.
Acknowledgments
All authors are indebted to Prof. You Minsheng (Fujian Agriculture and Forestry University, China) for helping us to get Barbarea vulgaris seeds from Denmark and providing insects (DBM) and insects rearing facility in his lab. We thank to Dr. Ba Trieu Nguyen (Vietnam Academy of Forest Science, Vietnam) for help to grow Barbarea vulgaris plants. We are also grateful to Prof. Thure Hauser (Department of Plant and Environmental Sciences, University of Copenhagen, Denmark) for kindly providing Barbarea.vulgaris seeds and technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Rowen, E.; Kaplan, I. Eco-evolutionary factors drive induced plant volatiles: A meta-analysis. New Phytol. 2016, 210. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M. Induced responses to herbivory by R. Karban and I.T. Baldwin. Trends Ecol. Evol. 1998, 13, 83. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Morales-Vargas, A.T.; Molina-Torres, J.; Adame-Alvarez, R.M.; Acosta-Gallegos, J.A.; Heil, M. Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J. Ecol. 2015, 103, 250–260. [Google Scholar] [CrossRef]
- Shinoda, T.; Nagao, T.; Nakayama, M.; Serizawa, H.; Koshioka, M.; Okabe, H.; Kawai, A. Identification of a triterpenoid saponin from a crucifer, Barbarea vulgaris, as a feeding deterrent to the diamondback moth, Plutella xylostella. J. Chem. Ecol. 2002, 28, 587–599. [Google Scholar] [CrossRef]
- Shelton, A.M.; Nault, B.A. Dead-end trap cropping: A technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop. Protion 2004, 23, 497–503. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R. Simultaneous Use of Barbarea vulgaris R. Br. (Brassicaceae) as a Trap Crop for Insect Pest Management and a Salad Vegetable. Acta Hortic. 2013, 979, 737–742. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Reichelt, M.; Gershenzon, J.; Heckel, D.G. Using plant chemistry and insect preference to study the potential of Barbarea (Brassicaceae) as a dead-end trap crop for diamondback moth (Lepidoptera: Plutellidae). Phytochemistry 2014, 98, 137–144. [Google Scholar] [CrossRef]
- Bruin, J.; Sabelis, M.W.; Dicke, M. Do plants tap SOS signals from their infested neighbours? Trends Ecol. Evol. 1995, 10, 167–170. [Google Scholar] [CrossRef]
- Meiners, T.; Wackers, F.; Lewis, W.J. The effect of molecular structure on olfactory discrimination by the parasitoid Microplitis croceipes. Chem. Senses 2002, 27, 811–816. [Google Scholar] [CrossRef]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef]
- Heckel, D.G.; Gahan, L.J.; Liu, Y.B.; Tabashnik, B.E. Genetic mapping of resistance to Bacillus thuringiensis toxins in diamondback moth using biphasic linkage analysis. Proc. Natl. Acad. Sci. USA 1999, 96, 8373–8377. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Huang, F.; Ghimire, M.N.; Leonard, B.R.; Siegfried, B.D.; Rangasamy, M.; Yang, Y.; Wu, Y.; Gahan, L.J.; Heckel, D.G.; et al. Efficacy of genetically modified Bt toxins against insects with different genetic mechanisms of resistance. Nat. Biotechnol. 2011, 29, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, A.; Weldegergis, B.T.; Colazza, S.; Dicke, M.; Fatouros, N.E. Attraction of egg-killing parasitoids toward induced plant volatiles in a multi-herbivore context. Oecologia 2015, 179, 163–174. [Google Scholar] [CrossRef]
- Spathe, A.; Reinecke, A.; Haverkamp, A.; Hansson, B.S.; Knaden, M. Host plant odors represent immiscible information entities—Blend composition and concentration matter in hawkmoths. PLoS ONE 2013, 8, e77135. [Google Scholar] [CrossRef] [PubMed]
- Allmann, S.; Spathe, A.; Bisch-Knaden, S.; Kallenbach, M.; Reinecke, A.; Sachse, S.; Baldwin, I.T.; Hansson, B.S. Feeding-induced rearrangement of green leaf volatiles reduces moth oviposition. eLife 2013, 2, e00421. [Google Scholar] [CrossRef]
- Poelman, E.H.; Zheng, S.J.; Zhang, Z.; Heemskerk, N.M.; Cortesero, A.M.; Dicke, M. Parasitoid-specific induction of plant responses to parasitized herbivores affects colonization by subsequent herbivores. Proc. Natl. Acad. Sci. USA 2011, 108, 19647–19652. [Google Scholar] [CrossRef]
- Dannon, E.A.; Tamo, M.; Van Huis, A.; Dicke, M. Effects of volatiles from Maruca vitrata larvae and caterpillar-infested flowers of their host plant Vigna unguiculata on the foraging behavior of the parasitoid Apanteles taragamae. J. Chem. Ecol. 2010, 36, 1083–1091. [Google Scholar] [CrossRef]
