Ultrasound Features and Ultrasound Scores in the Differentiation between Benign and Malignant Adnexal Masses
Abstract
:1. Introduction
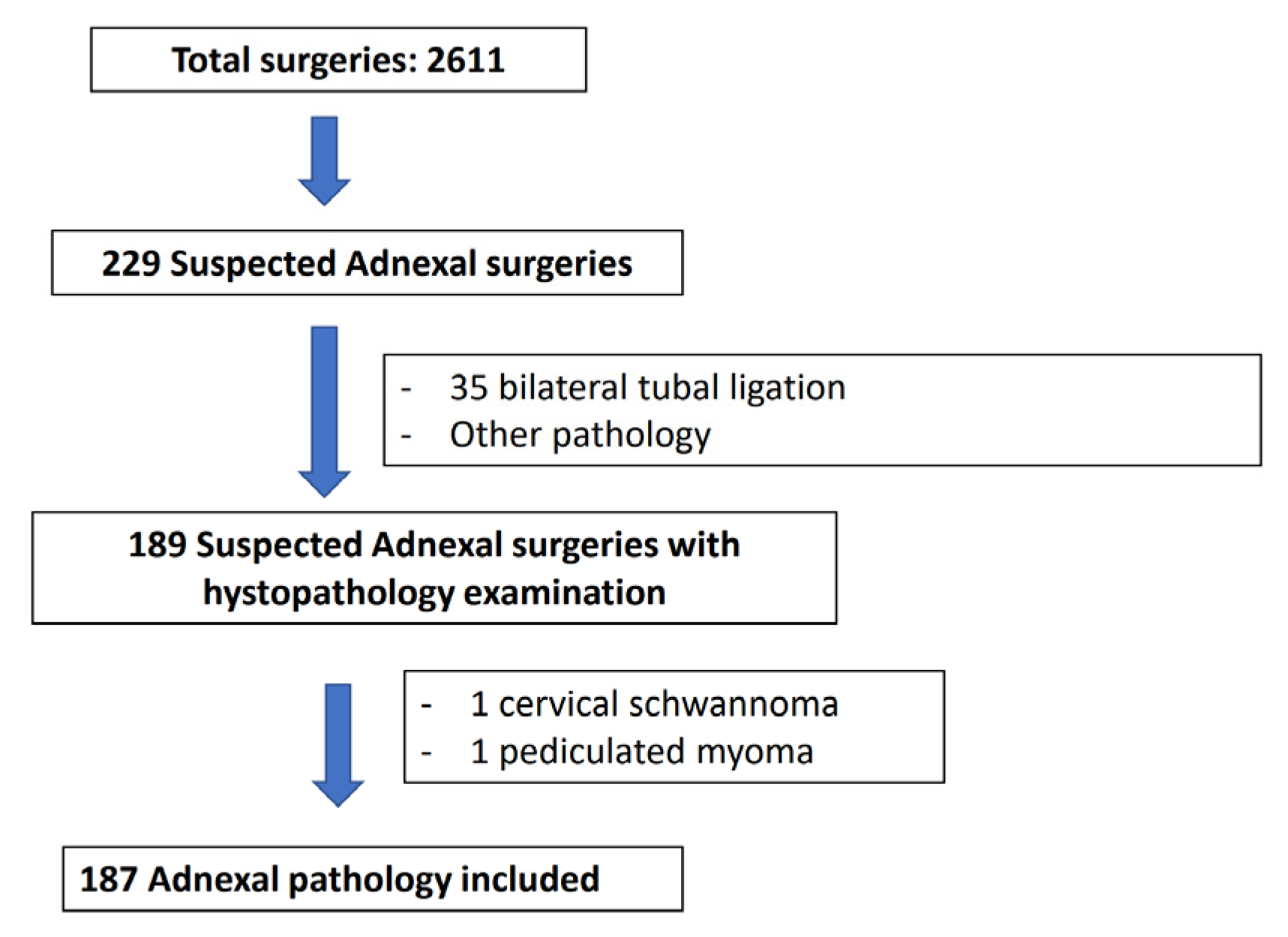
2. Materials and Methods
2.1. Criteria for Inclusion and Exclusion
2.2. Methodology
3. Results
4. Discussion
5. Limitations
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Summary Statement
Key Results
- -
- US scores (IOTA SRRA, ADNEX model and O-RADS) have high sensitivity classifying benign and malignant adnexal masses
- -
- There are certain US features related to malignancy (irregular contour, absence of acoustic shadowing, vascularized solid areas, ≥1 papillae, vascularized septum, moderate-severe ascites)
Abbreviations
| US | Ultrasound |
| SA | Subjective Assessment |
| IOTA | International Ovarian Tumor Analysis |
| SRRA | Simple Rules Risk Assessment |
| ADNEX model | Assessment of Different Neoplasias in the Adnexa |
| O-RADS | Ovarian Adnexal Reporting and Data System |
References
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viora, E.; Piovano, E.; Poma, C.B.; Cotrino, I.; Castiglione, A.; Cavallero, C.; Sciarrone, A.; Bastonero, S.; Iskra, L.; Zola, P. The ADNEX model to triage adnexal masses: An external validation study and comparison with the IOTA two-step strategy and subjective assessment by an experienced ultrasound operator. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Tavoraitė, I.; Kronlachner, L.; Opolskienė, G.; Bartkevičienė, D. Ultrasound Assessment of Adnexal Pathology: Standardized Methods and Different Levels of Experience. Medicina 2021, 57, 708. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Park, B.K.; Lee, Y.Y.; Kim, T.-J. Validation of IOTA-ADNEX Model in Discriminating Characteristics of Adnexal Masses: A Comparison with Subjective Assessment. J. Clin. Med. 2020, 9, 2010. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; Van Holsbeke, C.; Paladini, D.; Van Calster, B.; Vergote, I.; Van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Van Calster, B.; Testa, A.; Savelli, L.; Fischerova, D.; Froyman, W.; Wynants, L.; Van Holsbeke, C.; Epstein, E.; Franchi, D.; et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am. J. Obstet. Gynecol. 2016, 214, 424–437. [Google Scholar] [CrossRef] [Green Version]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef] [Green Version]
- Andreotti, R.F.; Timmerman, D.; Strachowski, L.M.; Froyman, W.; Benacerraf, B.R.; Bennett, G.L.; Bourne, T.; Brown, D.L.; Coleman, B.G.; Frates, M.C.; et al. O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology 2020, 294, 168–185. [Google Scholar] [CrossRef] [Green Version]
- Andreotti, R.F.; Timmerman, D.; Benacerraf, B.R.; Bennett, G.L.; Bourne, T.; Brown, D.L.; Coleman, B.G.; Frates, M.C.; Froyman, W.; Goldstein, S.R.; et al. Ovarian-Adnexal Reporting Lexicon for Ultrasound: A White Paper of the ACR Ovarian-Adnexal Reporting and Data System Committee. J. Am. Coll. Radiol. 2018, 15, 1415–1429. [Google Scholar] [CrossRef] [Green Version]
- Pelayo, M.; Pelayo-Delgado, I.; Sancho-Sauco, J.; Sanchez-Zurdo, J.; Abarca-Martinez, L.; Corraliza-Galán, V.; Martin-Gromaz, C.; Pablos-Antona, M.J.; Zurita-Calvo, J.; Alcázar, J.L. Comparison of Ultrasound Scores in Differentiating between Benign and Malignant Adnexal Masses. Diagnostics 2023, 13, 1307. [Google Scholar] [CrossRef]
