The Role of Video Capsule Endoscopy in Hereditary Polyposis Syndromes: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Inherited Polyposis Syndromes
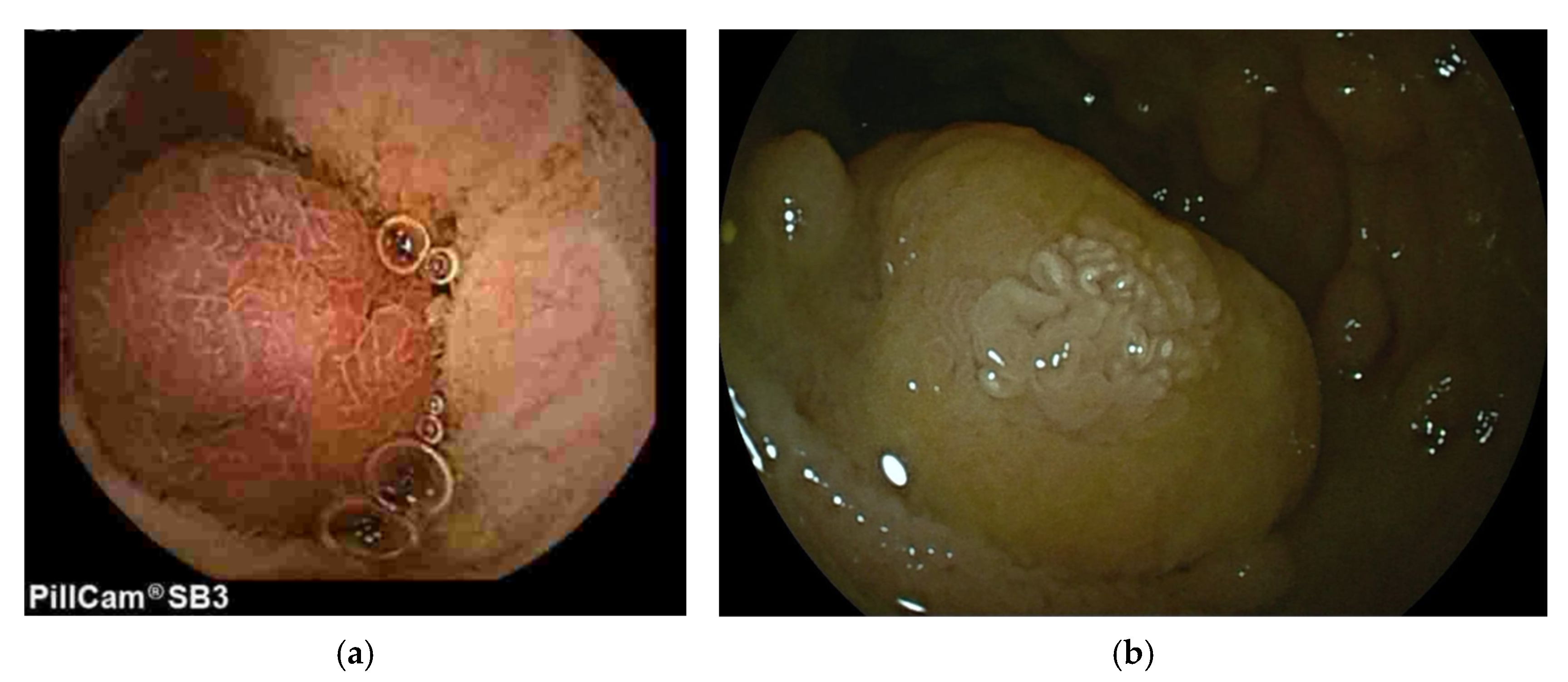
3.1. Peutz-Jeghers Syndrome (PJS)
3.2. Familial Adenomatous Polyposis (FAP)
3.3. Juvenile Polyposis Syndrome (JPS)
3.4. Phosphatase and Tensin Homolog Hamartoma Tumour Syndrome (PHTS)
3.5. Other Polyposis Syndromes (MAP, PPAP, NAP)
4. Discussion
5. AI and Future Perspectives
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| BMMRD | Biallelic Mismatch Repair Deficiency |
| BSG | British Society of Gastroenterology |
| CCE | Colon Capsule Endoscopy |
| CTE | Computed Tomography Enterograpy |
| ESGE | European Society of Gastrointestinal Endoscopy |
| HHT | Haemorrhagic Telangiectasia |
| FAP | Familiar Adenomatous Polyposis |
| FIT | Faecal Immunochemical Test |
| FPS | Frames Per Second |
| IBD | Inflammatory Bowel Disease |
| JPS | Juvenile Polyposis Syndrome |
| MAP | MUTYH-associated polyposis |
| MRE | Magnetic Resonance Enterography |
| NAP | NTHL-1 associated polyposis |
| PPAP | Polymerase Proofreading Associated Polyposis |
| PJS | Peutz-Jeghers Syndrome |
| PTEN-HS | Phosphatase and Tensin Homolog Hamartoma Syndrome |
| SBCE | Small Bowel Capsule Endoscopy |
| VCE | Video Capsule Endoscopy |
References
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless Capsule Endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef]
- Hong, S.M.; Jung, S.H.; Baek, D.H. Diagnostic Yields and Clinical Impacts of Capsule Endoscopy. Diagnostics 2021, 11, 1842. [Google Scholar] [CrossRef]
- Medtronic. PillCamTM SB 3 Capsule Endoscopy System. Available online: https://www.medtronic.com/en-us/healthcare-professionals/products/digestive-gastrointestinal/capsule-endoscopy/endoscopy-systems/pillcam-sb-3-capsule-endoscopy-system.html (accessed on 1 September 2025).
