Abstract
The exact etiopathogenesis of Kawasaki disease (KD), the most common childhood vasculitis, remains unknown; however, an aberrant immune response, possibly triggered by an infectious or environmental agent in genetically predisposed children, is believed to be the underlying pathogenetic mechanism. Patients with inborn errors of immunity (IEI) are predisposed to infections that trigger immune dysregulation due to an imbalance in various arms of the immune system. KD may develop as a complication in both primary and secondary immunodeficiencies. KD may occur either at disease presentation or have a later onset in IEIs. These include X-linked agammaglobulinemia (XLA), selective IgA deficiency, transient hypogammaglobulinemia of infancy; Wiskott–Aldrich syndrome (WAS), hyper IgE syndrome (HIES); chronic granulomatous disease (CGD), innate and intrinsic immunity defects, and autoinflammatory diseases, including PFAPA. Hitherto, the association between KD and IEI is confined to specific case reports and case series and, thus, requires extensive research for a comprehensive understanding of the underlying pathophysiological mechanisms. IEIs may serve as excellent disease models that would open new insights into the disease pathogenesis of children affected with KD. The current review highlights this critical association between KD and IEI supported by published literature.
1. Introduction
Kawasaki disease (KD) is the most typical childhood medium vessel vasculitis that usually affects children below five years of age [1]. However, older children and adolescents may also be affected. KD has a particular predilection for coronary arteries leading to coronary artery abnormalities (CAAs). The typical presentation of KD includes fever, erythematous rash, conjunctival injection, mucocutaneous involvement, and lymphadenopathy. Timely diagnosis and treatment with high-dose intravenous immunoglobulin (IVIG) are required in these children to prevent the development of CAAs and myocardial infarction. Hitherto, even after more than five decades of the initial description of the disease, the diagnosis of KD is clinical and based upon a set of clinical criteria [2]. The etiology of KD is still evolving, and there are no specific etiopathogenic mechanisms. Although the exact pathophysiology of KD is still under research, it is believed to be secondary to an abnormal/aberrant immune response possibly triggered by an infectious or environmental agent in genetically predisposed individuals [3].
Inborn Errors of Immunity (IEIs) are a heterogeneous group of monogenic disorders characterized by defects in the development or function of the immune system. These disorders can result in immune deficiencies, autoimmunity, autoinflammatory disorders, bone marrow failure, allergy, malignancy, and immune dysregulation [4]. Patients with IEIs are predisposed to develop an infection that triggers immune dysregulation, autoimmunity, or autoinflammatory disorder due to an imbalance in various arms of the immune system, such as phagocytic function, lymphoid system, humoral responses, and others [5]. The pathogenesis of KD revolves around a dysregulated immune system with a cytokine cascade in the acute phase [6]. The association between KD and specific IEIs is limited to certain case reports and case series and, thus, requires extensive research for a comprehensive understanding. In the present review, we aim to explore the interplay of the immune system in the pathogenesis of KD based on the published literature on the association of KD with IEI.
2. Methods
In this manuscript, we have reviewed the association of IEIs in the context of KD to understand the pathophysiology of KD. We searched the published literature online using the terms Kawasaki disease, mucocutaneous lymph node syndrome, primary immunodeficiency diseases, and inborn errors of immunity in PubMed, Embase, and Google Scholar databases. After an extensive search, we could retrieve 25 research articles on this topic, including case reports and case series, for inclusion in the present review.
3. Kawasaki Disease in Inborn Errors of Immunity
While KD is an immune-mediated condition, it is generally regarded as a complex multifactorial disorder with genetic and environmental factors contributing to its development [6]. KD may develop as a complication of an immunodeficiency disorder [7]. Patients with certain clinical types of IEI have shown KD during the disease course or at presentation [7]. These include antibody deficiency disorders, combined immunodeficiency disorders (CIDs), phagocytic defects, intrinsic and innate immunity defects, and autoinflammatory disorders as discussed below (Table 1).

Table 1.
Summary of clinical, laboratory findings, and treatment of KD in PID according to published literature.
3.1. KD and Predominantly Antibody Deficiencies
KD has been described in patients with antibody deficiencies such as X-linked agammaglobulinemia (XLA), selective immunoglobulin A (IgA) deficiency, and transient hypogammaglobulinemia of infancy. The first case report of the association of XLA and KD was by Behniafard et al., where-in a 15-month-old male infant suspected to have XLA because of absent tonsils, pan-hypogammaglobulinemia, markedly reduced B cells, and a family history of a confirmed diagnosis of XLA in sibling and maternal uncle presented with fever for five days with a neck mass, peripheral edema, and severe thrombocytosis with negative infectious work-up, diagnosed as atypical KD and the symptoms abated with high dose IVIG [8]. He also developed peri-ungual desquamation in 3rd week of illness. Another case report of a 3-year-old boy by Malekzadeh et al., who had several admissions due to autoimmune/immune dysregulation manifestations such as polyarthritis, and macrophage activation syndrome (MAS), presented with features of incomplete KD, and required methylprednisolone and IVIG for treatment, and was later diagnosed at six years to be XLA [9]. Both these cases had developed KD before any severe infectious complications and had not been on maintenance IVIG therapy. However, the case description by Sharma et al. of a 6-year-old boy diagnosed as a case of XLA at three years of age with a history of recurrent infections on regular maintenance IVIG infusion presented with manifestations of incomplete KD had a possible infectious trigger because of concomitant pneumonia during the episode [10]. Rivas-Larrauri et al. reported an 8-month-old boy with incomplete KD manifesting as fever, cough, rash, hoarseness of voice, thrombocytosis, and left coronary artery ectasia with concomitant XLA diagnosed based on Pseudomonas aeruginosa and rhinoviral infections, absence of Bruton tyrosine kinase (BTK) expression and a family history of XLA [7]. He responded well to IVIG, cyclosporine, and antibiotics.
