Phytohormone Cross-Talk Synthesizes Glycoalkaloids in Potato (Solanum tuberosum L.) in Response to Aphid (Myzus persicae Sulzer) Infestation under Drought Stress
Abstract
:Simple Summary
Abstract
1. Introduction
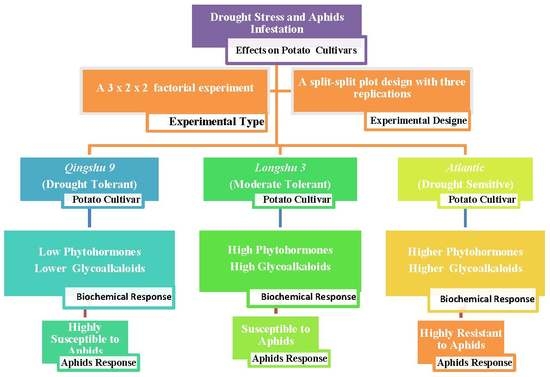
2. Materials and Methods
2.1. Growth Conditions and Plant Materials
2.2. Aphid Culture
2.3. Experimental Design and Treatments
2.4. Determination of Aphid Performance
2.5. Determination of Hydrogen Peroxide (H2O2), Malondialdehyde (MDA), and Protein Contents in Aphids
2.6. Leaf Abscission, Plant Stomatal Conductance, and Water Use Efficiency
2.7. Determination of Potato Aboveground Biomass
2.8. Determination of Hormones in Leaf and Root Samples
2.9. Determination of Glycoalkaloid Content in Potato
2.10. Statistical Analysis
3. Results
3.1. Aphid Performance
3.2. Effect of Drought Stress on Aphid H2O2 and MDA Contents
3.3. Genotypic Variation of Morphological and Physiological Response to Drought Stress and Aphid Treatments
3.4. Genotypic Variation of Pytohormone Defense Induced by Drought Stress and Aphid Treatments
3.5. Genotypic Variation of Glycoalkaloid Defense Induced by Drought Stress and Aphid Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Su, Z.; Ma, X.; Guo, H.; Sukiran, N.L.; Guo, B.; Assmann, S.M.; Ma, H. Flower development under drought stress: Morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 2013, 25, 3785–3807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flexas, J.; Medrano, H. Drought inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Goggin, F.L. Plant–aphid interactions: Molecular and ecological perspectives. Curr. Opin. Plant Biol. 2007, 10, 399–408. [Google Scholar] [CrossRef]
- Dao, L.; Friedman, M. Comparison of glycoalkaloid content of fresh and freeze-dried potato leaves determined by HPLC and colorimetry. J. Agric. Food Chem. 1996, 44, 2287–2291. [Google Scholar] [CrossRef]
- Mewis, I.; Khan, M.A.; Glawischnig, E.; Schreiner, M.; Ulrichs, C. Water stress and aphid feeding differentially influence metabolite composition in Arabidopsis thaliana (L.). PLoS ONE 2012, 7, 4–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Hill, L.; Crooks, C.; Doerner, P.; Lamb, C. Abscisic acid has a key role in modulating diverse plant–pathogen interactions. Plant Physiol. 2009, 150, 1750–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Maik, B.Ć. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Ann. Rev. Plant Biol. 2010, 61, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Mosher, S.; Moeder, W.; Nishimura, N.; Jikumaru, Y.; Joo, S.H.; Urquhart, W.; Klessig, D.F.; Kim, S.; Nambara, E.; Yoshioka, K. The lesion-mimic mutant cpr22 shows alterations in abscisic acid signaling and abscisic acid insensitivity in a salicylic acid-dependent manner. Plant Physiol. 2010, 152, 1901–1913. [Google Scholar] [CrossRef] [Green Version]
- Hillwig, M.S.; Chiozza, M.; Casteel, C.L.; Lau, S.T.; Hohenstein, J.; Hernández, E.; Jander, G.; MacIntosh, G.C. Abscisic acid deficiency increases defence responses against Myzus persicae in Arabidopsis. Mol. Plant Pathol. 2015, 34, 56–79. [Google Scholar] [CrossRef]
- Dicke, M.; Van Poecke, R.M.P. Signalling in plant–insect interactions: Signal transduction in direct and indirect plant defence. In Plant Signal Transduction; Oxford University Press: Oxford, UK, 2002; Volume 34, pp. 49–67. [Google Scholar]
- Wijesinha-Bettoni, R.; Mouillé, B. The Contribution of Potatoes to Global Food Security, Nutrition and Healthy Diets. Am. J. Pot. Res. 2019, 96, 139–149. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Michaud, A.D.; Cloutier, C. Proteomic profiling of aphid Macrosiphum euphorbiae response to host-plant–mediated stress induced by defoliation and water deficit. J. Insect Physiol. 2007, 53, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, J.; Liu, N.; Zhou, Q.; Ding, X.; Zhan, J.; Cheng, X.; Huang, J.; Lu, Y.; Yang, Y. Current status and management strategies for potato insect pests and diseases in China. Plant Prot. 2019, 45, 106–111. [Google Scholar]
