Abstract
Preventing, diagnosing, and controlling high blood pressure is a global health priority. The self-measurement of blood pressure is therefore fundamental and should be done with devices validated by recognized protocols, although most are not. The most widely used and current protocols are the 2010 European Society of Hypertension (ESH) revision and the 2018 Association for the Advancement of Medical Instrumentation (AAMI)/ ESH/ the International Organization for Standardization (ISO) universal standard, respectively. The aim of this study was to find out which blood pressure measuring devices have been adequately validated by the above protocols. A narrative review of blood pressure device validations was conducted by searching the PubMed database. From 52 records identified, 37 studies were included. Most validations follow the 2010 revision and only six follow the 2018 protocol, which is more demanding. Almost all validated sphygmomanometers are automated oscillometric sphygmomanometers in the general population. Wrist devices and devices combining new technologies are also validated, as well as in specific populations, such as the obese, pregnant women, or children. There is sufficient evidence to confirm that the universal AAMI/ ESH/ISO standard is considered the protocol of the century. However, it is necessary to increase the number of validations following it and, above all, validations of the new technologies that are invading the current market.
1. Introduction
After obesity, arterial hypertension (HBP) is the second most common cause of cardiovascular disease (CVD) [1]. If we add that CVD is the leading cause of morbidity and mortality [2,3], we can conclude that AHT carries a high risk of CV morbidity and mortality [4,5,6,7].
Therefore, one of the main objectives of health systems is to identify people with AHT and ensure that they have good control of their blood pressure (BP), since the higher the blood pressure levels, the greater the risk and morbidity and mortality from CV events [8,9,10]. Thus, preventing, diagnosing, treating, and controlling hypertension is a global health priority [4,5,7,9,10,11,12,13,14].
The detection and diagnosis of hypertension must be done by measuring BP. BP measurement is considered as one of the most frequently performed procedures at the clinical level, in primary or specialized care [15].
The first time BP was recorded in a consultation was in 1896, and since the end of the last century, out-of-office measurement has been established in two versions: ambulatory BP monitoring (ABPM) and home blood pressure monitoring (HBPM). The major advantage of these two methods is that by allowing BP to be measured on multiple occasions outside the healthcare environment, it provides a more reliable BP reading and has a higher prognostic value than doing it in the office [16,17,18].
If we focus on HBPM, it has become a very beneficial and increasingly used simple procedure, for highly consistent reasons [19,20,21]. The subject’s self-measurements are very useful for the monitoring of HBP, since it is estimated that about 80–90% of the doubts in the diagnosis and control of this pathology can be solved with this procedure, but, logically, these benefits can be obtained if the HBPM is done properly and with validated devices [15,17,22,23].
With regard to blood pressure validations, in the last 30 years, there have been several protocols for this purpose, such as the British Society of Hypertension (BSH) [24] protocol, the Association for the Advancement of Medical Instrumentation (AAMI) protocol, [25] and the international protocol published by the European Society of Hypertension (ESH) [26] and its review [27]. The latter [26,27] were the most current and widely used, but given the need to increase the validity of these devices, in 2018, experts from the AAMI, ESH, and the International Organization for Standardization (ISO) agreed to develop a universal standard for their validation. Today, it is considered as the single universal standard and replaces all other previous standards/protocols [25,28,29].
Given the relevance of HBPM and the need for increased monitoring of BP devices, according to the literature, the results of the present study will mainly serve to find out which devices can be validly used by subjects to measure and monitor their blood pressure, according to the most used and/or current protocols [27,29].
2. Materials and Methods
2.1. Study Design
A narrative review was carried out following the applicable recommendations of the Scale for the Assessment of Narrative Review Articles (SANRA) [30]. This scale contains six items to assess the quality of narrative review articles. It can be found in the Supplementary Material. The aim of this review was to update the data of the devices available on the market that are valid for measuring blood pressure since there are no recent previous systematic reviews on the issue.
2.2. Search Strategy
The database search was carried out during November–January 2021. Pubmed was the database used for this process.
The advanced search strategy was as follows, combining the terms with the Boolean and grouping operators that follow: (European Society of Hypertension [Title]) OR AAMI/ESH/ISO[Title]) AND validation [Title].
As for the search filters, only one of the publication dates is applied: Last 5 years.
The search strategy has been filtered by title because, by regulation, all validations following the “the revised 2010 European Society of Hypertension international protocol” and “the 2018 AAMI/ESH/ISO universal standard” should be named in a standardized way and, consequently, the titles of these publications should also be named in the same way.
2.3. Selection Criteria
Inclusion criteria comprised blood pressure device validation studies that followed the 2010 European Society of Hypertension international protocol review and/or the 2018 AAMI/ESH/ISO universal standard, conducted within the last 5 years.
Exclusion criteria included studies with a publication date prior to 2017, and those that were not device validations or did not follow the revised 2010 European Society of Hypertension international protocol and/or the 2018 AAMI/ESH/ISO universal standard.
2.4. Data Extraction
The data obtained were divided according to the validation protocol used. The common methodology governing the validation conditions was recorded, such as sample size, blood pressure range, and other variables, in accordance with the protocol used.
Study characteristics were also recorded, such as citation year and place of validation, types of devices used, population characteristics and origin, and validation conclusions.
Additional information was provided where necessary.
2.5. Flow Diagram
From 52 records identified through the searching process, 15 were removed due to exclusion, and finally, 37 studies were included in narrative synthesis (Figure 1).
