Personalized Low-Invasive Approach to Chronic Endometritis Evaluation in Premenopausal Women: Machine Learning-Based Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical, Instrumental, and Laboratory Parameters
2.3. Machine Learning and Statistical Analysis Methods
2.3.1. Data Pre-Processing
2.3.2. Selection of Features and Model
2.3.3. Model Evaluation and Interpretation
2.3.4. Software
- Programming language: Python 3.12.2
- Runtime environment: Jupyter Notebook 6.5.4
- Libraries used:
- •
- Pandas 2.3.2—for structured data analysis and manipulation (DataFrame operations).
- •
- NumPy 2.2.0—for multi-dimensional array computations and mathematical operations.
- •
- Missingno 0.5.2—for visualizing missing data patterns in datasets.
- •
- Matplotlib 3.10.5—for creating basic static visualizations.
- •
- Seaborn 0.13.2—for advanced statistical plotting.
- •
- SciPy 1.16.1—for statistical analysis and mathematical functions.
- •
- Miceforest 6.0.3—for multiple imputation by chained equations (MICE) to handle missing values.
- •
- Imbalanced-learn 0.14.0—for handling class imbalance using the SMOTENC algorithm.
- •
- Scikit-learn 1.7.1—for data preprocessing, model building, and validation.
- •
- SHAP 0.48.0—for model interpretability and explanation of predictions.
- •
- Mlxtend 0.23.4—for machine learning tools, including feature selection.
- •
- XGBoost 3.0.4—for gradient-boosting machine learning models.
- •
- MLstatkit 0.1.9—for performing DeLong’s test to compare ROC-AUC of two models.
3. Results
3.1. Characteristics of Patients
3.2. Prediction Models
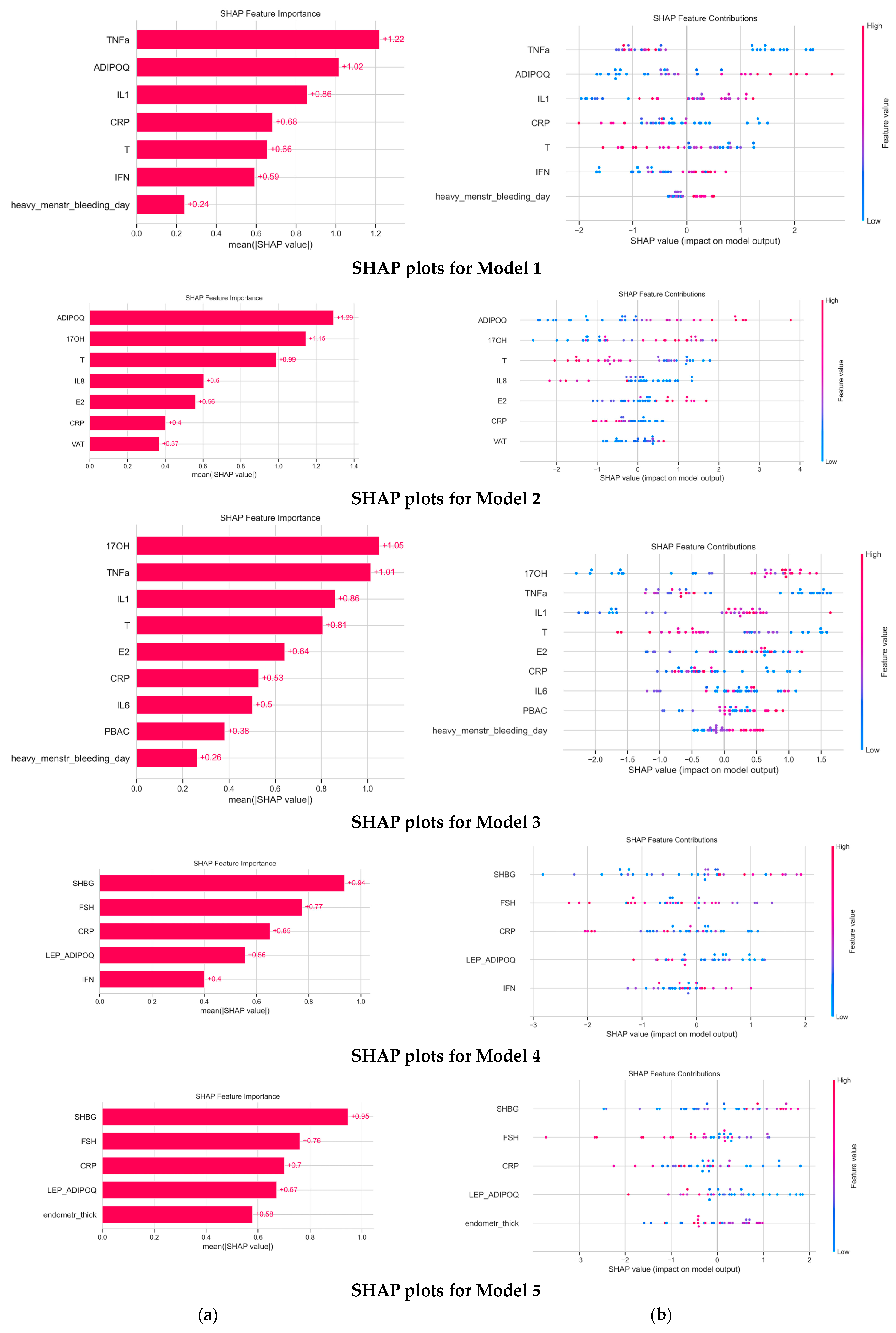
3.3. Model Evaluation and Interpretation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CE | Chronic Endometritis |
| RIF | Recurrent implantation failure |
| EP | Endometrial polyp |
| IL | Interleukin |
| TNF α | Tumor necrosis factor |
| ML | Machine learning |
| PCOS | Polycystic ovarian syndrome |
| HA | Hyperandrogenism |
| TSH | Thyroid-stimulating hormone |
| 17-OH | 17-Hydroxyprogesterone |
| FSH | Follicular-stimulating hormone |
| BMI | Body mass index |
| PBAC | Pictorial Blood Assessment Chart |
| CRP | C-reactive protein |
| AMH | Anti-Mullerian hormone |
| T | Total testosterone |
| SHBG | Sex hormone-binding globulin |
| LH | Luteinizing hormone |
| E2 | Estradiol |
| ADIPOQ | Adiponectin |
| IFN | Interferon |
| MICE | Multiple Imputation by Chained Equations |
| SMOTENC | Synthetic Minority Over-sampling Technique for Nominal and Continuous |
| ROC-AUC | Receiver Operating Characteristic—Area Under Curve |
| PR-AUC | Area Under the precision–recall curve |
| SHAP | Shapley additive explanations |
