Abstract
The growing demand for sustainable and high-performance lubricants has accelerated interest in biolubricants derived from renewable feedstocks. Vegetable oils are attractive candidates due to their biodegradability, low toxicity, and favorable viscosity index. However, their application is limited by poor oxidative and thermal stability. The epoxidation of unsaturated fatty acids offers a versatile route to address these drawbacks by enhancing stability and introducing reactive epoxy groups for further functionalization. This review highlights the advances in the use of epoxidized vegetable oils (EVOs), as platforms for lubricant design. Post-epoxidation modifications, such as ring-opening reactions, crosslinking, hybridization with additives, and click-type chemistries, are critically examined with emphasis on their impact on viscosity, polarity, tribofilm formation, and overall tribological behaviour. Structure–property relationships were discussed to establish design principles linking chemical modifications with lubrication regimes, wear resistance, and film-forming ability. In addition, sustainability aspects, including biodegradability, ecotoxicity, and life cycle assessment, are reviewed to evaluate the trade-offs between performance enhancement and environmental compatibility of these modifications. Current challenges and future perspectives are outlined, including the need for standardized testing protocols, the integration of multifunctional modifications, and predictive modelling tools. By bridging molecular engineering, tribological performance, and sustainability, this review provides a roadmap for the rational design of advanced epoxidized oil-based biolubricants.
1. Introduction
Lubricants have been indispensable since the Industrial Revolution, where they have played a critical role in reducing friction and wear, thereby extending the service life of machinery components. Today, tribological contacts account for nearly 23% of global energy consumption, with 20% consumed in overcoming friction and 3% linked to the remanufacturing of worn parts. The adoption of advanced tribological technologies could reduce energy losses by up to 40%, substantially lowering global CO2 emissions while generating significant economic savings [1]. This emphasizes the pivotal role of tribology in advancing industrial efficiency and sustainability.
Growing environmental concerns have accelerated the search for sustainable alternatives to conventional petroleum-based lubricants, which, despite their widespread use, have serious ecological and health risks. Their poor biodegradability and toxic components allow them to persist in soil, water, and air, leading to soil fertility loss, groundwater contamination, and long-term ecosystem disruption [2]. Coupled with increasing energy demand, finite fossil resources, and stricter environmental regulations, these challenges highlight the urgent need for greener lubricant technology. In this context, biolubricants have emerged as promising solutions to these problems. Produced primarily from renewable vegetable oils, such as soybean, rapeseed, sunflower, castor, and palm, they offer inherent biodegradability, higher viscosity indices, and often superior lubricating performance [3].
However, unmodified vegetable oils suffer from poor oxidative stability and thermal resistance, restricting their use in demanding operating conditions. To overcome these drawbacks, chemical modifications, including transesterification, epoxidation, epoxide ring-opening, hydrogenation, hydrolysis, and estolide formation, are employed to tailor molecular structures and improve functional performance [4]. Among these approaches, epoxidation has become a cornerstone. Epoxidation increases the stability and versatility of downstream functionalization by converting carbon–carbon double bonds in unsaturated oils into highly reactive epoxy groups [5]. Although epoxidation improves certain properties, it is the post-epoxidation functionalization, including amidation, esterification, and ring-opening reactions, that allows fine-tuning of tribological performance, such as friction reduction, wear resistance, and viscosity control [6].
In this review the post-epoxidation pathways and their impact on sustainable lubrication are critically examined. In contrast to previous reviews that focus primarily on epoxidation as a chemical transformation, this study considers epoxidation as a platform for the development of advanced lubricants. By systematically linking downstream chemical modifications to tribological outcomes and environmental benefits, this review establishes a unique roadmap for the development of next generation biolubricants. The integration of molecular design, performance metrics, and sustainability considerations sets this review apart from others and provides both scientific insight and practical guidance for the rational design of eco-friendly high-performance lubricants.
2. Epoxidation of Vegetable Oils: Fundamentals
Vegetable oils are primarily composed of triglycerides, which consist of a glycerol backbone esterified with three fatty acid chains (Figure 1a). The nature of these fatty acids, particularly the number and position of carbon–carbon double bonds, strongly determines the physical and chemical properties of oils [7]. However, the presence of unsaturated bonds renders vegetable oils highly susceptible to oxidation and thermal degradation, which restricts their direct application in demanding lubrication environments, such as engines.
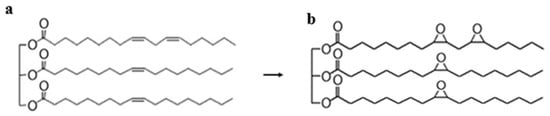
Figure 1.
(a) General structure of a triglyceride; (b) epoxidized triglyceride.
Epoxidation is a chemical reaction in which the carbon–carbon double bonds of unsaturated fatty acids are converted into three-membered oxirane (epoxy) rings (Figure 1b and Figure 2) [5]. The introduction of epoxy functionalities enhances oxidative stability by reducing the degree of unsaturation, while simultaneously providing highly reactive sites that can undergo further transformations, such as ring-opening, amidation, or esterification. This dual role makes epoxidation a critical step in the development of high-performance biolubricants, improving the intrinsic stability of vegetable oils and enabling tailored molecular architectures.

Figure 2.
Scheme of epoxidation reaction. Adapted from [5].
2.1. Methods of Epoxidation and Influence in Lubrication
Epoxidation of vegetable oils is a key chemical modification to enhance their applicability as biolubricants, mainly by improving oxidative stability, thermal resistance, and providing reactive sites for further functionalization [8]. However, the choice of epoxidation method strongly influences the environmental footprint, cost, and scalability of lubricant production.
The in situ peracid method, which is based on hydrogen peroxide and organic acids, remains the industrial standard. Its main strengths are its simplicity, low cost, and consistently high epoxide yields, which explain its dominance in industrial practice. For lubricants, this translates into the reliable large-scale availability of epoxidized oils with improved stability relative to unmodified triglycerides [9,10]. However, drawbacks include the generation of acidic byproducts, risks of oxirane ring-opening, and equipment corrosion, which not only complicate downstream processing but may also reduce the long-term stability of the lubricant.
Enzymatic epoxidation offers an attractive alternative because lipase-mediated systems operate under mild conditions with high selectivity and minimal side reactions. This provides oils with well-defined oxirane groups, which can be tailored for further derivatization into advanced lubricant structures [11,12]. From a sustainability perspective, this process is greener and safer, aligning well with the principles of green chemistry. However, the high cost of enzymes, limited productivity, and challenges in scaling-up hinder their industrial viability. Currently, this route is best viewed as a proof-of-concept approach with potential for specialty lubricants or high-value applications rather than bulk production.
Heterogeneous catalytic epoxidation using solid catalysts such as Ti-silicates, zeolites, or supported metal oxides represents a promising middle ground. It combines good catalytic performance with easier separation, reusability, and lower waste generation than peracid systems [13]. Importantly, its reduced environmental burden and potential for integration into continuous flow systems make it appealing for large-scale lubricant synthesis in the future. However, issues such as catalyst deactivation, mass transfer limitations, and relatively high costs must be addressed prior to industrial adoption.
Overall, each method presents a different balance between practicality, sustainability, and performance issues. Peracid epoxidation guarantees industrial relevance and supply security, enzymatic epoxidation represents the most sustainable but least mature route, and heterogeneous catalysis offers a forward-looking compromise with the potential to deliver both environmental and economic benefits once technological barriers are overcome. For lubricant production, a combined strategy may emerge, where peracid methods serve as the bulk manufacturing route, whereas enzymatic and heterogeneous processes evolve for specialized or greener applications [14].
Table 1 summarizes the main epoxidation methods applied to vegetable oils, outlining their mechanisms, benefits, and limitations in the context of lubricant development. In situ peracid epoxidation represents the established industrial standard, widely used for epoxidized vegetable oils production. Enzymatic epoxidation offers a greener, selective route but faces economic and scale-up barriers. Heterogeneous catalytic epoxidation provides improved catalyst recovery and reduced waste, though practical challenges remain. Together, these approaches highlight the trade-offs between efficiency, sustainability, and industrial applicability in designing high-performance, environmentally friendly biolubricants.

Table 1.
Principal epoxidation methods applied to vegetable oil.
2.2. Common Epoxidized Vegetable Oils
The selection of vegetable oil feedstocks for epoxidation strongly depends on their fatty acid composition and degree of unsaturation, which determine the number of reactive sites available for epoxidation. Soybean oil is the most widely used oil due to its abundance, low cost, and high linoleic acid content. Studies have shown that unmodified epoxidized soybean oil (ESBO) exhibits acceptable lubricating performance, including low wear and reduced friction, under certain conditions [22,23,24]. However, limitations such as a relatively high pour point, inadequate viscosity control, and limited thermal stability under severe conditions restrict their broader application in industrial lubrication systems [22].
