Comparison of mpMRI and 68Ga-PSMA-PET/CT in the Assessment of the Primary Tumors in Predominant Low-/Intermediate-Risk Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Measurements
2.2.1. MRI Protocol
2.2.2. PET/CT
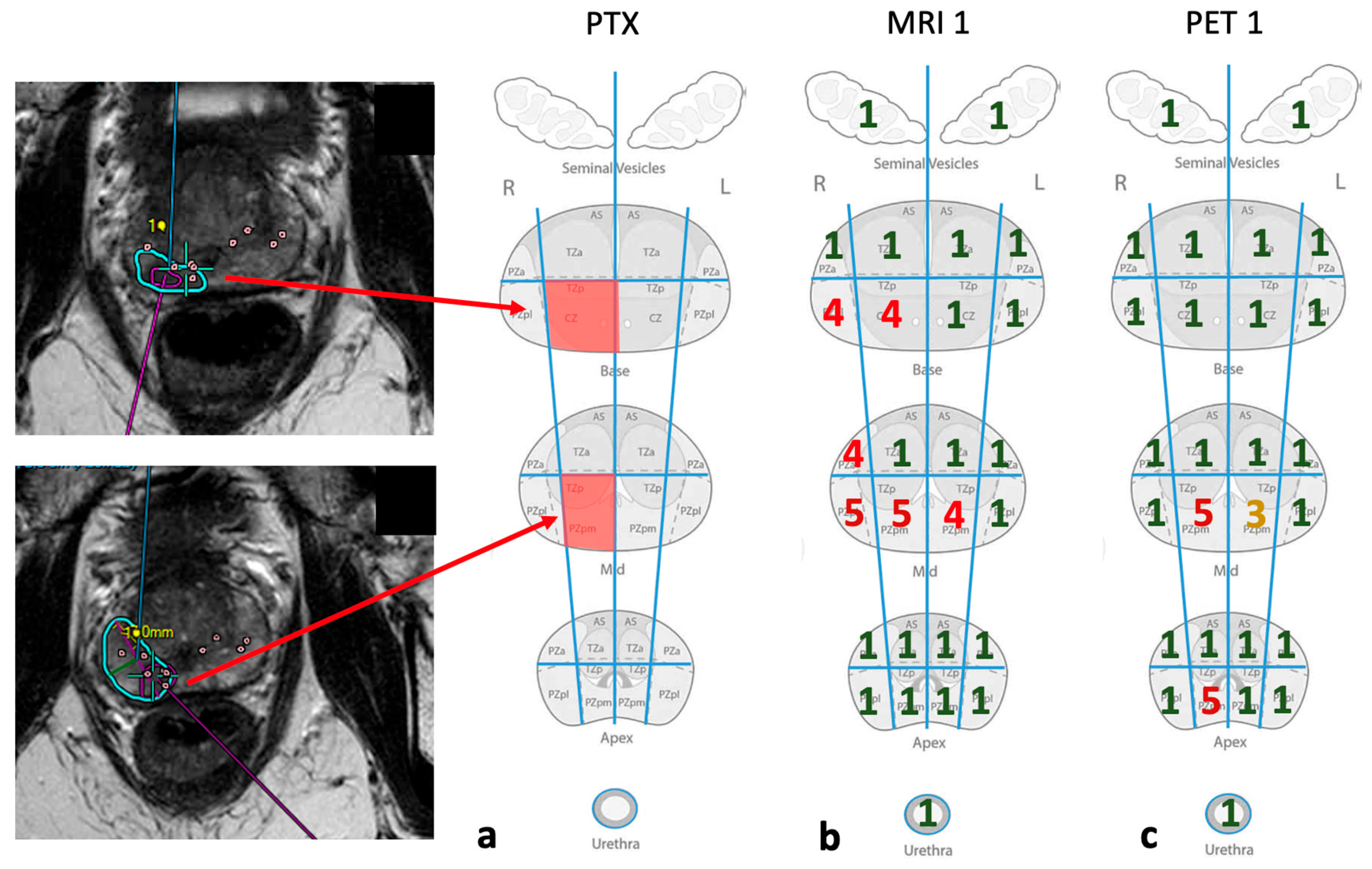
2.2.3. Image Analysis
2.2.4. Statistical Analysis
3. Results
3.1. ROC Analysis
3.2. Congruence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Contrast | TE [ms] | TR [ms] | FA [°] | NA | BW [Hz/px] | Matrix [pixels] | Voxel Size [mm3] | ETL | |
|---|---|---|---|---|---|---|---|---|---|
| T2w | tra | 110 | 5100 | 90 | 3 | 192 | 192 × 192 | 0.5 × 0.5 × 2.5 | 25 |
| T1w | tra | 8.00 | 584 | 90 | 2 | 250 | 176 × 130 | 0.7 × 0.7 × 2.5 | 10 |
| DWI | tra | 57.0 | 2885 | 90 | 1 | 1990 | 56 × 64 | 1.3 × 1.3 × 3 | 61 |
| DCE | tra | 1.27 | 3.67 | 20 | 2 | 1900 | 136 × 110 | 1.0 × 1.0 × 6.6 | 1 |
| Name | Slices | eff. mAs | kV | Scan Time | Delay | Pitch |
|---|---|---|---|---|---|---|
| Topo 120 min | 0.6 | 35.0 | 100 | 8.44 | 4 s | |
| AC CT WB 120 min | 5.0 | 30.0 | 120 | 28.64 | 2 s | 0.8 |
| Name | Method | Iteration | Subset | Output |
|---|---|---|---|---|
| PET WB 120 min | TrueX + TOF (ultraHD-PET) | 2 | 21 | corrected |
| PET WB 120 min Uncorrected | Iterativ | 3 | 24 | uncorrected |
| MRI 1/MRI 2 | PET 1/PET 2 | MRI/Biopsy | |
| n | 25 | 25 | 19 |
| Mean value | 91.11% | 87.11% | 83.18% |
| Median | 92.59% | 88.89% | 83.33% |
| Standard deviation | 4.66% | 8.50% | 12.98% |
| Variance | 21.79% | 72.17% | 168.54% |
| Minimum | 77.78% | 70.37% | 66.66% |
| Maximum | 100.0% | 100.0% | 100.0% |
| PET/biopsy | MRI/biopsyNTA | PET/biopsyNTA | |
| n | 19 | 19 | 19 |
| Mean value | 76.15% | 96.54% | 92.69% |
| Median | 79.17% | 100.0% | 95.83% |
| Standard deviation | 12.90% | 4.42% | 8.18% |
| Variance | 166.41% | 19.49% | 66.92% |
| Minimum | 50.0% | 87.5% | 72.72% |
| Maximum | 95.83% | 100.0% | 100.0% |
| Patient | Age | PSA (ng/mL) | Gleason | D’Amico | Activity in MBq (PET) | Time in min (PET) |
|---|---|---|---|---|---|---|
| 1 | 7.04 | 3 + 4 (7a) | INTER | 145 | 107 | |
| 2 | 75 | 15.96 | 3 + 3 | INTER | 140 | 115 |