Appendix A
Appendix A.1. Metrics of CE Prediction Models Included Gynecological History
| Model | Accuracy | Recall (CI 95%) | Precision | Specificity | F1 | ROC AUC | PR AUC |
| Model 1 | 0.545 | 0.696 (0.428, 0.923) | 0.450 | 0.450 | 0.545 | 0.615 | 0.453 |
| Model 2 | 0.515 | 0.618 (0.333, 0.889) | 0.421 | 0.450 | 0.500 | 0.588 | 0.483 |
| Model 2.5 | 0.545 | 0.538 (0.263, 0.818) | 0.438 | 0.550 | 0.483 | 0.562 | 0.400 |
| Model 3 | 0.545 | 0.538 (0.263, 0.818) | 0.438 | 0.550 | 0.483 | 0.562 | 0.400 |
| Model 4 | 0.576 | 0.614 (0.333, 0.875) | 0.471 | 0.550 | 0.533 | 0.600 | 0.439 |
Appendix A.2. Characteristics of the Models Included Gynecological History
| Model 1 | Model 2 | Model 2.5 | Model 3 | Model 4 |
| FSH IL-1 IL-8 SHBG Endometrial thickness Missed abortion | SHBG E2 IFN FSH CRP Missed abortion | SHBG FSH Leptin/ADPQ IFNγ Missed abortion | SHBG FSH Leptin/ADPQ IFNγ Missed abortion | Leptin/ADPQ SHBG FSH Endometrial thickness Missed abortion |
References
- Wang, C.; Lu, Y.; Ou, M.; Qian, L.; Zhang, Y.; Yang, Y.; Luo, L.; Wang, Q. Risk Factors for Recurrent Implantation Failure as Defined by the European Society for Human Reproduction and Embryology. Hum. Reprod. 2025, 40, 1138–1147. [Google Scholar] [CrossRef]
- Zargar, M.; Ghafourian, M.; Nikbakht, R.; Mir Hosseini, V.; Moradi Choghakabodi, P. Evaluating Chronic Endometritis in Women with Recurrent Implantation Failure and Recurrent Pregnancy Loss by Hysteroscopy and Immunohistochemistry. J. Minim. Invasive Gynecol. 2020, 27, 116–121. [Google Scholar] [CrossRef]
- Ticconi, C.; Inversetti, A.; Marraffa, S.; Campagnolo, L.; Arthur, J.; Zambella, E.; Di Simone, N. Chronic Endometritis and Recurrent Reproductive Failure: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1427454. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.D.; Schwartz, K.M.; Chan, M.; Cedars, M.I.; Cakmak, H.; Huang, D. Chronic Endometritis and Its Association with Implantation History, BCL6, and ERA in Infertility Patients. J. Assist. Reprod. Genet. 2025, 42, 3303–3310. [Google Scholar] [CrossRef] [PubMed]
- Yasuo, T.; Kitaya, K. Challenges in Clinical Diagnosis and Management of Chronic Endometritis. Diagnostics 2022, 12, 2711. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Fang, R.-L.; Luo, Y.-N.; Luo, C.-Q. Analysis of the Diagnostic Value of CD138 for Chronic Endometritis, the Risk Factors for the Pathogenesis of Chronic Endometritis and the Effect of Chronic Endometritis on Pregnancy: A Cohort Study. BMC Womens Health 2016, 16, 60. [Google Scholar] [CrossRef]