Research on rapeseed oil also highlights the improvements achieved with epoxidation, demonstrating that epoxidized rapeseed oil exhibits higher viscosity, improved oxidative stability, and better friction-reducing and load-bearing performance than unmodified oil, which is attributed to the formation of tribopolymerization films during friction [25]. Another study reported that epoxidized canola oil converted to a biolubricant showed high viscosity, excellent lubricity, and outstanding oxidative stability, emphasizing the benefits of removing unsaturation [19]. A comparative study between biolubricants from canola oil and biodiesel found that the canola-derived lubricant was highly viscous and thermally stable, making it suitable for high-temperature, heavy-load applications, whereas the biodiesel-derived lubricant exhibited better low-temperature performance, lower friction, and lower wear [26]. Collectively, these studies confirm that epoxidation enhances lubricant performance, whereas feedstock selection and final viscosity determine the suitability for specific operational conditions, from low-temperature automotive to high-temperature industrial applications.
Sunflower oil, which is also rich in linoleic acid, is highly suitable for epoxidation, although its limited availability restricts large-scale applications. The epoxidation of sunflower oil and its derivatives consistently improve their physicochemical and tribological properties, making them suitable as biolubricants. For example, epoxidized sunflower waste cooking oil demonstrating enhanced viscosity and structural stability [27]. Polyols derived from epoxidized sunflower oils via ring-opening with acetic acid or short-chain alcohols achieve high conversion and selectivity, yielding intermediates with predictable viscosities suitable as base stocks for low-temperature lubricant applications [28]. Another study, have also shown that epoxidized sunflower and rapeseed oils exhibit superior oxidative stability, lower pour points, and improves their overall performance as lubricants and mitigate the limitations [29].
Castor oil is unique because of its hydroxylated ricinoleic acid content, which enhances polarity, viscosity, and surface activity, making it particularly attractive for specialized lubricants. The epoxidation of castor oil has been widely studied as the first step toward bio-based lubricant development, as the introduction of epoxy groups improves the thermo-oxidative stability and increases the viscosity. Epoxidized castor oil fatty acid methyl esters exhibit significantly higher oxidative stability than unmodified base oil, and optimized epoxidation parameters for high free fatty acid feedstocks maximize oxirane oxygen content [20]. In another work, showed that viscosity can increase up to twenty-fold with higher degrees of epoxidation while retaining Newtonian flow behaviour, a desirable characteristic for lubricant formulation [30]. Collectively, these studies confirm that epoxidation alone provides a stable and viscous intermediate suitable for further chemical modification, making epoxidized castor oil an attractive precursor for advanced biolubricants.
Palm oil, the most widely produced vegetable oil globally, is characterized by good thermal stability and a relatively low level of unsaturation, which restricts its ability to form epoxy functionalities efficiently. A recent review emphasized the relevance of palm oil in lubricant applications owing to its abundance, favourable fatty acid profile, and crop sustainability. However, its inherent oxidative instability limits the formation of durable lubricant film. Chemical processing routes, such as epoxidation, have been shown to enhance the thermal and tribological properties of palm oil-based lubricants [31].
While the above studies provide valuable insights into the performance improvements achieved by epoxidation, a comparative analysis of these oils highlights important differences that influence their suitability for specific lubricant application. All major epoxidized vegetable oils, including soybean, rapeseed/canola, sunflower, castor, and palm oils, benefit from enhanced oxidative stability and viscosity relative to their unmodified counterparts. However, their performance profiles differ significantly. Soybean oil is abundant and inexpensive, making it a benchmark for industrial applications. However, its high pour point and moderate thermal stability may limit its use at extreme temperatures [32].
Rapeseed oil provides higher viscosity and stronger friction-reducing properties, making them better suited for high-load, high-temperature applications. However, availability and cost can be limiting factors [26].
Sunflower oil offers excellent lubricity and structural stability, particularly when chemically modified after epoxidation. However, large-scale supply constraints reduce their commercial relevance [27].
Castor oil, with its naturally hydroxylated structure, has a very high viscosity and polarity, supporting specialized high-performance lubricants. But, its high cost and lower abundance restrict its widespread adoption [33].
In practical terms, selecting a feedstock involves balancing the supply, cost, and inherent oil properties with the target lubricant performance.
For standard industrial biolubricants, epoxidized soybean oil (ESBO) remains dominant because of its scale and established processing methods. For high-temperature or specialty applications, rapeseed, canola, and castor oils are preferred because of their superior viscosity and oxidative stability.
To provide a clear comparison of the key features, lubricant-relevant advantages, and limitations of common vegetable oils, as well as the effects of epoxidation, Table 2 summarizes the properties of soybean, canola, sunflower, castor, and palm oils as lubricant feedstocks. This comparative overview highlights how the fatty acid composition, inherent viscosity, and polarity influence both the baseline lubricant performance and the improvements achieved through epoxidation. Epoxidation universally improves the oxidative and thermal stabilities of all feedstocks. The introduction of epoxide groups increases the polarity, enhancing adhesion and film formation on metal surfaces. Ultimately, the choice of vegetable oil depends on the target operating conditions, including the temperature, load, and required viscosity.

Table 2.
Comparative overview of Epoxidized Vegetable Oils for Lubricant Applications.
2.3. Post-Epoxidation Modifications for Biolubricant Production
Epoxidation of vegetable oils transforms unsaturated triglycerides into oxirane ring-containing reactive intermediates. These epoxides can undergo a wide range of chemical modifications that tailor their molecular structures and ultimately determine their performance as biolubricants [8]. The following section summarizes the principal reaction pathways reported in the literature, with an emphasis on the mechanism, reagents, and their influence on the tribological and physicochemical properties relevant to lubrication.
2.3.1. Ring Opening with Alcohols and Polyols
The ring-opening of epoxidized vegetable oils with alcohols and polyols is among the most frequently explored post-epoxidation routes in the literature. In the presence of acidic catalysts such as H2SO4, p-toluenesulfonic acid, or solid acid resins, alcohols attack the oxirane carbon to yield β-alkoxy alcohols. With multifunctional polyols (e.g., glycerol, ethylene glycol, and pentaerythritol), multiple hydroxyl groups are introduced, producing polyol-modified oils with higher polarity and viscosity [9,38,39].
From a lubrication perspective, hydroxyl incorporation enhances the metal surface affinity and film-forming capacity, which can reduce friction and wear in the boundary regime. For example, glycerol- and ethylene glycol-modified epoxidized soybean oils have demonstrated superior antiwear performance compared to the parent oil [40]. Hydroxylated oils also generally preserve biodegradability, making them attractive candidates for hydraulic fluids [41,42].
However, significant limitations persist. Hydroxyl functionalities are prone to oxidation, resulting in lower thermal-oxidative stability than esterified or hydrogenated analogues [43]. Increased viscosity can also compromise low-temperature flow, limiting applications where fluidity is essential. Moreover, ring-opening selectivity is strongly influenced by the catalyst type, solvent polarity, and distribution of epoxide groups, leading to variability in product properties and complicating scale-up for industrial use [44].
Thus, while alcohol and polyol ring-opening provide a versatile route to tailor polarity and tribological behaviour, further process optimization is required. Future research should focus on developing greener catalysts (e.g., solid acids and enzymes), improving selectivity, and systematically balancing the trade-offs between viscosity, oxidative stability, and low-temperature performance to enable broader industrial deployment.
2.3.2. Ring Opening with Amines and Aminoalcohols
The nucleophilic ring-opening of epoxides with amines and aminoalcohols provides a versatile route to β-amino alcohols and, in some cases, to secondary amides. These reactions are generally base-catalysed and can be accelerated by imidazole or tertiary amines. Common reagents include ethanolamine, diethanolamine, and N-methylamino ethanol [43].
The incorporation of nitrogen imparts several beneficial properties. Strong hydrogen bonding and increased cohesive energy density enhance thermal stability, whereas amino and hydroxyl groups provide high surface activity that favours the formation of persistent boundary films on metal surfaces. This leads to significant improvements in anti-wear performance and friction reduction, particularly under severe tribological conditions [45,46,47]. Therefore, such modifications show promise for demanding applications such as engine oils, gear lubricants, and chain oils.
However, this approach is not without drawbacks. While nitrogen functionalities are beneficial for adsorption, they are also prone to oxidation and nitration, which may reduce long-term lubricant stability and necessitate the use of antioxidants [28]. In addition, the increased polarity of aminoalcohol-modified oils often leads to reduced miscibility with nonpolar base stocks (e.g., PAOs), posing challenges in blending and formulation. Another concern is that the reaction can yield complex mixtures of β-amino alcohols and amides, depending on the reagent and conditions, which complicates the structure–property correlation and scale-up feasibility [48].
Overall, amine and aminoalcohol ring-opening represent powerful strategies for enhancing boundary lubrication performance and thermal stability; however, future work should focus on improving oxidative resistance, tailoring reaction selectivity, and exploring synergistic additive packages to offset stability concerns.
2.3.3. Ring Opening with Acids and Anhydrides (Esterification)
The reaction of epoxidized oils with carboxylic acids or acid anhydrides, produces β-hydroxy esters, which is a well-established route for tailoring lubricant properties. Typical reagents include short-chain acids (e.g., acetic acid), long-chain fatty acids (e.g., oleic and sebacic acids), and cyclic anhydrides (e.g., phthalic and succinic anhydrides) [9,19,41,49].