- Pelayo-Delgado, I.; Sancho, J.; Pelayo, M.; Corraliza, V.; Perez-Mies, B.; Del Valle, C.; Abarca, L.; Pablos, M.J.; Martin-Gromaz, C.; Pérez-Vidal, J.R.; et al. Contribution of Outpatient Ultrasound Transvaginal Biopsy and Puncture in the Diagnosis and Treatment of Pelvic Lesions: A Bicenter Study. Diagnostics 2023, 13, 380. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board (Ed.) Female genital tumours. In World Health Organization Classification of Tumours, 5th ed.; IARC Press: Lyon, France, 2020. [Google Scholar]
- McCluggage, W.G.; Singh, N.; Gilks, C.B. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition (2020). Histopathology 2022, 80, 762–778. [Google Scholar] [CrossRef]
- Nasioudis, D.; Mastroyannis, S.A.; Ko, E.M.; Haggerty, A.F.; Cory, L.; Giuntoli, R.L.; Kim, S.H.; Morgan, M.A.; Latif, N.A. Delay in adjuvant chemotherapy administration for patients with FIGO stage I epithelial ovarian carcinoma is associated with worse survival; an analysis of the National Cancer Database. Gynecol. Oncol. 2022, 166, 263–268. [Google Scholar] [CrossRef]
- Di Legge, A.; Testa, A.C.; Ameye, L.; Van Calster, B.; Lissoni, A.; Leone, F.; Savelli, L.; Franchi, D.; Czekierdowski, A.; Trio, D.; et al. Lesion size affects diagnostic performance of IOTA logistic regression models, IOTA simple rules and risk of malignancy index in discriminating between benign and malignant adnexal masses. Ultrasound Obstet. Gynecol. 2012, 40, 345–354. [Google Scholar] [CrossRef]
- Di Legge, A.; Pollastri, P.; Mancari, R.; Ludovisi, M.; Mascilini, F.; Franchi, D.; Jurkovic, D.; Coccia, M.E.; Timmerman, D.; Scambia, G.; et al. Clinical and ultrasound characteristics of surgically removed adnexal lesions with largest diameter ≤2.5 cm: A pictorial essay. Ultrasound Obstet. Gynecol. 2017, 50, 648–656. [Google Scholar]
- Bruno, M.; Capanna, G.; Di Florio, C.; Sollima, L.; Guido, M.; Ludovisi, M. Sonographic characteristics of ovarian Leydig cell tumor. Ultrasound Obstet. Gynecol. 2023. online ahead of printing. [Google Scholar] [CrossRef]
- Hack, K.; Gandhi, N.; Bouchard-Fortier, G.; Chawla, T.P.; Ferguson, S.E.; Li, S.; Kahn, D.; Tyrrell, P.N.; Glanc, P. External Validation of O-RADS US Risk Stratification and Management System. Radiology 2022, 304, 114–120. [Google Scholar] [CrossRef]
- Heremans, R.; Valentin, L.; Sladkevicius, P.; Timmerman, S.; Moro, F.; Van Holsbeke, C.; Epstein, E.; Testa, A.C.; Timmerman, D.; Froyman, W. Imaging in gynecological disease (24): Clinical and ultrasound characteristics of ovarian mature cystic teratomas. Ultrasound Obstet. Gynecol. 2022, 60, 549–558. [Google Scholar]
- Valentin, L.; Ameye, L.; Testa, A.; Lécuru, F.; Bernard, J.-P.; Paladini, D.; Van Huffel, S.; Timmerman, D. Ultrasound characteristics of different types of adnexal malignancies. Gynecol. Oncol. 2006, 102, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, Y.; Shen, L.-F.; Jiang, M.-J.; Yang, Z.-F.; Fang, G.-P. Ovarian thecoma-fibroma groups: Clinical and sonographic features with pathological comparison. J. Ovarian Res. 2016, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Paladini, D.; Testa, A.; Van Holsbeke, C.; Mancari, R.; Timmerman, D.; Valentin, L. Imaging in gynecological disease (5): Clinical and ultrasound characteristics in fibroma and fibrothecoma of the ovary. Ultrasound Obstet. Gynecol. 2009, 34, 188–195. [Google Scholar] [CrossRef]