- Hosseini, S.; Abbasi, H.; Salehpour, S.; Saharkhiz, N.; Nemati, M. Prevalence of Chronic Endometritis in Infertile Women Undergoing Hysteroscopy and Its Association with Intrauterine Abnormalities: A Cross-Sectional Study. JBRA Assist. Reprod. 2024, 28, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Kabodmehri, R.; Etezadi, A.; Sharami, S.H.; Ghanaei, M.M.; Hosseinzadeh, F.; Heirati, S.F.D.; Pourhabibi, Z. The Association between Chronic Endometritis and Uterine Fibroids. J. Family Med. Prim. Care 2022, 11, 653–659. [Google Scholar] [CrossRef]
- Vitagliano, A.; Cialdella, M.; Cicinelli, R.; Santarsiero, C.M.; Greco, P.; Buzzaccarini, G.; Noventa, M.; Cicinelli, E. Association between Endometrial Polyps and Chronic Endometritis: Is It Time for a Paradigm Shift in the Pathophysiology of Endometrial Polyps in Pre-Menopausal Women? Results of a Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 2182. [Google Scholar] [CrossRef]
- Ievleva, K.; Danusevich, I.; Atalyan, A.; Egorova, I.; Babaeva, N.; Rashidova, M.; Akhmedzyanova, M.R.; Sholokhov, L.; Nadeliaeva, I.; Lazareva, L.; et al. Diagnostic significance of interleukin levels in blood serum in premenopausal women with chronic endometritis and normal weight or overweight. Acta Biomed. Sci. 2024, 9, 38–48. [Google Scholar] [CrossRef]
- Ievleva, K.; Danusevich, I.; Atalyan, A.; Sharifulin, E.; Lazareva, L.; Nadeliaeva, I.; Rashidova, M.; Akhmedzyanova, M.R.; Belenkaya, L.; Sholokhov, L.; et al. Adipokine levels and their association with chronic endometritis in reproductive-aged women. Vopr. ginekol. akus. perinatol. Gynecol. Obstet. Perinatol. 2023, 22, 60–68. [Google Scholar] [CrossRef]
- Bays, H.E.; Bindlish, S.; Clayton, T.L. Obesity, Diabetes Mellitus, and Cardiometabolic Risk: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023. Obes. Pillars 2023, 5, 100056. [Google Scholar] [CrossRef]
- Mihara, M.; Yasuo, T.; Kitaya, K. Precision Medicine for Chronic Endometritis: Computer-Aided Diagnosis Using Deep Learning Model. Diagnostics 2023, 13, 936. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T.; Yamaguchi, T. Bridging the Diagnostic Gap between Histopathologic and Hysteroscopic Chronic Endometritis with Deep Learning Models. Medicina 2024, 60, 972. [Google Scholar] [CrossRef] [PubMed]
- Suturina, L.; Lizneva, D.; Lazareva, L.; Danusevich, I.; Nadeliaeva, I.; Belenkaya, L.; Atalyan, A.; Belskikh, A.; Bairova, T.; Sholokhov, L.; et al. Ethnicity and the Prevalence of Polycystic Ovary Syndrome: The Eastern Siberia PCOS Epidemiology and Phenotype Study. J. Clin. Endocrinol. Metab. 2024, 110, e32–e43. [Google Scholar] [CrossRef]
