Abstract
Background: Trismus, or restricted mouth opening, can present significant challenges in oral and maxillofacial surgery and trigger substantial functional and psychosocial disabilities. Intra-articular causes, such as temporomandibular joint ankylosis and arthritis, are thoroughly described; however, extra-articular pathologies like neoplastic, traumatic, infectious, and fibrotic conditions of adjacent soft and hard tissues are less frequently reported and present distinct diagnostic complexities and therapeutic hurdles. This retrospective study aims to investigate the difficulties encountered in diagnosis and surgical interventions associated with rare extra-articular causes of trismus. Material and Methods: This article describes five rare causes of extra-articular trismus. The cases range from benign pathologies like coronoid hyperplasia and osteomas to more complex diagnoses of myositis ossificans, external auditory canal abscess, and chronic osteomyelitis. A thorough diagnostic workup was performed for each patient, and specific surgical interventions were administered based on their pathology. Results: All five patients showed significant improvements in mouth opening after surgery. Diagnostic accuracy was ensured with advanced imaging modalities and innovative surgical techniques, and adequate postoperative care translated the favorable outcome. Conclusions: Although based on individual case descriptions, this study emphasizes the potential importance of early diagnosis, a multidisciplinary approach, and individualized treatment planning in managing rare extra-articular causes of trismus. These cases suggest a basis for a more organized system for the timely identification and treatment of such conditions. Additional research is needed to improve diagnostic accuracy, optimize surgical management, and develop evidence-based aftercare treatment to improve patient care and quality of life.
1. Introduction
Trismus, or restricted mouth opening, is a common problem encountered in oral and maxillofacial surgery []. The term originates from the Greek word “τρισμός” (trismos), meaning gnashing or grinding, and is described as a sustained, tetanic spasm of the mastication muscles that hinders normal jaw movement [,]. It is often considered both a symptom and a complication of temporomandibular disorders (TMDs), a collective term encompassing clinical conditions affecting the temporomandibular joint (TMJ), the masticatory muscles, and associated structures. TMDs are among the most prevalent musculoskeletal disorders in the craniofacial region, affecting approximately 31% to 34% of the population, with higher prevalence reported in women and individuals aged 20–40 years [,].
The normal range of mouth opening is variable, usually between 40 and 60 mm; an interincisal distance of less than 35 mm is often associated with severe functional disturbance []. Patients with trismus experience considerable difficulties in eating, speaking, and maintaining oral hygiene, which can severely impact their psychosocial well-being and quality of life [].
The etiology of trismus has a wide range, including both intra-articular and extra-articular causes. Intra-articular pathologies, such as temporomandibular joint ankylosis or arthritis, are well-documented and often recognized early, whereas extra-articular causes are rare and often present a challenge for the clinician in the diagnosis process due to their low prevalence and heterogeneity. The uncommon causes involve neoplastic, traumatic, infectious, or fibrotic processes in the surrounding soft and hard tissues [,].
Knowledge of the underlying pathology, along with appropriate management and treatment, is essential, and prolonged treatable delay and inappropriate treatment lead to progressive functional decline, decreased quality of life, and worse prognosis [,]. The previously available literature on this topic is limited and primarily consists of isolated case reports, probably due to the uncommonness of extra-articular causes, despite the advancements in imaging and surgical techniques. This discrepancy highlights the importance of a holistic perspective on the diagnosis, categorization, and management of such infrequent diagnoses [].
We describe several uncommon extra-articular causes of trismus and the challenges encountered while diagnosing and managing each entity. By presenting five rare clinical cases, we aim to contribute to understanding these unusual pathologies and enhance a systematic approach to their management. These conditions are individually infrequent, with the existing literature largely limited to isolated case reports or small case series. The scarcity of comprehensive epidemiological data reflects the inherent difficulty in studying such rare entities; nevertheless, their potential to cause significant functional impairment underscores the importance of early recognition and individualized treatment.
We hypothesize that such rare extra-articular causes of trismus are frequently underrecognized and that their structured presentation can contribute to earlier diagnosis and more effective treatment strategies.
2. Materials and Methods
A retrospective review was performed on patients diagnosed with rare extra-articular causes of trismus, treated at the Department of Oral and Maxillofacial Surgery from January 2018 to December 2024. The inclusion criteria discussed are those of cases with reduced mouth opening (defined as an interincisal distance of less than 35 mm) [,], secondary to extra-articular pathologies, which were confirmed by clinical assessment and imaging. This level of reduction was associated with significant functional impairment in daily activities, such as eating, speaking, and maintaining oral hygiene. Cases with intra-articular causes of trismus or incomplete medical records were excluded from the study.
Demographic details, clinical presentation, radiological findings, type of pathology, surgical approach performed, and postoperative outcome in mouth opening (interincisal distance) were available from the records. All patients underwent diagnostic imaging (CT or MRI) and received personalized surgical management depending on their specific diagnosis.
The study complied with the ethical principles of the Declaration of Helsinki (1964) (revised 2024).
- Case 1
A 48-year-old male patient was referred to the Department of Oral and Maxillofacial Surgery with a complaint of progressive limitation of mouth opening for the past six months. The main complaint of the patient was recurrent difficulty in eating, which significantly affected his daily life. Past medical history was unremarkable, and the patient had experienced no previous trauma to the oral or maxillofacial region, nor did he report any related symptoms, including pain, swelling, or joint noises. Clinical examination revealed a maximum interincisal mouth opening of 19 mm, which is less than normal. No other abnormalities were found in the intraoral examination with respect to the mucosa (i.e., no lesions or masses) or signs of infection.
A panoramic radiograph was performed to investigate the limited mouth opening, which showed a well-defined radiopaque mass at the right coronoid process. A computed tomography (CT) scan was performed to further characterize the lesion and its relationship with surrounding structures. CT images showed an enlarged and elongated right coronoid process with its superior margin extending posteriorly and directly posterior to the zygomatic bone (Figure 1). This abnormal positioning indicated a mechanical obstruction in jaw movement, reducing mouth opening. Based on the imaging findings and the clinical history of slow symptom progression, a provisional diagnosis of a benign osseous neoplasm, likely an osteoma of the right mandibular coronoid process, was made.
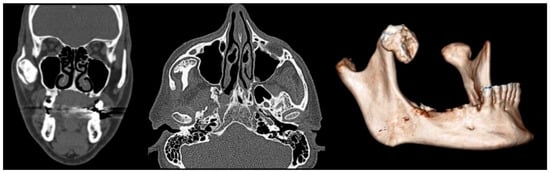
Figure 1.
Coronal (left), axial (center), and 3D reconstructed (right) CT images demonstrating an enlarged and elongated right coronoid process.
Due to a marked restriction of mouth opening, a surgical reconstruction was planned after detailed preoperative planning. The procedure was conducted under general anesthesia with nasotracheal intubation for an unobstructed surgical field. An intraoral approach, combined with an extraoral approach, was deemed appropriate to access the lesion and obtain complete excision of the affected coronoid process.
The intraoral approach began with a similar incision to that performed for the sagittal split osteotomy. This technique offered direct access to the coronoid process. The periosteum and masseter muscle were meticulously elevated on the ascending ramus of the mandible to provide access to the coronoid process. For coronoid process detachment, the temporalis muscle insertion along the anterior border of the ramus was released, and the tendon was dissected and separated from the coronoid process. One channel retractor was introduced into the sigmoid notch to stabilize the field, and a ramus clamp was applied to push and secure the coronoid process. A low coronoidectomy was completed using a reciprocating saw from the sigmoid notch to the anterior oblique ridge. Despite these attempts, the coronoid process could not be resected due to the proximity to the zygomatic arch and lack of intraoral access.
Given the intraoperative difficulties, an additional extraoral approach was undertaken. Direct access to the zygomatic arch was achieved with a hemi-coronal incision with preauricular extension (Figure 2). Following the elevation of the periosteum, osteotomy of the zygomatic arch was performed at two different points to obtain broad access to the coronoid process. Prior to the osteotomy, a 1.5 mm titanium plate was placed to stabilize the anatomical positioning of the arch during the postoperative period. Once the coronoid process was fully exposed, it was successfully removed. The zygomatic arch was then repositioned anatomically and stabilized using the preplaced titanium plate.