The incorporation of ester groups provides an effective means to tune the viscosity, pour point, and polarity [50]. Long-chain esters contribute to improved low-temperature fluidity and lubricity, whereas branched esters enhance oxidative stability by sterically hindering radical propagation [40,49]. Furthermore, ester functionalities are inherently biodegradable and promote favourable tribological properties, making them attractive for environmentally adapted hydraulic and gear lubricants [51].
Despite these advantages, esterification strategies have important limitations. β-hydroxy esters are susceptible to hydrolysis, particularly under humid or acidic conditions, which can reduce lubricant lifetime and produce corrosive byproducts [52]. Moreover, the trade-off between low-temperature fluidity and oxidative stability remains a key challenge, as the introduction of bulky ester groups may improve cold flow properties but simultaneously increase volatility and reduce high-temperature film strength [53,54]. Future research should emphasize process optimization to balance these competing effects, explore synergistic combinations with antioxidants or nanoparticles, and assess the long-term tribological stability of esterified oils under realistic conditions.
2.3.4. Polymerization
Epoxidized vegetable oils (EVOs), such as epoxidized soybean oil (ESO), can undergo cationic polymerization or curing with multifunctional reagents, such as anhydrides and amines, forming partially or fully crosslinked networks. These transformations significantly increase the viscosity, yielding materials ranging from high-molecular-weight oligomers to gel-like networks [55,56].
These polymerized structures are particularly relevant for biolubricants that require an enhanced load-bearing capacity. For example, curing ESBO with anhydrides produces viscoelastic materials with mechanical integrity that are suitable for improving viscosity and lubrication performance [57].
Challenges include:
- Flowability vs. Network Strength: Increasing the crosslink density enhances viscosity and mechanical integrity; however, excessive structuring can lead to gel-like or solid-like materials that lose the fluidity required for lubrication [58].
- Processability: Once cured, crosslinked EVO derivatives become insoluble, complicating their blending with other base oils or additives and limiting their applicability in multi-component lubricant systems [59].
- Recyclability and Reversibility: Permanent covalent networks hinder reprocessing and recycling. This is a concern for sustainable lubricant design, where reversible or dynamic crosslinking chemistries (e.g., Diels–Alder and hydrogen bonding) could offer more circular solutions [60].
To summarize the main post-epoxidation pathways, Figure 3 provides a conceptual overview of the principal ring-opening and polymerization routes applied to epoxidized vegetable oils. These transformations diversify the chemical landscape of oxirane intermediates, allowing fine-tuning of the polarity, viscosity, and tribological performance through the targeted incorporation of hydroxyl, amino, ester, or polymeric functionalities. Each route introduces distinct structural features that dictate the balance between lubricity, oxidative stability, and biodegradability, which are key parameters for designing high-performance biolubricants.
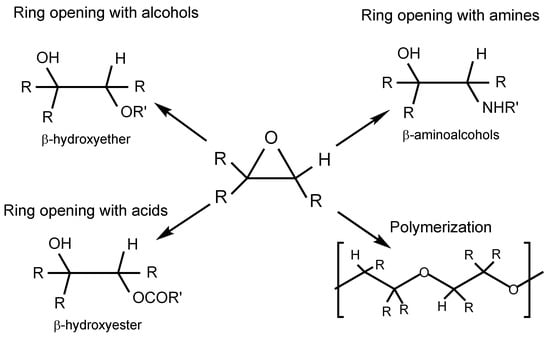
Figure 3.
Schematic representation of the main post-epoxidation modification routes of vegetable-oil based epoxides.
Future research should focus on controlled crosslinking strategies, such as dynamic covalent chemistries or partial network formation, to balance structural stability and adaptability under tribological stress.
2.4. Additional Reported Routes Relevant to Biolubricants
In addition to the widely studied alcohol-, amine-, and acid-mediated ring-opening routes, several specialized post-epoxidation transformations have been reported, each offering unique advantages and challenges.
- CO2 carbonation: The conversion of epoxides into cyclic carbonates provides a direct route to more polar oils with enhanced oxidative stability and tuneable viscosity [61]. However, these reactions often require high pressures and specific catalysts, which can hinder scalability. Moreover, while cyclic carbonates improve biodegradability, their stability under hydrolytic conditions remains to be explored.
- Thiol–epoxy click chemistry: This reaction efficiently incorporates sulphur-containing groups that enhance the extreme-pressure (EP) performance without relying on conventional ZnDTP-type additives. The method is fast and selective; however, the introduction of sulphur moieties raises concerns about odour, oxidative stability, and possible environmental persistence [62].
- Phosphorus-based ring opening: formation of phosphate esters imparts both anti-wear and fire-resistant properties. Despite their strong performance, their environmental acceptability is debated because phosphorus-containing compounds may contribute to aquatic toxicity and catalyst poisoning in engines [63].
Taken together, these specialized chemistries expand the toolbox for tailoring EVO-based lubricants, enabling precise control over the viscosity, polarity, tribological film strength, and oxidative resistance. However, most of these methods remain at the proof-of-concept stage, and their industrial uptake will depend on solving scalability, cost, and environmental safety challenges.
2.5. Hybridization with Other Oils and Additives
In addition to chemical modification, hybridization strategies, such as blending epoxidized vegetable oils (EVOs) with other base oils or functional additives, have been widely explored. Reported systems include ionic liquids (ILs), nanoparticles (graphene, MoS2, nano clays) [64,65] and biodegradable antiwear (AW) and extreme pressure (EP) additives. Such hybrids frequently demonstrate synergistic improvements in friction reduction, oxidative stability, and wear resistance while maintaining biodegradability when eco-friendly additives are selected [66,67].
Despite these promising results, several limitations remain.
- Dispersion and stability: Nanoparticles often aggregate or sediment in polar-modified EVO matrices, reducing their long-term reliability [68].
- Interfacial compatibility: The polarity mismatch between EVOs and certain ILs or nanoparticles can hinder uniform blending, sometimes requiring the use of surfactants or surface modification [69].
- Cost and scalability: ILs and advanced nanomaterials can substantially increase formulation costs, restricting industrial uptake.
Nonetheless, hybrid systems offer a practical pathway to overcome EVO limitations without extensive chemical synthesis, especially for tailoring properties for automotive, industrial, and marine lubricants. Future research should emphasize long-term dispersion stability, synergistic additive design, and life cycle assessment (LCA) to balance performance gains with sustainability goals.
Table 3 summarizes the key reaction types, their influence on lubricant properties, and their typical applications. This comparative summary highlights how different chemical and physical modification routes—ranging from alcohol/polyol and amine ring-opening to hybridization with functional additives—can be employed to tailor the viscosity, polarity, oxidative stability, tribological performance, and biodegradability. The table illustrates the versatility of epoxidized vegetable oils as tuneable biolubricant base stocks and provides a practical guide for selecting modification routes based on specific-performance requirements.

Table 3.
Summary of epoxide ring-opening and modification strategies for lubricant applications.
3. Structure–Tribological Property Relationship of Post-Epoxidation Derivatives
The tribological performance of epoxidized vegetable oil (EVO) derivatives is dictated by the interplay between the molecular structure, physicochemical properties, and surface interactions. Post-epoxidation modifications, including alcohol/polyol and amine ring-opening, esterification, phosphorylation, and crosslinking, directly influence viscosity, polarity, tribochemical activity, and tribofilm formation [70]. Understanding these relationships is essential for the rational design of biolubricants across boundary, mixed, and hydrodynamic lubrication regimes.
3.1. Integrative Summary
From these structure–property relationships, several design principles have emerged.
- Polar groups (–OH, –NH2, –PO4): enhance boundary lubrication but may reduce the oxidative stability [71].
- Long aliphatic chains: improve oxidative resistance and VI but reduce surface adsorption [72].
- Reactive functionalities (amines, phosphates, sulphur groups): promote tribochemical film formation and enhance antiwear performance [73,74].
- Crosslinking: increases load-bearing capacity but reduces biodegradability and flowability [75].
Quantitative comparisons from the literature highlight how specific chemical modifications tune the viscosity, friction, and wear resistance of the EVO derivatives. Table 4 summarizes the representative data extracted from tribological studies, showing that polarity-enhancing modifications (amines and phosphates) dominate boundary lubrication, whereas chain extension and esterification primarily improve mixed-to-hydrodynamic performance.

Table 4.
Typical tribological properties of modified epoxidized vegetable oils (EVOs), including viscosity, coefficient of friction, wear reduction, and dominant lubrication regime reported in literature.
In essence, molecular polarity and surface reactivity dominate boundary lubrication, whereas viscosity and chain architecture control the performance in mixed and hydrodynamic regimes. This framework provides a molecular-level rationale for designing EVO derivatives with targeted tribological properties, guiding the development of biodegradable oils, greases, and high-performance lubricants.