- Suturina, L.; Lizneva, D.; Atalyan, A.; Lazareva, L.; Belskikh, A.; Bairova, T.; Sholokhov, L.; Rashidova, M.; Danusevich, I.; Nadeliaeva, I.; et al. Establishing Normative Values to Determine the Prevalence of Biochemical Hyperandrogenism in Premenopausal Women of Different Ethnicities from Eastern Siberia. Diagnostics 2022, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Sharifulin, E.; Igumnov, I.; Krusko, O.; Atalyan, A.; Suturina, L. Chronic Endometritis in Women of Reproductive Age with Polycystic Ovary Syndrome. Acta Biomed. Sci. 2020, 5, 27–36. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Çorbacıoğlu, Ş.K.; Aksel, G. Receiver Operating Characteristic Curve Analysis in Diagnostic Accuracy Studies: A Guide to Interpreting the Area under the Curve Value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar] [CrossRef]
- Carrington, A.M.; Manuel, D.G.; Fieguth, P.W.; Ramsay, T.; Osmani, V.; Wernly, B.; Bennett, C.; Hawken, S.; Magwood, O.; Sheikh, Y.; et al. Deep ROC Analysis and AUC as Balanced Average Accuracy, for Improved Classifier Selection, Audit and Explanation. IEEE Trans. Pattern Anal. Mach. Intell. 2023, 45, 329–341. [Google Scholar] [CrossRef]
- Dos Santos, E.; Pecquery, R.; de Mazancourt, P.; Dieudonné, M.-N. Adiponectin and Reproduction. Vitam. Horm. 2012, 90, 187–209. [Google Scholar] [CrossRef]
- Widmann, G.; Luger, A.K.; Sonnweber, T.; Schwabl, C.; Cima, K.; Gerstner, A.K.; Pizzini, A.; Sahanic, S.; Boehm, A.; Coen, M.; et al. Machine Learning Based Multi-Parameter Modeling for Prediction of Post-Inflammatory Lung Changes. Diagnostics 2025, 15, 783. [Google Scholar] [CrossRef]
- Giltay, E.J.; van Schaardenburg, D.; Gooren, L.J.; Popp-Snijders, C.; Dijkmans, B.A. Androgens and Ankylosing Spondylitis: A Role in the Pathogenesis? Ann. N. Y. Acad. Sci. 1999, 876, 340–364. [Google Scholar] [CrossRef] [PubMed]
- Meis, P.J.; Klebanoff, M.; Thom, E.; Dombrowski, M.P.; Sibai, B.; Moawad, A.H.; Spong, C.Y.; Hauth, J.C.; Miodovnik, M.; Varner, M.W.; et al. Prevention of Recurrent Preterm Delivery by 17 Alpha-Hydroxyprogesterone Caproate. N. Engl. J. Med. 2003, 348, 2379–2385. [Google Scholar] [CrossRef]
- Yamagata, K.; Mizuno, Y.; Mizuno, Y.; Tamaru, S.; Kajihara, T. Androgens Modulate Endometrial Function. Med. Mol. Morphol. 2025, 58, 93–99. [Google Scholar] [CrossRef]
- Di Stasi, V.; Maseroli, E.; Rastrelli, G.; Scavello, I.; Cipriani, S.; Todisco, T.; Marchiani, S.; Sorbi, F.; Fambrini, M.; Petraglia, F.; et al. SHBG as a Marker of NAFLD and Metabolic Impairments in Women Referred for Oligomenorrhea and/or Hirsutism and in Women With Sexual Dysfunction. Front. Endocrinol. 2021, 12, 641446. [Google Scholar] [CrossRef]