Nishikawa et al. reported a patient with complete KD at two years of age with concomitant selective IgA deficiency [11]. The patient underwent serum IgA level assessment before initiating IVIG therapy and had low IgA levels, normal immunoglobulin G (IgG) levels, and high anti-IgG antibody titers, with low IgA levels in the sister. His symptoms resolved with the initiation of methylprednisolone [11]. Anzai et al. successfully treated a 5-year-old male with complete KD with intravenous cyclosporine A instead of IVIG who was diagnosed with selective IgA deficiency at the time of work-up for KD [12]. Şanlıdağ et al. presented a 4-year-old boy diagnosed with transient hypogammaglobulinemia of infancy at 3.5 years of age who developed incomplete KD [13]. He was treated with IVIG and aspirin and showed a good response.
3.2. KD and Immunodeficiency Disorders Affecting Cellular and Humoral Immunity
Among combined immunodeficiency disorders, an association between Wiskott–Aldrich Syndrome (WAS) and Hyper IgE syndrome (HIES), and KD has been reported.
WAS is a combined immune deficiency characterized by eczema, thrombocytopenia, and immune deficiency. Autoimmune manifestations have been reported in 25–70% of children with WAS, with vasculitis being the second most common manifestation found in up to 30% of patients with WAS [27]. Large vessel vasculitis resembling Takayasu arteritis has been very well described with WAS in the literature, besides small vessel vasculitis like IgA vasculitis [14]. Kawakami et al. described a male infant with a typical triad of WAS carrying an intron six variant in WAS gene, who presented with classical features of KD in the form of fever, rash, dorsal edema, conjunctival injection, strawberry tongue, and cervical lymphadenopathy at six months of age [14]. His platelet count increased transiently in the acute phase of KD and decreased after IVIG therapy. He postulated that the acute phase of KD is associated with increased interleukin-6 (IL-6) levels with a thrombopoietic effect and could have led to a transient increase in platelet count in WAS.
Hyper IgE syndrome (HIES) is a rare IEI characterized by a triad of recurrent staphylococcal skin and lung infections, eczema, and elevated immunoglobulin E (IgE) levels. Autoimmune manifestations have been described in HIES [28]. Lupus-like phenotype with predominant renal involvement has been reported in patients with signal transducer and activator of transcription-3 (STAT-3) mutated HIES with anti-double stranded deoxyribonucleic acid antibodies (anti-dsDNA) positivity [29]. Association of HIES with KD has been described by Kimata et al. in two children from the same family, including a 10-year-old boy and his elder brother at three years of age, both of whom had a history of recurrent staphylococcal infections [15]. It is important to note that STAT-3 loss-of-function mutation is associated with connective tissue abnormalities, including vasculopathy involving medium-sized arteries, particularly cerebral and coronary arteries, in the form of coronary ectasias and aneurysms [30]. Ling et al. described two adult patients with HIES with coronary artery ectasia and aneurysms detected by angiography following myocardial infarction [16]. Yared et al. reported a 15-day-old neonate diagnosed with HIES with a family history of KD in the elder brother [17]. Whether autoimmune phenomena play a role or an undiagnosed episode of KD in childhood, or connective tissue abnormality, causing ectasias in such cases needs to be understood. It can be accepted that there is an overlap between the HIES and KD, such as association with microorganisms like Staphylococcus aureus and Candida albicans, dysfunctional T cells, and dysregulated regulatory T cells (Tregs). Both autosomal recessive forms of HIES due to dedicator of cytokinesis 8 (DOCK-8) and tyrosine-kinase 2 (TYK-2) mutations), and autosomal dominant HIES due to STAT3 loss-of-function mutation may lead to vasculitis [16]. Young et al. investigated a 30-year-old with HIES and CAAs. They showed a deficiency of cluster of differentiation 4 (CD4) + T central memory (CD4 + TCM) cells and memory B cells with an expansion of CD4 + terminally differentiated effector memory cells expressing CD45RA (TEMRA) cells [18].
3.3. KD and Phagocytic Defects
Chronic granulomatous disease (CGD) is an IEI characterized by recurrent infections by catalase-positive organisms and granulomatous inflammation with visceral abscesses due to defective nicotinamide adenine dinucleotide phosphate (NADPH) oxidase required for oxidative burst within phagocytes. These patients have an increased frequency of autoimmunity such as inflammatory bowel disease, lupus, rheumatoid arthritis, and IgA nephropathy [31]. KD has also been reported in patients with CGD. Yamazaki et al. described a 1-year-old boy with CGD who presented with atypical KD and CAAs and was treated with IVIG and steroids [19]. The authors hypothesized that defective NADPH oxidase causes impaired apoptotic debris clearance leading to persistent antigenic stimulation and exaggerated immune response. The hyperactivated neutrophils release proinflammatory cytokines. NADPH oxidase deficient T-cells show skewed cytokine production, T helper type 1 (T1) immune response, granuloma formation, and autoimmunity. The overlapping features between KD and X-linked CGD include immune-mediated generalized vasculitis and Staphylococcus aureus and Candida infections [19]. Muneuchi et al. described a 2-year-old male child diagnosed with X-linked CGD at four months of age due to glycoprotein 91 phagocyte oxidase (gp91-phox) gene mutation who presented with incomplete KD [20]. Tsuge et al. showed a 10-month-old male infant diagnosed with gp91-phox-mutated X-linked CGD at eight months of age who had complete KD [21]. Hule et al. reported two siblings from the same family who had an autosomal recessive form of CGD due to neutrophil cytosolic factor 1 (NCF1) gene mutation and developed KD [22].