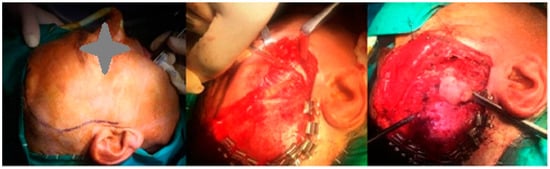
Figure 2.
Intraoperative photographs demonstrating the additional extraoral approach. (Left) Marking of the hemi-coronal incision with preauricular extension. (Middle) Dissection to expose the zygomatic arch. (Right) Direct visualization and access to the coronoid process through the extended approach, allowing improved surgical access due to intraoperative limitations encountered during the initial phase.
The resected specimen was a bony mushroom-shaped mass (approximately 2.5 cm × 3 cm × 3 cm) (Figure 3). Histopathological examination of the lesion showed a highly cellular collagenized stroma in continuity with interspersed bony trabeculae. At higher magnification, cancellous lamellated bone was evident, with lacunae containing osteocytes and osteoblastic rimming. These findings were consistent with the diagnosis of an osteoma, a benign bone-forming tumor.
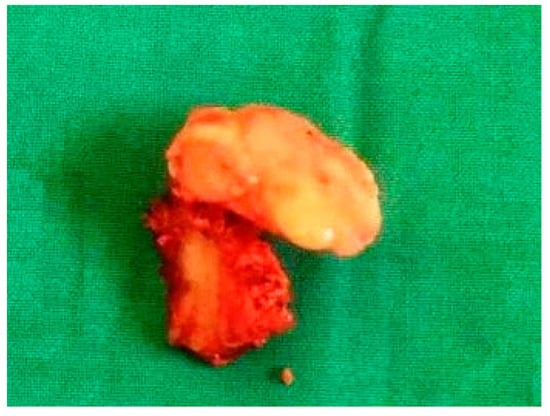
Figure 3.
The resected specimen shows a bony, mushroom-shaped mass.
The postoperative recovery was uneventful, with no immediate complications like infection, nerve injury, or malocclusion. The patient showed a marked improvement in mouth opening, which increased to 36 mm. He was also counseled to initiate physiotherapy and mouth-opening exercises to improve the function of the mandible and to prevent fibrosis postoperatively. At the three-month follow-up, the patient’s mouth opening improved to 39 mm (Figure 4). A 1-year follow-up examination revealed stability of surgical results without signs of recurrence or functional impairment.

Figure 4.
Postoperative clinical photographs showing improved mouth opening. (Left) Measurement of interincisal distance at three-month follow-up demonstrates an opening of 39 mm. (Right) One-year follow-up confirms stability of surgical results with maintained mouth opening and no signs of recurrence or functional limitation.
- Case 2
A 10-year-old female patient was referred to the Department of Oral and Maxillofacial Surgery by her pediatrician due to a progressive limitation in mouth opening. The patient had difficulty with activities like eating and speaking without any associated pain. Intraoral examination showed a maximum interincisal opening (MIO) of 17 mm, which was remarkably limited for her age. Her medical history was unremarkable, and no injuries, infections, or systemic illnesses were documented. There was no family history of similar symptoms, and an examination of the temporomandibular joints showed no sounds, pain, or signs of dysfunction.
Initial panoramic radiography showed bilateral elongation of the coronoid processes (Figure 5), indicating structural abnormalities as the cause of her restricted mouth opening.
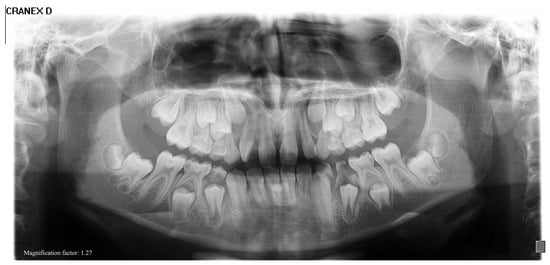
Figure 5.
Initial panoramic radiograph showing bilateral elongation of the coronoid processes. The structural abnormality is evident on both sides and correlates clinically with the patient’s restricted mouth opening.
Computed tomography (CT) imaging offered enhanced visualization, confirming the elongation of the bilateral coronoid processes and revealing the presence of heterotopic bone on the medial and inferior surfaces of the bilateral zygomatic arches (Figure 6). These findings indicated mechanical interference between the coronoid processes and the zygomatic arches during mandibular movement. Based on the clinical and imaging findings, as well as the history of slow symptom progression, a diagnosis of coronoid impingement syndrome (CIS) was established.
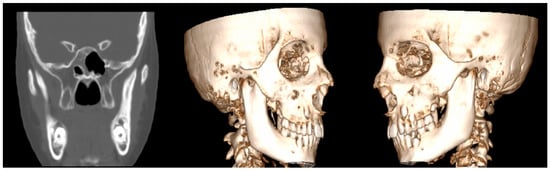
Figure 6.
Coronal CT slice (left) and 3D reconstructed views (center and right) demonstrating bilateral elongation of the coronoid processes. Additionally, heterotopic bone formation is visible on the medial and inferior surfaces of the bilateral zygomatic arches, contributing to the patient’s limited mouth opening.
Surgical treatment was planned in order to restore functional mouth opening. Nasotracheal intubation was expected to be challenging because of the significant restriction of the mouth opening. General anesthesia was achieved, and nasotracheal intubation was conducted with a fiberoptic scope because the head-scope-guided approach was impossible. An intraoral approach was used for the bilateral coronoidectomies to reduce external scarring and postoperative morbidity. Bilateral buccal mucosa incisions at the level of the ascending ramus of the mandible were made to expose the coronoid processes. The periosteum and surrounding soft tissues were elevated, exposing the elongated coronoid processes. Performing the procedure with a reciprocating saw, the coronoid processes were resected (Figure 7) entirely on both sides to remove mechanical interference with the zygomatic arches. Adequate hemostasis was obtained during the procedure.
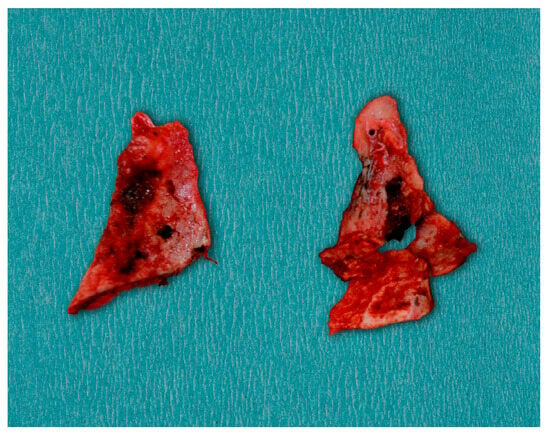
Figure 7.
Resected bilateral coronoid processes.
Immediately following surgery, the operating room’s maximal interincisal opening increased to 37 mm. This immediate improvement confirmed the restriction’s mechanical nature and the intervention’s success. Postoperative recovery was uneventful, without any complications. The patient was discharged with instructions to begin a structured physiotherapy program, including mouth-opening exercises, starting four weeks postoperatively to prevent fibrosis and maintain functional improvement.
At the ten-month follow-up, the maximal interincisal opening had improved to 45 mm, reflecting sustained functional gains. At the one-year follow-up, the patient exhibited stable and satisfactory mouth opening with no signs of recurrence or postoperative complications. The surgical outcome was highly successful, and the patient had fully recovered.
- Case 3
A 34-year-old male patient was referred by his dentist to the Department of Oral and Maxillofacial Surgery, complaining of reduced mouth opening that had started about a month earlier. The clinical examination showed a maximal incisal opening (MIO) of 15 mm (Figure 8). The patient’s medical history was notable for a facial injury on the right side sustained three months prior. There was no past medical history of systemic disease or other pertinent conditions.