- Günther, V.; Allahqoli, L.; Deenadayal-Mettler, A.; Maass, N.; Mettler, L.; Gitas, G.; Andresen, K.; Schubert, M.; Ackermann, J.; von Otte, S.; et al. Molecular Determinants of Uterine Receptivity: Comparison of Successful Implantation, Recurrent Miscarriage, and Recurrent Implantation Failure. Int. J. Mol. Sci. 2023, 24, 17616. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, B.; Yalti, S.; Ozden, S.; Ficicioglu, C. High Basal Estradiol Level and FSH/LH Ratio in Unexplained Recurrent Pregnancy Loss. Arch. Gynecol. Obstet. 2004, 270, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Kitajima, M.; Inoue, T.; Fujishita, A.; Nakashima, M.; Masuzaki, H. 17β-Estradiol and Lipopolysaccharide Additively Promote Pelvic Inflammation and Growth of Endometriosis. Reprod. Sci. 2015, 22, 585–594. [Google Scholar] [CrossRef]
- Holesh, J.E.; Bass, A.N.; Lord, M. Physiology, Ovulation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Brosens, J.; Verhoeven, H.; Campo, R.; Gianaroli, L.; Gordts, S.; Hazekamp, J.; Hägglund, L.; Mardesic, T.; Varila, E.; Zech, J.; et al. High Endometrial Aromatase P450 mRNA Expression Is Associated with Poor IVF Outcome. Hum. Reprod. 2004, 19, 352–356. [Google Scholar] [CrossRef]
- Hagag, H.M.; Ismail, K.A.; Almutairi, M.M.; Alnefaie, B.I.; Alajmani, S.H.; Altalhi, A.M.; Alkhamash, A.H.; Althobaiti, N.S.; Alhumaidi, M.A.; Bawahab, A.A.; et al. Clinicopathological Aspects of Dilation and Curettage (D&C) Biopsies Taken from Patients Living at High Altitude in Taif, KSA, with a Special Emphasis on Chronic Endometritis. Life 2024, 14, 1021. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Sarankhuu, B.-E.; Jeon, H.J.; Jeong, D.-U.; Park, S.-R.; Kim, T.-H.; Lee, S.K.; Han, A.R.; Yu, S.-L.; Kang, J. Adiponectin Receptor 1 Regulates Endometrial Receptivity via the Adenosine Monophosphate–Activated Protein Kinase/E–Cadherin Pathway. Mol. Med. Rep. 2024, 30, 184. [Google Scholar] [CrossRef]
- Brezovec, N.; Perdan-Pirkmajer, K.; Čučnik, S.; Sodin-Šemrl, S.; Varga, J.; Lakota, K. Adiponectin Deregulation in Systemic Autoimmune Rheumatic Diseases. Int. J. Mol. Sci. 2021, 22, 4095. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lui, D.T.W.; Cheung, C.Y.Y.; Fong, C.H.Y.; Yuen, M.M.A.; Chow, W.S.; Woo, Y.C.; Xu, A.; Lam, K.S.L. Higher Circulating Adiponectin Concentrations Predict Incident Cancer in Type 2 Diabetes—The Adiponectin Paradox. J. Clin. Endocrinol. Metab. 2020, 105, e1387–e1396. [Google Scholar] [CrossRef]