3.4. KD and Defects in Intrinsic and Innate Immunity
KD has been reported in children with innate immune defects, including Warts, Hypogammaglobulinemia, Infections, Myelokathexis (WHIM) syndrome, and Signal transducer and activator of transcription 2 (STAT 2) deficiency [23,24]. Ma et al. reported a case of WHIM syndrome due to gain-of-function C-X-C motif chemokine receptor 4 (CXCR4) gene mutation with a history of leukopenia, neutropenia, and low immunoglobulin levels at birth who developed incomplete KD at one year of age without CAAs with increase/normalization of the neutrophil count during the KD episode [23]. His symptoms associated with KD, including coronary artery dilatation, improved after high-dose IVIG; however, leukopenia and neutropenia due to underlying CXCR4 mutation persisted. Hambleton et al. described a 5-year-old male with STAT2 deficiency who developed disseminated vaccine strain measles and presented as KD six days post-vaccination at 18 months of life [24]. The child responded to supplemental oxygen and supportive therapy.
3.5. KD and Autoinflammatory Disorders
Broderick et al. reported four children aged 10–36 months diagnosed with KD and treated with IVIG [25]. Post-IVIG, each child developed recurrent fever episodes with pharyngitis, aphthous ulcers, cervical lymphadenopathy, ocular symptoms, abdominal pain, and rash that responded to steroid therapy. Based on the signs and symptoms and response to steroid therapy with afebrile periods in between, they were diagnosed with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome that responded to steroid therapy. It was suggested that genetic predisposition leading to dysregulated innate immune response could be responsible for the pathogenesis of KD in PFAPA. Ninomiya et al. reported a 2-year-old girl diagnosed with PFAPA at one year of age who developed KD [26].
4. Discussion
According to the published literature, we could retrieve 25 cases, including 22 children and three adults affected with different clinical subtypes of IEI and developed KD during their disease. These include predominantly antibody deficiencies comprising XLA (n = 4), selective IgA deficiency (n = 2), and transient hypogammaglobulinemia of infancy (n = 1); combined immunodeficiencies including WAS (n = 1), and HIES (n = 6); phagocyte defects including CGD (n = 4); innate immune defects including WHIM syndrome (n = 1), and STAT2 defect (n = 1); and autoinflammatory diseases including PFAPA (n = 5). Of these, complete KD was documented in four patients, while incomplete KD was documented in nine patients. CAAs were seen in 9/25 (36%) patients with KD and IEIs.
4.1. Pathogenetic Mechanisms of KD in XLA
In XLA, patients develop subclinical infections leading to chronic inflammation and immune dysregulation. In response to infectious organisms, the expression of major histocompatibility complex (MHC) antigens increases via molecular mimicry on antigen-presenting cells (APCs), leading to T helper type 2 (T2) immune response and production of cytokines, including interleukins (IL-4, IL-5, and IL-10). The immune system shifts towards an autoimmune phenotype with aberrant B-cell function [8]. Additionally, overactive toll-like receptor-9 (TLR-9) and nuclear factor-kappa B (NF-κB)-mediated immune stimulation leads to the production of natural immunoglobulin M (IgM) antibodies from innate B-1 cells and T1 type of immune responses. The impairment of Tregs could also explain this association of KD in XLA [8]. Autoimmune mechanisms that may play a role in the pathogenesis of KD in XLA include leaky antibody production, defective BTK signaling leading to abnormal myeloid maturation and aberrant toll-like receptor (TLR) signaling, and autoreactive T cells [10]. XLA has been associated with inflammatory diseases with elevated levels of tumor necrosis factor- α (TNF-α) and interleukins (IL-10, IL-6, and IL-1α) secreted by peripheral blood mononuclear cells (PBMCs). The activity of inflammasome, nucleotide-binding domain, leucine-rich–containing family, and pyrin domain–containing-3 (NLRP3) activity is impaired in XLA as BTK is a regulator of NLRP3. Some infections, such as Pseudomonas aeruginosa, are found to be a common feature of XLA and infection-triggered KD [7].
In KD, B cells increase, whereas natural killer (NK) and T cells decrease in the acute phase. Serum IgG levels are significantly lower than normal age-matched controls (pre-IVIG), which has been found to correlate with disease severity, including CAAs and IVIG resistance [32,33]. Reduced B cell receptor (BCR) repertoire and B cell diversity have been detected through next-generation sequencing (NGS) in acute KD than febrile controls and post-IVIG therapy in KD [34,35]. Genome-wide association studies (GWAS) revealed down-regulation with reduced expression of B-lymphoid tyrosine kinase (BLK) and B-cell lymphoma 2-like 11 (BCL2L11) genes and upregulation with enhanced expression of CD40, Fc Gamma Receptor Iia (FCGR2A) and immunoglobulin heavy chain variable region (IGHV) genes leading to impaired function, activation and development of B cells [36]. The fact that the incidence of KD is extremely low in infants below six months of age and it increases after that due to declining maternal IgG levels also points towards the role of B-cell development and function in the pathogenesis of KD [36] (Table 2).