- Hale, B.K.; Bale, J.S.; Pritchard, J.; Masters, G.J. Effects of host plant drought stress on the performance of the bird cherry-oat aphid, Rhopalosiphum padi (L.): A mechanistic analysis. Ecol. Entom. 2003, 28, 666–677. [Google Scholar] [CrossRef]
- Agele, S.O.; Ofuya, T.I.; James, P.O. Effects of watering regimes on aphid infestation and performance of selected varieties of cowpea (Vigna unguiculata L. Walp) in a humid rain forest zone of Nigeria. Crop Prot. 2006, 25, 73–78. [Google Scholar] [CrossRef]
- Koricheva, J.; Larsson, S. Insect performance on experimentally stressed woody plants: A meta-analysis. Annu. Rev. Entomol. 1998, 43, 195–216. [Google Scholar] [CrossRef] [Green Version]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Simpson, K.L.S.; Jackson, G.E.; Grace, J. The response of aphids to plant water stress—the case of Myzus persicae and Brassica oleracea var. capitata. Entomol. Exp. Appl. 2012, 142, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Quandahor, P.; Lin, C.; Gou, Y.; Coulter, J.A.; Liu, C. Leaf morphological and biochemical responses of three potato (Solanum tuberosum L.) cultivars to drought stress and aphid (Myzus persicae Sulzer) infestation. Insects 2019, 10, 435. [Google Scholar] [CrossRef] [Green Version]
- Turtola, S.; Rousi, M.; Pusenius, J.; Yamaji, K.; Heiska, S.; Tirkkonen, V.; Meier, B.; Julkunen-Tiitto, R. Clone-specific responses in leaf phenolics of willows exposed to enhanced UVB radiation and drought stress. Glob. Chang. Biol. 2005, 11, 1655–1663. [Google Scholar] [CrossRef]
- Mody, K.; Eichenberger, D.; Dorn, S. Stress magnitude matters: Different intensities of pulsed water stress produce non-monotonic resistance responses of host plants to insect herbivores. Ecol. Entomol. 2009, 34, 133–143. [Google Scholar] [CrossRef]
- Zhu, G.; Xue, M.; Luo, J.; Guixia, G.; Liu, F.; Zhao, H.; Sun, X. Effects of short-term heat shock and physiological responses to heat stress in two Bradysia adults, Bradysia odoriphaga and Bradysia difformis. Sci. Rep. 2017, 7, 13381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Des Marais, D.L.; Auchincloss, L.C.; Sukamtoh, E.; McKay, J.K.; Logan, T.; Richards, J.H.; Juenger, T.E. Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proc. Natl. Acad. Sci. USA 2014, 111, 2836–2841. [Google Scholar] [CrossRef] [Green Version]
- Bañón, S.; Ochoa, J.; Franco, J.A.; Alarcón, J.J.; Sánchez-Blanco, M.J. Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ. Exp. Bot. 2006, 56, 36–43. [Google Scholar] [CrossRef]
- Stingl, N.; Krischke, M.; Fekete, A.; Mueller, M.J. Analysis of defense signals in Arabidopsis thaliana leaves by ultra-performance liquid chromatography/tandem mass spectrometry: Jasmonates, salicylic acid, abscisic acid. Methods Mol. Biol. 2013, 1009, 103–113. [Google Scholar]
- White, T.C.R. A hypothesis to explain outbreaks of looper caterpillars, with special reference to Selidosema suavis in a plantation of Pinus radiate in New Zealand. Oecologia 1974, 16, 279–301. [Google Scholar] [CrossRef]
- White, T.C.R. Plant vigour versus plant stress: A false dichotomy. Oikos 2009, 118, 807–808. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Karley, A.J.; Douglas, A.E.; Parker, W.E. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J. Exp. Bot. 2002, 205, 3009–3018. [Google Scholar]