- Oh, D.J.; Kim, K.S.; Lim, Y.J. A New Active Locomotion Capsule Endoscopy under Magnetic Control and Automated Reading Program. Clin. Endosc. 2020, 53, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Capsovision. Instructions for Use CapsoCam Plus. Available online: https://capsovision.com/wp-content/uploads/IFU-2796F_CapsoCam-Plus_Eng.pdf (accessed on 1 September 2025).
- O’Hara, F.J.; Mc Namara, D. Capsule Endoscopy with Artificial Intelligence-Assisted Technology: Real-World Usage of a Validated AI Model for Capsule Image Review. Endosc. Int. Open 2023, 11, E970–E975. [Google Scholar] [CrossRef]
- Schulmann, K.; Hollerbach, S.; Kraus, K.; Willert, J.; Vogel, T.; Moslein, G.; Pox, C.; Reiser, M.; Reinacher-Schick, A.; Schmiegel, W. Feasibility and Diagnostic Utility of Video Capsule Endoscopy for the Detection of Small Bowel Polyps in Patients with Hereditary Polyposis Syndromes. Am. J. Gastroenterol. 2005, 100, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.A.; Santisi, J.; Church, J.; Levinthal, G. The Utility of Capsule Endoscopy Small Bowel Surveillance in Patients with Polyposis. Am. J. Gastroenterol. 2005, 100, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Postgate, A.; Hyer, W.; Phillips, R.; Gupta, A.; Burling, D.; Bartram, C.; Marshall, M.; Taylor, S.; Brown, G.; Schofield, G.; et al. Feasibility of Video Capsule Endoscopy in the Management of Children with Peutz-Jeghers Syndrome: A Blinded Comparison with Barium Enterography for the Detection of Small Bowel Polyps. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 417–423. [Google Scholar] [CrossRef]
- Ohmiya, N.; Nakamura, M.; Takenaka, H.; Morishima, K.; Yamamura, T.; Ishihara, M.; Miyahara, R.; Kawashima, H.; Itoh, A.; Hirooka, Y.; et al. Management of Small-Bowel Polyps in Peutz-Jeghers Syndrome by Using Enteroclysis, Double-Balloon Enteroscopy, and Videocapsule Endoscopy. Gastrointest. Endosc. 2010, 72, 1209–1216. [Google Scholar] [CrossRef]
- Soares, J.; Lopes, L.; Vilas Boas, G.; Pinho†, C. Wireless Capsule Endoscopy for Evaluation of Phenotypic Expression of Small-Bowel Polyps in Patients with Peutz-Jeghers Syndrome and in Symptomatic First-Degree Relatives. Endoscopy 2004, 36, 1060–1066. [Google Scholar] [CrossRef]
- Gupta, A.; Postgate, A.J.; Burling, D.; Ilangovan, R.; Marshall, M.; Phillips, R.K.S.; Clark, S.K.; Fraser, C.H. A Prospective Study of MR Enterography Versus Capsule Endoscopy for the Surveillance of Adult Patients with Peutz-Jeghers Syndrome. Am. J. Roentgenol. 2010, 195, 108–116. [Google Scholar] [CrossRef]
- Eliakim, R. Where Do I See Minimally Invasive Endoscopy in 2020: Clock Is Ticking. Ann. Transl. Med. 2017, 5, 202. [Google Scholar] [CrossRef]
- CapsoVision Inc. Announces Successful Launch of CapsoCam SV-1 at UEGW in Stockholm, Sweden. Available online: https://capsovision.com/capsovision-inc-announces-successful-launch-of-capsocam-sv-1-at-uegw-in-stockholm-sweden/ (accessed on 1 September 2025).
- IntroMedic Co., Ltd. MiroCam® Capsule Endoscope System MC100. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf14/K140751.pdf (accessed on 1 September 2025).
- Capsovision. CapsoVision Announces the Launch of CapsoCam SV-2. Available online: https://capsovision.com/capsovision-announces-the-launch-of-capsocam-sv-2/ (accessed on 1 September 2025).
- Department of Health & Human Services. MiroCam® Capsule Endoscope System MC1200. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf14/K143663.pdf (accessed on 1 September 2025).
- US Food and Drug Administration. MiroCam® Capsule Endoscope System MC1600. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf18/K180732.pdf (accessed on 1 September 2025).
- FDA: US Food and Drug Administration. MiroCam® MC2000 Capsule Endoscope System. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K170438.pdf (accessed on 1 September 2025).
- AnX Robotica. NaviCam® SB with ProScanTM. Available online: https://www.anxrobotics.com/products/navicam-sb-capsule-system/ (accessed on 17 August 2025).