Table 2.
B-cell mediated humoral immunity in KD and pathogenetic mechanisms involved in KD associated with XLA.
Thus, we hypothesize that XLA provides an excellent disease model for understanding the role of humoral immunity in the pathogenesis of KD. As B cell development and function are impaired in KD despite an increase in B cell number, this suggests that B-cell immune response plays a significant role in KD [36]. In XLA, the absence of B cells leading to severely reduced IgG levels may predispose these children to KD with CAAs, which needs further exploration (Figure 1).
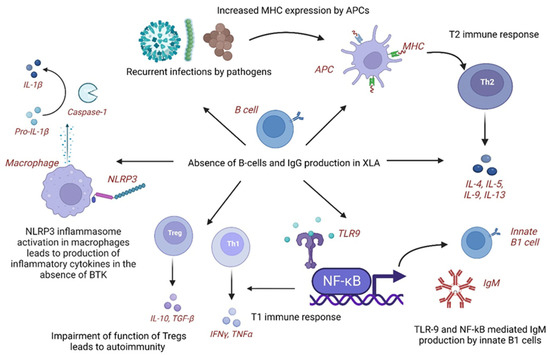
Figure 1.
Cellular and molecular mechanisms underlying pathogenesis of Kawasaki disease in X-linked agammaglobulinemia. APCs: Antigen-presenting cells; BTK: Bruton tyrosine kinase; IgG: Immunoglobulin G; IgM: Immunoglobulin M; IL: interleukin; IFNγ: Interferon γ; KD: Kawasaki disease; MHC: Major histocompatibility complex; NLRP3: Nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3; NF-kB: Nuclear factor KB; T1: T helper type 1 immune response; T2: T helper type 2 (Th2) immune response; TLR-9: Toll-like receptor-9; Tregs: Regulatory T cells; TGF-β: Transforming growth factor-β; TNFα: Tumor necrosis factorα; Th1: T helper type 1 cells; Th2: T helper type 2 cells; XLA: X-linked agammaglobulinemia. Created with BioRender.com. https://www.biorender.com (accessed on 20 June 2023).
4.2. Pathogenetic Mechanisms of KD in Selective IgA Deficiency
Autoimmune complications are reported in 7–36% of patients with selective IgA deficiency, including systemic lupus erythematosus (SLE), Sjogren’s syndrome, and juvenile idiopathic arthritis following sinopulmonary infections [11]. In patients with KD and concurrent selective IgA deficiency, IgM plasma cells might replace the function of IgA plasma cells that otherwise infiltrate coronary arteries and other tissues in KD. IgA plasma cells play an essential role in the inflammatory process associated with KD [12].
4.3. Pathogenetic Mechanisms of KD in Transient Hypogammaglobulinemia of Infancy
In transient hypogammaglobulinemia of infancy, incomplete KD could be due to partial humoral response due to hypogammaglobulinemia as B-cell-mediated antibody response plays a significant pathogenic role in complete KD [13].
4.4. Pathogenetic Mechanisms of KD in WAS
IL-6, a proinflammatory and thrombopoietic cytokine, is increased in the acute phase of KD [37]. Thrombocytopenia in WAS may respond to the stimulatory effects of IL-6 in acute KD. Other vasculitic disorders like Takayasu arteritis and IgA vasculitis have been reported in patients with WAS, indicating an increased predisposition to vasculitic diseases [14].
4.5. Pathogenetic Mechanisms of KD in HIES
High-dose IVIG is an effective treatment for KD with HIES, HIES, and atopic dermatitis, as it significantly improves severe eczema. It reduces in-vivo and in-vitro IgE production from normal B-cells after stimulation with interleukin-4 (IL-4) and anti-CD40 antibody reagents [15]. Association between HIES and KD may exist due to the clinical overlap, especially fever, rash, and CAAs. Autosomal recessive (AR)-HIES patients may develop vasculitis, stenosis, and aneurysms [16]. AD or sporadic cases (STAT-3 loss-of-function) may develop carotid and cerebral aneurysms secondary to connective tissue abnormalities. Thus, CAAs could be due to connective tissue abnormalities in HIES. Diagnosis of KD may be missed because of similar features between the two disorders [16]. KD and HIES share clinical features, including onset in childhood, CAAs, mucocutaneous involvement, lymphadenopathy, multiple genetic variations, and infection susceptibility (Table 3). AR-HIES (DOCK-8 or TYK-2 mutations) may also present as vasculitis like KD [17]. In KD, circulating CD4+ and CD8+ effector memory T cells with the proinflammatory phenotype and increased cytokine production have been detected in the acute phase with a rapid increase in the subacute phase followed by a decline in convalescent phase, indicating that antigenic exposure might occur days to weeks before the onset of acute phase [38]. HIES patients may show expanded terminally differentiated CD4+ effector memory T cells expressing CD45RA (TEMRA cells) associated with increased cytokine production leading to increased IgE levels, reduced CD4+ central memory T cells associated with decreased T-cell proliferation, and reduced memory B cells associated with impaired antibody responses in HIES [18]. CAAs could develop in HIES due to undiagnosed and untreated underlying KD [18]. Whether CAAs in patients with HIES are due to the primary disease or an episode of undiagnosed KD remains conjectural (Figure 2).