- Rajabaskar, D.; Ding, H.; Wu, Y.; Eigenbrode, S.D. Behavioral Responses of Green Peach Aphid, Myzus persicae (Sulzer), to the Volatile Organic Compound Emissions from Four Potato Varieties. Am. J. Potato Res. 2013, 90, 171–178. [Google Scholar] [CrossRef]
- Oswald, C.J.; Brewer, M.J. Aphid-barley interactions mediated by water stress and barley resistance to Russian wheat aphid (Homoptera: Aphididae). Environ. Entomol. 2002, 26, 591–602. [Google Scholar] [CrossRef]
- Bethke, J.A.; Redak, R.A.; Schuch, U.K. Melon aphid performance on chrysanthemum as mediated by cultivar, and differential levels of fertilization and irrigation. Entomol. Exp. Appl. 1998, 88, 41–47. [Google Scholar] [CrossRef]
- Lees, A.D. The role of photoperiod and temperature in the determination of parthenogenetic and sexual forms in the aphid Megoura viciae Buckton—III. Further properties of the maternal switching mechanism in apterous aphids. J. Insect Physiol. 1963, 9, 153–164. [Google Scholar] [CrossRef]
- Dixon, A.F.G.; Glen, D.M. Laboratory studies on aggregation, size and fecundity in the black bean aphid, Aphis fabae Scop. Bul. Entomol. Res. 1971, 61, 97–111. [Google Scholar] [CrossRef]
- Asin, L.; Pons, X. Effect of High Temperature on the Growth and Reproduction of Corn Aphids (Homoptera: Aphididae) and Implications for Their Population Dynamics on the Northeastern Iberian Peninsula. Environ. Entomol. 2001, 6, 1127–1134. [Google Scholar] [CrossRef]
- Sinden, S.L.; Deahl, K.L.; Aulenbach, B.B. Effect of glycoalkaloids and phenolics on potato flavor. J. Food Sci. 1976, 41, 520–523. [Google Scholar] [CrossRef]
- Nitithamyong, A.; Vonelbe, J.H.; Wheeler, R.M.; Tibbitts, T.W. Glycoalkaloids in potato tubers grown under controlled environments. Am. J. Potato Res. 1999, 76, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kozukue, N.; Kozukue, E.; Mizuno, S. Glycoalkaloids in potato plants and tubers. HortScience 1987, 22, 294–296. [Google Scholar]
- Friedman, M.; Levin, C.E. Dehydrotomatine content in tomatoes. J. Agric. Food Chem. 1998, 46, 4571–4576. [Google Scholar] [CrossRef]
- Bale, J.S.; Ponder, K.L.; Pritchard, J. Coping with Stress. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CAB International: Wallingford, UK, 2007; Volume 287, pp. 309–310. [Google Scholar]
- Johnson, S.N.; Staley, J.T.; Mcleod, F.A.L.; Hartley, S.E. Plant-mediated effects of soil invertebrates and summer drought on above-ground multitrophic interactions. J. Ecol. 2011, 99, 57–65. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quandahor, P.; Gou, Y.; Lin, C.; Mujitaba Dawuda, M.; A. Coulter, J.; Liu, C. Phytohormone Cross-Talk Synthesizes Glycoalkaloids in Potato (Solanum tuberosum L.) in Response to Aphid (Myzus persicae Sulzer) Infestation under Drought Stress. Insects 2020, 11, 724. https://doi.org/10.3390/insects11110724
Quandahor P, Gou Y, Lin C, Mujitaba Dawuda M, A. Coulter J, Liu C. Phytohormone Cross-Talk Synthesizes Glycoalkaloids in Potato (Solanum tuberosum L.) in Response to Aphid (Myzus persicae Sulzer) Infestation under Drought Stress. Insects. 2020; 11(11):724. https://doi.org/10.3390/insects11110724
Chicago/Turabian StyleQuandahor, Peter, Yuping Gou, Chunyan Lin, Mohammed Mujitaba Dawuda, Jeffrey A. Coulter, and Changzhong Liu. 2020. "Phytohormone Cross-Talk Synthesizes Glycoalkaloids in Potato (Solanum tuberosum L.) in Response to Aphid (Myzus persicae Sulzer) Infestation under Drought Stress" Insects 11, no. 11: 724. https://doi.org/10.3390/insects11110724
APA StyleQuandahor, P., Gou, Y., Lin, C., Mujitaba Dawuda, M., A. Coulter, J., & Liu, C. (2020). Phytohormone Cross-Talk Synthesizes Glycoalkaloids in Potato (Solanum tuberosum L.) in Response to Aphid (Myzus persicae Sulzer) Infestation under Drought Stress. Insects, 11(11), 724. https://doi.org/10.3390/insects11110724