Molecular modifications directly tune the physicochemical properties and tribological performance of the lubricant. Polar groups enhance adsorption and boundary film stability, esterification increases viscosity and supports hydrodynamic films, reactive moieties such as amines and phosphates promote tribochemical protection, and crosslinking increases load-bearing capacity [84]. Collectively, these relationships provide a molecular-level rationale for balancing friction reduction, wear protection, and film formation. To illustrate these relationships, Figure 4 represents how specific molecular modifications translate into changes in physicochemical properties and ultimately tribological effects. This schematic highlights the direct links between chemical design, lubricant behaviour, and performance in the service.
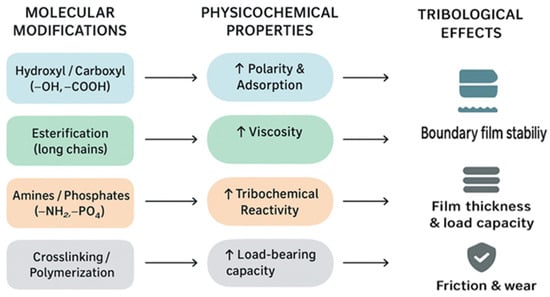
Figure 4.
Structure–property–tribological relationship. Upward arrows in the figure indicate an increase.
To better compare how different vegetable oils respond to epoxidation and subsequent chemical modification, their key lubricant-relevant properties were ranked on a semi-quantitative scale (Table 5). The values ranged from 1 to 5, where 1 = very poor, 2 = poor, 3 = moderate, 4 = good, and 5 = excellent performance for each property. The ranges (e.g., 4–5) indicate tunability depending on the specific modification route and reaction conditions. This comparative framework highlights the intrinsic strengths of each oil and the improvements achievable through post-epoxidation strategies.

Table 5.
Comparative performance of vegetable oils before epoxidation (pure), after epoxidation and after post-modification reactions *.
Considering viscosity, post-epoxidation modifications generally allow for fine-tuning of viscosity. The esterification of epoxidized canola or soybean oil increases the viscosity for load-bearing applications, whereas polyols derived from castor oil maintain their already high viscosity (scored 5 across all stages) [78,86].
For oxidative stability, conversion or elimination of residual double bonds significantly improves oxidative stability. Canola, soybean, and castor oils all achieved long OIT values (>50 h in many studies), reflected by scores increasing from 3–4 in the pure state to 5 after modification. Palm oil consistently scores high (5) because of its natural saturation [50,85].
In terms of low-temperature fluidity, selective esterification and short-chain alcohol modifications preserve or enhance the low-temperature performance. Canola- and soybean-based lubricants achieved good to excellent scores (4–5). Despite its high viscosity, castor oil retains functional cold flow (4), whereas palm oil remains limited (2–3) [89].
Finally considering tribological Performance polar functionalities introduced by epoxidation and subsequent modifications enhanced the adsorption and film formation on metal surfaces. The modified castor and canola oils exhibited excellent antiwear and friction-reducing properties (score 5). Soybean and sunflower oils improved the effectiveness but remained slightly less effective (scores 3–4) [90].
Considering the overall suitability of different vegetable oils:
- Castor oil: Best suited for high-load, high-friction, or industrial applications because of its consistently high viscosity and strong tribological behaviour.
- Canola oil: Offers an excellent balance of properties, making it highly versatile for automotive and general-purpose lubricants.
- Soybean oil: A strong general-purpose base oil that is suitable for moderate-load applications after modification.
- Sunflower oil: Limited by moderate oxidative stability and lower tribological performance but is tuneable.
- Palm oil: Highly thermally stable but constrained by poor low-temperature fluidity, restricting its use to high-temperature applications.
Post-epoxidation modifications provide a versatile toolbox for tailoring viscosity, cold-flow, and tribological properties, effectively transforming oils with inherent limitations (e.g., sunflower and soybean oils) into competitive candidates. Castor and canola oils have emerged as the most promising bases for high-performance, sustainable biolubricants, whereas palm oil remains niche owing to cold-flow restrictions.
3.2. Viscosity and Viscosity Index (VI)
Viscosity is a critical factor that controls the transition from mixed to hydrodynamic lubrication [91,92]. Ring-opening reactions that introduce hydroxyl or ester groups, increase both the viscosity (from around 100–200 cSt to 300 cSt at 40 °C) and viscosity index (VI) from approximately 120 to 160, enhancing film formation under shear [28]. However, excessive viscosity can hinder flow in high-speed applications, emphasizing the need to balance film stability and energy efficiency [93].
3.3. Coefficient of Friction (COF) Across Lubrication Regimes
The coefficient of friction depends on the bulk viscosity and surface affinity. In the boundary regime, polar functional groups (–OH, –COOH, –CONH–, and–PO4) are strongly adsorbed onto metallic surfaces, forming thin protective films that reduce friction [94]. Amines and phosphorus-based modifications can decrease the COF because of tribochemical reactions that reinforce the boundary layers [75,76]. Conversely, esterification with long chains favours mixed-to-hydrodynamic performance, improving the COF in high-speed sliding but contributing less to direct surface protection [95].
3.4. Wear Resistance and Tribofilm Formation
Wear protection arises from both physical adsorption and chemical reactivity. Alcohol and ester-modified oils primarily reduce mild wear via adsorption, producing relatively thin and less durable films [96]. Amine- and phosphorus-based derivatives form robust tribofilm through tribochemical mechanisms, drastically reducing the wear volume and depth [97,98]. Hybrid systems incorporating nanoparticles (graphene, MoS2, nano clays) create solid lubrication phases that synergistically reinforce tribofilm [99]. However, dispersion stability remains a challenge, particularly under thermal or mechanical stresses [82].
3.5. Effect of Polarity and Surface Chemistry
Polarity governs the adsorption strength on metal and oxide surfaces. Highly polar modifications enhance boundary lubrication but can compromise oxidative stability and accelerate degradation [45,83]. In contrast, long-chain esterification reduces polarity, improving oxidation resistance and VI but diminishing surface adsorption [100]. Thus, a careful balance between polarity and oxidative stability is essential for tailoring the performance to the intended lubrication regime [26].
4. Sustainability and Biodegradability
The sustainable development of biolubricants must extend beyond their tribological performance to encompass environmental compatibility, biodegradability, and life cycle impact. As lubricants are inevitably released into the environment through leakage, volatilization, wear debris, or disposal, their end-of-life fate, ecotoxicity, and overall life-cycle footprint are essential for assessing sustainability. These three pillars—biodegradability, ecotoxicity, and life cycle impact—constitute the fundamental framework for environmental evaluation [21,101,102].
Biodegradable lubricants derived from epoxidized, and post-modified vegetable oils have been explored for use in various applications, including automotive engines, gear systems, hydraulic fluids, and marine or agricultural equipment operating in environmentally sensitive environments. Their high lubricity, renewability, and low toxicity make them suitable substitutes for mineral-based oils in both high-load and moderate-speed conditions [41,51,103].
For instance, epoxidized soybean oil derivatives have shown promising performance as base fluids for gear and hydraulic systems [38], while polyol and amide-modified esters have been proposed for industrial and high-temperature applications [9,49].
4.1. Biodegradability Assessment
Standardized protocols, including OECD 301 [104], ISO 9439 [105], and ASTM D5864 [106], are widely used to quantify the biodegradability of lubricants in aquatic environments. Lubricants that achieve more than 60% degradation within 28 days are considered readily biodegradable. Native vegetable oils typically meet this criterion because of their triglyceride structure, whereas chemical modifications can alter degradation rates depending on the introduced functionalities [107,108].
Unmodified vegetable oils, such as soybean or rapeseed oil, exhibit biodegradability values between 65 and 90% (28 days). Epoxidized vegetable oils (EVOs) are moderately biodegradable (50–70%), but their biodegradability is slightly reduced by from the presence of the oxirane ring [102]. Esterification with long-chain fatty alcohols or polyols generally preserves biodegradability (60–85%) while improving viscosity index and oxidative stability [109]. In contrast, hydroxylation and amination increase polarity and boundary lubrication but reduce oxidative stability and, in some cases, lower biodegradability to 40–60% because of the introduction of nitrogen or oxygen [110].
Crosslinked or aromatic structures present the greatest challenges. Polyesters can fall below 30% biodegradation, and aromatic substitutions result in less than 20% biodegradability, raising concerns regarding persistence and toxicity [101]. Recent LCA studies on trimethylolpropane (TMP) esters from palm-based feedstocks reported biodegradability above 70%, combined with a seven-fold lower global warming potential (GWP ≈ 6 kg CO2-eq/kg) than that of mineral-based lubricants [111].
4.2. Ecotoxicity and Environmental Trade-Offs
Despite their renewable origin, some chemically modified oils or additives can release degradation products that are ecotoxicologically concerning. Nitrogen- and sulfur-containing derivatives may promote oxidation and generate harmful byproducts that affect aquatic life [110]. Similarly, nanoparticle-based additives, while improving anti-wear properties, may raise concerns about bioaccumulation and environmental persistence [106,112].
Therefore, ecotoxicity tests, such as OECD 201 (algal growth inhibition) and OECD 202 (Daphnia immobilization), are essential to complement biodegradation assessments. For example, biolubricants based on TMP esters from high-oleic feedstocks show EC50 values greater than100 mg/L in Daphnia tests, indicating low toxicity, whereas metal-based additives can reduce EC50 to less than10 mg/L [109]. Thus, the environmental trade-off depends not only on the chemical structure but also on the choice and concentration of additives.