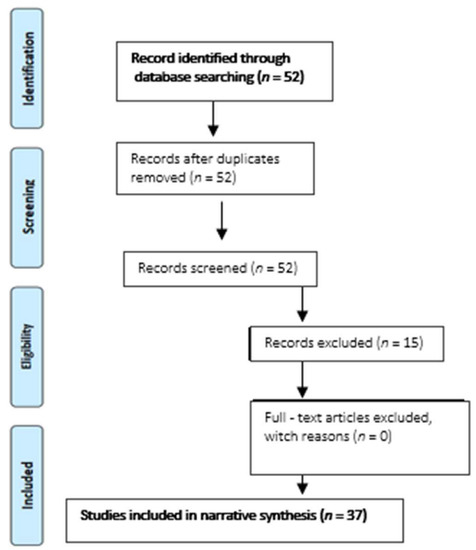
Figure 1.
Flow diagram of the narrative review in the Pubmed database.
15 studies were excluded for the following reasons:
- − 7 articles discussed the validation standard itself and/or revisions of the validation standard
- − 2 studies commented with the proper use of validation protocols
- − 4 publications were corrections of previously included articles.
- − 1 paper was about validation but only followed the ESH 2002 protocol.
- − 1 study did not analyze validity but reproducibility.
3. Results
3.1. Characteristics of the Validation Protocols
The studies of the last five years on blood pressure measuring devices mainly apply two validation protocols for BP devices, which are the review of the international ESH protocol [27] and the international universal standard AAMI/ESH/ISO 81160-2:2018 [29], the latter being the most recent.
Thus, 31 articles following the ESH review [27] and six based on the AAMI/ESH/ISO universal standard [29] are found in the literature. The methodology of these studies has common characteristics, depending on the validation protocol they follow (Table 1). The characteristics of the sample, the sample recruitment criteria, the blood pressure measurement method, or the analyses required to validate the device in question will differ.

Table 1.
Characteristics of the main validation protocols.
Both protocols use a similar sequential BP measurement procedure by alternating two devices (reference and test). Validated devices are already used as a reference, but the 2010 ESH prefers these to be two mercury sphygmomanometers with stethoscopes and the AAMI/ESH/ISO standard indicates that the reference can be as stated above, but can also be non-mercury sphygmomanometers, aneroid manometers, or other types. The measurement conditions are similar, where the subject being measured must remain relaxed and calm in a certain position. Also, the human validation equipment is almost identical, and it has a similar tolerable BP measurement error. In both protocols, Bland–Altman charts are required.
On the other hand, they vary substantially in the range of BP recruitment, sample characteristics and size, and validation criteria.
For a device to pass the ESH [27], it must pass two phases. To pass the first phase, two conditions must be met: a minimum of 65, 81, and 93 comparisons falling within 5, 10, and 15 mm/Hg, respectively; a minimum of two of the following three requirements: 73, 87, and 96 differences must be within the category of 5, 10, and 15 mm/Hg, respectively. In the second phase, a minimum of 24 subjects are required to have two of their three differences in the 5 mm/Hg category, and a maximum of three individuals with the three differences greater than 5 mm/Hg is allowed.
To pass the universal standard [29], the device must also pass two phases. In the first phase, the mean BP difference must be 5 mm/hg or less, and its standard deviations 8 mm/hg or less for SBP and DBP. In the second phase, the standard deviation of 85 averaged BP differences (test minus reference BP per subject) must be within a threshold defined by the mean test-reference BP difference listed for SBP and DBP.
In terms of specific populations, the standards proposed by the three societies as a whole are more explicit.
Thus, although the ESH [27] protocol improved on previous protocols [24,26,27] by eliminating some validation steps and reducing the sample size, it is simpler to apply than the most recent protocol. The international universal validation protocol (AAMI/ESH/ISO) of 2018 [29] is currently the most complete but more complex than the previous one.
3.2. Characteristics of the Studies
All the studies collected have the same quality in terms of study type, as they all follow the same standards and have the same design: prospective cross-sectional observational studies [31].
Having analyzed the common and different aspects in terms of the validation conditions governing the respective protocols (Table 1), we now turn to the rest of the characteristics, which are summarized in Table 2.

Table 2.
Comparative table of validation studies.
4. Discussion
We found positive results in most studies, exceeding the protocol in question and validating the test devices for their recommended use, as well as in the population analyzed, with the exception of two studies with four test devices, Omron RS6 (hem-6221-E), Microlife WatchBP O3 [33], Yuwell YE680a, and Cofoe KF-65B [67].
The validated devices are different depending on the measurement method, the area of the body where they are applied, their characteristics, or their function: self-measurement, BP monitoring, or professional use, among others. Only one study has validated a device that uses the auscultatory method to measure BP [66]. Typically, oscillometric upper arm sphygmomanometers are validated as the conventional devices for measuring BP and heart rate (HR). These consist of an automatic monitor with an LCD display and a cuff connected by rubber tubes, with some exceptions, which do not have tubes connecting the pump to the cuff; rather, the device itself is embedded in the cuff [42,47,53,55]. They are usually battery operated and some have various cuff sizes.
The cuff used by most authors in their test device is usually the standard or medium size (22–32 cm), except for some who have tested more than one cuff, such as small (18–22 cm) [65], or large (32–42 cm) [65,66].
There are authors who opt for other wrist devices of the same type [33,35,38,44,50,61] and therefore do not have rubber tubes. These are more applicable to obese subjects because they have a larger arm circumference, giving erroneous measurement results if a standard upper cuff is used [33].
Of the above, many are recommended devices for HBPM but some are designed for ABPM [32,36,46,48,53], while there are few validated sphygmomanometers that are used only in a professional manner [58,59,65,66]. According to the ESH protocol, the validation of ABPM devices is performed only in static conditions and does not require testing in ambulatory conditions.