| 3 | 69 | 6.98 | 3 + 3 | LOW | 119 | 124 |
| 4 | 64 | 10.20 | 3 + 4 (7a) | INTER | 151 | 128 |
| 5 | 73 | 13.30 | 4 + 3 (7b) | INTER | 150 | 122 |
| 6 | 72 | 5.87 | 4 + 3 (7b) | INTER | 139 | 120 |
| 7 | 62 | 7.06 | 3 + 3 | LOW | 134 | 122 |
| 8 | 73 | 9.86 | 3 + 4 (7a) | INTER | 132 | 120 |
| 9 | 83 | 14.10 | 3 + 3 | INTER | 143 | 120 |
| 10 | 71 | 3.51 | 3 + 3 | LOW | 138 | 110 |
| 11 | 72 | 12.50 | 3 + 3 | INTER | 140 | 115 |
| 12 | 84 | 6.00 | 3 + 3 | LOW | 151 | 115 |
| 13 | 73 | 9.64 | 3 + 3 | LOW | 145 | 115 |
| 14 | 75 | 10.20 | 3 + 3 | INTER | 154 | 121 |
| 15 | 84 | 8.24 | 3 + 4 (7a) | INTER | 152 | 116 |
| 16 | 68 | 5.70 | 3 + 3 | LOW | ||
| 17 | 77 | 5.90 | 3 + 3 | LOW | 152 | 118 |
| 18 | 58 | 4.56 | 3 + 4 (7a) | INTER | 151 | 115 |
| 19 | 71 | 17.90 | 3 + 3 | INTER | 146 | 124 |
| 20 | 74 | 6.65 | 3 + 3 | LOW | ||
| 21 | 71 | 9.00 | 3 + 4 (7a) | INTER | 150 | 134 |
| 22 | 78 | 9.56 | 3 + 3 | LOW | 142 | 142 |
| 23 | 68 | 11.90 | 3 + 4 (7a) | INTER | 147 | 128 |
| 24 | 63 | 14.50 | 3 + 3 | INTER | 131 | 141 |
| 25 | 83 | 23.70 | 3 + 4 (7a) | HIGH | 144 | 116 |
| 26 | 76 | 15.30 | 3 + 3 | INTER | 152 | 130 |
| 27 | 64 | 3 + 4 (7a) | INTER | 153 | 119 | |
| 28 | 71 | 11.03 | 154 | 118 | ||
| Median | 72 | 9.64 | 145.5 | |||
| Mean | 72.23 | 10.01 | 144.42 |


References
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.M.; Papa, N.; Buteau, J.; Ho, B.; Liu, V.; Roberts, M.; Thompson, J.; Moon, D.; Sheehan-Dare, G.; Alghazo, O.; et al. The PRIMARY Score: Using intra-prostatic PSMA PET/CT patterns to optimise prostate cancer diagnosis. J. Nucl. Med. 2022, 63, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Haring, A. (AZQ) S3-Leitlinie Prostatakarzinom. 2021, p. 371. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom (accessed on 24 May 2025).