- Baker, J.F.; Newman, A.B.; Kanaya, A.; Leonard, M.B.; Zemel, B.; Miljkovic, I.; Long, J.; Weber, D.; Harris, T.B. The Adiponectin Paradox in the Elderly: Associations With Body Composition, Physical Functioning, and Mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 247–253. [Google Scholar] [CrossRef]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Ievleva, K.D.; Danusevich, I.N.; Suturina, L.V. The role of leptin in endometrium disorders: Literature review. Probl. Endokrinol. 2024, 70, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X. Role of Leptin and Adiponectin in Immune Response and Inflammation. Int. Immunopharmacol. 2025, 161, 115082. [Google Scholar] [CrossRef] [PubMed]
- Tate, A.R.; Rao, G.H.R. Inflammation: Is It a Healer, Confounder, or a Promoter of Cardiometabolic Risks? Biomolecules 2024, 14, 948. [Google Scholar] [CrossRef]
- Arefi, S.; Babashamsi, M.; Shariat Panahi, P.; Asgharpour Saruiy, L.; Zeraati, H. C-reactive protein level and pregnancy rate in patients undergoing IVF/ICSI. Int. J. Reprod. Biomed. 2010, 8, 197–202. [Google Scholar]
- Gaskins, A.J.; Wilchesky, M.; Mumford, S.L.; Whitcomb, B.W.; Browne, R.W.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. Endogenous Reproductive Hormones and C-Reactive Protein across the Menstrual Cycle: The BioCycle Study. Am. J. Epidemiol. 2012, 175, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sethi, A. Endometritis—Diagnosis, Treatment and Its Impact on Fertility—A Scoping Review. JBRA Assist. Reprod. 2022, 26, 538–546. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Without CE | CE | p |
|---|---|---|---|
| Age, years | |||
| M ± SD | 32.92 ± 5.88 | 35.02 ± 6.06 | 0.05 |
| Me (Q1; Q3) | 33.0 (28.0; 38.0) | 36.0 (32.75; 39.25) | |
| BMI, kg/m2 | |||
| M ± SD | 24.5 ± 3.12 | 24.31 ± 3.49 | 0.786 |
| Me (Q1; Q3) | 24.84 (21.82; 26.77) | d24.3 (21.18; 27.24) | |
| VAT, % | |||
| M ± SD | d5.31 ± 1.6 | 5.34 ± 1.72 | 0.891 |
| Me (Q1; Q3). | 6.0 (4.0; 6.25) | 5.0 (4.0; 7.0) | |
| Number of heavy menstrual bleeding days | 0.646 | ||
| M ± SD | 2.11 ± 1.14 | 2.2 ± 1.0 | |
| Me (Q1; Q3). | 2.0 (1.0; 3.0) | 2.0 (1.0; 3.0) | |
| Endometrial polyp, n(%) | 0/64 (0%) | 0/44 (0%) | 1.000 |
| Uterine fibroids, n (%) | 4/64 (6.25%) | 4/44 (9.09%) | 0.713 |
| Spontaneous_abortions, n (%) | 12/62 (19.35%) | 7/43 (16.28%) | 0.687 |
| missed_abortion, n (%) | 4/64 (6.25%) | 0/44 (0%) | 0.242 |
| extrauterine_pregnancy, n (%) | 2/62 (3.23%) | 2/43 (4.65%) | 1.000 |
| C-section, n (%) | 13/41 (31.71%) | 5/34 (14.71%) | 0.086 |
| Endometrial thickness, mm | 0.512 | ||
| M ± SD | 8.73 ± 2.89; | 8.55 ± 2.99; | |
| Me (Q1; Q3). | 8.5 (7.0; 11.0) | 8.0 (7.0; 10.0) | |
| FSH, mIU/L | 0.639 | ||
| M ± SD | 5.15 ± 2.74; | 4.94 ± 2.71; | |
| Me (Q1; Q3). | 4.85 (3.3; 6.55) | 4.35 (3.38; 5.8) | |
| T, ng/dL | 0.007 | ||
| M ± SD | 298.68 ± 144.88; | 225.9 ± 118.9; | |