Among those used for HBPM, other validated devices differ more from the above. The Inbody BPBIO320 [49] and the Inbody BPBIO750 [62] are for public use as well as being automatic right upper arm oscillometric devices, but in this case, it is a kiosk type with a fixed hole for the user to insert their arm to measure their BP. Other devices have also been validated that measure BP oscillometrically and can be connected wirelessly to electronic devices allowing for better visual recording and monitoring [46,51,63]. Other units have similar behavior but are operated directly from an app connected to the oscillometric cuff via Bluetooth [42,47,55]. To this effect, one study tests an app that measures BP without a cuff through fingerprints [68] but it is difficult to follow the validation protocol with it.
Therefore, of all the validations, only one device without a cuff to measure BP is attempted to be validated [68], and the results are unsuccessful.
It should be noted that the use of new technologies for health promotion and disease prevention is booming and, in particular, BP control using these has gained importance, adding additional advantages to HBPM, which results in a more active subject with better adherence and follow-up [68,69,70,71]. Despite this and its widespread use and downloading by society, only six apps are being validated [42,46,47,51,55,63,68], considering that one of them, Qardioarm, has been validated on several occasions, by different teams and in different populations [42,47,55]. This is not a unique case, since it draws our attention to the fact that the Omron RS6 is tested on obese subjects and is found to be invalid by some authors [33] and valid by others [61]. This may be due to the fact that both studies follow the ESH review and do not have specific criteria for vulnerable populations [27].
As for the parameters assessed in the populations, all studies include recruitment SBP and DBP, age (in years), and circumference of the upper arm [32,34,36,37,39,40,41,42,43,45,46,47,48,49,51,52,53,54,55,56,57,58,59,60,62,63,64,65,66,67], wrist [38,40], or both [25,33,44,61] (in mm), depending on the device to be tested. Only one study does not measure any circumference of its participants [68]. BMI is also assessed by some authors [33,34,38,45,46,47,51,55,57,61], although only some of these [47,51,55,61] reflect the weight and height of their participants.
These measured characteristics are generally similar as they all meet the recruitment criteria of the protocols [27,29], except for special populations.
Most of these studies are conducted in the general population, which are healthy adults over 25 years of age for one protocol [27], and 12 years of age for another [29]. In all of them, the number of women recruited tends to be higher than that of men.
Some studies look at other specific populations prone to HBP, such as the obese [33,47,61], children [36,65], diabetics [42], pregnant women [48], or subjects with chronic kidney disease [55]. Furthermore, the difference between the recruited and tested subjects is often greater in these specific populations, as more failures encountered tend to emerge. Most of the reasons for exclusion are full BP ranges. The above are necessary because the results obtained in the general population cannot be extrapolated to specific populations, as they do not follow the same recruitment conditions, requiring a larger sample in most cases. Hence, the universal standard already applies explicit criteria for such populations [29] where even stricter BP control is necessary.
As for the validation team, it also follows the rules of the protocols [27,29], reflected in Table 1, so it usually corresponds to two observers and a supervisor, except for others that have additional explanations, such as teams formed by three licensed physicians [32,48,58], three nurses [47,55], four physicians [49], or three medical technologists [50,59]. All teams were trained in BP measurement.
Although the universal standard is considered the protocol of the century [29], only seven articles have been seen to follow it [56,58,59,62,65,66,68] compared to the large number of studies that follow the ESH [27], almost all of them with positive results [72,73], because although they share conditions, it is less strict (Table 1).
For example, it was recommended to the journals that, from November 2019 onward, they no longer accept articles following the ESH to validate the devices [74] and only accept the most recent protocol [29], so it is considered necessary to increase the monitoring of this type of device, especially their apps, following the same protocol. In addition, a mercury-free sphygmomanometer can be used as the gold standard in this protocol and is used by the ESH.
To conclude this section, it is worth mentioning that one of the main limitations of our study is that we have only used one database, Pubmed. We were guided by a study that analysed optimal database combinations for literature searches and found that “Sixteen percent of the included references (291 articles) were only found in a single database” [75].
5. Conclusions
Since 2017, 37 devices have been attempted to be validated to measure BP with reputable protocols, with most obtaining positive results. It is necessary to increase the number of studies according to what is considered the protocol of the century, the universal AAMI/ESH/ISO standard (ISO 81160-2:2018), and especially using new technologies, as few validations have been found on them.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm13010009/s1. Scale S1: Scale for the Assessment of Narrative Review Articles (SANRA).
Author Contributions
Conceptualization, V.M.-P. and M.R.-J.; formal analysis, V.M.-P. and M.I.U.-G.; data curation, V.M.-P., S.G.-C., E.N.-F. and M.R.-J.; writing—original draft preparation, V.M.-P. and M.R.-J.; writing—review and editing, V.M.-P., S.G.-C., M.R.-J., E.N.-F. and M.I.U.-G.; supervision, V.M.-P., S.G.-C., M.R.-J., E.N.-F. and M.I.U.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bolaji, A.A. Simulation of a Real-time Mobile Health Monitoring System Model for Hypertensive Patient in Rural Nigeria. Afr. J. Comput. Ict. 2014, 7, 95–100. [Google Scholar]
- Wang, H.; Dwyer-Lindgren, L.; Lofgren, K.T.; Rajaratnam, J.K.; Marcus, J.R.; Levin-Rector, A.; Levitz, C.E.; Lopez, A.D.; Murray, C.J.L. Age-specific and sex-specific mortality in 187 countries, 1970-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2071–2094. [Google Scholar] [CrossRef] [PubMed]
- Raghu, A.; Praveen, D.; Peiris, D.; Tarassenko, L.; Clifford, G. Engineering a mobile health tool for resource-poor settings to assess and manage cardiovascular disease risk: SMARThealth study. BMC Med. Inf. Decis. 2015, 15, 36. Available online: https://pubmed.ncbi.nlm.nih.gov/25924825/ (accessed on 12 December 2021). [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [PubMed]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013, 310, 959–968. Available online: https://pubmed.ncbi.nlm.nih.gov/24002282/ (accessed on 12 December 2021). [CrossRef]
- Levin, M.G.; Klarin, D.; Walker, V.M.; Gill, D.; Lynch, J.; Hellwege, J.N.; Keaton, J.M.; Lee, K.M.; Assimes, T.L.; Natarajan, P.; et al. Association between genetic variation in blood pressure and increased lifetime risk of peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2027–2034. Available online: https://www.ahajournals.org/doi/abs/10.1161/ATVBAHA.120.315482 (accessed on 12 December 2021). [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffo, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2021, 29, 5–115. Available online: https://pubmed.ncbi.nlm.nih.gov/34558602/ (accessed on 12 December 2021). [CrossRef]
- BP lowering and cardiovascular risk reduction. Drug Ther. Bull. 2021, 60, 3. Available online: https://pubmed.ncbi.nlm.nih.gov/34893501/ (accessed on 12 December 2021).