- El-Shater Bosaily, A.; Parker, C.; Brown, L.C.; Gabe, R.; Hindley, R.G.; Kaplan, R.; Emberton, M.; Ahmed, H.U. PROMIS—Prostate MR imaging study: A paired validating cohort study evaluating the role of multi-parametric MRI in men with clinical suspicion of prostate cancer. Contemp. Clin. Trials 2015, 42, 26–40. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Kwan, T.N.; Spremo, S.; Teh, A.Y.M.; McHarg, D.; Thangasamy, I.; Woo, H.H. Performance of Ga-68 PSMA PET/CT for diagnosis and grading of local prostate cancer. Prostate Int. 2021, 9, 107–112. [Google Scholar] [CrossRef]
- Privé, B.M.; Israël, B.; Janssen, M.J.R.; Van Der Leest, M.M.G.; De Rooij, M.; Van Ipenburg, J.A.; Jonker, M.; Peters, S.M.B.; De Groot, M.; Zámecnik, P.; et al. Multiparametric MRI and18 F-PSMA-1007 PET/CT for the Detection of Clinically Significant Prostate Cancer. Radiology 2024, 311, e231879. [Google Scholar] [CrossRef]
- Taneja, S.S.; Bennett, J.; Coleman, J.; Grubb, R.; Andriole, G.; Reiter, R.E.; Marks, L.; Azzouzi, A.-R.; Emberton, M. Final Results of a Phase I/II Multicenter Trial of WST11 Vascular Targeted Photodynamic Therapy for Hemi-Ablation of the Prostate in Men with Unilateral Low Risk Prostate Cancer Performed in the United States. J. Urol. 2016, 196, 1096–1104. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; Ng, C.-F.; Yee, C.-H.; Chiu, P.K.-F. High-intensity focused ultrasound strategies for treating prostate cancer. Asian J. Androl. 2024, 26, 595–599. [Google Scholar] [CrossRef]
- Cosset, J.-M.; Cathelineau, X.; Wakil, G.; Pierrat, N.; Quenzer, O.; Prapotnich, D.; Barret, E.; Rozet, F.; Galiano, M.; Vallancien, G. Focal brachytherapy for selected low-risk prostate cancers: A pilot study. Brachytherapy 2013, 12, 331–337. [Google Scholar] [CrossRef]
- Suárez, J.F.; Zamora, V.; Garin, O.; Gutiérrez, C.; Pont, À.; Pardo, Y.; Goñi, A.; Mariño, A.; Hervás, A.; Herruzo, I.; et al. Mortality and biochemical recurrence after surgery, brachytherapy, or external radiotherapy for localized prostate cancer: A 10-year follow-up cohort study. Sci. Rep. 2022, 12, 12589. [Google Scholar] [CrossRef]
- The World Medical Association. Wma Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Philips. Philips Uro Nav 2019. Available online: https://www.documents.philips.com/assets/20191120/bf5ebcae009944e9b01aab0b00121cc3.pdf (accessed on 15 September 2023).