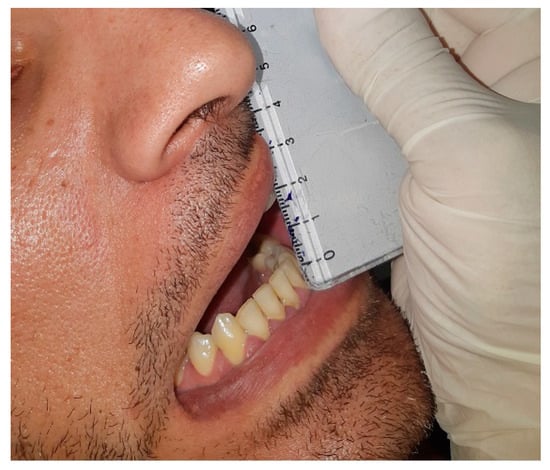
Figure 8.
Preoperative clinical photograph showing a maximal incisal opening (MIO) of 15 mm, indicating a significant limitation in mouth opening.
Imaging studies were performed to assess the restricted mouth opening. Orthopantomography and computed tomography (CT) showed a well-defined calcified mass in the right masseter muscle (Figure 9). There was no evidence of fractures or abnormality of the cranial visceral bones. Laboratory tests showed normal serum calcium, phosphorus, and parathyroid hormone levels, excluding systemic metabolic causes of calcification. Given the clinical history and imaging findings, along with the background of previous trauma with gradual symptom development, a provisional diagnosis of myositis ossificans traumatica (MOT) was made.
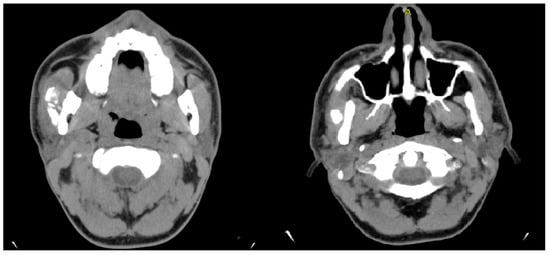
Figure 9.
Axial CT images revealing a well-defined calcified mass within the right masseter muscle.
Surgical intervention was indicated, and the patient was transferred to the operating room. Under general anesthesia, fiberoptic-assisted nasotracheal intubation was performed owing to the limited opening of the mouth. The calcified mass in the right masseter muscle was excised through the intraoral approach to avoid excessive visible scarring. The lesion was circumscribed and measured 2.6 cm at its largest diameter, allowing for excision. Histopathological examination showed alternating lamellar bone with fat cells, fibrous tissue, and thin-walled vascular spaces, consistent with a diagnosis of MOT.
The patient had an uneventful immediate postoperative recovery without any complications. Upon discharge, the MIO had improved to approximately 34 mm. A regimen of forced physical therapy and mouth-opening exercises was recommended to prevent fibrosis and maintain an improved range of motion.
Two months after surgery, the patient returned to the department complaining of a new reduction in mouth opening. Clinical examination revealed an MIO of 20 mm. Imaging studies, including CT scans, identified a well-defined calcified lesion within the left masseter muscle, distinct from the previous surgical site (Figure 10). Given the recurrence of symptoms, a second surgical intervention was planned. Surgery was performed under general anesthesia with fiberoptic-assisted nasotracheal intubation, and the calcified mass in the left masseter muscle was excised by the intraoral approach.
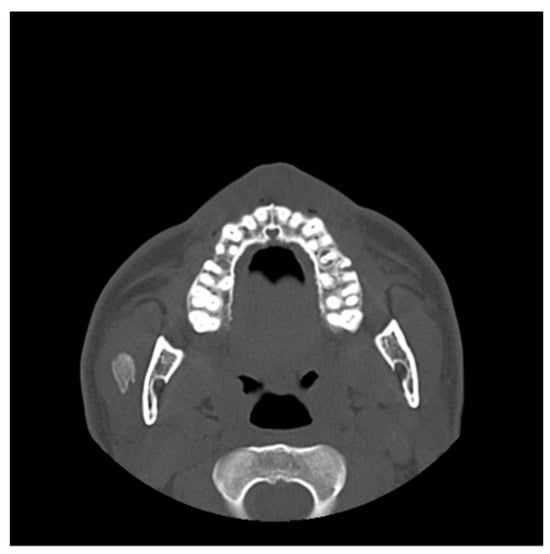
Figure 10.
An axial CT scan showed a well-defined calcified lesion within the left masseter muscle, anatomically distinct from the previous surgical site. The radiologic appearance is consistent with recurrent or new-onset myositis ossificans.
Histological analysis of the excised lesion described a central zone of bone tissue with abundant osteoblasts surrounded by mature bone, findings consistent with myositis ossificans. The edges of the specimen were free of tumor or other pathological findings, ensuring complete excision.
The postoperative course was again uneventful, and the patient demonstrated a significant improvement in mouth opening, with an MIO of 36 mm achieved soon after surgery. He was once more advised to perform mouth-opening exercises and continue physiotherapy. At the six-month follow-up, the patient’s MIO had increased to 52 mm (Figure 11), reflecting excellent functional recovery. At the one-year follow-up, the patient remained asymptomatic, with no signs of recurrence and stable mouth-opening capacity, indicating a successful surgical outcome and long-term resolution of the condition.
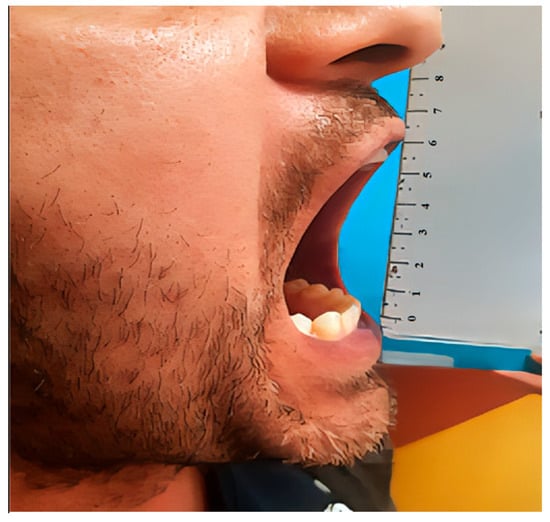
Figure 11.
Clinical photograph at six-month follow-up showing a maximal incisal opening (MIO) of 52 mm, demonstrating excellent functional recovery and restoration of normal mandibular mobility.
- Case 4
A 44-year-old healthy male patient was referred to the Craniomaxillofacial Surgery Department by his physiotherapist due to severe restriction of mouth opening. The patient reported a history of extraction of an impacted mandibular third molar (#38) three years prior. According to the patient, the extraction was straightforward and without immediate complications. As per his medical history, the onset of reduced mouth opening occurred suddenly and without apparent cause. Initial clinical and imaging evaluations by the patient’s dentist prompted a referral to a specialized physiotherapy center within our hospital.
Despite undergoing nine physiotherapy sessions, there was no improvement in the patient’s condition. During this period, the patient developed episodic intermittent hypoesthesia of the left inferior alveolar nerve. Given the inadequate response following therapy, he was referred to our clinic for further evaluation and management. On presentation, the patient exhibited no abnormal swelling, facial asymmetry, or hypertrophy of the masticatory muscles. The temporomandibular joint (TMJ) showed no frictional sounds, and no compression pain was elicited upon palpation of the joint areas bilaterally. The maximal incisal opening (MIO) was limited to 13 mm, and the posterior dental contacts were symmetrical.
A panoramic radiograph demonstrated normal TMJ articulation bilaterally, with no evidence of odontogenic infection or structural abnormalities (Figure 12). Magnetic resonance imaging (MRI) revealed mild bilateral anterior disc dislocation with arthropathy, more pronounced on the left side, and incomplete translation of the disc on mouth opening. No intracranial pathology or secondary cause of head and facial pain was identified. A computed tomography (CT) scan ruled out any pathological findings in the mandible.
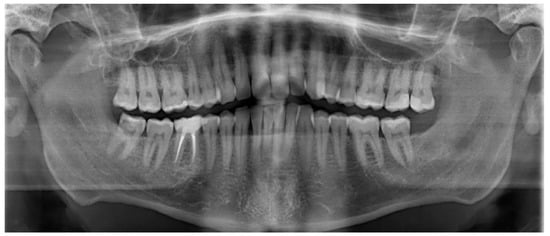
Figure 12.