- Siu, A.L.; Bibbins-Domingo, K.; Grossman, D.; Baumann, L.C.; Davidson, K.W.; Ebell, M. US Preventive Services Task Force. Screening for high blood pressure in adults: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2015, 163, 778–786. [Google Scholar] [CrossRef]
- Casey, D.E., Jr.; Daniel, D.M.; Bhatt, J.; Carey, R.M.; Commodore-Mensah, Y.; Holmes, A.; Smith, A.P.; Wozniak, G.; Wright, J.T., Jr. Controlling High Blood Pressure: An Evidence-Based Blueprint for Change. Am. J. Med. Qual. 2021, 37, 22–31. [Google Scholar] [CrossRef]
- Khatib, R.; Schwalm, J.D.; Yusuf, S.; Haynes, R.B.; McKee, M.; Khan, M.; Nieuwlaat, R. Patient and healthcare provider barriers to hypertension awareness, treatment and follow up: A systematic review and meta-analysis of qualitative and quantitative studies. PLoS ONE 2014, 9, e84238. Available online: https://pubmed.ncbi.nlm.nih.gov/24454721/ (accessed on 12 December 2021). [CrossRef]
- Rodgers, A.; Chow, C.K.; Jackson, R.T.; Patel, A.; Usherwood, T. Guideline for the diagnosis and management of hypertension in adults—2016. Med. J. Aust. 2017, 206, 141. Available online: https://pubmed.ncbi.nlm.nih.gov/28208048/ (accessed on 13 December 2021). [CrossRef]
- Carey, R.M.; Wright, J.T.; Taler, S.J.; Whelton, P.K. Guideline-Driven Management of Hypertension: An Evidence-Based Update. Circ. Res. 2021, 128, 827–846. Available online: https://pubmed.ncbi.nlm.nih.gov/33793326/ (accessed on 13 December 2021).
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. Available online: http://ahajournals.org (accessed on 13 December 2021). [CrossRef]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Misra, S.; Myers, M.G.; Ogedegbe, G.; Schwawartz, J.; Townsend, R.R.; et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension 2019, 73, e35–e66. Available online: https://www.ahajournals.org/doi/abs/10.1161/HYP.0000000000000087 (accessed on 13 December 2021). [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leuuw, P.; Dolan, E.; et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. Available online: https://pubmed.ncbi.nlm.nih.gov/24886823/ (accessed on 14 December 2021). [CrossRef]
- Parati, G.; Stergiou, G.S.; Bilo, G.; Kollias, A.; Pengo, M.; Ochoa, J.E.; Agarwal, R.; Asayama, K.; Asmar, R.; Burnier, M.; et al. Home blood pressure monitoring: Methodology, clinical relevance and practical application: A 2021 position paper by the Working Group on Blood Pressure Monitoring and Cardiovascular Variability of the European Society of Hypertension. J. Hypertens. 2021, 39, 1742–1767. Available online: https://pubmed.ncbi.nlm.nih.gov/34269334/ (accessed on 14 December 2021). [CrossRef]
- O’Brien, E.; Parati, G.; Stergiou, G.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J. Hypertens. 2013, 31, 1731–1768. Available online: https://pubmed.ncbi.nlm.nih.gov/24029863/ (accessed on 14 December 2021). [CrossRef]
- Zhang, S.; Zhou, X.; Chen, Y.; Wang, L.; Zhu, B.; Jiang, Y.; Bu, P.; Liu, W.; Li, D.; Li, Y.; et al. Changes in Home Blood Pressure Monitored among Elderly Patients with Hypertension during the COVID-19 Outbreak: A Longitudinal Study in China Leveraging a Smartphone-Based Application. Circ. Cardiovasc. Qual. Outcomes 2021, 14, 605–612. Available online: https://www.ahajournals.org/doi/abs/10.1161/CIRCOUTCOMES.120.007098 (accessed on 14 December 2021). [CrossRef]
- Feitosa, F.G.A.M.; Feitosa, A.D.M.; Paiva, A.M.G.; Mota-Gomes, M.A.; Barroso, W.S.; Miranda, R.D.; Barbosa, E.C.D.; Brandão, A.A.; Lima-Filho, J.L.; Sposito, A.C.; et al. Impact of the COVID-19 pandemic on blood pressure control: A nationwide home blood pressure monitoring study. Hypertens. Res. 2021, 45, 364–368. Available online: https://pubmed.ncbi.nlm.nih.gov/34857897/ (accessed on 14 December 2021). [CrossRef]
- Citoni, B.; Figliuzzi, I.; Presta, V.; Volpe, M.; Tocci, G. Home Blood Pressure and Telemedicine: A Modern Approach for Managing Hypertension During and After COVID-19 Pandemic. High Blood Press Cardiovasc. Prev. 2021, 29, 1–14. Available online: https://pubmed.ncbi.nlm.nih.gov/34855154/ (accessed on 14 December 2021). [CrossRef]
- Narita, K.; Hoshide, S.; Kario, K. Nighttime Home Blood Pressure Is Associated with the Cardiovascular Disease Events Risk in Treatment-Resistant Hypertension. Hypertension 2022, 79, e18–e20. Available online: https://www.ahajournals.org/doi/abs/10.1161/HYPERTENSIONAHA.121.18534 (accessed on 14 December 2021). [CrossRef] [PubMed]