4.3. Life Cycle Assessment (LCA)
Life Cycle Assessment (LCA) provides a quantitative evaluation of the cradle-to-grave environmental burden of lubricants. LCAs consistently show that vegetable oil–derived lubricants have 50–70% lower global warming potential (GWP) and 40–60% lower non-renewable energy demand than mineral oils [105,106]. For example, unmodified vegetable oils have GWP values of 1.5–1.8 kg CO2-eq/kg, compared with 4.5 kg CO2-eq/kg for mineral oils.
Among the modified oils, epoxidation and esterification maintained a reduced GWP (2.0–2.3 kg CO2-eq/kg) while enhancing oxidation stability. In contrast, crosslinking or aromatic modifications can increase the GWP to 2.5–3.0 kg CO2-eq./kg due to higher energy consumption and solvent use. Recent process simulations for palm-based TMP esters indicate a seven fold reduction in GWP (approximately 6 kg CO2-eq/kg lubricant) compared with mineral lubricants, achieving an internal rate of return above 140% and strong energy efficiency [111].
As discussed in Section 2.1, epoxidation methods differ significantly in terms of environmental performance. In situ peracid epoxidation remains the industrial standard but generates acidic waste, whereas enzymatic and heterogeneous catalytic routes offer greener alternatives with varying economic and scalability constraints [109].
4.4. Sustainability–Performance Balance
As detailed in Section 2.3, post-epoxidation modifications control polarity and film-forming ability. From a sustainability perspective, esterification and epoxidation provide the most favorable trade-off between oxidative stability and biodegradability [102].
Quantitative LCAs show that epoxidation and esterification offer the best compromise between tribological performance (up to 50% reduction in friction coefficient) and environmental safety (50–65% reduction in GWP). In contrast, crosslinked polyester or aromatic-functionalized oils achieve excellent stability and load-bearing capacity but have poor biodegradability (<30%) and higher energy consumption.
Future research should prioritize multifunctional synthesis routes that combine durability and eco-safety. Examples include the use of deep eutectic solvents, bio-based ionic liquids, and natural nanofillers (e.g., clays, cellulose, and lignin), as well as green-catalyzed or solvent-free processes. These strategies can significantly reduce the GWP while maintaining high lubrication efficiency, bringing biolubricants closer to fully sustainable formulations.
To summarize and compare the environmental and functional impacts of the key modification routes, Table 6 presents the quantitative biodegradability and life cycle indicators derived from recent literature. The data illustrates that unmodified and esterified vegetable oils maintain high biodegradability (>60%) and substantial GWP reductions (>60%), whereas crosslinked or aromatic derivatives, despite their improved stability, exhibit markedly reduced biodegradation rates and higher carbon footprints. TMP esters derived from high-oleic feedstocks are promising benchmarks that balance tribological efficiency, oxidative stability, and environmental performance.

Table 6.
Summary of biodegradability (OECD 301, 28 days), global warming potential (GWP) reduction, and the main environmental and performance trade-offs associated with various chemical modifications of vegetable oils. n.d.: not determined (data not reported in the cited references).
5. Gaps and Future Work
Although significant advances have been made in the chemical modification of epoxidized vegetable oils (EVOs), several scientific and technological challenges remain unresolved. One key limitation is the lack of integrated frameworks linking the molecular structure, tribological performance, and environmental behavior. Most studies still address these aspects separately, limiting understanding of the structure–property–sustainability relationships. Future research should adopt standardized multi-parameter testing protocols that simultaneously evaluate tribological behaviour, biodegradability, and ecotoxicity under realistic operating conditions [28]. This approach will enable a more holistic understanding of lubricant functionality [113].
Another critical issue concerns standardization and scalability. Variations in tribometers, applied loads, and testing environments across laboratories hinder comparability and model development. Establishing harmonized testing standards specifically tailored to biolubricants, including high-load, high-temperature, and aqueous conditions, is essential to ensure reproducibility [114]. From a synthesis perspective, scalable green chemistry approaches, such as enzymatic epoxidation, solvent-free esterification, and solid acid catalysis, require optimization to minimize cost and energy intensity [111].
Emerging research directions focus on biobased catalysts and dynamic covalent systems, which offer both sustainability and adaptability. Catalysts derived from biopolymers, amino acids, or polyphenolic frameworks enable recyclable, metal-free transformations compatible with green chemistry principles [10,115]. Dynamic covalent networks, capable of reversible bond exchange under mild conditions, creates opportunities for self-healing, recyclable, and adaptive lubricants that can adjust their structure in response to shear or temperature changes [116]. These materials could bridge the current gap between stability and biodegradability, introducing a new paradigm for “intelligent” sustainable lubricants.
Finally, comprehensive Life Cycle Assessment (LCA) and Techno-Economic Analysis (TEA) remain crucial to quantify sustainability gains, identify bottlenecks, and evaluate industrial feasibility. Incorporating LCA and TEA at early design stages will ensure that laboratory innovations translate into scalable, economically viable, and environmentally responsible lubricant technologies [101].
Future research should prioritize:
- Developing integrated and standardized protocols linking tribology, biodegradability, and ecotoxicity.
- Advancing bio-based and recyclable catalytic systems for greener EVO modification.
- Exploring dynamic covalent and self-healing lubricants to combine durability and environmental compatibility.
- Leveraging computational and AI-based modeling to predict structure–property–performance relationships.
- Embedding LCA and TEA frameworks to assess sustainability and scalability from the design stage.
By combining molecular innovation, computational design, and sustainability metrics, future EVO-based lubricants can evolve toward intelligent, circular, and high-performance materials capable of reducing frictional energy losses while safeguarding environmental integrity.
6. Conclusions
Epoxidized vegetable oils (EVOs) are a versatile platform for developing sustainable lubricants. Epoxidation enhances oxidative stability while creating reactive sites that can be functionalized via ring-opening, esterification, crosslinking, or hybridization. These post-modifications allow the fine-tuning of viscosity, polarity, tribofilm formation, and biodegradability, leading to lubricants that can compete with or surpass petroleum-based formulations.
The key conclusions are as follows:
- Structure–property correlation: Polar functionalities (–OH, –NH2, –PO4) improve boundary lubrication and wear resistance, whereas esterification and long-chain modifications enhance viscosity index (VI) and oxidative stability.
- Performance–sustainability trade-offs: Modifications that increase tribological performance (e.g., crosslinking and amine incorporation) may reduce biodegradability or increase ecotoxicity.
- Versatility of EVOs: They can serve as base oils, reactive intermediates, or components in hybrid systems with nanoparticles, ionic liquids, and multifunctional additives.
- Environmental promise: EVO-based lubricants derived from renewable feedstocks offer a reduced carbon footprint and improved biodegradability compared to petroleum oils.
Among the different EVO modification routes, epoxidized soybean oil (ESBO) and epoxidized linseed oil (ELO) are the most technologically mature, being produced at an industrial scale and available as reactive intermediates in coatings, polymers, and lubricants. Ring-opening with polyols or amino alcohols offers a balance between tribological enhancement and biodegradability, whereas esterification with long-chain fatty acids provides improved oxidative stability and viscosity control suitable for high-temperature applications. Processes employing heterogeneous or enzymatic catalysis are emerging as scalable and eco-efficient alternatives to conventional acid catalysis, aligning with the goals of industrial green chemistry. The continued integration of Life Cycle Assessment (LCA) and Techno-Economic Analysis (TEA) is crucial for identifying pathways with both technical feasibility and environmental advantages, paving the way for the commercialization of high-performance EVO-based lubricants.
Author Contributions
D.C.M.R.: Conceptualization, Data curation, Formal analysis, Investigation, Writing—original draft and Writing—review and editing. A.R.: Conceptualization, Supervision, Resources, Project administration, Funding acquisition, Writing—review and editing. A.C.S.: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing—review and editing. J.C.: Conceptualization, Supervision, Resources, Project administration, funding acquisition, Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research is sponsored by national funds through FCT- Fundação para a Ciência e Tecnologia, under projects UID/00285/2025—Centre for Mechanical Engineering, Materials and Processes and LA/P/0112/2020.