Postoperative panoramic radiograph showing normal bilateral temporomandibular joint (TMJ) articulation. No signs of odontogenic infection, recurrence, or structural abnormalities are present.
Based on the findings, a provisional diagnosis of tendomyopathy of the masseter muscles was established. The patient was started on tizanidine (4 mg) and re-referred for ongoing physiotherapy. After 1 week, the patient stopped taking the medication because of dizziness and muscle limpness. He also did not want to continue with physiotherapy. Tolperisone (150 mg) replaced tizanidine, which slightly improved trismus. Three months later, the patient presented with worsening symptoms. Mouth opening had further reduced to 2 mm, and he presented with swelling in the region of the left mandibular angle. The hypoesthesia of the inferior alveolar nerve, which had been intermittent, was now persistent. The patient was afebrile and reported no difficulty swallowing. An MRI revealed findings suggesting a developing abscess adjacent to focal cortical defects on the lateral side of the distal mandibular ramus, with possible incipient abscess formation medially (Figure 13). The masticatory musculature was associated with diffuse edematous or phlegmonous swelling, particularly the masseter and medial pterygoid muscles on the left side. The distal mandibular ramus also showed an increased bone marrow signal, corresponding to acute osteomyelitis.
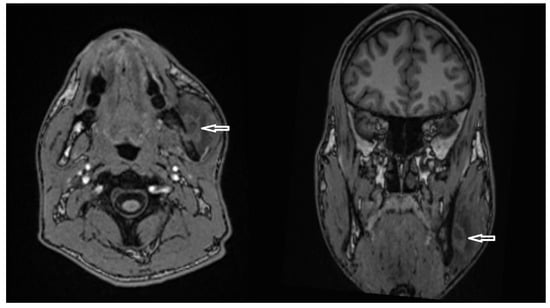
Figure 13.
Axial (left) and coronal (right) T1-weighted MRI images showing a hyperintense lesion adjacent to the lateral aspect of the distal mandibular ramus (white arrows), suggestive of a developing abscess.
Surgical management by incision and drainage of the abscess and surgical curettage was performed under local anesthesia. Gram stain, culture, and sensitivity testing of swabs taken during the procedure showed a polymicrobial anaerobic population primarily composed of the Streptococcus milleri group. The histopathological study showed foci of chronic inflammatory infiltration and medullary fibrosis, leading to the diagnosis of late-diagnosed chronic osteomyelitis of the mandible. Pharmaceutical management consisted of a combination of amoxicillin (875 mg)/clavulanic acid (125 mg) and ibuprofen (600 mg) for anti-inflammatory. Fifteen days postoperatively, during a follow-up examination, the patient presented with a marked mouth opening measuring 39 mm, lower swelling in the mandibular angle region, and significant pain relief.
Further CT imaging disclosed a localized osteolysis area in the left mandibular corpus at the height of the mandibular canal (Figure 14), along with slight bony dehiscence of the medial and lateral compacta. No residual or adjacent soft tissue swelling was evident. The antibiotic was continued for another 15 days, and the patient showed marked improvement. At the end of treatment, the patient showed a mouth opening of 48 mm and remained pain-free.
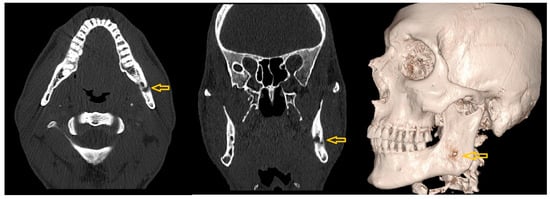
Figure 14.
Axial (left), coronal (middle), and 3D reconstructed (right) CT images showing a localized area of osteolysis in the left mandibular body at the level of the mandibular canal (yellow arrows).
- Case 5
A 45-year-old female patient was referred to the Craniomaxillofacial Surgery Department by the otorhinolaryngology (ENT) team with severe restriction of mouth opening and temporomandibular joint (TMJ) dysfunction. The patient presented with left otalgia and reduced mouth opening for three days and was initially diagnosed with otitis externa. Her symptoms persisted despite being treated with local antibiotic drops, leading to further evaluation. On examination, there was no evidence of swelling, erythema, or fluctuation near the TMJ. Severe tenderness was noted over the left TMJ. There was the restriction of maximal incisal opening (MIO) to 10 mm with right-sided mandibular deviation on occlusion. There were no visible mucosal abnormalities or evidence of odontogenic infection.
Panoramic radiographs indicated bilateral normal TMJ articulation without signs of odontogenic infection or structural changes (Figure 15).
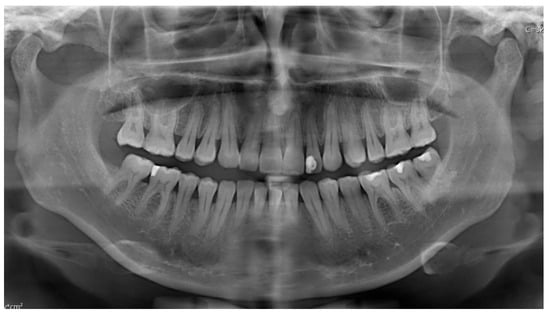
Figure 15.
Panoramic radiograph demonstrating normal bilateral temporomandibular joint (TMJ) articulation. No evidence of odontogenic infection, pathological lesions, or structural abnormalities is observed.
A CT scan shows subluxation of the left TMJ (Figure 16), with soft tissue swelling up to the external auditory canal, but no evidence of abscess or joint arthrosis was found.
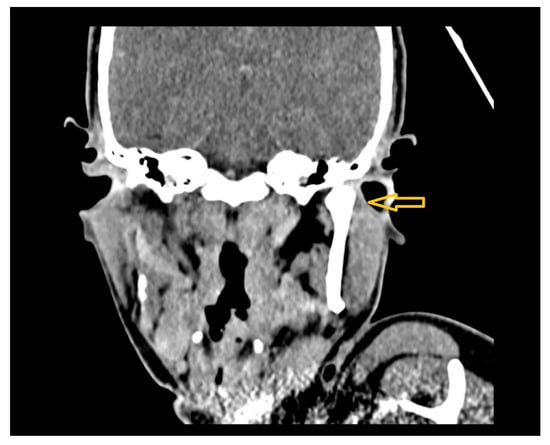
Figure 16.
Coronal CT image showing subluxation of the left temporomandibular joint (TMJ), accompanied by surrounding soft tissue swelling extending toward the external auditory canal (yellow arrow).
Magnetic resonance imaging (MRI) revealed TMJ effusion with phlegmonous changes posterior to the mandibular condyle and a small suspected abscess measuring 4 × 5 mm (Figure 17).
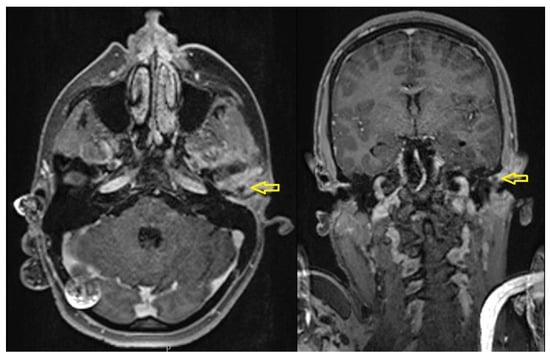
Figure 17.
Axial (left) and coronal (right) contrast-enhanced MRI images showing effusion in the left temporomandibular joint (TMJ) and surrounding phlegmonous inflammatory changes (yellow arrows).
Surgical intervention was performed under local anesthesia, including incision and drainage of an abscess in the anterior external ear canal wall (Figure 18), followed by placement of a medicated Meche with gentamicin cream. Systemic antibiotics were initiated with Co-Amoxicillin. Swabs collected during the procedure revealed methicillin-resistant Staphylococcus aureus (MRSA), prompting a switch to Clindamycin (600 mg) thrice daily.