- Bryant, K.B.; Green, M.B.; Shimbo, D.; Schwartz, J.E.; Kronish, I.M.; Zhang, Y.; Sheppard, J.P.; McManus, R.J.; Moran, A.E.; Bellows, B.K. Home Blood Pressure Monitoring for Hypertension Diagnosis by Current Recommendations: A Long Way to Go. Hypertension 2021, 79, e15–e17. Available online: https://pubmed.ncbi.nlm.nih.gov/34852639/ (accessed on 15 December 2021). [CrossRef] [PubMed]
- O’Brien, E.; Petrie, J.; Littler, W.; De Swiet, M.; Padfield, P.L.; O’Malley, K.; Jamieson, M.; Altman, D.; Bland, M.; Atkins, N. The British Hypertension Society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J. Hypertens. 1990, 8, 607–619. Available online: https://pubmed.ncbi.nlm.nih.gov/2168451/ (accessed on 15 December 2021). [CrossRef] [PubMed]
- Stergiou, G.S.; Alpert, B.; Mieke, S.; Asmar, R.; Atkins, N.; Eckert, S.; Frick, G.; Friedman, B.; Graßl, T.; Ichikawa, T.; et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J. Hypertens. 2018, 71, 368–374. Available online: https://pubmed.ncbi.nlm.nih.gov/29386350/ (accessed on 15 December 2021). [CrossRef] [PubMed]
- O’Brien, E.; Pickering, T.; Asmar, R.; Myers, M.; Parati, G.; Staessen, J.; Mengden, T.; Imai, Y.; Waeber, B.; Palatini, P.; et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press. Monit. 2002, 7, 3–17. Available online: https://pubmed.ncbi.nlm.nih.gov/12040236/ (accessed on 15 December 2021). [CrossRef] [PubMed]
- O’Brien, E.; Atkins, N.; Stergiou, G.; Karpettas, N.; Parati, G.; Asmar, R.; Imai, Y.; Wang, J.; Mengden, T.; Shennan, A.; et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press. Monit. 2010, 5, 23–38. Available online: https://pubmed.ncbi.nlm.nih.gov/20110786/ (accessed on 15 December 2021). [CrossRef] [PubMed]
- Stergiou, G.S.; Alpert, B.; Mieke, S.; Asmar, R.; Atkins, N.; Eckert, S.; Frick, G.; Friedman, B.; Graßl, T.; Ichikawa, T.; et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J. Hypertens. 2018, 36, 472–478. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Palatini, P.; Asmar, R.; Ioannidis, J.P.; Kollias, A.; Lacy, P.; McManus, R.J.; Myers, M.G.; Parati, G.; Shennan, A.; et al. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J. Hypertens. 2019, 37, 459–466. Available online: https://pubmed.ncbi.nlm.nih.gov/30702492/ (accessed on 15 December 2021).
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. Available online: https://pubmed.ncbi.nlm.nih.gov/30962953/ (accessed on 15 December 2021). [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Abou-Dakn, M.; Döhmen, C.; Wenzel, S. Validation of the TONOPORT VI ambulatory blood pressure monitor in adults according to the European Society of Hypertension International Protocol revision 2010. J. Hum. Hypertens. 2017, 31, 89–92. Available online: https://pubmed.ncbi.nlm.nih.gov/27411300/ (accessed on 8 January 2022). [CrossRef]
- Azaki, A.; Diab, R.; Harb, A.; Asmar, R.; Chahine, M.N. Questionable accuracy of home blood pressure measurements in the obese population—Validation of the Microlife WatchBP O3® and Omron RS6® devices according to the European Society of Hypertension-International Protocol. Vasc. Health Risk Manag. 2017, 13, 61–69. Available online: https://pubmed.ncbi.nlm.nih.gov/28280348/ (accessed on 9 January 2022). [CrossRef]
- Chen, Q.; Kang, Y.Y.; Li, Y.; Wang, J.G. Validation of the BPUMP BF1112 upper-arm blood pressure monitor for home blood pressure monitoring according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2017, 22, 105–108. Available online: https://pubmed.ncbi.nlm.nih.gov/28072599/ (accessed on 9 January 2022). [CrossRef]
- Liu, Z.Y.; Zhang, Q.H.; Ye, X.L.; Liu, D.P.; Cheng, K.; Zhang, C.H.; Wan, Y. Validation of the G.LAB MD2200 wrist blood pressure monitor according to the European Society of Hypertension, the British Hypertension Society, and the International Organization for Standardization Protocols. Blood Press. Monit. 2017, 22, 101–104. Available online: https://https://pubmed.ncbi.nlm.nih.gov/28177942/ (accessed on 9 January 2022). [CrossRef]
- Beime, B.; Deutsch, C.; Krüger, R.; Wolf, A.; Müller, P.; Hammel, G.; Bramlage, P. Validation of the custo screen pediatric blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Eur. J. Pediatr. 2017, 176, 573–580. Available online: https://pubmed.ncbi.nlm.nih.gov/28236027/ (accessed on 9 January 2022). [CrossRef]
- Fania, C.; Albertini, F.; Palatini, P. Validation of the A&D UM-201 device for office blood pressure measurement according to the European Society of Hypertension International Protocol Revision 2010. Blood Press. Monit. 2017, 22, 234–237. Available online: https://pubmed.ncbi.nlm.nih.gov/28362645/ (accessed on 10 January 2022).