- Cortegoso Valdivia, P.; Elosua, A.; Houdeville, C.; Pennazio, M.; Fernández-Urién, I.; Dray, X.; Toth, E.; Eliakim, R.; Koulaouzidis, A. Clinical Feasibility of Panintestinal (or Panenteric) Capsule Endoscopy: A Systematic Review. Eur. J. Gastroenterol. Hepatol. 2021, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.B.; Rosa, B.; Castro, F.D.D.; Moreira, M.J.; Cotter, J. PillCam COLON 2 © in Crohn’s Disease: A New Concept of Pan-Enteric Mucosal Healing Assessment. World J. Gastroenterol. 2015, 21, 7233–7241. [Google Scholar] [CrossRef] [PubMed]
- Tai, F.W.D.; Ellul, P.; Elosua, A.; Fernandez-Urien, I.; Tontini, G.E.; Elli, L.; Eliakim, R.; Kopylov, U.; Koo, S.; Parker, C.; et al. Panenteric Capsule Endoscopy Identifies Proximal Small Bowel Disease Guiding Upstaging and Treatment Intensification in Crohn’s Disease: A European Multicentre Observational Cohort Study. United Eur. Gastroenterol. J. 2021, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Spada, C.; Hassan, C.; Galmiche, J.; Neuhaus, H.; Dumonceau, J.; Adler, S.; Epstein, O.; Gay, G.; Pennazio, M.; Rex, D.; et al. Colon Capsule Endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2012, 44, 527–536. [Google Scholar] [CrossRef]
- Rex, D.K.; Boland, R.C.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Hosoe, N.; Kamiya, K.J.L.; Takabayashi, K.; Ogata, H.; Kanai, T. A Narrative Review of Recent Progress and Current Perspectives on Video Capsule Endoscopy. Dig. Med. Res. 2021, 4, 5. [Google Scholar] [CrossRef]
- Goenka, M.K. Capsule Endoscopy: Present Status and Future Expectation. World J. Gastroenterol. 2014, 20, 10024. [Google Scholar] [CrossRef]
- Blanco-Velasco, G.; Hernández-Mondragón, O.V.; Solórzano-Pineda, O.M.; García-Contreras, L.F.; Martínez-Camacho, C.; Murcio-Pérez, E. Which Model of Small Bowel Capsule Endoscopy Has a Better Diagnostic Yield? A Systematic Review and Meta-Analysis. Acta Gastro Enterol. Belg. 2022, 85, 509–517. [Google Scholar] [CrossRef]
- Sanchez-Mete, L.; Stigliano, V. Update on Small Bowel Surveillance in Hereditary Colorectal Cancer Syndromes. Tumori J. 2019, 105, 12–21. [Google Scholar] [CrossRef]
- Kim, E.R. Roles of Capsule Endoscopy and Device-Assisted Enteroscopy in the Diagnosis and Treatment of Small-Bowel Tumors. Clin. Endosc. 2020, 53, 410–416. [Google Scholar] [CrossRef]
- Pennazio, M.; Spada, C.; Eliakim, R.; Keuchel, M.; May, A.; Mulder, C.; Rondonotti, E.; Adler, S.; Albert, J.; Baltes, P.; et al. Small-Bowel Capsule Endoscopy and Device-Assisted Enteroscopy for Diagnosis and Treatment of Small-Bowel Disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015, 47, 352–386. [Google Scholar] [CrossRef]
- Cheung, D.Y.; Kim, J.S.; Shim, K.-N.; Choi, M.-G. Korean Gut Image Study Group The Usefulness of Capsule Endoscopy for Small Bowel Tumors. Clin. Endosc. 2016, 49, 21–25. [Google Scholar] [CrossRef]
- Iaquinto, G.; Fornasarig, M.; Quaia, M.; Giardullo, N.; D’Onofrio, V.; Iaquinto, S.; Di Bella, S.; Cannizzaro, R. Capsule Endoscopy Is Useful and Safe for Small-Bowel Surveillance in Familial Adenomatous Polyposis. Gastrointest. Endosc. 2008, 67, 61–67. [Google Scholar] [CrossRef]
- Gorji, L.; Albrecht, P. Hamartomatous Polyps: Diagnosis, Surveillance, and Management. World J. Gastroenterol. 2023, 29, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Phen, C.; Attard, T.M. The Role of Capsule Endoscopy in the Management of Pediatric Hereditary Polyposis Syndromes. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Dal Buono, A.; Gaiani, F.; Poliani, L.; Laghi, L. Juvenile Polyposis Syndrome: An Overview. Best Pract. Res. Clin. Gastroenterol. 2022, 58–59, 101799. [Google Scholar] [CrossRef]
- Levi, Z.; Baris, H.N.; Kedar, I.; Niv, Y.; Geller, A.; Gal, E.; Gingold, R.; Morgenstern, S.; Baruch, Y.; Leach, B.H.; et al. Upper and Lower Gastrointestinal Findings in PTEN Mutation–Positive Cowden Syndrome Patients Participating in an Active Surveillance Program. Clin. Transl. Gastroenterol. 2011, 2, e5. [Google Scholar] [CrossRef]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the Management of Hereditary Colorectal Cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.-J.; Kallenberg, F.G.J.; Clark, S.K.; Dekker, E.; Latchford, A. Frequency and Features of Duodenal Adenomas in Patients With MUTYH-Associated Polyposis. Clin. Gastroenterol. Hepatol. 2016, 14, 986–992. [Google Scholar] [CrossRef]