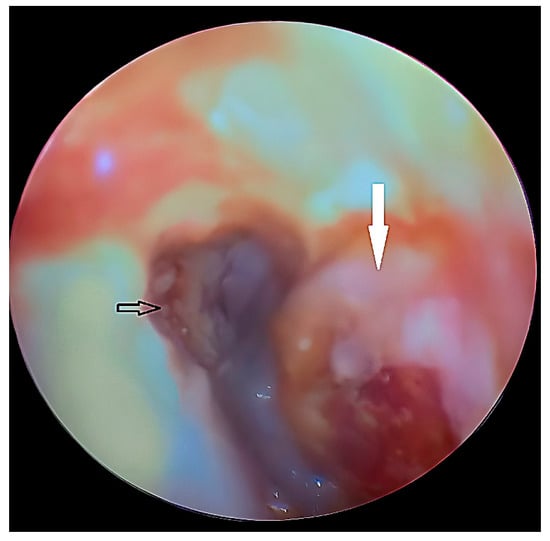
Figure 18.
Otoscopic view showing the tympanic membrane (black arrow) and a bulging abscess (white arrow) in the anterior wall of the external auditory canal.
Gradually, over the subsequent weeks, the patient’s pain intensity decreased, and mouth opening improved to 25 mm. At 15 days postoperatively, the patient had marked improvement in pain, and mouth opening had improved to 42 mm. She was advised to perform mouth-opening exercises and continue physiotherapy. Mouth opening was further improved at the end of treatment to 51 mm while the patient remained pain-free with no evidence of residual infection or complications.
3. Results
Five rare causes of extra-articular trismus were included. The pathologies were coronoid process hyperplasia and osteoma, myositis ossificans traumatica, chronic suppurative osteomyelitis of the ramus mandible, and deep-space abscess originating from the external auditory canal. Detailed diagnosis was made through various high-resolution imaging modalities, including computed tomography (CT) and magnetic resonance imaging (MRI), to localize and characterize the lesions appropriately. Surgical management was guided by each case’s etiology and anatomical complexity and included coronoidectomy, local excision of ossified or infected tissue, and drainage of deep-seated abscesses. All five patients in this study showed significant improvement in mouth opening following surgery, with the interincisal distances increasing from less than 20 mm before the operation to more than 35 mm after it. Structured physiotherapy and regular follow-up further strengthened this functional recovery to achieve gradual and sustained recovery (Table 1).

Table 1.
Patients with extra-articular causes of trismus.
4. Discussion
Trismus, known as “locked jaw,” is frequently encountered in oral and maxillofacial surgery and can have a devastating impact on the quality of life of a patient [,]. Although the normal range of interincisal distance varies among individuals and has been found to be between 40 and 60 mm [,], any significant decrease in this should be considered as a possible underlying disease and should be treated accordingly [].
Trismus has a broad etiologic spectrum, which can be classified as intra-articular and extra-articular [,,]. Intra-articular causes include pathologies of the temporomandibular joint (TMJ) itself, such as ankylosis, arthritis, synovitis, and meniscal derangements. These include several structural abnormalities or inflammatory processes directly affecting the joint []. The extra-articular causes, however, are numerous and may affect the adjacent structures, such as the masticatory muscles, coronoid processes, and soft tissues of the oral cavity [,]. These can be categorized further into congenital [] and acquired conditions, with the acquired causes typically being trauma [,], infection [,], tumors [,], and fibrotic changes [,]. The table below (Table 2) summarizes the most common causes for trismus based on accepted classifications.

Table 2.
Patients with extra-articular causes of trismus.
Less commonly, trismus could be caused by tumors that involve the coronoid process of the mandible []. Notably, osteomas, benign bone tumors, can develop in this area and lead to restricted mouth opening by physically preventing mandibular movement against the zygomatic arch []. To date, only a few cases of coronoid osteoma have been reported in the literature since Lewars’ first report in 1959 []. Petronis et al. (2024) recently addressed the difficulty in diagnosing coronoid osteomas because they are often asymptomatic in the early stages []. These tumors are generally diagnosed by clinical examination and imaging studies, including computed tomography (CT) and magnetic resonance imaging (MRI), supported by histopathological evaluation [,,]. In our patient, the tumor was classified as a peripheral and cancellous variant of osteoma, originating from the periosteum of the coronoid process. Notably, no association with Gardner’s syndrome []—identified by colorectal polyposis, skeletal abnormalities, and supernumerary teeth—was recognized in this patient.
Osteomas usually appear in adults older than 40 years and show a male predominance (a male-to-female ratio of approximately 2:1) []. The pathogenesis of etiology is still a matter of debate. Some investigators regard οsteomas to be true neoplasms, while others believe they are developmental anomalies or reactive lesions in response to trauma or infection []. However, in the absence of trauma or infection, as in our case, the etiology is unknown. Differential diagnoses for osteomas are osteochondroma, fibrous dysplasia, Paget’s disease, and ossifying fibroma [,]. They are generally benign and asymptomatic, but the surgery is often indicated if they cause functional disturbance, as in our patient who underwent intraoral coronoidectomy. This approach minimizes scarring and postoperative morbidity []. At the one-year follow-up, the patient had fully recovered without any recurrence.
Another rare etiology of trismus is bilateral coronoid process hyperplasia []. This condition, which is the exception, is seen in approximately 5% of mandibular hypomobility cases and is described by the abnormal elongation of the coronoid processes [,]. This elongation protrudes against the temporal surface of the zygomatic bone or the medial surface of the zygomatic arch while moving the mandible [,]. According to the study by Goh et al. (2020), most cases occur in men, and the average age of onset is 23 years []. However, it is rare in children under the age of 10 []. Our case of a 10-year-old female patient with bilateral coronoid process hyperplasia underscores the progressive and painless nature associated with the condition. Reduced mouth opening can be insidious, often undetected, until the function is seriously impaired and identified, in this case, during a routine pediatric check-up.
Coronoid hyperplasia has an obscure etiology. Possible explanations include genetics, trauma, overactivity of the temporalis muscle, and hormone ratios [,]. However, none of these factors were found in our patient. The most common treatment is to surgically remove the coronoid processes by performing a coronoidectomy through intraoral or extraoral approaches []. Intraoral coronoidectomy is often preferred due to its lower risk of complications, like facial nerve injury and external scarring []. These findings are consistent with those reported by Nogueira et al. (2015) in their retrospective analysis of similar cases []. The authors emphasized the importance of early intervention and physiotherapy. Postoperative physiotherapy is important to maintain an adequate range of motion and prevent recurrence [,]. In our case, the patient also reported considerable improvement and absence of recurrence at the one-year follow-up.
Myositis ossificans traumatica (MOT) is another rare etiology of trismus. MOT is described as the ectopic ossification of skeletal muscle and usually occurs after trauma []. Although it most often presents in the quadriceps or brachialis muscles, the masseter muscle in the head and neck region is not an unprecedented involvement []. Similar cases have been reported in the literature [,,,] discussing the diagnostic and therapeutic dilemmas of MOT. Trauma is a commonly accepted primary precipitating factor, although the exact pathogenesis of MOT is unclear. Proposed mechanisms include migrating osteoprogenitor cells to soft tissues and detaining periosteal fragments or differentiated cells exposed to bone morphogenic proteins []. Histopathological assessment of MOT lesions commonly indicates a three-zone arrangement, encompassing a peripheral layer of mature lamellar bone, an intermediate zone of immature osteoid and cartilage, and a central zone of granulation tissue and muscle necrosis [].
In our case, a patient presented with trismus following trauma to the right side of the face. Imaging studies showed a calcified mass in the masseter muscle, and MOT was confirmed histopathologically. Subsequently, the lesion was surgically excised, and intensive physiotherapy was pursued. However, the patient experienced recurrence two months later, necessitating a second surgical intervention. This fact underscores the difficulty in managing MOT, including the best time for surgical intervention and how to maximize compliance with postoperative physiotherapy to prevent recurrence. Jović et al. (2021) also reported a high recurrence rate in cases of incomplete excision or insufficient rehabilitation []. Other treatments for MOT, including non-steroidal anti-inflammatory drugs, bisphosphonates, and low-dose radiation therapy, have also been investigated, but the evidence of their effectiveness is inconsistent [,,,,,,,].