- Kang, Y.Y.; Chen, Q.; Liu, C.Y.; Li, Y.; Wang, J.G. Validation of the AVITA BPM17 wrist blood pressure monitor for home blood pressure monitoring according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2017, 22, 230–233. Available online: https://pubmed.ncbi.nlm.nih.gov/28383290/ (accessed on 10 January 2022). [CrossRef]
- Fania, C.; Albertini, F.; Palatini, P. Validation of the A&D UM-211 device for office blood pressure measurement according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2017, 22, 302–305. Available online: https://pubmed.ncbi.nlm.nih.gov/28520592/ (accessed on 10 January 2022).
- Chen, Q.; Lei, L.; Li, Y.; Wang, J.G. Validation of the YuWell YE690A upper-arm blood pressure monitor, for clinic use and self-measurement, according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2017, 22, 295–297. Available online: https://pubmed.ncbi.nlm.nih.gov/28617717/ (accessed on 10 January 2022). [CrossRef]
- Grover-Páez, F.; Cardona-Muñoz, E.G.; Cardona-Müller, D.; Guzmán-Saldívar, V.H.; Rodríguez-De La Cerda, M.; Jiménez-Cázarez, M.B.; Totsuka-Sutto, S.E.; Alanis-Sánchez, G.A.; Ramos-Becerra, C.G. Validation of the Omron HEM-7320-LA, upper arm blood pressure monitor with Intelli Wrap Technology Cuff HEM-FL1 for self-measurement and clinic use according to the European Society of Hypertension International Protocol revision 2010 in the Mexican population. Blood Press. Monit. 2017, 22, 375–378. Available online: https://pubmed.ncbi.nlm.nih.gov/28945665/ (accessed on 15 January 2022).
- Chahine, M.N.; Topouchian, J.; Zelveian, P.; Hakobyan, Z.; Melkonyan, A.; Azaki, A.; Diab, R.; Harb, A.; Asmar, R. Validation of BP devices QardioArm® in the general population and Omron M6 Comfort® in type II diabetic patients according to the European Society of Hypertension International Protocol (ESH-IP). Med. Devices 2017, 11, 11–20. Available online: https://pubmed.ncbi.nlm.nih.gov/29343992/ (accessed on 15 January 2022). [CrossRef] [PubMed][Green Version]
- Chen, L.; Li, J.; Wen, J.; Guo, C.; Zhang, J.; Yu, Z. Validation of the Pangao PG-800B26 upper arm blood pressure monitor in the general population according to the European Society of Hypertension and the British Hypertension Society protocols. Blood Press. Monit. 2018, 23, 41–44. Available online: https://pubmed.ncbi.nlm.nih.gov/28902676/ (accessed on 15 January 2022). [CrossRef] [PubMed]
- Zhao, H.; Qiao, W.; Zhang, R.; Cui, P.; Hou, F.; Zhang, W. Validation of the Pangao PG-800A36 automatic wrist blood pressure monitor according to the European Society of Hypertension and the British Hypertension Society protocols. Blood Press. Monit. 2018, 23, 37–40. Available online: https://pubmed.ncbi.nlm.nih.gov/28926362/ (accessed on 15 January 2022). [CrossRef] [PubMed]
- Kang, Y.Y.; Chen, Q.; Liu, C.Y.; Li, Y.; Wang, J.G. Validation of the AVITA BPM64 upper-arm blood pressure monitor for home blood pressure monitoring according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2018, 23, 45–48. Available online: https://pubmed.ncbi.nlm.nih.gov/29049094/ (accessed on 15 January 2022). [CrossRef] [PubMed]
- Pereira, T.; Guimarães, J. Validation of the Beneware model ABP-021 ambulatory blood pressure monitor according to the revised 2010 European Society of hypertension international protocol. Blood Press. Monit. 2018, 23, 210–213. Available online: https://pubmed.ncbi.nlm.nih.gov/29894314/ (accessed on 16 January 2022). [CrossRef]
- Mazoteras-Pardo, V.; Becerro-De-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; López-López, D.; Palomo-López, P.; Rodríguez-Sanz, D.; Calvo-Lobo, C. The QardioArm Blood Pressure App for Self-Measurement in an Obese Population: Validation Study Using the European Society of Hypertension International Protocol Revision 2010. JMIR mHealth uHealth. 2018, 6, e11632. Available online: https://pubmed.ncbi.nlm.nih.gov/30361193/ (accessed on 16 January 2022). [CrossRef]
- Abou-Dakn, M.; Wenzel, S. Validation of the PHYSIO-PORT UP ambulatory blood pressure monitor in pregnant women according to the European Society of Hypertension International Protocol revision 2010. J. Hum. Hypertens. 2018, 32, 770–774. Available online: https://pubmed.ncbi.nlm.nih.gov/30232401/ (accessed on 16 January 2022). [CrossRef]
- Kollias, A.; Stambolliu, E.; Kyriakoulis, K.G.; Papadatos, S.S.; Stergiou, G.S. Validation of the single-cuff oscillometric blood pressure monitor InBody BPBIO320 for public use according to the 2010 European Society of Hypertension International Protocol. Blood Press. Monit. 2019, 24, 30–32. Available online: https://pubmed.ncbi.nlm.nih.gov/30531495/ (accessed on 17 January 2022). [CrossRef]