- The CORGI Consortium; Palles, C.; The WGS500 Consortium; Cazier, J.-B.; Howarth, K.M.; Domingo, E.; Jones, A.M.; Broderick, P.; Kemp, Z.; Spain, S.L.; et al. Germline Mutations Affecting the Proofreading Domains of POLE and POLD1 Predispose to Colorectal Adenomas and Carcinomas. Nat. Genet. 2013, 45, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, R.P.; Hoogerbrugge, N. NTHL1 Defines Novel Cancer Syndrome. Oncotarget 2015, 6, 34069–34070. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Zhao, Z.-Y.; Li, B.-R.; Li, J.; Jin, X.-W.; Yu, E.-D.; Xu, X.-D.; Ning, S.-B. Early Screening the Small Bowel Is Key to Protect Peutz-Jeghers Syndrome Patients from Surgery: A Novel Mutation c.243delG in STK11 Gene. BMC Gastroenterol. 2019, 19, 70. [Google Scholar] [CrossRef]
- Maluenda, C.; Bodas, A.; Paredes, C.; Fernández, S.; Asteinza, M. Capsule Endoscopy in a 15-Year-Old Boy with Peutz-Jeghers Syndrome. Eur. J. Pediatr. 2007, 166, 1087–1088. [Google Scholar] [CrossRef]
- Louro-da-Ponte, A.I.; Taveira-Pinho, R.; Rodrigues, M.A.; Pinto-Pais, M.T.; Pinho Fernandes, C.D.; Ribeiro, I.C.; Silva, J.I.; Rodrigues-Carvalho, J. Advances and Pitfalls in the Management of Small Bowel Polyps in Peutz-Jeghers Syndrome. Rev. Esp. Enferm. Dig. 2015, 107, 390–391. [Google Scholar]
- Kirakosyan, E.; Lokhmatov, M. High-Tech Diagnostic Methods and Enteroscopic Treatment of Children with Peutz-Jeghers Syndrome. Eur. J. Pediatr. Surg. 2020, 30, 529–535. [Google Scholar] [CrossRef]
- Van Lier, M.G.F.; Mathus-Vliegen, E.M.H.; Wagner, A.; Van Leerdam, M.E.; Kuipers, E.J. High Cumulative Risk of Intussusception in Patients With Peutz–Jeghers Syndrome: Time to Update Surveillance Guidelines? Am. J. Gastroenterol. 2011, 106, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; Fleishman, N.R.; Staggs, V.S.; Thomson, M.; Stoecklein, N.; Lawson, C.E.; Washburn, M.P.; Umar, S.; Attard, T.M. Small Intestinal Polyp Burden in Pediatric Peutz–Jeghers Syndrome Assessed through Capsule Endoscopy: A Longitudinal Study. Children 2023, 10, 1680. [Google Scholar] [CrossRef]
- Latchford, A.; Cohen, S.; Auth, M.; Scaillon, M.; Viala, J.; Daniels, R.; Talbotec, C.; Attard, T.; Durno, C.; Hyer, W. Management of Peutz-Jeghers Syndrome in Children and Adolescents: A Position Paper From the ESPGHAN Polyposis Working Group. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 442–452. [Google Scholar] [CrossRef]
- Van Leerdam, M.E.; Roos, V.H.; Van Hooft, J.E.; Dekker, E.; Jover, R.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Pellisé, M.; Saurin, J.-C.; et al. Endoscopic Management of Polyposis Syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Aretz, S.; Auranen, A.; Bruno, M.J.; Cavestro, G.M.; Crosbie, E.J.; Goverde, A.; Jelsig, A.M.; Latchford, A.R.; Van Leerdam, M.E.; et al. The Management of Peutz–Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline. J. Clin. Med. 2021, 10, 473. [Google Scholar] [CrossRef]
- Gastineau, S.; Viala, J.; Caldari, D.; Mas, E.; Darviot, E.; Le Gall, C.; Maurage, C.; Michaud, L.; Dabadie, A. Contribution of Capsule Endoscopy to Peutz-Jeghers Syndrome Management in Children. Dig. Liver Dis. 2012, 44, 839–843. [Google Scholar] [CrossRef]
- De Palma, G.D.; Rega, M.; Ciamarra, P.; Di Girolamo, E.; Patrone, F.; Mastantuono, L.; Simeoli, I. Small-Bowel Polyps in Peutz-Jeghers Syndrome: Diagnosis by Wireless Capsule Endoscopy. Endoscopy 2004, 36, 1039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parsi, M.A.; Burke, C.A. Utility of Capsule Endoscopy in Peutz-Jeghers Syndrome. Gastrointest. Endosc. Clin. N. Am. 2004, 14, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Tomas, C. Update on Imaging of Peutz-Jeghers Syndrome. World J. Gastroenterol. 2014, 20, 10864. [Google Scholar] [CrossRef]
- Brown, G.; Fraser, C.; Schofield, G.; Taylor, S.; Bartram, C.; Phillips, R.; Saunders, B. Video Capsule Endoscopy in Peutz-Jeghers Syndrome: A Blinded Comparison with Barium Follow-Through for Detection of Small-Bowel Polyps. Endoscopy 2006, 38, 385–390. [Google Scholar] [CrossRef]
- Urquhart, P.; Grimpen, F.; Lim, G.J.; Pizzey, C.; Stella, D.L.; Tesar, P.A.; Macrae, F.A.; Appleyard, M.A.; Brown, G.J. Capsule Endoscopy versus Magnetic Resonance Enterography for the Detection of Small Bowel Polyps in Peutz–Jeghers Syndrome. Fam. Cancer 2014, 13, 249–255. [Google Scholar] [CrossRef]