| Me (Q1; Q3). | 274.61 (212.0; 370.53) | 239.64 (123.24; 299.09) | |
| SHBG, nmol/L | 0.082 | ||
| M ± SD | 84.89 ± 63.58; | 97.98 ± 58.31; | |
| Me (Q1; Q3). | 70.05 (44.05; 91.05) | 83.5 (52.22; 128.25) | |
| Leptin, ng/mL | 0.109 | ||
| M ± SD | 21.75 ± 17.29; | 14.81 ± 8.57; | |
| Me (Q1; Q3). | 15.95 (10.0; 26.48) | 14.35 (9.07; 19.73) | |
| Adiponectin, ng/mL | 0.055 | ||
| M ± SD | 12.84 ± 10.51; | 18.93 ± 14.51; | |
| Me (Q1; Q3). | 10.0 (6.9; 15.2) | 14.35 (8.28; 29.1) | |
| Leptin/adiponectin | 0.009 | ||
| M ± SD | 2.62 ± 2.47; | 2.06 ± 3.65; | |
| Me (Q1; Q3). | 1.72 (0.86; 4.04) | 0.63 (0.31; 1.88) | |
| CRP, IU/L | 0.084 | ||
| M ± SD | 2.18 ± 2.52; | 1.83 ± 2.77; | |
| Me (Q1; Q3). | 1.3 (0.8; 2.45) | 0.8 (0.52; 2.45) | |
| IL1, ng/mL | 0.015 | ||
| M ± SD | 1.37 ± 1.43; | 2.75 ± 5.23; | |
| Me (Q1; Q3). | 0.9 (0.5; 1.9) | 1.55 (0.95; 2.3) | |
| TNFα, ng/mL | 0.004 | ||
| M ± SD | 2.65 ± 2.07; | 2.04 ± 2.29; | |
| Me (Q1; Q3). | 2.0 (1.5; 2.7) | 1.3 (0.88; 1.82) | |
| IL1/TNFα | <0.001 | ||
| M ± SD | 1.67 ± 6.81; | 1.61 ± 1.21; | |
| Me (Q1; Q3). | 0.63 (0.28; 0.9) | 1.16 (0.86; 2.28) | |
| IFNγ, ng/mL | 0.233 | ||
| M ± SD | 0.82 ± 0.81 | 1.65 ± 3.74 | |
| Me (Q1; Q3). | 0.7 (0.2; 1.0) | 0.9 (0.3; 1.3) |
| Model | Accuracy | Recall (CI 95%) | Precision | Specificity | F1 | ROC AUC | PR AUC |
|---|---|---|---|---|---|---|---|
| Model 1 | 0.697 | 0.537 | 0.629 | 0.800 | 0.583 | 0.704 | 0.565 |
| (0.25, 0.8) | (0.333, 0.909) | ||||||
| Model 2 | 0.606 | 0.690 | 0.502 | 0.550 | 0.581 | 0.673 | 0.608 |
| (0.417, 0.923) | (0.267, 0.733) | ||||||
| Model 3 | 0.545 | 0.614 | 0.439 | 0.500 | 0.516 | 0.677 | 0.537 |
| (0.333, 0.9) | (0.222, 0.667) | ||||||
| Model 4 | 0.667 | 0.615 | 0.568 | 0.700 | 0.593 | 0.758 | 0.704 |
| (0.333, 0.9) | (0.308, 0.818) | ||||||
| Model 5 | 0.697 | 0.696 | 0.600 | 0.700 | 0.643 | 0.769 | 0.680 |
| (0.438, 0.929) | (0.357, 0.833) |
| Model A vs. Model B | AUC (Model A) | CI 95% (Model A) | AUC (Model B) | CI 95% (Model B) | Z | p |
|---|---|---|---|---|---|---|
| Model 1 vs. Model 2 | 0.70 | 0.5170, 0.8907 | 0.67 | 0.4716, 0.8745 | −0.347 | 0.7285 |
| Model 1 vs. Model 3 | 0.70 | 0.5170, 0.8907 | 0.68 | 0.4916, 0.8622 | −0.314 | 0.7537 |
| Model 1 vs. Model 4 | 0.70 | 0.5170, 0.8907 | 0.76 | 0.5913, 0.9241 | −0.605 | 0.5452 |
| Model 1 vs. Model 5 | 0.70 | 0.5170, 0.8907 | 0.77 | 0.6049, 0.9336 | −0.678 | 0.4980 |
| Model 2 vs. Model 3 | 0.67 | 0.4716, 0.8745 | 0.68 | 0.4916, 0.8622 | −0.033 | 0.9739 |
| Model 2 vs. Model 4 | 0.67 | 0.4716, 0.8745 | 0.76 | 0.5913, 0.9241 | −0.795 | 0.4269 |
| Model 2 vs. Model 5 | 0.67 | 0.4716, 0.8745 | 0.77 | 0.6049, 0.9336 | −0.771 | 0.4405 |