- Saito, K.; Hishiki, Y.; Takahashi, H. Validation of two automatic devices, Omron HEM-6232T and HEM-6181, for self-measurement of blood pressure at the wrist according to the ANSI/AAMI/ISO 81060-2:2013 protocol and the European Society of Hypertension International Protocol revision 2010. Vasc. Health Risk Manag. 2019, 15, 47–55. Available online: https://pubmed.ncbi.nlm.nih.gov/30881007/ (accessed on 17 January 2022). [CrossRef]
- Mazoteras-Pardo, V.; Becerro-De-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; López-López, D.; Palomo-López, P.; Rodríguez-Sanz, D.; Calvo-Lobo, C. Validation in the General Population of the iHealth Track Blood Pressure Monitor for Self-Measurement According to the European Society of Hypertension International Protocol Revision 2010: Descriptive Investigation. JMIR mHealth uHealth. 2019, 7, e13137. Available online: https://pubmed.ncbi.nlm.nih.gov/30888331/ (accessed on 17 January 2022). [CrossRef]
- Reshetnik, A.; Gohlisch, C.; Abou-Dakn, M.; Tölle, M.; Zidek, W.; Van Der Giet, M. Validation of noninvasive oscillometric blood pressure 2020 up pressure upper arm blood pressure monitoring technology according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2019, 24, 99–101. Available online: https://pubmed.ncbi.nlm.nih.gov/30762596/ (accessed on 17 January 2022). [CrossRef]
- Fania, C.; Vezzù, L.; Lazzaretto, I.; Palatini, P. Validation of the Hingmed WBP-02A device for ambulatory blood pressure monitoring according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2019, 24, 151–154. Available online: https://pubmed.ncbi.nlm.nih.gov/30807305/ (accessed on 17 January 2022). [CrossRef]
- Liu, Z.; Chen, L. Validation of Transtek TMB-1776 according to European Society of Hypertension International Protocol revision 2010 in adults. Blood Press. Monit. 2019, 24, 319–322. Available online: https://pubmed.ncbi.nlm.nih.gov/31567185/ (accessed on 18 January 2022). [CrossRef]
- Mazoteras-Pardo, V.; Becerro-De-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; López-López, D.; Rodríguez-Sanz, D.; Casado-Hernández, I.; Calvo-Lobo, C.; Palomo-López, P. QardioArm Upper Arm Blood Pressure Monitor Against Omron M3 Upper Arm Blood Pressure Monitor in Patients With Chronic Kidney Disease: A Validation Study According to the European Society of Hypertension International Protocol Revision 2010. J. Med. Internet Res. 2019, 21, e14686. Available online: https://pubmed.ncbi.nlm.nih.gov/31789600/ (accessed on 18 January 2022). [CrossRef]
- Kollias, A.; Anagnostopoulos, I.; Gravvani, A.; Stambolliu, E.; Bountzona, I.; Menti, A.; Stergiou, G.S. Validation of the InBody BP170 oscillometric home blood pressure monitor in general population according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization Universal Standard. Blood Press. Monit. 2020, 25, 50–52. Available online: https://pubmed.ncbi.nlm.nih.gov/31633518/ (accessed on 18 January 2022).
- Zhang, W.; Lei, L.; Li, Y.; Wang, J.G. Validation of the HL868ED upper-arm blood pressure monitor for clinical use and self-measurement according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2020, 25, 53–57. Available online: https://pubmed.ncbi.nlm.nih.gov/31658108/ (accessed on 18 January 2022). [CrossRef]
- Kollias, A.; Gravvani, A.; Anagnostopoulos, I.; Kyriakoulis, K.G.; Bountzon, I.; Menti, A.; Stergiou, G.S. Validation of the InBody BPBIO250 oscillometric blood pressure monitor for professional office use in general population according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization Universal Standard. Blood Press. Monit. 2020, 15, 115–117. Available online: https://pubmed.ncbi.nlm.nih.gov/31913149/ (accessed on 18 January 2022).
- Saito, K.; Hishiki, Y.; Takahashi, H. Validation of the Omron HBP-1320 for professional use according to the ANSI/AAMI/ISO 81060-2: 2013 protocol and the 2010 revision of the European Society of Hypertension International Protocol. Blood Press. Monit. 2020, 25, 162–166. Available online: https://pubmed.ncbi.nlm.nih.gov/32118675/ (accessed on 18 January 2022). [CrossRef]
- Song, C.; Yu, Y.; Lu, B.C.; Yan, X.L. Validation of the Globalcare GCE603 automated blood pressure monitor for self-measurement according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2020, 25, 291–294. Available online: https://pubmed.ncbi.nlm.nih.gov/32898351/ (accessed on 18 January 2022). [CrossRef]
- Omar, S.M.; Ali, E.A.; Ibrahim, Y.; Al-Wutayd, O.; Adam, I. Validation of the wrist blood pressure measuring device Omron RS6 (HEM-6221-E) among obese Sudanese patients according to the European Society of Hypertension International Protocol Revision 2010. F1000Resarch 2020, 9, 1284. Available online: https://pubmed.ncbi.nlm.nih.gov/34691394/ (accessed on 20 January 2022).