Chronic osteomyelitis of the mandible is a challenging and often underdiagnosed condition that can significantly impact a patient’s quality of life, particularly when diagnosed late []. Its insidious onset and nonspecific symptoms (pain, swelling, and restricted mouth opening) make an early diagnosis and treatment difficult []. In our case, a patient was presented initially with unexplained trismus and intermittent hypoesthesia of the inferior alveolar nerve, which are both red flags for more profound pathological processes. Over time, the disease progressed to include swelling, abscess formation, and cortical bone defects on imaging modalities. In the current case, magnetic resonance imaging (MRI) was instrumental in demonstrating inflammatory changes in the masticatory muscles and changes in the mandibular bone marrow signal that suggest acute-on-chronic osteomyelitis []. These results, together with findings from CT imaging, provide further evidence that comprehensive imaging plays an important role in the diagnosis and management of patients with mandibular osteomyelitis. The delay in diagnosis that is common in such cases illustrates the need for osteomyelitis to be part of the differential diagnosis in people who present with progressive trismus, once more common causes have been excluded [].
Management of chronic osteomyelitis requires a multimodal approach. Surgery seeks to contain the infection and eliminate sources of persistent inflammation []. In this case, surgical management encompasses the exact methods for draining abscesses and curettage of the involved area, all of which match standard practices, such as sequestrectomy, saucerization, and decortication. Resection and subsequent reconstruction may be indicated in refractory cases []. The microbiological analysis in our case identified Streptococcus milleri as the predominant organism. This abscess-forming pathogen belongs to the Streptococcus anginosus group and is characterized by its tendency for deep infections, such as osteomyelitis []. Identification of S. milleri guided antibiotic therapy, which was critical in the eventual resolution of infection.
This case illustrates the importance of a high index of suspicion for chronic osteomyelitis in patients presenting with progressive trismus and nonspecific symptoms. Trismus is a clinical entity that needs a comprehensive diagnostic workup involving clinical assessment and advanced imaging. Panoramic radiography, CT, and MRI are important for detecting the underlying cause and for treatment planning. Physiotherapy following surgery is vital for the return of mandibular function and prevention of recurrence. Imaging studies, like open-mouth panoramic radiography, should be repeated periodically to follow recovery with time [,].
External auditory canal abscess or inflammation can be a rare but clinically significant cause of temporomandibular joint (TMJ) dysfunction and trismus []. Both structures are susceptible to secondary involvement due to their close anatomical relationship, with the external auditory canal neighboring the mandibular condyle. Infection in the external auditory canal can directly extend to the TMJ or compress adjacent tissues as a result of abscess expansion, resulting in pain, decreased movement, and dysfunction of the jaw []. Such cases often require advanced imaging to assess the extent of the pathology and ascertain the diagnosis to allow for appropriate management [,].
In the presented case, microbiological analysis revealed methicillin-resistant Staphylococcus aureus (MRSA) as the causative pathogen. MRSA’s virulent behavior, resistance mechanisms, and association with healthcare settings are well described. The ability to create biofilms and infiltrate host tissues makes treatment challenging, often necessitating a dual method of surgical drainage and specific antibiotic therapy []. In our case, MRSA identification after the first 48 h of the Co-Amoxicillin led to changing the antibiotic to Clindamycin; this choice is a frequent option for its excellent effectiveness against MRSA and good penetration in bone and soft tissues. Therefore, this specific antimicrobial approach, together with immediate surgical treatment, is necessary to control the infections of the TMJ and adjacent parts [].
Imaging was crucial to exposing the extent of the disease. Computed tomography (CT) demonstrated subluxation of the TMJ with surrounding soft tissue swelling. At the same time, magnetic resonance imaging (MRI) showed joint effusion, phlegmonous change, and a small abscess adjacent to the anterior wall of the external auditory canal, with no abnormality noted on panoramic radiographs. This emphasizes the role of CT and MRI in head and neck infections with complex inflammatory anatomical microenvironments [].
Management consisted of incision and drainage of the abscess, followed by medicated dressing and systemic antibiotics. That approach effectively reduced infection and restored function. The resolution of the symptoms and the improvement in maximal incisal opening to 51 mm at the end of the treatment emphasize the effectiveness of early aggressive intervention.
Rehabilitation of trismus is the major phase after its treatment, and it requires a multidisciplinary approach to manage and achieve optimal recovery along with comprehensive prevention of future recurrences. These multidisciplinary teams usually consist of oral and maxillofacial surgeons, physiotherapists, and speech therapists, all of whom are integral in managing the functional and structural consequences of trismus [].
The mainstay of rehabilitation is physiotherapy, which consists of active and passive stretching exercises to enhance the range of mandibular movement progressively []. These exercises are normally augmented through the use of dynamic devices, similar to continuous passive motion machines, and specialized implements, together with bite blocks, jaw stretchers, or mouth screws []. Individualized splints are specialized aids that help apply uniform lengthening to the masticatory muscles and prevent re-apposition. Regular physiotherapy and following a prescribed routine are essential for having a better recovery, and relapse can be prevented as it tends to fix the recovery in the jaw. For certain conditions, such as hyperplasia of the coronoid process or myositis ossificans, early initiation of physiotherapy—often immediately after surgical fixation—has been shown to improve outcomes. Early mobilization preserves the functional mobility of the temporomandibular joint and surrounding structures and prevents postoperative fibrosis or scarring, limiting recovery [,].
Speech therapy may then also be initiated, especially when trismus impairs swallowing, speech articulation, or oral functions. A holistic approach that covers functional and mechanical aspects while rehabbing a patient from trismus is vital to ensure the patient regains function and quality of life [].
5. Conclusions
In conclusion, trismus may result from various aetiologies, including benign osteomas, coronoid process hyperplasia, myositis ossificans, and chronic osteomyelitis. The cases presented in this study emphasize the clinical complexity associated with such rare extra-articular causes and highlight the importance of early diagnosis, multidisciplinary evaluation, and customized surgical planning. Early intervention and structured postoperative care are essential in achieving functional outcomes. Although the results are derived from selected clinical scenarios, these findings report pivotal management areas and advocate the requirement for continued research to improve diagnostic care, develop surgical interventions, and standardize rehabilitation protocols to improve patient management.