- Aparicio, T.; Pachev, A.; Laurent-Puig, P.; Svrcek, M. Epidemiology, Risk Factors and Diagnosis of Small Bowel Adenocarcinoma. Cancers 2022, 14, 2268. [Google Scholar] [CrossRef] [PubMed]
- Katsinelos, P.; Kountouras, J.; Chatzimavroudis, G.; Zavos, C.; Pilpilidis, I.; Fasoulas, K.; Paroutoglou, G. Wireless Capsule Endoscopy in Detecting Small-Intestinalpolyps in Familial Adenomatous Polyposis. World J. Gastroenterol. 2009, 15, 6075. [Google Scholar] [CrossRef]
- Günther, U.; Bojarski, C.; Buhr, H.-J.; Zeitz, M.; Heller, F. Capsule Endoscopy in Small-Bowel Surveillance of Patients with Hereditary Polyposis Syndromes. Int. J. Colorectal Dis. 2010, 25, 1377–1382. [Google Scholar] [CrossRef]
- Bouchard, S. Video Capsule Endoscopy: Perspectives of a Revolutionary Technique. World J. Gastroenterol. 2014, 20, 17330. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Watabe, H.; Iwama, T.; Obi, S.; Omata, M.; Koike, K. The Prevalence of Small Intestinal Polyps in Patients with Familial Adenomatous Polyposis: A Prospective Capsule Endoscopy Study. Fam. Cancer 2014, 13, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Nakajima, T.; Kakugawa, Y.; Sakamoto, T.; Kuribayashi, S.; Otake, Y.; Matsuda, T.; Kanemitsu, Y.; Taniguchi, H.; Saito, Y. Surveillance Using Capsule Endoscopy Is Safe in Post-Colectomy Patients with Familial Adenomatous Polyposis: A Prospective Japanese Study. Fam. Cancer 2016, 15, 75–83. [Google Scholar] [CrossRef]
- Akin, E.; Demirezer Bolat, A.; Buyukasik, S.; Algin, O.; Selvi, E.; Ersoy, O. Comparison between Capsule Endoscopy and Magnetic Resonance Enterography for the Detection of Polyps of the Small Intestine in Patients with Familial Adenomatous Polyposis. Gastroenterol. Res. Pract. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Tescher, P.; Macrae, F.A.; Speer, T.; Stella, D.; Gibson, R.; Tye-Din, J.A.; Srivatsa, G.; Jones, I.T.; Marion, K. Surveillance of FAP: A Prospective Blinded Comparison of Capsule Endoscopy and Other GI Imaging to Detect Small Bowel Polyps. Hered. Cancer Clin. Pract. 2010, 8, 3. [Google Scholar] [CrossRef]
- Plum, N.; May, A.; Manner, H.; Ell, C. Small-Bowel Diagnosis in Patients with Familial Adenomatous Polyposis: Comparison of Push Enteroscopy, Capsule Endoscopy, Ileoscopy, and Enteroclysis. Z. Gastroenterol. 2009, 47, 339–346. [Google Scholar] [CrossRef]
- Wong, R.F.; Tuteja, A.K.; Haslem, D.S.; Pappas, L.; Szabo, A.; Ogara, M.M.; DiSario, J.A. Video Capsule Endoscopy Compared with Standard Endoscopy for the Evaluation of Small-Bowel Polyps in Persons with Familial Adenomatous Polyposis (with Video). Gastrointest. Endosc. 2006, 64, 530–537. [Google Scholar] [CrossRef]
- Caspari, R.; Von Falkenhausen, M.; Krautmacher, C.; Schild, H.; Heller, J.; Sauerbruch, T. Comparison of Capsule Endoscopy and Magnetic Resonance Imaging for the Detection of Polyps of the Small Intestine in Patients with Familial Adenomatous Polyposis or with Peutz-Jeghers’ Syndrome. Endoscopy 2004, 36, 1054–1059. [Google Scholar] [CrossRef]
- Mata, A.; Llach, J.; Castells, A.; Rovira, J.M.; Pellisé, M.; Ginès, A.; Fernández-Esparrach, G.; Andreu, M.; Bordas, J.M.; Piqué, J.M. A Prospective Trial Comparing Wireless Capsule Endoscopy and Barium Contrast Series for Small-Bowel Surveillance in Hereditary GI Polyposis Syndromes. Gastrointest. Endosc. 2005, 61, 721–725. [Google Scholar] [CrossRef]
- Cavallo, D.; Ballardini, G.; Ferrari, A.; Delconte, G.; Signoroni, S.; Sala, P.; Chiaravalli, S.; Massimino, M.; Bertario, L.; Vitellaro, M. Wireless Capsule Endoscopy in Adolescents with Familial Adenomatous Polyposis. Tumori J. 2016, 102, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Borsotti, E.; Nava, F.L.; Benedicenti, F.; Cini, L.; Magarotto, A.; Ferrari, D.; Cantù, P.; Vitellaro, M.; Rausa, E.; Cavalcoli, F. Hereditary Colorectal Cancer Syndromes: Small Bowel Cancer Risk and Endoscopic Surveillance Strategies. Diagnostics 2025, 15, 819. [Google Scholar] [CrossRef] [PubMed]
- Ocaña Jiménez, J.; López Buenadicha, A.; Nuño Vázquez-Garza, J. Surgical Management of Familial Adenomatous Polyposis: Pancreas-Sparing Duodenectomy or Pancreaticoduodenectomy (Whipple Procedure). Rev. Esp. Enfermedades Dig. 2019, 111, 572–573. [Google Scholar] [CrossRef]