- Turlings, T.C.; Fritzsche, M.E. Attraction of parasitic wasps by caterpillar-damaged plants. In Proceedings of the Novartis Foundation Symposium on the Neuronal and Cognitive Effects of Oestrogens, London, UK, 7–9 September 1999; Volume 223, pp. 21–31. [Google Scholar]
- Mithen, R.; Raybould, A.; Giamoustaris, A. Divergent selection for secondary metabolites between wild populations of Brassica oleracea and its implications for plant-herbivore interactions. Heredity 1995, 75, 472. [Google Scholar] [CrossRef]
- Dimock, M.B.; Renwick, J.A.; Radke, C.D.; Sachdev-Gupta, K. Chemical constituents of an unacceptable crucifer, Erysimum cheiranthoides, deter feeding by Pieris rapae. J. Chem. Ecol. 1991, 17, 525–533. [Google Scholar] [CrossRef]
- Renwick, J.A.; Haribal, M.; Gouinguene, S.; Stadler, E. Isothiocyanates stimulating oviposition by the diamondback moth, Plutella xylostella. J. Chem. Ecol. 2006, 32, 755–766. [Google Scholar] [CrossRef]
- You, M.; Yue, Z.; He, W.; Yang, X.; Yang, G.; Xie, M.; Zhan, D.; Baxter, S.W.; Vasseur, L.; Gurr, G.M. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013, 45, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, S.; Akutse, K.S.; Hussain, M.; Wang, L. Diaphorina citri induces huanglongbing-infected citrus plant volatiles to repel and reduce the performance of Propylaea japonica. Front. Plant Sci. 2016, 7, 1969. [Google Scholar] [CrossRef] [PubMed]
- Pineda, A.N.A.; Soler, R.; Weldegergis, B.T.; Shimwela, M.M.; Van Loon, J.J.; Dicke, M. Non-pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via jasmonic acid signaling. Plant Cell Environ. 2013, 36, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Hountondji, F.C.; Sabelis, M.W.; Hanna, R.; Janssen, A. Herbivore-induced plant volatiles trigger sporulation in entomopathogenic fungi: The case of Neozygites tanajoae infecting the cassava green mite. J. Chem. Ecol. 2005, 31, 1003–1021. [Google Scholar] [CrossRef]
- Lucas-Barbosa, D. Integrating Studies on Plant-Pollinator and Plant-Herbivore Interactions. Trends Plant Sci. 2016, 21, 125–133. [Google Scholar] [CrossRef]
- Seidl-Adams, I.; Richter, A.; Boomer, K.B.; Yoshinaga, N.; Degenhardt, J.; Tumlinson, J.H. Emission of herbivore elicitor-induced sesquiterpenes is regulated by stomatal aperture in maize (Zea mays) seedlings. Plant Cell Environ. 2015, 38, 23–34. [Google Scholar] [CrossRef]
- Bruinsma, M.; Posthumus, M.A.; Mumm, R.; Mueller, M.J.; van Loon, J.J.; Dicke, M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: Effects of time and dose, and comparison with induction by herbivores. J. Exp. Bot. 2009, 60, 2575–2587. [Google Scholar] [CrossRef]
- Coleman, R.A.; Ramchunder, S.J.; Davis, K.M.; Moody, A.J.; Foggo, A. Herbivore-induced infochemicals influence foraging behaviour in two intertidal predators. Oecologia 2007, 151, 454–463. [Google Scholar] [CrossRef]
- Arimura, G.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta 2005, 1734, 91–111. [Google Scholar] [CrossRef]
- Dicke, M. Specificity of herbivore-induced plant defences. In Proceedings of the Novartis Foundation Symposium on the Neuronal and Cognitive Effects of Oestrogens, London, UK, 7–9 September 1999; Volume 223, pp. 43–54. [Google Scholar]
- Sachdev-Gupta, K.; Renwick, J.A.; Radke, C.D. Isolation and identification of oviposition deterrents to cabbage butterfly, Pieris rapae, from Erysimum cheiranthoides. J. Chem. Ecol. 1991, 17, 247. [Google Scholar] [CrossRef]
- Renwick, J.A.; Radke, C.D.; Sachdev-Gupta, K. Chemical constituents of Erysimum cheiranthoides deterring oviposition by the cabbage butterfly, Pieris rapae. J. Chem. Ecol. 1989, 15, 2161–2169. [Google Scholar] [CrossRef]
- Renwick, J.A.; Radke, C.D. Chemical stimulants and deterrents regulating acceptance or rejection of crucifers by cabbage butterflies. J. Chem. Ecol. 1987, 13, 1771–1776. [Google Scholar] [CrossRef]
- Kuzina, V.; Ekstrom, C.T.; Andersen, S.B.; Nielsen, J.K.; Olsen, C.E.; Bak, S. Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol. 2009, 151, 1977–1990. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E.; Nielsen, J.K. Seasonal variation in leaf glucosinolates and insect resistance in two types of Barbarea vulgaris ssp. arcuata. Phytochemistry 2001, 58, 91–100. [Google Scholar] [CrossRef]