Table 3.
Clinical and laboratory features shared between KD and HIES.
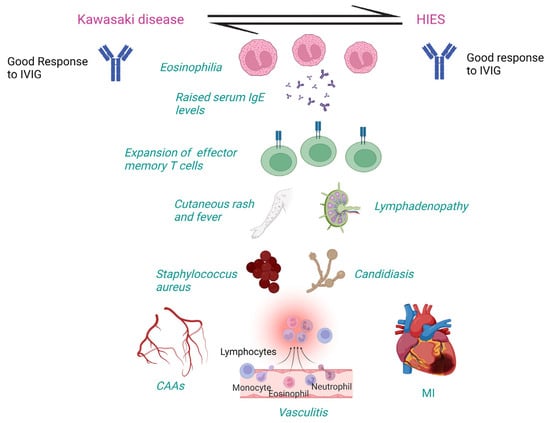
Figure 2.
Clinical and laboratory features shared between Kawasaki disease and hyper IgE syndrome. CAAs: Coronary artery abnormalities, HIES: Hyper IgE syndrome; IgE: Immunoglobulin E; IVIG: Intravenous immunoglobulin; MI: Myocardial infarction. Created with BioRender.com https://www.biorender.com (accessed on 20 June 2023).
4.6. Pathogenetic Mechanisms of KD in CGD
NADPH-oxidase deficient T cells in CGD show skewed T1 immune response with the production of T1 cytokines leading to autoimmunity [39]. Polyclonal hypergammaglobulinemia and autoantibody production are reported in patients with CGD and murine models, respectively [40]. Defective apoptosis of neutrophils and inefficient clearance of apoptotic debris in CGD may result in an exaggerated inflammatory response [40]. The ineffective removal of microbial pathogens and their antigens causes autoimmunity. Candida albicans and Staphylococcus aureus are common pathogens associated with KD and CGD [19]. Activated neutrophils infiltrate the coronary arteries with higher granulocyte-colony stimulating factor (G-CSF) levels detected in patients with CAAs than in patients without CAAs in the early phase of KD eliciting vasculitis due to mechanisms other than reactive oxygen species (ROS) generation. However, incomplete KD in CGD could be due to defective ROS production by neutrophils [19]. Predisposition to infections in CGD may delay the diagnosis of KD and cause vascular damage [20]. Insufficient ROS production in hyperactivated neutrophils and monocytes reduces immunoregulatory response leading to skewing of the immune system towards hyperinflammatory phenotype that may lead to KD. T cells are hyperactivated in CGD, evident by sustained elevation of soluble interleukin-2 receptor (sIL-2R) levels, leading to poor response to initial high-dose IVIG therapy [21]. Non-functional residual ROS generation leads to ineffective microbial clearance and persistent infection, triggering an abnormal immune response in AR-CGD. Activation of innate immunity and neutrophils with the release of proinflammatory cytokines such as interleukins (IL-1, IL-6) and TNF and activation of signaling pathways in CGD leads to an overwhelming immune response predisposing to KD [22] (Figure 3).
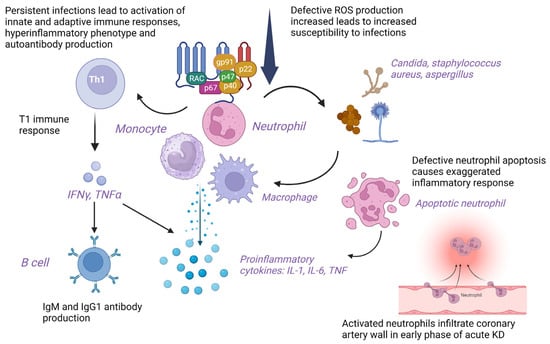
Figure 3.
Cellular and molecular mechanisms underlying pathogenesis of Kawasaki disease in chronic granulomatous disease. gp91: 91-kilodalton phagocytic oxidase glycoprotein, IgM: Immunoglobulin M; IgG1: Immunoglobulin G subclass 1; IL: Interleukin; IFNγ: Interferon γ; KD: Kawasaki disease; p22: 22-kilodalton phagocytic oxidase protein (p22phox); p40: 40-kilodalton phagocytic oxidase protein (p40phox); p47: 47-kilodalton phagocytic oxidase protein (p47phox); p67: 67-kilodalton phagocytic oxidase protein (p67phox); T1 immune response: T helper type 1 immune response; Th1: T helper type 1 cells; TNF: Tumor necrosis factor; RAC: Rho-related C3 botulinum toxin substrate; ROS: Reactive oxygen species. Created with BioRender.com. https://www.biorender.com (accessed on 19 June 2023).
4.7. Pathogenetic Mechanisms of KD in WHIM Syndrome
Since the arterial endothelial cells regulate the expression of CXCR4, the up-regulation or down-regulation of CXCR4 or defective CXCR4/C-X-C motif chemokine ligand 12 (CXCL12) axis signaling can cause abnormal coronary artery development and endothelial cell dysfunction and endothelial injury leading to KD. Other autoimmune diseases reported in WHIM syndrome include diabetes and hypothyroidism [23]. Disseminated viral infection may mimic KD in patients with STAT2 deficiency [24].