Acknowledgments
Diana C. M. Ribeiro acknowledges to FCT for the funding of her research degree grant UI/BD/151490/2021.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EVOs | Epoxidized vegetable oils |
| ESBO | Epoxidized soybean oil |
| PAO | Polyalphaolefin |
| VI | Viscosity index |
| OIT | Oxidation induction time |
| EP | Extreme pressure |
| LCA | Life cycle assessment |
| TEA | Techno-economic analysis |
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Plata, D.B.; Saboya, R.M.A.; de Luna, F.M.T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An overview of the biolubricant production process: Challenges and future perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Zeng, Y.; Shang, Z.; Zheng, Z.; Shi, N.; Yang, B.; Han, S.; Yan, J. A Review of Chemical Modification of Vegetable Oils and Their Applications. Lubricants 2024, 12, 180. [Google Scholar] [CrossRef]
- Panchal, T.M.; Patel, A.; Chauhan, D.D.; Thomas, M.; Patel, J.V. A methodological review on bio-lubricants from vegetable oil based resources. Renew. Sustain. Energy Rev. 2017, 70, 65–70. [Google Scholar] [CrossRef]
- Woma, T.Y.; Lawal, S.A.; Abdulrahman, A.S.; Olutoye, M.A.; Ojapah, M.M. Vegetable oil based lubricants: Challenges and prospects. Tribol. Online 2019, 14, 60–70. [Google Scholar] [CrossRef]
- Saurabh, T.; Patnaik, M.; Bhagt, S.L.; Renge, V.C. Epoxidation of vegetable oils: A review. Int. J. Adv. Eng. Technol. 2011, 17, 302. [Google Scholar]
- Lathi, P.S.; Mattiasson, B. Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil. Appl. Catal. B Environ. 2007, 69, 207–212. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [Google Scholar] [CrossRef]
- Gryglewicz, S.; Muszyński, M.; Nowicki, J. Enzymatic synthesis of rapeseed oil-based lubricants. Ind. Crops Prod. 2013, 45, 25–29. [Google Scholar] [CrossRef]
- Kerman, C.O.; Gaber, Y.; Ghani, N.A.; Lämsä, M.; Hatti-Kaul, R. Clean synthesis of biolubricants for low temperature applications using heterogeneous catalysts. J. Mol. Catal. B Enzym. 2011, 72, 263–269. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Luo, C.; Xia, X.; Liu, Z.; Zhao, Y.; Du, F.; Jin, X. Opportunities and Emerging Challenges of the Heterogeneous Metal-Based Catalysts for Vegetable Oil Epoxidation. ACS Sustain. Chem. Eng. 2022, 10, 7426–7446. [Google Scholar] [CrossRef]
- Cogliano, T.; Russo, V.; Eränen, K.; Tesser, R.; Di Serio, M.; Salmi, T. Comparison of continuous technologies for vegetable oils epoxidation. Chem. Eng. Sci. 2025, 301, 120726. [Google Scholar] [CrossRef]
- Roslan, A.; Jalil, M.J.; Azmi, I.S.; Aznizam, N.S.; Mustapha, S.A.; Masri, A.N.; Mubarak, N.M.; Hosseini-Bandegharaei, A. In situ epoxidation of hybrid waste cooking oil and oleic acid via peracid mechanism. Sci. Rep. 2025, 15, 19304. [Google Scholar] [CrossRef]
- Janković, M.R.; Govedarica, O.M.; Sinadinović-Fišer, S.V. The epoxidation of linseed oil with in situ formed peracetic acid: A model with included influence of the oil fatty acid composition. Ind. Crops Prod. 2020, 143, 111881. [Google Scholar] [CrossRef]
- Corrêa, F.D.A.; Sutili, F.K.; Miranda, L.S.M.; Leite, S.G.F.; De Souza, R.O.M.A.; Leal, I.C.R. Epoxidation of oleic acid catalyzed by PSCI-Amano lipase optimized by experimental design. J. Mol. Catal. B Enzym. 2012, 81, 7–11. [Google Scholar] [CrossRef]
- Sustaita-Rodríguez, A.; Ramos-Sánchez, V.H.; Camacho-Dávila, A.A.; Zaragoza-Galán, G.; Espinoza-Hicks, J.C.; Chávez-Flores, D. Lipase catalyzed epoxidation of fatty acid methyl esters derived from unsaturated vegetable oils in absence of carboxylic acid. Chem. Cent. J. 2018, 12, 39. [Google Scholar] [CrossRef]
- Sharma, R.V.; Dalai, A.K. Synthesis of bio-lubricant from epoxy canola oil using sulfated Ti-SBA-15 catalyst. Appl. Catal. B Environ. 2013, 142–143, 604–614. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Epoxidation of castor oil fatty acid methyl esters (COFAME) as a lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef]
- Wai, P.T.; Jiang, P.; Shen, Y.; Zhang, P.; Gu, Q.; Leng, Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019, 9, 38119–38136. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z. Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind. Crops Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Hwang, H.S.; Adhvaryu, A.; Erhan, S.Z. Preparation and properties of lubricant basestocks from epoxidized soybean oil and 2-ethylhexanol. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 811–815. [Google Scholar] [CrossRef]
- Ribeiro Filho, P.R.C.F.; do Nascimento, M.R.; Cavalcante, C.L.; de Luna, F.M.T. Synthesis and tribological properties of bio-based lubricants from soybean oil. Biomass Convers. Biorefinery 2024, 14, 20509–20521. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Yang, S.; Chen, H.; Wang, D. Study of epoxidized rapeseed oil used as a potential biodegradable lubricant. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 561–563. [Google Scholar] [CrossRef]
- Sharma, R.V.; Somidi, A.K.R.; Dalai, A.K. Preparation and properties evaluation of biolubricants derived from canola oil and canola biodiesel. J. Agric. Food Chem. 2015, 63, 3235–3242. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghobadian, B.; Dehghani Soufi, M.; Gorjian, S. Chemical modification of sunflower waste cooking oil for biolubricant production through epoxidation reaction. Mater. Sci. Energy Technol. 2021, 4, 119–127. [Google Scholar] [CrossRef]
- Campanella, A.; Rustoy, E.; Baldessari, A.; Baltanás, M.A. Lubricants from chemically modified vegetable oils. Bioresour. Technol. 2010, 101, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Sriram, G.; Subadhra, L. Synthesis, chemical modification and tribological evaluation of plant oil as bio-degradable low temperature lubricant. Procedia Eng. 2012, 38, 1508–1517. [Google Scholar] [CrossRef]
- Campos Flexa Ribeiro Filho, P.R.; Rocha do Nascimento, M.; Otaviano da Silva, S.S.; Tavares de Luna, F.M.; Rodríguez-Castellón, E.; Loureiro Cavalcante, C. Synthesis and Frictional Characteristics of Bio-Based Lubricants Obtained from Fatty Acids of Castor Oil. Lubricants 2023, 11, 57. [Google Scholar] [CrossRef]
- Durango-Giraldo, G.; Zapata-Hernandez, C.; Santa, J.F.; Buitrago-Sierra, R. Palm oil as a biolubricant: Literature review of processing parameters and tribological performance. J. Ind. Eng. Chem. 2022, 107, 31–44. [Google Scholar] [CrossRef]
- Parente, E.J.; Marques, J.P.C.; Rios, I.C.; Cecilia, J.A.; Rodríguez-Castellón, E.; Luna, F.M.T.; Cavalcante, C.L. Production of biolubricants from soybean oil: Studies for an integrated process with the current biodiesel industry. Chem. Eng. Res. Des. 2021, 165, 456–466. [Google Scholar] [CrossRef]
- Antunes, Q.G.; Santos, A.L.A.; Siqueira, J.I.P.; Lima, R.G.S.; da Silva, G.F.; dos Santos, J.P.L. Study of the stability and physicochemical behavior of a novel biolubricant based on castor oil used in cutting operations. Biomass Convers. Biorefinery 2025, 15, 20331–20345. [Google Scholar] [CrossRef]
- Hwang, H.S.; Erhan, S.Z. Synthetic lubricant basestocks from epoxidized soybean oil and Guerbet alcohols. Ind. Crops Prod. 2006, 23, 311–317. [Google Scholar] [CrossRef]
- Castro, W.; Perez, J.M.; Erhan, S.Z.; Caputo, F. A study of the oxidation and wear properties of vegetable oils: Soybean oil without additives. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 47–52. [Google Scholar] [CrossRef]
- Madankar, C.S.; Dalai, A.K.; Naik, S.N. Green synthesis of biolubricant base stock from canola oil. Ind. Crops Prod. 2013, 44, 139–144. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, J.; Li, C.; Xu, H.; Sun, M.; Xu, L.; Yu, L. Synthesis and Lubricating Properties of Bio-Based Lubricants from Palm Oil. ChemPlusChem 2025, 90, 202500013. [Google Scholar] [CrossRef]
- Stradolini, P.; Gryczak, M.; Petzhold, C.L. Polyols from castor oil (Ricinus communis) and epoxidized soybean oil (Glycine max) for application as a lubricant base. JAOCS J. Am. Oil Chem. Soc. 2024, 101, 321–334. [Google Scholar] [CrossRef]