Author Contributions
Conceptualization: I.K. and M.G.; methodology, I.K.; validation, I.K.; investigation, I.K. and M.G.; resources, I.K.; data curation, I.K., M.G., S.K., C.S., S.D.M., S.W. and D.T.; writing—original draft preparation, I.K., M.G. and D.T.; writing—review and editing, I.K. and D.T.; supervision, I.K. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This manuscript presents a retrospective description of five clinical cases combined with a literature review. According to our institution’s guidelines and applicable regulations, such retrospective case series that do not constitute systematic investigations aimed at contributing to generalizable knowledge are not considered research and therefore do not require approval by an Institutional Review Board (IRB) or Ethics Committee. Nevertheless, written informed consent for participation and publication was obtained from all patients involved.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CT | Computed tomography |
| MRI | Magnetic Resonance Imaging |
| OPG | Orthopantomogramm |
| TMJ | Temporomandibular joint |
| MIO | Maximum interincisal opening |
| CIS | Coronoid impingement syndrome |
| MOT | Myositis ossificans traumatica |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| SAG | Streptococcus anginosus group |
| ENT | Otorhinolaryngology (ear, nose, throat) |
References
- Poornima, G.; Poornima, C. Trismus. J. Health Sci. Res. 2014, 5, 15–20. [Google Scholar] [CrossRef]
- Dhanrajani, P.J.; Jonaidel, O. Trismus: Aetiology, Differential Diagnosis and Treatment. Diagnosis and Treatment. Dent. Update 2002, 29, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Rapidis, A.D.; Dijkstra, P.U.; Roodenburg, J.L.N.; Rodrigo, J.P.; Rinaldo, A.; Strojan, P.; Ferlito, A. Trismus in patients with head and neck cancer: Etiopathogenesis, diagnosis and management. Clin. Otolaryngol. 2015, 40, 516–526. [Google Scholar] [CrossRef]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef]
- Mezitis, M.; Rallis, G.; Zacharides, N. The normal range of mouth opening. J. Oral Maxillofac. Surg. 1984, 47, 1028–1029. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Johansson, M.; Rydén, A.; Houltz, E.; Finizia, C. Impact of trismus on health-related quality of life and mental health. Head Neck 2015, 37, 1672–1679. [Google Scholar] [CrossRef]
- Luyk, N.H.; Steinberg, B. Aetiology and diagnosis of clinically evident jaw trismus. Aust. Dent. J. 1990, 35, 523–529. [Google Scholar] [CrossRef]
- Amin, B.; Chandra, S.B. Recognizing trismus symptoms, prevention, and treatment. Med. Sci. 2019, 8, 766–769. [Google Scholar] [CrossRef]
- Tveterås, K.; Kristense, S. The aetiology and pathogenesis of trismus. Clin. Otolaryngol. Allied Sci. 1986, 11, 383–387. [Google Scholar] [CrossRef]
- Siddiqui, L.; Khan, H.F.; Kanwal, S.S.; Mustafa, K.M. Evaluating the Impact of temporo-mandibular joint disorders on oral health related quality of life; A literature review. Int. Ann. Health Sci. 2024, 3, 4–11. [Google Scholar] [CrossRef]
- Małgorzata, P.; Małgorzata, K.M.; Karolina, C.; Gala, A. Diagnostic of Temporomandibular Disorders and Other Facial Pain Conditions-Narrative Review and Personal Experience. Medicina 2020, 56, 472. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Hilaire, H.; Weber, W.; Ramer, M.; Lumerman, H. Clinicopathologic conference: Trismus following dental treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 261–266. [Google Scholar] [CrossRef]
- Skinner, A.M.; Rees, M.J. Congenital trismus secondary to masseteric fibrous bands: A 7-year follow-up report as an approach to management. J. Craniofac. Surg. 2004, 15, 709–713. [Google Scholar] [CrossRef]
- Hong, C.J.; Caulley, L.; Kohlert, S.; Graham, G.E.; McMillan, H.J.; Michaud, J.; Vaccani, J.P. Congenital Trismus From Brainstem Dysgenesis: Case Report and Review of Literature. Pediatrics 2016, 138, e20154605. [Google Scholar] [CrossRef]
- Campanella, G.; Artuso, G.; Murgia, M.S.; Orrù, G.; Casu, C. Severe Post-Traumatic Trismus Unresponsive to Drug Therapy in a 12-Year-Old Patient Treated with a Capacitive-Resistive Electrical Transfer Therapy: A Case Report. Oral 2022, 2, 173–181. [Google Scholar] [CrossRef]
- Managutti, A.; Patel, N.; Menat, S.; Kamala, R.; Patel, H. Post-traumatic-Zygomaticocoronoid Ankylosis: A Rare Clinical Case Report. IJSS Case Rep. Rev. 2015, 1, 4–6. [Google Scholar]
- Obradovic, B. Intraoral management of odontogenic infection associated with severe trismus under local anesthesia. Ann. Ital. Chir. 2021, 92, 116–118. [Google Scholar]
- Kim, H.-Y.; Chung, J.-W. Infectious Myositis of the Jaw Presenting as Trismus of Unknown Origin. J. Oral Med. Pain 2020, 45, 115–119. [Google Scholar] [CrossRef]
- Satheeshkumar, P.S.; Mohan, M.P.; Jacob, J. Restricted mouth opening and trismus in oral oncology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Mathew, A.; Balasubramanian, D. Trismus in Head and Neck Cancer: Causes and Management. In Dysphagia Management in Head and Neck Cancers; Thankappan, K., Iyer, S., Menon, J., Eds.; Springer: Singapore, 2018; pp. 161–172. [Google Scholar] [CrossRef]
- Bouman, M.A.; Dijkstra, P.U.; Reintsema, H.; Roodenburg, J.L.; Werker, P.M. Surgery for extra-articular trismus: A systematic review. Br. J. Oral Maxillofac. Surg. 2016, 54, 253–259. [Google Scholar] [CrossRef]
- Shen, Y.W.; Shih, Y.H.; Fuh, L.J.; Shieh, T.M. Oral Submucous Fibrosis: A Review on Biomarkers, Pathogenic Mechanisms, and Treatments. Int. J. Mol. Sci. 2020, 21, 7231. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Lin, L.-M.; Lin, C.-C. Osteoma of the mandibular coronoid process. Int. J. Oral Maxillofac. Surg. 1998, 27, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Saikrishna, D.; Das, A.; Jha, C. Management of a case of osteoma of coronoid: A rare case report. Natl. J. Maxillofac. Surg. 2021, 12, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Lewars, L.J. Osteomas of the coronoid process. Oral Surg. Oral Med. Oral Pathol. 1959, 12, 490–496. [Google Scholar]
- Petronis, Z.; Janovskiene, A.; Leketas, M. Cancellous osteoma of the coronoid process: A literature review and rare case report. J. Oral Facial Pain Headache 2024, 38, 126–130. [Google Scholar] [CrossRef]
- Vashishth, S.; Garg, K.; Patil, P.; Sreenivasan, V. An unusual cause for trismus caused by mandibular coronoid osteoma: A case report. Imaging Sci. Dent. 2013, 43, 45–48. [Google Scholar] [CrossRef]
- Nah, K.S. Osteomas of the craniofacial region. Imaging Sci. Dent. 2011, 41, 107–113. [Google Scholar] [CrossRef]
- Jundt, G.; Bertoni, F.; Unni, K.K.; Saito, K.; Dehne, L.P. Benign tumours of bone and cartilage. In WHO Classification of Tumors: Pathology and Genetics of Head and Neck Tumours; Barnes, L., Eveson, J.W., Reichart, P., Sidransky, D., Eds.; IARC: Lyon, France, 2005; pp. 54–55. [Google Scholar]
- Wesley, R.K.; Cullen, C.L.; Bloom, W.S. Gardner’s syndrome with bilateral osteomas of coronoid process resulting in limited opening. Pediatr. Dentl. 1987, 9, 53–57. [Google Scholar]
- Woldenberg, Y.; Nash, M.; Bodner, L. Peripheral osteoma of the maxillofacial region. Diagnosis and management: A study of 14 cases. Med. Oral Patol. Oral Cir. Bucal. 2005, 10 (Suppl. S2), E139–E142. [Google Scholar] [PubMed]
- Dandriyal, R.; Giri, K.Y.; Pant, S.; Alam, S.; Joshi, A. Giant osteochondroma of the coronoid process. J. Maxillofac. Oral Surg. 2015, 14 (Suppl. S1), 412–416. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Liu, X.; Liang, P.S.; Tao, Q. Osteochondroma of the coronoid process: A case report and review of the literature. Oncol. Lett. 2019, 18, 2270–2277. [Google Scholar] [CrossRef]
- Ghazizadeh, M.; Sheikhi, M.; Salehi, M.; Khaleghi, A. Bilateral coronoid hyperplasia causing painless limitation of mandibular movement. Radiol Case Rep. 2017, 13, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Chung, J.W. Coronoid Process Hyperplasia: A Rare Case of Restricted Mouth Opening Masquerading as Temporomandibular Disorder. J. Oral Med. Pain 2023, 48, 112–117. [Google Scholar] [CrossRef]
- Tavassol, F.; Spalthoff, S.; Essig, H.; Bredt, M.; Gellrich, N.C.; Kokemüller, H. Elongated coronoid process: CT-based quantitative analysis of the coronoid process and review of literature. Int. J. Oral Maxillofac. Surg. 2012, 41, 331–338. [Google Scholar] [CrossRef]
- Goh, Y.C.; Tan, C.C.; Lim, D. Coronoid hyperplasia: A review. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Farronato, M.; Lucchina, A.G.; Mortellaro, C.; Fama, A.; Galbiati, G.; Farronato, G.; Maspero, C. Bilateral Hyperplasia of the Coronoid Process in Pediatric Patients: What is the Gold Standard for Treatment? J. Craniofac. Surg. 2019, 30, 1058–1063. [Google Scholar] [CrossRef]
- Puche, M.; Guijarro-Martínez, R.; Pérez-Herrezuelo, G.; Miragall, L.; Iglesias, M.E.; Martínez-Costa, C. The hypothetical role of congenital hypotonia in the development of early coronoid hyperplasia. J. Craniomaxillofac. Surg. 2012, 40, e155–e158. [Google Scholar] [CrossRef]
- Khandavilli, S.D.; Pattni, N.; Naredla, P.R.; Williams, R. First case of bilateral coronoid hyperplasia in monozygotic twin sisters-a new aetiological perspective? Oral Maxillofac. Surg. 2016, 20, 441–443. [Google Scholar] [CrossRef]
- Parmentier, G.I.; Nys, M.; Verstraete, L.; Politis, C. A systematic review of treatment and outcomes in patients with mandibular coronoid process hyperplasia. J. Korean Assoc. Oral Maxillofac. Surg. 2022, 48, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.F.C.; Maranhão, C.M.C.T.; Almeida, R.A.C.; Torres, B.C.A. Treatment of hyperplasia of the coronoid process of the mandible in adults: Analysis of 42 literature reports and illustrative case. RGO Rev. Gaúch Odontol. 2021, 69, e20210033. [Google Scholar] [CrossRef]
- Ozkaya, O.; Colak, O.; Sutcu, M.; Akan, M. The outcome of coronoidectomy in bilateral coronoid process hyperplasia. Cranio 2018, 36, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Fernández Ferro, M.; Fernández Sanromán, J.; Sandoval Gutierrez, J.; Costas López, A.; López de Sánchez, A.; Etayo Pérez, A. Treatment of bilateral hyperplasia of the coronoid process of the mandible. Presentation of a case and review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, E595–E598. [Google Scholar]
- Hanisch, M.; Hanisch, L.; Fröhlich, L.F.; Werkmeister, R.; Bohner, L.; Kleinheinz, J. Myositis ossificans traumatica of the masticatory muscles: Etiology, diagnosis and treatment. Head Face Med. 2018, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Palla, B.; Han, M.D.; Callahan, N. Myositis Ossificans Traumatica of the Head and Neck in a Child. J. Dent. Child. 2020, 87, 120–123. [Google Scholar]
- Anshika, R.; Hoogar, M.B.; Shilpi, S. Myositis ossificans of masseter muscle: A rare case. Int. J. Health Sci. Res. 2021, 11, 105–108. Available online: https://www.ijhsr.org/IJHSR_Vol.11_Issue.2_Feb2021/IJHSR015.pdf (accessed on 2 February 2021).