4.8. Pathogenetic Mechanisms of KD in PFAPA
Both KD and PFAPA may co-occur and are associated with activation of the innate immune system with similar cytokine profiles, including TNF-α and interleukins (IL1β and IL6). Levels of interleukin-1 receptor alpha (IL-1Rα), an inhibitor of IL-1β, are increased in response to IVIG [25]. The association between KD and PFAPA could be attributed to genetic predisposition leading to dysregulated innate immunity. NOD-like receptor (NLR) messenger RNA (mRNA) levels increase in patients with KD. NLR-ligand injection causes KD-like CAAs in mice [26].
4.9. Multisystem Inflammatory Syndrome in Children (MIS-C) and IEIs
Recently, a multisystem inflammatory syndrome in children, also known as MIS-C, has been identified as a rare but fatal complication of otherwise mild severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and young adults [41]. It is characterized by a hyperinflammatory state that closely mimics KD. Unlike KD, which usually affects children below five years of age, MIS-C affects slightly older children aged between 7 to 12 years of age and has a more severe disease course with features of hemophagocytic lymphohistiocytosis (HLH) leading to multiorgan dysfunction syndrome (MODS), cytopenia, shock, and myocarditis [41,42,43]. Cytokine storm underlies the hyperinflammatory state. Thus, IEIs were hypothesized to be the underlying predisposing factors of this severe condition in affected children. Genetic defects, including defects in suppressor of cytokine signaling 1 (SOCS1), X-linked inhibitor of apoptosis (XIAP), and cytochrome B-245 b-chain (CYBB) genes, have been identified in children with MIS-C using a targeted NGS approach. Autosomal recessive, bi-allelic loss-of-function or hypomorphic variants in 2’-5’-oligoadenylate synthetase 1 and 2 (OAS1OAS2) and ribonuclease L (RNASEL) genes involved in OAS-RNase L anti-viral signaling pathway have been identified in 5 children in a cohort of 558 children diagnosed with MIS-C using whole genome or exome sequencing approach by Lee et al. [44]. These children fulfilled the criteria of MIS-C and were recruited from 16 countries worldwide, with ages ranging from 3 months to 19 years and a male-to-female ratio of 1.5:1. In addition to these, variants in several genes implicated in IEIs, including HLH, such as perforin (PRF1), syntaxin binding protein 2 (STXBP2), unc-13 homolog D (UNC13D), lysosomal trafficking regulator (LYST), adaptor related protein complex three subunit Beta 1 (AP3B1), and dedicator of cytokinesis 8 (DOCK8) and genes involved in the interferon responses have been identified. However, the pathogenicity of these rare variants needs to be functionally validated. These studies suggest that children with IEIs involving the interferon response and regulatory genes and signaling pathways are predisposed to MIS-C [41]. Similar mechanisms might be operable in children with KD.
5. Future Directions
The significant interplay of the pathophysiological mechanisms between IEIs and KD is supported by the published literature. Although IEIs are considered rare disorders, they are being increasingly reported worldwide in different populations and ethnicities, having different clinical presentations, KD being one of them. Further research in this area may identify other types of IEI in KD patients. Targeted NGS panels, whole exome, and whole genome sequencing and transcriptome studies may unravel monogenic IEI defects in patients with KD; however, this may require larger patient cohorts. With recent advancements in precision medicine, such as targeted therapies and hematopoietic stem cell transplant (HSCT), the treatment of monogenic diseases has improved. Identification of monogenic defects in KD could provide newer insights into these patients’ pathophysiology, diagnosis, and management.
6. Conclusions
The reported data suggest that underlying IEIs are associated with an increased predisposition to the development of KD. Since IEI and KD have an early onset during childhood and share features attributed to immune deficiency and immune dysregulation with hyperinflammation, it would be interesting to investigate the patients with KD for monogenic IEIs. Thus, the significance of these case reports needs further evaluation and validation by analyzing larger patient cohorts.
Author Contributions
S.S. (Saniya Sharma) and R.K.P. designed the manuscript. S.S. (Saniya Sharma), P.L.N., R.K.P., K.S. and M.D. wrote the manuscript. R.K.P., A.R. and S.S. (Surjit Singh) reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare there are no conflict of interest.