- Philips. Philips DynaCad 2019. Available online: https://www.documents.philips.com/assets/20230505/1ceb087b34934e809099aff90162436c.pdf (accessed on 15 September 2023).
- Beyer, T.; Schlemmer, H.-P.; Weber, M.-A.; Thierfelder, K.M. PI-RADS 2.1—Image Interpretation: The Most Important Updates and Their Clinical Implications. Rofo 2020, 193, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, E.; Glazer, D.I.; Dunne, R.M.; Fennessy, F.M.; Harisinghani, M.G.; Tempany, C.M. Prostate imaging reporting and data system version 2 (PI-RADS v2): A pictorial review. Abdom. Radiol. 2017, 42, 278–289. [Google Scholar] [CrossRef]
- Giesel, F.L.; Sterzing, F.; Schlemmer, H.P.; Holland-Letz, T.; Mier, W.; Rius, M.; Afshar-Oromieh, A.; Kopka, K.; Debus, J.; Haberkorn, U.; et al. Intra-individual comparison of (68)Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1400–1406. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Yildirim, O.; Woo, S.; Vargas, H.A.; Hricak, H. The role of MRI in prostate cancer: Current and future directions. Magn. Reson. Mater. Phy 2022, 35, 503–521. [Google Scholar] [CrossRef]
- Franklin, A.; Yaxley, W.J.; Raveenthiran, S.; Coughlin, G.; Gianduzzo, T.; Kua, B.; McEwan, L.; Wong, D.; Delahunt, B.; Egevad, L.; et al. Histological comparison between predictive value of preoperative 3-T multiparametric MRI and 68 Ga-PSMA PET/CT scan for pathological outcomes at radical prostatectomy and pelvic lymph node dissection for prostate cancer. BJU Int. 2021, 127, 71–79. [Google Scholar] [CrossRef]
- Sonni, I.; Felker, E.R.; Lenis, A.T.; Sisk, A.E.; Bahri, S.; Allen-Auerbach, M.; Armstrong, W.R.; Suvannarerg, V.; Tubtawee, T.; Grogan, T.; et al. Head-to-Head Comparison of 68 Ga-PSMA-11 PET/CT and mpMRI with a Histopathology Gold Standard in the Detection, Intraprostatic Localization, and Determination of Local Extension of Primary Prostate Cancer: Results from a Prospective Single-Center Imaging Trial. J. Nucl. Med. 2022, 63, 847–854. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen–Targeted PET Imaging: PSMA-RADS Version 1.0. J. Nucl. Med. 2018, 59, 479–485. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Israël, B.; van der Leest, M.; Sedelaar, M.; Padhani, A.R.; Zámecnik, P.; Barentsz, J.O. Multiparametric Magnetic Resonance Imaging for the Detection of Clinically Significant Prostate Cancer: What Urologists Need to Know. Part 2: Interpretation. Eur. Urol. 2020, 77, 469–480. [Google Scholar] [CrossRef]