- Senatore, F.; D’Agostino, M.; Dini, I. Flavonoid glycosides of Barbarea vulgaris L. (Brassicaceae). J. Agric. Food Chem. 2000, 48, 2659–2662. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Van Leur, H.; Vet, L.E.; van der Putten, W.H.; van Dam, N.M. Barbarea vulgaris glucosinolate phenotypes differentially affect performance and preference of two different species of lepidopteran herbivores. J. Chem. Ecol. 2008, 34, 121–131. [Google Scholar] [CrossRef]
- Van Leur, H.; Raaijmakers, C.E.; van Dam, N.M. A heritable glucosinolate polymorphism within natural populations of Barbarea vulgaris. Phytochemistry 2006, 67, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Salerno, G.; Leombruni, B.; Frati, F.; Bin, F. Short-range allelochemicals from a plant-herbivore association: A singular case of oviposition-induced synomone for an egg parasitoid. J. Exp. Biol. 2010, 213, 3911–3919. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ye, W.; Hossain, M.A.; Okuma, E.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 977–983. [Google Scholar] [CrossRef]
- Khokon, M.A.; Jahan, M.S.; Rahman, T.; Hossain, M.A.; Muroyama, D.; Minami, I.; Munemasa, S.; Mori, I.C.; Nakamura, Y.; Murata, Y. Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Kriksunov, K.L.; Jander, G. Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiol. 2008, 146, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Barillari, J.; Iori, R.; Rollin, P.; Hennion, F. Glucosinolates in the subantarctic crucifer Kerguelen cabbage (Pringlea antiscorbutica). J. Nat. Prod. 2005, 68, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Ahman, I. Toxicities of host secondary compounds to eggs of the Brassica specialist Dasineura brassicae. J. Chem. Ecol. 1986, 12, 1481–1488. [Google Scholar] [CrossRef]
- Tollsten, L.; Bergström, G. Headspace volatiles of whole plants and macerated plant parts of Brassica and Sinapis. Phytochemistry 1988, 27, 2073–2077. [Google Scholar] [CrossRef]
- Bukovinszky, T.; Gols, R.; Posthumus, M.A.; Vet, L.E.; Van Lenteren, J.C. Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellen). J. Chem. Ecol. 2005, 31, 461–480. [Google Scholar] [CrossRef]
- Kergunteuil, A.; Dugravot, S.; Mortreuil, A.; Le Ralec, A.; Cortesero, A.M. Selecting volatiles to protect brassicaceous crops against the cabbage root fly, Delia radicum. Entomol. Exp. Appl. 2012, 144, 69–77. [Google Scholar] [CrossRef]
- Marazzi, C.; Patrian, B.; Stadler, E. Secondary metabolites of the leaf surface affected by sulphur fertilisation and perceived by the diamondback moth. Chemoecology 2004, 14, 81–86. [Google Scholar] [CrossRef]
- Lu, J.H.; Liu, S.S.; Shelton, A.M. Laboratory evaluations of a wild crucifer Barbarea vulgaris as a management tool for the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Bull. Entomol. Res. 2004, 94, 509–516. [Google Scholar] [CrossRef]
- Badenes-Perez, F.R.; Shelton, A.M.; Nault, B.A. Evaluating trap crops for diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2004, 97, 1365–1372. [Google Scholar] [CrossRef]
- Muniappan, R.; Cruz, J.; Bamba, J. Trap crops for diamondback moth and other crucifer pests in Guam. In Proceedings of the Fourth International Workshop—Management of Diamondback Moth and Other Crucifer Pests, Melbourne, Australia, 26–29 November 2001; pp. 141–146. [Google Scholar]
- Badenes-Perez, F.R.; Reichelt, M.; Heckel, D.G. Can sulfur fertilisation improve the effectiveness of trap crops for diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae)? Pest Manag. Sci. 2010, 66, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.K.; Nagao, T.; Okabe, H.; Shinoda, T. Resistance in the plant, Barbarea vulgaris, and counter-adaptations in flea beetles mediated by saponins. J. Chem. Ecol. 2010, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Badenes-Perez, F.R.; Shelton, A.M.; Nault, B.A. Using yellow rocket as a trap crop for diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2005, 98, 884–890. [Google Scholar] [CrossRef]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.; Qiu, D. Role of saponins in plant defense against specialist herbivores. Molecules 2019, 24, 2067. [Google Scholar] [CrossRef] [PubMed]
- Badenes-Perez, F.R.; Nault, B.A.; Shelton, A.M. Manipulating the attractiveness and suitability of hosts for diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2005, 98, 836–844. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).