- Ma, T.; Jang, E.J.; Zukerberg, L.R.; Odze, R.; Gala, M.K.; Kelsey, P.B.; Forcione, D.G.; Brugge, W.R.; Casey, B.W.; Syngal, S.; et al. Recurrences Are Common after Endoscopic Ampullectomy for Adenoma in the Familial Adenomatous Polyposis (FAP) Syndrome. Surg. Endosc. 2014, 28, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Drini, M.; Speer, A.; Dow, C.; Collier, N.; Bhathal, P.; Macrae, F.A. Management of Duodenal Adenomatosis in FAP: Single Centre Experience. Fam. Cancer 2012, 11, 167–173. [Google Scholar] [CrossRef]
- Langers, A.; De Vos Tot Nederveen Cappel, W.; Veenendaal, R.; Bonsing, B.; Hardwick, J.; Vasen, H. Double Balloon Endoscopy for Detection of Small-Bowel Adenomas in Familial Adenomatous Polyposis after Pancreaticoduodenectomy According to Whipple. Endoscopy 2008, 40, 773–774. [Google Scholar] [CrossRef]
- Soravia, C.; Berk, T.; Haber, G.; Cohen, Z.; Gallinger, S. Management of Advanced Duodenal Polyposis in Familial Adenomatous Polyposis. J. Gastrointest. Surg. 1997, 1, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Iwamuro, M.; Kawano, S.; Otsuka, M. Differential Diagnoses and Management Approaches for Gastric Polyposis. Gastroenterol. Insights 2024, 15, 122–144. [Google Scholar] [CrossRef]
- Postgate, A.; Will, O.; Fraser, C.; Fitzpatrick, A.; Phillips, R.; Clark, S. Capsule Endoscopy for the Small Bowel in Juvenile Polyposis Syndrome: A Case Series. Endoscopy 2009, 41, 1001–1004. [Google Scholar] [CrossRef]
- Matsumoto, T.; Umeno, J.; Jimbo, K.; Arai, M.; Iwama, I.; Kashida, H.; Kudo, T.; Koizumi, K.; Sato, Y.; Sekine, S.; et al. Clinical Guidelines for Diagnosis and Management of Juvenile Polyposis Syndrome in Children and Adults-Secondary Publication. J. Anus Rectum Colon 2023, 7, 115–125. [Google Scholar] [CrossRef]
- Pavone, P.; Praticò, A.D.; Campisi, C.; Falsaperla, R. A Mild Phenotype Associated with a de Novo Microdeletion 10q23.1-Q23.2: A New Patient with a Novel Feature. BMJ Case Rep. 2016, 2016, bcr2016214388. [Google Scholar] [CrossRef]
- Saito, K.; Nomura, E.; Sasaki, Y.; Abe, Y.; Kanno, N.; Mizumoto, N.; Shibuya, R.; Sakuta, K.; Yagi, M.; Yoshizawa, K.; et al. Characteristics of Small Bowel Polyps Detected in Cowden Syndrome by Capsule Endoscopy. Case Rep. Gastrointest. Med. 2015, 2015, 1–4. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Colas, C.; Pouwels, S.; Hoogerbrugge, N.; PHTS Guideline Development Group; Bisseling, T.; Bubien, V.; Caux, F.; Chabbert-Buffet, N.; Colas, C.; et al. Cancer Surveillance Guideline for Individuals with PTEN Hamartoma Tumour Syndrome. Eur. J. Hum. Genet. 2020, 28, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Muguruma, N.; Igarashi, M.; Ohsumi, S.; Oka, S.; Kakuta, F.; Kubo, Y.; Kumagai, H.; Sasaki, M.; Sugai, T.; et al. Clinical Guidelines for Diagnosis and Management of Cowden Syndrome/PTEN Hamartoma Tumor Syndrome in Children and Adults-Secondary Publication. J. Anus Rectum Colon 2023, 7, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Hatogai, K.; Hosoe, N.; Imaeda, H.; Rey, J.-F.; Okada∥, S.; Ishibashi∥, Y.; Kimura∥, K.; Yoneno∥, K.; Usui∥, S.; Ida∥, Y.; et al. Role of Enhanced Visibility in Evaluating Polyposis Syndromes Using a Newly Developed Contrast Image Capsule Endoscope. Gut Liver 2012, 6, 218–222. [Google Scholar] [CrossRef]
- Zagorowicz, E.S. Small Bowel Tumors Detected and Missed during Capsule Endoscopy: Single Center Experience. World J. Gastroenterol. 2013, 19, 9043. [Google Scholar] [CrossRef]
- Rex, D.K.; Adler, S.N.; Aisenberg, J.; Burch, W.C.; Carretero, C.; Chowers, Y.; Fein, S.A.; Fern, S.E.; Fernandez-Urien Sainz, I.; Fich, A.; et al. Accuracy of Capsule Colonoscopy in Detecting Colorectal Polyps in a Screening Population. Gastroenterology 2015, 148, 948–957.e2. [Google Scholar] [CrossRef]
- Pecere, S.; Senore, C.; Hassan, C.; Riggi, E.; Segnan, N.; Pennazio, M.; Sprujievnik, T.; Rondonotti, E.; Baccarin, A.; Quintero, E.; et al. Accuracy of Colon Capsule Endoscopy for Advanced Neoplasia. Gastrointest. Endosc. 2020, 91, 406–414.e1. [Google Scholar] [CrossRef]
- Turvill, J.; Haritakis, M.; Pygall, S.; Bryant, E.; Cox, H.; Forshaw, G.; Musicha, C.; Allgar, V.; Logan, R.; McAlindon, M. Multicentre Study of 10,369 Symptomatic Patients Comparing the Diagnostic Accuracy of Colon Capsule Endoscopy, Colonoscopy and CT Colonography. Aliment. Pharmacol. Ther. 2025, 61, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- PillCamTM Software V9. Available online: https://www.medtronic.com/covidien/en-gb/products/capsule-endoscopy/pillcam-software.html (accessed on 1 September 2025).