- Ntineri, A.; Prapa, S.; Bountzona, I.; Menti, A.; Stergiou, G.S. Validation of the single-cuff oscillometric blood pressure monitor InBody BPBIO750 for public spaces according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization Universal Standard. Blood Press. Monit. 2021, 26, 146–148. Available online: https://pubmed.ncbi.nlm.nih.gov/33323723/ (accessed on 20 January 2022). [PubMed]
- Chahine, M.N.; Harb, S.B.; Saad, A.R.; Sarkis, P.; Azaki, A.; Harb, A.; Allouch, A.; Asmar, R. Validation of the PHILIPS DL8760 upper arm blood pressure monitor, in oscillometry mode, for self-measurement in a general population, according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2021, 26, 237–241. Available online: https://pubmed.ncbi.nlm.nih.gov/33661139/ (accessed on 20 January 2022). [CrossRef] [PubMed]
- Deutsch, C.; Bramlage, C.; Botta, B.; Krüger, R.; Forstner, K.; Bramlage, P.; Beime, B. Validation of the blood pressure measurement device Beurer BM 28 according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2021, 26, 292–298. Available online: https://pubmed.ncbi.nlm.nih.gov/33741775/ (accessed on 20 January 2022). [CrossRef] [PubMed]
- Zhang, H.-J.; Zhang, J.; Wang, S.-L.; Zhang, J.; Teng, L.-N.; Zhang, S.-J.; Zhou, D.-J.; Long, M.-Z. Validation of the YuWell YE900 oscillometric blood pressure monitor for professional office use in adults and children according to the AAMI/ESH/ISO Universal Standard (ISO 81060-2:2018). Blood Press. Monit. 2021, 26, 396–399. Available online: https://pubmed.ncbi.nlm.nih.gov/34480474/ (accessed on 20 January 2022). [CrossRef] [PubMed]
- Ntineri, A.; Menti, A.; Konstantinos, G.; Bountzona, K.J.; Prapa, S.; Kollias, G.S.S. Validation of the InBody BPBIO210 manual auscultatory hybrid device for professional office use in a general population according to the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization. Blood Press. Monit. 2021, 27, 135–138. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Fang, Z.; Lu, Y.; Cui, J.; Du, X.; Hu, R. Smartphone application-supported validation of three automatic devices for self-measurement of blood pressure according to the European Society of Hypertension International Protocol revision 2010: The Omron HEM-7120, Yuwell YE680A and Cofoe KF-65B. Blood Press. Monit. 2021, 26, 435–440. Available online: https://pubmed.ncbi.nlm.nih.gov/34001755/ (accessed on 20 January 2022). [CrossRef]
- Degott, J.; Ghajarzadeh-Wurzner, A.; Hofmann, G.; Proença, M.; Bonnier, G.; Lemkaddem, A.; Lemay, M.; Christen, U.; Knebel, J.-F.; Durgnat, V.; et al. Smartphone based blood pressure measurement: Accuracy of the OptiBP mobile application according to the AAMI/ESH/ISO universal validation protocol. Blood Press. Monit. 2021, 26, 441–448. Available online: https://pubmed.ncbi.nlm.nih.gov/34139747/ (accessed on 20 January 2022). [CrossRef]
- Taylor, P.; Berg, C.; Thompson, J.; Dean, K.; Yuan, T.; Nallamshetty, S.; Tong, I. Effective Access to Care in a Crisis Period: Hypertension Control During the COVID-19 Pandemic by Telemedicine. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 19–26. Available online: https://pubmed.ncbi.nlm.nih.gov/34805763/ (accessed on 23 January 2022). [CrossRef]
- Wu, D.; Huyan, X.; She, Y.; Hu, J.; Duan, H.; Deng, N. Exploring and Characterizing Patient Multibehavior Engagement Trails and Patient Behavior Preference Patterns in Pathway-Based mHealth Hypertension Self-Management: Analysis of Use Data. JMIR mHealth uHealth 2022, 10, e33189. Available online: https://pubmed.ncbi.nlm.nih.gov/35113032/ (accessed on 23 January 2022). [CrossRef]
- Bricca, A.; Pellegrini, A.; Zangger, G.; Ahler, J.J.; Jäger, M.; Skou, S.T. The Quality of Health Apps and Their Potential to Promote Behavior Change in Patients With a Chronic Condition or Multimorbidity: Systematic Search in App Store and Google Play. JMIR mHealth uHealth 2022, 10, e33168. Available online: https://pubmed.ncbi.nlm.nih.gov/35119367/ (accessed on 23 January 2022). [CrossRef]
- Stergiou, G.S.; Asmar, R.; Myers, M.; Palatini, P.; Parati, G.; Shennan, A.; Wang, J.; O’Brien, E.; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. Improving the accuracy of blood pressure measurement: The influence of the European Society of Hypertension International Protocol (ESH-IP) for the validation of blood pressure measuring devices and future perspectives. J. Hypertens. 2018, 36, 479–487. Available online: https://pubmed.ncbi.nlm.nih.gov/29384984/ (accessed on 23 January 2022). [CrossRef]
- O’Brien, E.; Stergiou, G.; Palatini, P.; Asmar, R.; Ioannidis, J.P.; Kollias, A.; Lacy, P.; McManus, R.J.; Myers, M.G.; Shennan, A.; et al. Validation protocols for blood pressure measuring devices: The impact of the European Society of Hypertension International Protocol and the development of a Universal Standard. Blood Press. Monit. 2019, 24, 163–166. Available online: https://pubmed.ncbi.nlm.nih.gov/31116156/ (accessed on 23 January 2022). [CrossRef]
- Alpert, B.S.; Sarkis, J.; Dart, R.A.; Quinn, D.; Friedman, B.; Townsend, R.R.; Shimbo, D. Future use of the European Society of Hypertension International Protocol for validation of automated sphygmomanometers. Blood Press. Monit. 2019, 24, 161–162. Available online: https://pubmed.ncbi.nlm.nih.gov/31116154/ (accessed on 23 January 2022). [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).