References
- Singh, S.; Vignesh, P.; Burgner, D. The epidemiology of Kawasaki disease: A global update. Arch. Dis. Child. 2015, 100, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [PubMed]
- Agarwal, S.; Agrawal, D.K. Kawasaki disease: Etiopathogenesis and novel treatment strategies. Expert Rev. Clin. Immunol. 2017, 13, 247–258. [Google Scholar] [CrossRef]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef] [PubMed]
- Marrani, E.; Burns, J.C.; Cimaz, R. How Should We Classify Kawasaki Disease? Front. Immunol. 2018, 9, 2974. [Google Scholar] [CrossRef]
- Takahashi, K.; Oharaseki, T.; Yokouchi, Y. Pathogenesis of Kawasaki disease. Clin. Exp. Immunol. 2011, 164 (Suppl. S1), 20–22. [Google Scholar] [CrossRef]
- Rivas-Larrauri, F.; Aguilar-Zanela, L.; Castro-Oteo, P.; Rosales-Hernandez, L.A.; Otero-Mendoza, F.; López-Herrera, G.; Ordoñez-Ortega, J.; Garrido-García, M.; Yamazaki-Nakashimada, M.A. Kawasaki disease and immunodeficiencies in children: Case reports and literature review. Rheumatol. Int. 2019, 39, 1829–1838. [Google Scholar] [CrossRef]
- Behniafard, N.; Aghamohammadi, A.; Abolhassani, H.; Pourjabbar, S.; Sabouni, F.; Rezaei, N. Autoimmunity in X-linked agammaglobulinemia: Kawasaki disease and review of the literature. Expert Rev. Clin. Immunol. 2012, 8, 155–159. [Google Scholar] [CrossRef]
- Malekzadeh, I.; Moradinejad, M.H.; Ziaee, V.; Malek, A.; Khalili, A. Autoimmunity in X-linked agammaglobulinemia; a patient with several episodes of autoimmunity. Iran J. Ped. 2013, 23, 75. [Google Scholar]
- Sharma, D.; Guleria, S.; Suri, D.; Rawat, A.; Garg, R.; Singh, S. A child with X-linked agammaglobulinemia and Kawasaki disease: An unusual association. Rheumatol. Int. 2017, 37, 1401–1403. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nomura, Y.; Kono, Y.; Kawano, Y. Selective IgA deficiency complicated by Kawasaki syndrome. Pediatr. Int. 2008, 50, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Anzai, T.; Minami, T.; Sato, T.; Furui, S.; Yamagata, T. Treatment of a patient with Kawasaki disease associated with selective IgA deficiency by continuous infusion of cyclosporine A without intravenous immunoglobulin. Turk. J. Pediatr. 2016, 58, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Şanlıdağ, B.; Balkan, C.; Bahçeciler, N. A case report: Incomplete Kawasaki disease in a hypogammaglobulinémie child. Arch. Argent Pediatr. 2018, 116, e322–e324. [Google Scholar] [PubMed]
- Kawakami, C.; Miyake, M.; Tamai, H. Kawasaki disease in a patient with Wiskott-Aldrich syndrome: An increase in the platelet count. Int. J. Hematol. 2003, 77, 199–200. [Google Scholar] [CrossRef]
- Kimata, H. High-dose intravenous gamma-globulin treatment for hyperimmunoglobulinemia E syndrome. J. Allergy Clin. Immunol. 1995, 95, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.C.; Freeman, A.F.; Gharib, A.M.; Arai, A.E.; Lederman, R.J.; Rosing, D.R.; Holland, S.M. Coronary artery aneurysms in patients with hyper IgE recurrent infection syndrome. Clin. Immunol. 2007, 122, 255–258. [Google Scholar] [CrossRef]
- Yared, T.; Mohsen, S. Job’s Syndrome with a Family History of Kawasaki Disease: A Case Presentation and Review of Literature. Dis. Diagn. 2021, 10, 19–22. [Google Scholar] [CrossRef]
- Young, T.Y.; Jerome, D.; Gupta, S. Hyperimmunoglobulinemia E syndrome associated with coronary artery aneurysms: Deficiency of central memory CD4+ T cells and expansion of effector memory CD4+ T cells. Ann. Allergy Asthma Immunol. 2007, 98, 389–392. [Google Scholar] [CrossRef]
- Yamazaki-Nakashimada, M.A.; Ramírez-Vargas, N.; De Rubens-Figueroa, J. Chronic granulomatous disease associated with atypical Kawasaki disease. Pediatr. Cardiol. 2008, 29, 169–171. [Google Scholar] [CrossRef]
- Muneuchi, J.; Ishimura, M.; Takada, H.; Hoshina, T.; Utsunomiya, R.; Ikeda, K.; Yamaguchi, K.; Ohga, S.; Kusuhara, K.; Hara, T. Incomplete Kawasaki disease in a patient with chronic granulomatous disease. Pediatr. Int. 2010, 52, e134–e136. [Google Scholar] [CrossRef]
- Tsuge, M.; Shigemitsu, Y.; Yano, Y.; Fujiwara, M.; Miyai, T.; Ueda, K.; Takata, K.; Moriwake, T. Immunoglobulin resistance in Kawasaki disease with chronic granulomatous disease. Pediatr. Int. 2012, 54, e32–e34. [Google Scholar] [CrossRef]
- Hule, G.P.; Kanvinde, P.R.; Kulkarni, M.A.; van Leeuwen, K.; de Boer, M.; Bargir, U.A.; Taur, P.D.; Desai, M.M.; Madkaikar, M.R. p47phox−/− Chronic Granulomatous Disease Patient with Incomplete Kawasaki Disease. J. Clin. Immunol. 2018, 38, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Y.; Wu, P.; Kang, M.; Hong, Y.; Xue, Y.; Chen, C.; Li, H.; Fang, Y. Case Report: A Novel CXCR4 Mutation in a Chinese Child With Kawasaki Disease Causing WHIM Syndrome. Front. Immunol. 2022, 13, 857527. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, S.; Goodbourn, S.; Young, D.F.; Dickinson, P.; Mohamad, S.M.; Valappil, M.; McGovern, N.; Cant, A.J.; Hackett, S.J.; Ghazal, P.; et al. STAT2 deficiency and susceptibility to viral illness in humans. Proc. Natl. Acad. Sci. USA 2013, 19, 3053–3058. [Google Scholar] [CrossRef]