- Ramos Moreira, D.; Worman, M.; Nery Ferreira, E.; Roberto Campos Flexa Ribeiro Filho, P.; Morais Ribeiro da Silva, L.; Bezerra Mota Gomes Arruda, T.; Eduardo Arruda Rodrigues, F.; Murilo Tavares de Luna, F.; Liberato Petzhold, C.; Maier, M.E.; et al. Development of polyols analogous to neopentyl glycol and trimethylolpropane for the production of oleic acid-based biolubricants. Fuel 2025, 381, 133156. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Perez, J.M. Oxidation and low temperature stability of vegetable oil-based lubricants. Ind. Crops Prod. 2006, 24, 292–299. [Google Scholar] [CrossRef]
- Abdel-Hameed, H.S.; El-Saeed, S.M.; Ahmed, N.S.; Nassar, A.M.; El-Kafrawy, A.F.; Hashem, A.I. Chemical transformation of Jojoba oil and Soybean oil and study of their uses as bio-lubricants. Ind. Crops Prod. 2022, 187, 115256. [Google Scholar] [CrossRef]
- Rios, L.; Echeverri, D.; Cardeño, F. Hydroxylation of vegetable oils using acidic resins as catalysts. Ind. Crops Prod. 2013, 43, 183–187. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants-A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Ribeiro, D.C.M.; Serra, C.; Coelho, J.; Ramalho, A.L. A biolubricant based on epoxidized soybean oil modified with aminoalcohols for enhanced tribological performance. Fuel 2026, 403, 136092. [Google Scholar] [CrossRef]
- Biswas, A.; Adhvaryu, A.; Gordon, S.H.; Erhan, S.Z.; Willett, J.L. Synthesis of diethylamine-functionalized soybean oil. J. Agric. Food Chem. 2005, 53, 9485–9490. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Liu, Z.; Adhvaryu, A. Lubricant base stock potential of chemically modified vegetable oils. J. Agric. Food Chem. 2008, 56, 8919–8925. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Perez, J.M. Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 2004, 257, 359–367. [Google Scholar] [CrossRef]
- Murru, C.; Badía-Laíño, R.; Díaz-García, M.E. Oxidative Stability of Vegetal Oil-Based Lubricants. ACS Sustain. Chem. Eng. 2021, 9, 1459–1476. [Google Scholar] [CrossRef]
- Cañellas, G.; Emeric, A.; Combarros, M.; Navarro, A.; Beltran, L.; Vilaseca, M.; Vives, J. Tribological Performance of Esters, Friction Modifier and Antiwear Additives for Electric Vehicle Applications. Lubricants 2023, 11, 109. [Google Scholar] [CrossRef]
- Boyde, S. Hydrolytic stability of synthetic ester lubricants. J. Synth. Lubr. 2000, 16, 297–312. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, J.; Ni, H.; Wang, X.; Zhu, Y.; Chen, L. Study on the coupling relationship of low temperature fluidity and oxidation stability of biodiesel. Appl. Sci. 2020, 10, 1757. [Google Scholar] [CrossRef]
- Hu, C.; Ai, J.; Ma, L.; Wen, P.; Fan, M.; Zhou, F.; Liu, W. Ester Oils Prepared from Fully Renewable Resources and Their Lubricant Base Oil Properties. ACS Omega 2021, 6, 16343–16355. [Google Scholar] [CrossRef]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. Coatings 2019, 133, 131–138. [Google Scholar] [CrossRef]
- Vermiglio, A.L.; Alarcon, R.T.; Cavalheiro, É.T.G.; Bannach, G.; Farmer, T.J.; North, M. Highly crosslinked polyesters prepared by ring-opening copolymerization of epoxidized baru nut and macaw palm oils with cyclic anhydrides. RSC Sustain. 2023, 1, 987–993. [Google Scholar] [CrossRef]
- Barros, A.; Nepomuceno, N.; Nicácio, P.; Souza, M.; Silva, I.; Luna, C.; Fook, M.; Araújo, E.; Wellen, R. Enhancement of Biobased Epoxy Through the Curing and Thermal Stability Control with Carboxylic Acids. J. Polym. Environ. 2024, 32, 2431–2447. [Google Scholar] [CrossRef]
- La Scala, J.; Wool, R.P. Property analysis of triglyceride-based thermosets. Polymer 2005, 46, 61–69. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deléglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Perez-Sena, W.Y.; Ciccarelli, F.; Eränen, K.; Di Serio, M.; Russo, V.; Salmi, T. A pathway to cyclic carbonates: Cycloaddition of carbon dioxide to epoxidized methyl oleate on grafted heterogeneous catalysts. J. CO2 Util. 2025, 91, 103005. [Google Scholar] [CrossRef]
- Sammaiah, A.; Padmaja, K.V.; Prasad, R.B.N. Tribology and oxidation studies of fatty acid sulfide derivatives synthesized via thiol-ene “Click” additions. Eur. J. Lipid Sci. Technol. 2016, 118, 495–502. [Google Scholar] [CrossRef]
- Illy, N.; Fache, M.; Ménard, R.; Negrell, C.; Caillol, S.; David, G. Phosphorylation of bio-based compounds: The state of the art. Polym. Chem. 2015, 6, 6257–6291. [Google Scholar] [CrossRef]
- Avilés, M.D.; Mostaza, P.; Bermúdez, M.D.; Carrión-Vilches, F.J. Epoxidized vegetable lubricant enhanced by ionic liquid. Wear 2025, 564–565, 205740. [Google Scholar] [CrossRef]
- Lai, C.M.; How, H.G.; Jason, Y.J.J.; Teoh, Y.H.; Yaqoob, H.; Zhang, S.; Rafe Hatshan, M.; Sher, F. Tribological characterisation of graphene hybrid nanolubricants in biofuel engines. Fuel 2024, 357, 129654. [Google Scholar] [CrossRef]
- Kulkarni, T.; Toksha, B.; Chatterjee, A.; Naik, J.; Autee, A. Anti-wear (AW) and extreme-pressure (EP) behavior of jojoba oil dispersed with green additive CaCO3 nanoparticles. J. Eng. Appl. Sci. 2023, 70, 29. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, C.; Zhou, Z.; Nie, X.; Chen, Y.; Cao, H.; Liu, B.; Zhang, N.; Said, Z.; et al. Extreme pressure and antiwear additives for lubricant: Academic insights and perspectives. Int. J. Adv. Manuf. Technol. 2022, 120, 1–27. [Google Scholar] [CrossRef]
- Azman, N.F.; Samion, S. Dispersion Stability and Lubrication Mechanism of Nanolubricants: A Review. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 393–414. [Google Scholar] [CrossRef]
- Waheed, S.; Ahmed, A.; Abid, M.; Mufti, R.A.; Ferreira, F.; Bashir, M.N.; Shah, A.U.R.; Jafry, A.T.; Zulkifli, N.W.; Fattah, I.M.R. Ionic liquids as lubricants: An overview of recent developments. J. Mol. Struct. 2024, 1301, 137307. [Google Scholar] [CrossRef]
- Kurre, S.K.; Yadav, J. A review on bio-based feedstock, synthesis, and chemical modification to enhance tribological properties of biolubricants. Ind. Crops Prod. 2023, 193, 116122. [Google Scholar] [CrossRef]
- He, X.; Lu, J.; Desanker, M.; Invergo, A.M.; Lohr, T.L.; Ren, N.; Lockwood, F.E.; Marks, T.J.; Chung, Y.W.; Wang, Q.J. Boundary Lubrication Mechanisms for High-Performance Friction Modifiers. ACS Appl. Mater. Interfaces 2018, 10, 40203–40211. [Google Scholar] [CrossRef]
- Gusain, R.; Khan, A.; Khatri, O.P. Fatty acid-derived ionic liquids as renewable lubricant additives: Effect of chain length and unsaturation. J. Mol. Liq. 2020, 301, 112322. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Wang, J.; Yao, X.; Xiong, X.; Huang, H. Synergistic Tribological Performance of Phosphorus- and Sulfur-Based Extreme Pressure and Anti-Wear Additives. Lubricants 2025, 13, 55. [Google Scholar] [CrossRef]
- Luiz, J.F.; Spikes, H. Tribofilm Formation, Friction and Wear-Reducing Properties of Some Phosphorus-Containing Antiwear Additives. Tribol. Lett. 2020, 68, 1–24. [Google Scholar] [CrossRef]
- Najman, M.N.; Kasrai, M.; Bancroft, G.M.; Miller, A. Study of the chemistry of films generated from phosphate ester additives on 52100 steel using X-ray absorption spectroscopy. Tribol. Lett. 2002, 13, 209–218. [Google Scholar] [CrossRef]
- Turco, R.; Tesser, R.; Vitiello, R.; Russo, V.; Andini, S.; Di Serio, M. Synthesis of biolubricant basestocks from epoxidized soybean oil. Catalysts 2017, 7, 309. [Google Scholar] [CrossRef]
- Hu, W.; Xu, Y.; Zeng, X.; Li, J. Alkyl-Ethylene Amines as Effective Organic Friction Modifiers for the Boundary Lubrication Regime. Langmuir 2020, 36. [Google Scholar] [CrossRef]
- Lasch, G.; Stradolini, P.; Gehlen, G.S.; Barros, L.Y.; Poletto, J.C.; Ramalho, A.; Fernandes, C.M.C.G.; Romio, P.C.; Petzhold, C.L.; Ferreira, N.F.; et al. Comparative tribological investigation of castor oil and its transesterified and aminolyzed derivatives. Tribol. Int. 2024, 196. [Google Scholar] [CrossRef]