- Rácz, I.; Jánoki, M.; Kovács, V. Measurement of Small-Bowel Polyp Size in Patients with Peutz-Jeghers Syndrome by Using Reference Granules during Video Capsule Endoscopy. Endoscopy 2007, 39, E41. [Google Scholar] [CrossRef][Green Version]
- Hosoe, N.; Horie, T.; Tojo, A.; Sakurai, H.; Hayashi, Y.; Limpias Kamiya, K.J.-L.; Sujino, T.; Takabayashi, K.; Ogata, H.; Kanai, T. Development of a Deep-Learning Algorithm for Small Bowel-Lesion Detection and a Study of the Improvement in the False-Positive Rate. J. Clin. Med. 2022, 11, 3682. [Google Scholar] [CrossRef]
- Lee, J.; Wallace, M.B. State of the Art: The Impact of Artificial Intelligence in Endoscopy 2020. Curr. Gastroenterol. Rep. 2021, 23, 7. [Google Scholar] [CrossRef]
- Deding, U.; Herp, J.; Havshoei, A.; Kobaek-Larsen, M.; Buijs, M.; Nadimi, E.; Baatrup, G. Colon Capsule Endoscopy versus CT Colonography after Incomplete Colonoscopy. Application of Artificial Intelligence Algorithms to Identify Complete Colonic Investigations. United Eur. Gastroenterol. J. 2020, 8, 782–789. [Google Scholar] [CrossRef]
- Sui, Z.; Wan, C.; Cheng, H.; Yang, B. Micro/Nanorobots for Gastrointestinal Tract. Front. Chem. 2024, 12, 1423696. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.W.; Gordon, M.; Sinopolou, V.; Radford, S.J.; Darie, A.-M.; Vuyyuru, S.K.; Alrubaiy, L.; Arebi, N.; Blackwell, J.; Butler, T.D.; et al. British Society of Gastroenterology Guidelines on Inflammatory Bowel Disease in Adults: 2025. Gut 2025, 74, s1–s101. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Veraldi, S.; Russo, G.; Aloi, M.; Rizzello, F.; Gionchetti, P.; Alvisi, P.; Labriola, F.; Vecchi, M.; Eidler, P.; et al. Pan-Enteric Capsule Endoscopy to Characterize Crohn’s Disease Phenotypes and Predict Clinical Outcomes in Children and Adults: The Bomiro Study. Inflamm. Bowel Dis. 2025, 31, 636–646. [Google Scholar] [CrossRef]
- Lei, I.I.; O’Connell, N.; Adu-Darko, M.A.; Parambil, J.; Suresh, V.; Mc Donnell, K.; Newville, J.; Chaplin, K.; Siyambalapityage, D.; Khan, A.; et al. From Stool to Scope: Optimising FIT Thresholds to Guide Future Panenteric Capsule Endoscopy and Reduce Colonoscopy Burden in Iron Deficiency Anaemia. Cancers 2025, 17, 1951. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, S. The Cost-Effectiveness of Video Capsule Endoscopy. Gastrointest. Endosc. Clin. N. Am. 2021, 31, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Zhou, H.-C.; Ma, M.; Zhang, H.-X.; Jia, X. Comparison of Magnetic Resonance Enterography, Capsule Endoscopy and Gastrointestinal Radiography of Children with Small Bowel Crohn’s Disease. Exp. Ther. Med. 2013, 6, 115–120. [Google Scholar] [CrossRef]
- Taylor, S.A.; Mallett, S.; Bhatnagar, G.; Morris, S.; Quinn, L.; Tomini, F.; Miles, A.; Baldwin-Cleland, R.; Bloom, S.; Gupta, A.; et al. Magnetic Resonance Enterography Compared with Ultrasonography in Newly Diagnosed and Relapsing Crohn’s Disease Patients: The METRIC Diagnostic Accuracy Study. Health Technol. Assess. 2019, 23, 1–162. [Google Scholar] [CrossRef]
- NHS. England 2022/23 National Tariff Workbook (Annex A). Available online: https://www.england.nhs.uk/wp-content/uploads/2020/11/22-23NT_Annex-DtA-National-tariff-workbook.xlsx (accessed on 12 October 2025).
| Capsule Name | Manufacturer | Year Released |
|---|---|---|
| PillCam SB [7,8,9,10] | Given Imaging Ltd. (Yokneam, Israel) | 2001 |
| EndoCapsule [10] | Olympus (Tokyo, Japan) | 2005 |
| Pillcam SB2 [11,12] | Medtronic (Minneapolis, MN, USA) | 2007 |
| OMOM Capsule [13] | Jinshan Science & Technology (Chongqing, China) | 2008 |
| Capsocam SV-1 [14] | Capsovision (Saratoga, NY, USA) | 2011 |
| PillCam SB3 [3] | Medtronic (Minneapolis, MN, USA) | 2013 |
| MiroCam MC1000 [15] | Intromedic (Seoul, Republic of Korea) | 2014 |
| Endocapsule 10 [2] | Olympus (Tokyo, Japan) | 2014 |
| Capsocam SV-2 [16] | Capsovision (Saratoga, NY, USA) | 2014 |
| Mirocam MC1200 [17] | Intromedic (Seoul, Republic of Korea) | 2015 |
| OMOM Capsule 2 [13] | Jinshan Science & Technology (Chongqing, China) | 2018 |
| MiroCam MC 1600 [18] | Intromedic (Seoul, Republic of Korea) | 2018 |
| Mirocam MC2000 [19] | Intromedic (Seoul, Republic of Korea) | 2018 |
| CapsoCam Plus [5] | Capsovision (Saratoga, NY, USA) | 2019 |
| OMOM HD [6] | Jinshan Science and Technology (Yubei, China) | 2020 |
| MiroCam 4000 [4] | Intromedic (Seoul, Republic of Korea) | 2020 |
| NaviCam SB with ProScan™ [20] | AnX Robotica (Plano, TX, USA) | 2024 |
| Polyposis Syndrome | Inheritance Pattern | Polyp Type | Small Bowel Involvement | Use of VCE for Surveillance | Additional Comments |
|---|---|---|---|---|---|
| PJS | Autosomal dominant [7] | Hamartomatous [7] | Invariably [30] | Start at the age of 8 and repeat every 3 years [31]. | VCE versus MRE: a. VCE better in the detection of jejuno-ileal polyps less than 10 mm [30] b. VCE may miss larger polyps [32] |
| FAP | Autosomal dominant [33] | Adenomatous [33] | 80%, especially in duodenum [33] | Not routinely [2]. | N/A |
| JPS | Autosomal dominant [34] | Juvenile [34] | 7% in jejunum, ileum or duodenum [34] | Not routinely (Only in symptomatic patients and in those with SMAD4 variants) [35]. | VCE suggested to rule out HHT [36]. |
| PTEN-HS | Autosomal dominant [34] | Hyperplastic/Hamartomatous/Juvenile/Adenomatous [34] | 90% duodenum [37] | Not routinely [35]. | N/A |