- Jović, S.; Brajković, D.; Borilović, M.; Marjanović, U.; Brkić, M.; Kozomara, R.; Stošić, S. Recurring myositis ossificans traumatica of temporal muscle: A case report. Vojnosanit. Pregl. 2021, 78, 255–260. [Google Scholar] [CrossRef]
- Spinzia, A.; Moscato, G.; Broccardo, E.; Castelletti, L.; Maglitto, F.; Orabona, G.D.; Piombino, P. A rare isolated unilateral myositis ossificans traumatica of the lateral pterygoid muscle: A case report. J. Med. Case Rep. 2014, 8, 230. [Google Scholar] [CrossRef]
- Torres, A.M.; Nardis, A.C.; da Silva, R.A.; Savioli, C. Myositis ossificans traumatica of the medial pterygoid muscle following a third molar extraction. Int. J. Oral Maxillofac. Surg. 2015, 44, 488–490. [Google Scholar] [CrossRef]
- Abbasi, A.J.; Taheri, M.M.; Asadi, A.; Bahrami, R.; Nikparto, N. Myositis ossificans traumatica of masticatory muscles: A case report and review of the literature. Oral Maxillofac. Surg. Cases 2024, 10, 100361. [Google Scholar] [CrossRef]
- Fité-Trepat, L.; Martos-Fernández, M.; Alberola-Ferranti, M.; Romanini-Montecino, C.; Saez-Barba, M.; Bescós-Atín, C. Myositis ossificans of the masseter muscle: A rare location. Report of a case and review of literature. J. Clin. Exp. Dent. 2016, 8, e210–e213. [Google Scholar] [CrossRef]
- Baur, D.A.; Altay, M.A.; Flores-Hidalgo, A.; Ort, Y.; Quereshy, F.A. Chronic osteomyelitis of the mandible: Diagnosis and management--an institution’s experience over 7 years. J. Oral Maxillofac. Surg. 2015, 73, 655–665. [Google Scholar] [CrossRef]
- Chun, J.Y.; Shim, Y.J. Osteomyelitis of the Mandibular Coronoid Process Mimicking a Temporomandibular Joint Disorder: A Case Report. J. Oral Med. Pain 2024, 49, 35–39. [Google Scholar] [CrossRef]
- Muraoka, H.; Ito, K.; Hirahara, N.; Ichiki, S.; Kondo, T.; Kaneda, T. Magnetic resonance imaging texture analysis in the quantitative evaluation of acute osteomyelitis of the mandibular bone. Dentomaxillofac. Radiol. 2022, 51, 20210321. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, T.; Torkov, P.; Thygesen, T.H. Diagnosis and Treatment of Osteomyelitis of the Jaw—A Systematic Review (2002–2015) of the Literature. J. Dent. Oral Disord. 2017, 3, 1066. [Google Scholar] [CrossRef]
- Vargas-Rojas, D.C.; Rodriguez-Flores, A.; Moreno-Villalobos, D.; Chumpitaz-Cerrate, V.; Chávez-Rimache, L. Radical Surgical Approach to Chronic Suppurative Osteomyelitis: Case Report. Odovtos-Int. J. Dent. Sci. 2023, 26, 14–20. [Google Scholar] [CrossRef]
- Griffin, A.Τ.; Timbrook, Τ.; Harting, J.; Christensen, D. Streptococcus anginosus group and osteomyelitis: A single centre clinical experience. Postgrad. Med. J. 2013, 89, 262–265. [Google Scholar] [CrossRef]
- Tiwari, P.; Bera, R.N.; Kanojia, S.; Chauhan, N.; Hirani, M.S. Assessing the optimal imaging modality in the diagnosis of jaw osteomyelitis. A meta-analysis. Br. J. Oral Maxillofac. Surg. 2021, 59, 982–992. [Google Scholar] [CrossRef]
- Park, M.S.; Eo, M.Y.; Myoung, H.; Kim, S.M.; Lee, J.H. Early diagnosis of jaw osteomyelitis by easy digitalized panoramic analysis. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 6. [Google Scholar] [CrossRef]
- Salimi, F.; Motter, D.; Salimi, Z. Temporomandibular joint (TMJ) disorders as first clinical manifestations in external auditory canal cholesteatoma. A case report. Ann. Med. Surg. 2022, 74, 103287. [Google Scholar] [CrossRef] [PubMed]
- Mardinger, O.; Rosen, D.; Minkow, B.; Tulzinsky, Z.; Ophir, D.; Hirshberg, A. Temporomandibular joint involvement in malignant external otitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Tsuno, N.S.G.; Tsuno, M.Y.; Coelho Neto, C.A.F.; Noujaim, S.E.; Decnop, M.; Pacheco, F.T.; Souza, S.A.; Fonseca, A.P.A.; Garcia, M.R.T. Imaging the External Ear: Practical Approach to Normal and Pathologic Conditions. Radiographics 2022, 42, 522–540. [Google Scholar] [CrossRef]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef]
- Weden, A.; Haig, H. Current thinking in physiotherapy for the management of idiopathic and postsurgical temporomandibular disorders: A narrative review. Br. J. Oral Maxillofac. Surg. 2024, 62, 588–593. [Google Scholar] [CrossRef]
- Sidebottom, A.J. How do I manage restricted mouth opening secondary to problems with the temporomandibular joint? Br. J. Oral Maxillofac. Surg. 2013, 51, 469–472. [Google Scholar] [CrossRef]
- Shulman, D.H.; Shipman, B.; Willis, F.B. Treating trismus with dynamic splinting: A cohort, case series. Adv. Ther. 2008, 25, 9–16. [Google Scholar] [CrossRef]
- Harris, B.N.; Kuhn, M.; Evangelista, L.; Davis, S. Speech and Swallow Therapy. In Complex Head and Neck Microvascular Surgery; Quimby, A., Parmar, S., Fernandes, R., Eds.; Springer: Cham, Switzerland, 2023; pp. 231–248. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).