- Nakai, H.; Nagayama, H.; Takahashi, H.; Froemming, A.T.; Kawashima, A.; Bolan, C.W.; Adamo, D.A.; Carter, R.E.; Fazzio, R.T.; Tsuji, S.; et al. Cancer Detection Rate and Abnormal Interpretation Rate of Prostate MRI in Patients With Low-Grade Cancer. J. Am. Coll. Radiol. 2024, 21, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Koerber, S.A.; Utzinger, M.T.; Kratochwil, C.; Kesch, C.; Haefner, M.F.; Katayama, S.; Mier, W.; Iagaru, A.H.; Herfarth, K.; Haberkorn, U.; et al. 68Ga-PSMA-11 PET/CT in Newly Diagnosed Carcinoma of the Prostate: Correlation of Intraprostatic PSMA Uptake with Several Clinical Parameters. J. Nucl. Med. 2017, 58, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, A.; Phung, T.H.; O Soares, M.; Shepherd, L.; Glynn, D.; Harden, M.; Walker, R.; Duarte, A.; Dias, S. MRI software and cognitive fusion biopsies in people with suspected prostate cancer: A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol. Assess. 2024, 28, 1. [Google Scholar] [CrossRef]
- Watts, K.L.; Frechette, L.; Muller, B.; Ilinksy, D.; Kovac, E.; Sankin, A.; Aboumohamed, A. Systematic review and meta-analysis comparing cognitive vs. image-guided fusion prostate biopsy for the detection of prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 734.e19–734.e25. [Google Scholar] [CrossRef]






| MRI1 | MRI2 | PET1 | PET2 | MRI mv | PET mv | MRI/ Biopsy | PET/ Biopsy | MRI/ Biopsy NTA | PET/ Biopsy NTA | |
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | 0.77 | 0.78 | 0.68 | 0.61 | 0.82 | 0.69 | ||||
| p-value (DeLong) | 0.748 | <0.001 | 0.006 | |||||||
| Congruence mv | 92.59% | 88.89% | 83.18% | 76.15% | 96.54% | 92.69% | ||||
| p-value (Wilcoxon) | 0.051 | 0.034 | 0.024 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argow, M.J.; Hupfeld, S.; Schenke, S.A.; Neumann, S.; Damm, R.; Vogt, J.; Guer, M.; Wuestemann, J.; Schostak, M.; Fischbach, F.; et al. Comparison of mpMRI and 68Ga-PSMA-PET/CT in the Assessment of the Primary Tumors in Predominant Low-/Intermediate-Risk Prostate Cancer. Diagnostics 2025, 15, 1358. https://doi.org/10.3390/diagnostics15111358
Argow MJ, Hupfeld S, Schenke SA, Neumann S, Damm R, Vogt J, Guer M, Wuestemann J, Schostak M, Fischbach F, et al. Comparison of mpMRI and 68Ga-PSMA-PET/CT in the Assessment of the Primary Tumors in Predominant Low-/Intermediate-Risk Prostate Cancer. Diagnostics. 2025; 15(11):1358. https://doi.org/10.3390/diagnostics15111358
Chicago/Turabian StyleArgow, Moritz J., Sebastian Hupfeld, Simone A. Schenke, Sophie Neumann, Romy Damm, Johanna Vogt, Melis Guer, Jan Wuestemann, Martin Schostak, Frank Fischbach, and et al. 2025. "Comparison of mpMRI and 68Ga-PSMA-PET/CT in the Assessment of the Primary Tumors in Predominant Low-/Intermediate-Risk Prostate Cancer" Diagnostics 15, no. 11: 1358. https://doi.org/10.3390/diagnostics15111358
APA StyleArgow, M. J., Hupfeld, S., Schenke, S. A., Neumann, S., Damm, R., Vogt, J., Guer, M., Wuestemann, J., Schostak, M., Fischbach, F., & Kreissl, M. C. (2025). Comparison of mpMRI and 68Ga-PSMA-PET/CT in the Assessment of the Primary Tumors in Predominant Low-/Intermediate-Risk Prostate Cancer. Diagnostics, 15(11), 1358. https://doi.org/10.3390/diagnostics15111358