- Broderick, L.; Tremoulet, A.H.; Burns, J.C.; Bastian, J.F.; Hoffman, H.M. Recurrent fever syndromes in patients after recovery from Kawasaki syndrome. Pediatrics 2011, 127, e489–e493. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, T.; Takada, H.; Nagatomo, Y.; Nanishi, E.; Nagata, H.; Yamamura, K.; Doi, T.; Ikeda, K.; Hara, T. Development of Kawasaki disease in a patient with PFAPA. Pediatr. Int. 2013, 55, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.; Rikhi, R.; Loganathan, S.K.; Suri, D.; Singh, S. Autoimmunity in Wiskott-Aldrich Syndrome: Updated Perspectives. Appl. Clin. Genet. 2021, 14, 363–388. [Google Scholar] [CrossRef]
- Saikia, B.; Rawat, A.; Minz, R.W.; Suri, D.; Pandiarajan, V.; Jindal, A.; Sahu, S.; Karim, A.; Desai, M.; Taur, P.D.; et al. Clinical Profile of Hyper-IgE Syndrome in India. Front. Immunol. 2021, 12, 626593. [Google Scholar] [CrossRef]
- Goel, R.R.; Nakabo, S.; Dizon, B.L.P.; Urban, A.; Waldman, M.; Howard, L.; Darnell, D.; Buhaya, M.; Carmona-Rivera, C.; Hasni, S.; et al. Lupus-like autoimmunity and increased interferon response in patients with STAT3-deficient hyper-IgE syndrome. J. Allergy Clin. Immunol. 2021, 147, 746–749.e9. [Google Scholar] [CrossRef]
- Yong, P.F.; Freeman, A.F.; Engelhardt, K.R.; Holland, S.; Puck, J.M.; Grimbacher, B. An update on the hyper-IgE syndromes. Arthritis Res. Ther. 2012, 14, 228. [Google Scholar] [CrossRef]
- Henrickson, S.E.; Jongco, A.M.; Thomsen, K.F.; Garabedian, E.K.; Thomsen, I.P. Noninfectious Manifestations and Complications of Chronic Granulomatous Disease. J. Pediatr. Infect. Dis. Soc. 2018, 7, S18–S24. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Ohashi, Y.; Harada, K.; Asai, T.; Okawa, S.; Nagashima, M.; Katoh, T.; Baba, K.; Frusho, K.; Okuni, M.; et al. Coronary risks after high-dose gamma-globulin in children with Kawasaki disease. Pediatr. Int. 2000, 42, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki-Nakashimada, M.A.; Gámez-González, L.B.; Murata, C.; Honda, T.; Yasukawa, K.; Hamada, H. IgG levels in Kawasaki disease and its association with clinical outcomes. Clin. Rheumatol. 2019, 38, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Pan, C.T.; Huang, Y.H.; Huang, F.C.; Lin, Y.S.; Li, S.C.; Huang, L.H. Global Investigation of Immune Repertoire Suggests Kawasaki Disease Has Infectious Cause. Circ. J. 2019, 83, 2070–2078. [Google Scholar] [CrossRef]
- Ko, T.M.; Kiyotani, K.; Chang, J.S.; Park, J.H.; Yin Yew, P.; Chen, Y.T.; Wu, J.Y.; Nakamura, Y. Immunoglobulin profiling identifies unique signatures in patients with Kawasaki disease during intravenous immunoglobulin treatment. Hum. Mol. Genet. 2018, 27, 2671–2677. [Google Scholar] [CrossRef]
- Lee, J.K. Hygiene Hypothesis as the Etiology of Kawasaki Disease: Dysregulation of Early B Cell Development. Int. J. Mol. Sci. 2021, 22, 12334. [Google Scholar] [CrossRef]
- Ueno, Y.; Takano, N.; Kanegane, H.; Yokoi, T.; Yachie, A.; Miyawaki, T.; Taniguchi, N. The acute phase nature of interleukin-6: Studies in Kawasaki disease and other febrile illnesses. Clin. Exp. Immunol. 1989, 76, 337–342. [Google Scholar]
- Franco, A.; Shimizu, C.; Tremoulet, A.H.; Burns, J.C. Memory T-cells and characterization of peripheral T-cell clones in acute Kawasaki disease. Autoimmunity 2010, 43, 317–324. [Google Scholar] [CrossRef]
- Jackson, S.H.; Devadas, S.; Kwon, J.; Pinto, L.A.; Williams, M.S. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 2004, 5, 818–827. [Google Scholar] [CrossRef]
- Sanford, A.N.; Suriano, A.R.; Herche, D.; Dietzmann, K.; Sullivan, K.E. Abnormal apoptosis in chronic granulomatous disease and autoantibody production characteristic of lupus. Rheumatology 2006, 45, 178–181. [Google Scholar] [CrossRef]
- Bucciol, G.; Meyts, I.; COVID Human Genetic Effort. Inherited and acquired errors of type I interferon immunity govern susceptibility to COVID-19 and multisystem inflammatory syndrome in children. J. Allergy Clin. Immunol. 2023, 151, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Le Pen, J.; Yatim, A.; Dong, B.; Aquino, Y.; Ogishi, M.; Pescarmona, R.; Talouarn, E.; Rinchai, D.; Zhang, P.; et al. Inborn errors of OAS-RNase L in SARS-CoV-2-related multisystem inflammatory syndrome in children. Science 2023, 379, eabo3627. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).