| MAP | Autosomal recessive [38] | Adenomatous [38] | Duodenal involvement 34% [39] | Not routinely [38]. | N/A |
| PPAP | Autosomal dominant [40] | Adenomatous [40] | N/A (association with colorectal cancer) [40] | N/A | N/A |
| NAP | Autosomal recessive [41] | Adenomatous [41] | N/A (association with colorectal cancer) [41] | N/A | N/A |
| Author | Study Design | Year | Population | No of Patients | Capsule Type | Comparator | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| Postgate et al. | Prospective | 2009 | Paediatric | 11 | SB1 | Barium enterography | More polyps were found by SB1 compared with barium enterography (p = 0.02), but there was no difference for polyps larger than 10 mm. | [9] |
| Brown et al. | Prospective | 2006 | Adults | 19 | SB1 | Barium enterography | SB1 detected more polyps larger than 10 mm when compared with barium studies (p = 0.008). | [55] |
| Burke et al. | Prospective | 2005 | Adults post-colectomy (mixed population with PJS/FAP) | 3 with PJS | SB1 | SB radiography | In two cases, SB1 revealed diffuse polyposis while radiography was normal. In another patient, SB1 detected more than 20 diffuse small bowel polyps larger than 10 mm, but only 1 polyp (less than 1 cm) was found during radiography [8]. | [8] |
| Ohmiya et al. | Retrospective | 2010 | Adults | 18 | SB1 | Fluoroscopic enteroclysis/ Double-balloon enteroscopy | SB1 polyp detection rates were similar to enteroscopy, while less polyps were found by enteroclysis | [10] |
| Schulmann et al. | Prospective | 2005 | Adults | 11 | SB1 | MRE/Push enteroscopy | Among symptomatic individuals, both MRE and SB1 detected distal ileal polyps, while push-enteroscopy failed to detect a large lesion. In the asymptomatic group, SB1 revealed jejunal or ileal polyps that went undetected by push-enteroscopy in four patients. | [7] |
| Urquhart et al. | Prospective | 2014 | Adults | 20 | SB1 | MRE | The total number of polyps ≥ 10 mm detected by SB1 was greater compared with MRE. | [56] |
| Gupta et al. | Prospective | 2010 | Adults | 19 | SB2 | MRE | SB2 detected more polyps smaller than 10 mm compared with MRE (p = 0.03). | [12] |
| Criterion | Points | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Polyp number | 1–4 | 5–20 | >20 |
| Polyp size (mm) | 1–4 | 5–10 | >10 |
| Histology | Tubular | Tubulovillous | Villous |
| Dysplasia | Mild | Moderate | Severe |
| Author | Study Design | Year | Population | No of FAP Patients | Capsule Type | Comparator | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|
| Caspari et al. | Prospective | 2004 | Mixed patients (FAP/PJS) | 16 FAP | SB1 | MRE | SB1 and MRE detection rates for polyps larger than 15 mm were similar, whereas smaller polyps were seen more often with SB1 and polyps smaller than 5 mm were exclusively seen with SB1. | [67] |
| Akin et al. | Prospective | 2012 | Only FAP | 6 | SB1 | MRE | Four patients had polyps ≤ 10 mm, seen only by SB1. | [63] |
| Mata et al. | Prospective | 2005 | Mixed patients (FAP/PJS) | 20 FAP | SB1 | SB follow-through | In 4 FAP patients, polyps detected by SB1 but not reported in radiographic series. | [68] |
| Tescher et al. | Prospective | 2010 | Only FAP | 20 | SB1 | MRE/SBFT/Side-viewing gastroscopy | SB1 was the only imaging modality that identified polyps in all bowel segments, demonstrating a significantly higher total number of polyp findings in the jejunum, ileum and caecum than MRE and SBFT. | [64] |
| Plum et al. | Prospective | 2009 | Only FAP | 25 | SB1 | Push enteroscopy/ Ileoscopy/ Enteroclysis | Thirteen of the patients had adenomas in regions not accessible to push enteroscopy or ileoscopy. SB1 was a safe and convenient method for evaluating the small bowel in these patients, while enteroclysis wasinferior to the endoscopic procedures for evaluation of the small bowel in FAP. | [65] |
| Wong et al. | Prospective | 2006 | Only FAP | 32 | SB1 | Push enteroscopy /Ileocolonoscopy | Compared to SB1, the combination of push enteroscopy and lower endoscopy also detected significantly more polyps throughout the entire examined small bowel (p < 0.001). | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manti, M.; Laskaratos, F.-M.; Latchford, A.; Monahan, K.; Epstein, O.; Humphries, A. The Role of Video Capsule Endoscopy in Hereditary Polyposis Syndromes: A Narrative Review. Diagnostics 2025, 15, 2813. https://doi.org/10.3390/diagnostics15212813
Manti M, Laskaratos F-M, Latchford A, Monahan K, Epstein O, Humphries A. The Role of Video Capsule Endoscopy in Hereditary Polyposis Syndromes: A Narrative Review. Diagnostics. 2025; 15(21):2813. https://doi.org/10.3390/diagnostics15212813
Chicago/Turabian StyleManti, Magdalini, Faidon-Marios Laskaratos, Andrew Latchford, Kevin Monahan, Owen Epstein, and Adam Humphries. 2025. "The Role of Video Capsule Endoscopy in Hereditary Polyposis Syndromes: A Narrative Review" Diagnostics 15, no. 21: 2813. https://doi.org/10.3390/diagnostics15212813
APA StyleManti, M., Laskaratos, F.-M., Latchford, A., Monahan, K., Epstein, O., & Humphries, A. (2025). The Role of Video Capsule Endoscopy in Hereditary Polyposis Syndromes: A Narrative Review. Diagnostics, 15(21), 2813. https://doi.org/10.3390/diagnostics15212813