- de Andrade Souza, L.; Moreira, D.R.; Ricardo, N.M.P.S.; Maier, M.E.; Filho, P.R.C.F.R.; Luna, F.M.T.; Petzhold, C.L. Esters from castor oil functionalized with aromatic amines as a potential lubricant. JAOCS J. Am. Oil Chem. Soc. 2023, 100, 175–184. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Valencia, C.; Franco, J.M.; Gallegos, C. Viscosity modification of high-oleic sunflower oil with polymeric additives for the design of new biolubricant formulations. Environ. Sci. Technol. 2009, 43, 2060–2065. [Google Scholar] [CrossRef]
- Zulkifli, W.M.; Azman, S.S.N.; Kalam, M.A.; Masjuki, H.H.; Yunus, R.; Gulzar, M. Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol. Int. 2016, 93, 555–562. [Google Scholar] [CrossRef]
- Loza, K.; Epple, M.; Maskos, M. Stability of nanoparticle dispersions and particle agglomeration. In Biological Responses to Nanoscale Particles; NanoScience and Technology; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 85–100. [Google Scholar] [CrossRef]
- Guegan, J.; Southby, M.; Spikes, H. Friction Modifier Additives, Synergies and Antagonisms. Tribol. Lett. 2019, 67, 83. [Google Scholar] [CrossRef]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Melo Neta, M.M.F.; Lima, G.R.; Tavares, P.D.O.; Figueredo, I.D.M.; Rocha, W.D.S.; Ribeiro Filho, P.R.; Cavalcante, C.L., Jr.; Luna, F.M.T. Thermo-Oxidative Stability and Tribological Properties of Biolubricants Obtained from Castor Oil Fatty Acids and Isoamyl Alcohol. Lubricants 2023, 11, 490. [Google Scholar] [CrossRef]
- Acevedo-Serrano, M.; Nogales-Delgado, S.; González, J.F.G. Catalytic Biolubricant Production from Canola Oil Through Double Transesterification with Methanol and Neopentyl Glycol. Catalysts 2024, 14, 748. [Google Scholar] [CrossRef]
- Saka, A.; Abor, T.K.; Okafor, A.C.; Okoronkwo, M.U. Thermo-rheological and tribological properties of low- and high-oleic vegetable oils as sustainable bio-based lubricants. RSC Sustain. 2025, 3, 1461–1476. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhang, Y.; Li, B.; Yang, M.; Zhang, X.; Guo, S.; Liu, G.; Zhai, M. Comparative evaluation of the lubricating properties of vegetable-oil-based nanofluids between frictional test and grinding experiment. J. Manuf. Process. 2017, 26, 94–104. [Google Scholar] [CrossRef]
- Filho, P.R.C.F.R.; Santos, L.D.S.E. The influence of unsaturation modifications on the tribological characteristics of bio-based lubricants obtained from vegetable oils: A review. J. Brazilian Soc. Mech. Sci. Eng. 2025, 47, 1–14. [Google Scholar] [CrossRef]
- de Melo Neta, M.M.F.; de Oliveira Tavares, P.; Filho, P.R.C.F.R.; Cavalcante, C.L.; de Luna, F.M.T. Lubricants basestock oil obtained from residual fatty acids: Evaluation of tribological properties and thermo-oxidative stability. J. Brazilian Soc. Mech. Sci. Eng. 2025, 47, 1–12. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Benyajati, C.N.; Sritham, W.; Soparat, J.; Limprayoon, N.; Seetapan, N.; Fuongfuchat, A. Roles of viscosity, applied load and surface wettability on the lubrication behaviour of model liquid/semi-solid foods: Measurements with a bespoke tribo-cell fixture and rotational rheometer. Curr. Res. Food Sci. 2022, 5, 57–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Biboulet, N.; Venner, C.H.; Lubrecht, A.A. Prediction of the Stribeck curve under full-film Elastohydrodynamic Lubrication. Tribol. Int. 2020, 149, 105569. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Reddyhoff, T.; Gallegos, C.; Spikes, H.A. Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol. Int. 2014, 69, 110–117. [Google Scholar] [CrossRef]
- Hsu, S.M.; Gates, R.S. Boundary lubricating films: Formation and lubrication mechanism. Tribol. Int. 2005, 38, 305–312. [Google Scholar] [CrossRef]
- Havet, L.; Blouet, J.; Valloire, F.R.; Brasseur, E.; Slomka, D. Tribological characteristics of some environmentally friendly lubricants. Wear 2001, 248, 140–146. [Google Scholar] [CrossRef]
- Spikes, H. Friction Modifier Additives. Tribol. Lett. 2015, 60, 1–26. [Google Scholar] [CrossRef]
- Spikes, H. Mechanisms of ZDDP—An Update. Tribol. Lett. 2025, 73. [Google Scholar] [CrossRef]
- Nalam, P.C.; Pham, A.; Castillo, R.V.; Espinosa-Marzal, R.M. Adsorption Behavior and Nanotribology of Amine-Based Friction Modifiers on Steel Surfaces. J. Phys. Chem. C 2019, 123, 13672–13680. [Google Scholar] [CrossRef]
- Ali, Z.A.; Takhakh, A.M.; Al-Waily, M. A review of use of nanoparticle additives in lubricants to improve its tribological properties. Mater. Today Proc. 2022, 52, 1442–1450. [Google Scholar] [CrossRef]
- Salimon, J.; Abdullah, B.M.; Yusop, R.M.; Salih, N. Synthesis, reactivity and application studies for different biolubricants. Chem. Cent. J. 2014, 8, 1–11. [Google Scholar] [CrossRef]
- Raimondi, A.; Girotti, G.; Blengini, G.A.; Fino, D. LCA of petroleum-based lubricants: State of art and inclusion of additives. Int. J. Life Cycle Assess. 2012, 17, 987–996. [Google Scholar] [CrossRef]
- Malik, M.A.I.; Kalam, M.A.; Mujtaba, M.A.; Almomani, F. A review of recent advances in the synthesis of environmentally friendly, sustainable, and nontoxic bio-lubricants: Recommendations for the future implementations. Environ. Technol. Innov. 2023, 32, 103366. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Biresaw, G.; Sharma, B.K.; Erhan, S.Z. Friction behavior of some seed oils: Biobased lubricant applications. Ind. Eng. Chem. Res. 2006, 45, 3735–3740. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. OECD Guideline 301-Ready Biodegrability; Organisation for Economic Co-operation and Development: Paris, France, 1992. [Google Scholar]
- ISO 9439; Water Quality-Evaluation of Ultimate Aerobic Biodegradability of Organic Compounds in Aqueous Medium-Method by Analysis of Released Carbon Dioxide. International Organization for Standardization: Geneva, Switerland, 1999.
- ASTM D5864; Standard Test Method for Determining Aerobic Aquatic Biodegradation of Lubricants. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Uppar, R.; Dinesha, P.; Kumar, S. A Critical Review on Vegetable Oil-Based Bio-Lubricants: Preparation, Characterization, and Challenges; Springer: Dordrecht, Netherlands, 2023; Volume 25. [Google Scholar]
- Vag, C.; Marby, A.; Kopp, M.; Furberg, L.; Norrby, T. A comparative life cycle assessment of the manufacture of base fluids for lubricants. J. Synth. Lubr. 2002, 19, 39–57. [Google Scholar] [CrossRef]
- Patel, J.R.; Chauhan, K.V.; Rawal, S.; Patel, N.P.; Subhedar, D.G. Advances and Challenges in Bio-Based Lubricants for Sustainable Tribological Applications: A Comprehensive Review of Trends, Additives, and Performance Evaluation. Lubricants 2025, 13, 440. Available online: https://www.preprints.org/manuscript/202509.0365/v1 (accessed on 20 October 2025).
- Moussa, H. Life Cycle Assessment of a Hybrid Poly Butylene Succinate Composite. 2014. Available online: https://core.ac.uk/reader/144147184 (accessed on 20 October 2025).
- Thanahiranya, P.; Sadhukhan, J.; Charoensuppanimit, P.; Vacharanukrauh, T.; Chuetor, S.; Chanthanumataporn, M.; Assabumrungrat, S. Design and assessment of biolubricant production processes utilizing various feedstocks in palm oil-based biodiesel industry. J. Clean. Prod. 2025, 518, 145949. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Willing, A. Lubricants based on renewable resources—An environmentally compatible alternative to mineral oil products. Chemosphere 2001, 43, 89–98. [Google Scholar] [CrossRef]
- Deleanu, L.; Georgescu, C.; Cristea, G.C.; Ionescu, T.F.; Dionis, G.; Ojoc, G.G.; Dima, D.; Paduraru, I. Standardization in Tribology. Tribol. Ind. 2024, 46, 398–417. [Google Scholar] [CrossRef]
- Liu, S.; Saha, B.; Vlachos, D.G. Catalytic production of renewable lubricant base oils from bio-based 2-alkylfurans and enals. Green Chem. 2019, 21, 3606–3614. [Google Scholar] [CrossRef]
- Xue, H.; Wang, C.; Liang, F.; He, J.; He, Q.; Cai, M.; Tian, Q.; Chang, G.; Huang, Q.; Siddiq, M.; et al. Dynamic Covalent Polymer-Nanoparticle Networks as High-Performance Green Lubricants: Synergetic Effect in Load-Bearing Capacity. Macromolecules 2024, 57, 8383–8391. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).