| Model 3 vs. Model 4 | 0.68 | 0.4916, 0.8622 | 0.76 | 0.5913, 0.9241 | −0.734 | 0.4627 |
| Model 3 vs. Model 5 | 0.68 | 0.4916, 0.8622 | 0.77 | 0.6049, 0.9336 | −0.794 | 0.4273 |
| Model 4 vs. Model 5 | 0.76 | 0.5913, 0.9241 | 0.77 | 0.6049, 0.9336 | −0.200 | 0.8415 |
| Models | PR-AUC (Model A) | PR-AUC (Model B) | CI 95% Differences | p-Value |
|---|---|---|---|---|
| Model 1 vs. Model 2 | 0.565 | 0.608 | −0.283, 0.265 | 0.828 |
| Model 1 vs. Model 3 | 0.565 | 0.537 | −0.193, 0.324 | 0.720 |
| Model 1 vs. Model 4 | 0.565 | 0.704 | −0.312, 0.110 | 0.388 |
| Model 1 vs. Model 5 | 0.565 | 0.680 | −0.279, 0.121 | 0.360 |
| Model 2 vs. Model 3 | 0.608 | 0.537 | −0.227, 0.370 | 0.604 |
| Model 2 vs. Model 4 | 0.608 | 0.704 | −0.306, 0.117 | 0.392 |
| Model 2 vs. Model 5 | 0.608 | 0.680 | −0.358, 0.231 | 0.604 |
| Model 3 vs. Model 4 | 0.537 | 0.704 | −0.444, 0.112 | 0.240 |
| Model 3 vs. Model 5 | 0.537 | 0.680 | −0.432, 0.157 | 0.372 |
| Model 4 vs. Model 5 | 0.704 | 0.680 | −0.144, 0.201 | 0.828 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ievleva, K.D.; Atalyan, A.V.; Baintuev, T.G.; Nadeliaeva, I.G.; Lazareva, L.M.; Sharifulin, E.M.; Akhmedzyanova, M.R.; Sholokhov, L.F.; Danusevich, I.N.; Suturina, L.V. Personalized Low-Invasive Approach to Chronic Endometritis Evaluation in Premenopausal Women: Machine Learning-Based Modeling. Diagnostics 2025, 15, 2929. https://doi.org/10.3390/diagnostics15222929
Ievleva KD, Atalyan AV, Baintuev TG, Nadeliaeva IG, Lazareva LM, Sharifulin EM, Akhmedzyanova MR, Sholokhov LF, Danusevich IN, Suturina LV. Personalized Low-Invasive Approach to Chronic Endometritis Evaluation in Premenopausal Women: Machine Learning-Based Modeling. Diagnostics. 2025; 15(22):2929. https://doi.org/10.3390/diagnostics15222929
Chicago/Turabian StyleIevleva, Kseniia D., Alina V. Atalyan, Timur G. Baintuev, Iana G. Nadeliaeva, Ludmila M. Lazareva, Eldar M. Sharifulin, Margarita R. Akhmedzyanova, Leonid F. Sholokhov, Irina N. Danusevich, and Larisa V. Suturina. 2025. "Personalized Low-Invasive Approach to Chronic Endometritis Evaluation in Premenopausal Women: Machine Learning-Based Modeling" Diagnostics 15, no. 22: 2929. https://doi.org/10.3390/diagnostics15222929
APA StyleIevleva, K. D., Atalyan, A. V., Baintuev, T. G., Nadeliaeva, I. G., Lazareva, L. M., Sharifulin, E. M., Akhmedzyanova, M. R., Sholokhov, L. F., Danusevich, I. N., & Suturina, L. V. (2025). Personalized Low-Invasive Approach to Chronic Endometritis Evaluation in Premenopausal Women: Machine Learning-Based Modeling. Diagnostics, 15(22), 2929. https://doi.org/10.3390/diagnostics15222929