- Valentin, L.; Ameye, L.; Jurkovic, D.; Metzger, U.; Lécuru, F.; Van Huffel, S.; Timmerman, D. Which extrauterine pelvic masses are difficult to correctly classify as benign or malignant on the basis of ultrasound findings and is there a way of making a correct diagnosis? Ultrasound Obstet. Gynecol. 2006, 27, 438–444. [Google Scholar] [PubMed]
- Valentin, L.; Ameye, L.; Savelli, L.; Fruscio, R.; Leone, F.P.G.; Czekierdowski, A.; Lissoni, A.A.; Fischerová, D.; Guerriero, S.; Van Holsbeke, C.; et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: Logistic regression models do not help. Ultrasound Obstet. Gynecol. 2011, 38, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Marko, J.; Marko, K.I.; Pachigolla, S.L.; Crothers, B.A.; Mattu, R.; Wolfman, D.J. Mucinous Neoplasms of the Ovary: Radiologic-Pathologic Correlation. Radiographics 2019, 39, 982–997. [Google Scholar] [CrossRef]
- Pascual, A.; Guerriero, S.; Rams, N.; Juez, L.; Ajossa, S.; Graupera, B.; Hereter, L.; Cappai, A.; Pero, M.; Perniciano, M.; et al. Clinical and ultrasound features of benign, borderline, and malignant invasive mucinous ovarian tumors. Eur. J. Gynaecol. Oncol. 2017, 38, 382–386. [Google Scholar] [PubMed]
- Valentin, L.; Ameye, L.; Franchi, D.; Guerriero, S.; Jurkovic, D.; Savelli, L.; Fischerova, D.; Lissoni, A.; Van Holsbeke, C.; Fruscio, R.; et al. Risk of malignancy in unilocular cysts: A study of 1148 adnexal masses classified as unilocular cysts at transvaginal ultrasound and review of the literature. Ultrasound Obstet. Gynecol. 2012, 41, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, V.; Minář, L.; Felsinger, M.; Ovesná, P.; Bednaříková, M.; Číhalová, M.; Jandáková, E.; Hausnerová, J.; Chaloupková, B.; Zikán, M. Brenner tumor of the ovary—Ultrasound features and clinical management of a rare ovarian tumor mimicking ovarian cancer. Ginekol. Pol. 2018, 89, 357–363. [Google Scholar]
- Moro, F.; Zannoni, G.F.; Arciuolo, D.; Pasciuto, T.; Amoroso, S.; Mascilini, F.; Mainenti, S.; Scambia, G.; Testa, A.C. Imaging in gynecological disease (11): Clinical and ultrasound features of mucinous ovarian tumors. Ultrasound Obstet. Gynecol. 2017, 50, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Moro, F.; Poma, C.B.; Zannoni, G.F.; Urbinati, A.V.; Pasciuto, T.; Ludovisi, M.; Moruzzi, M.C.; Carinelli, S.; Franchi, D.; Scambia, G.; et al. Imaging in gynecological disease (12): Clinical and ultrasound features of invasive and non-invasive malignant serous ovarian tumors. Ultrasound Obstet. Gynecol. 2017, 50, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Esquivel Villabona, A.L.; Rodríguez, J.N.; Ayala, N.; Buriticá, C.; Gómez, A.C.; Velandia, A.M.; Rodríguez, N.; Alcázar, J.L. Two-Step Strategy for Optimizing the Preoperative Classification of Adnexal Masses in a University Hospital, Using International Ovarian Tumor Analysis Models: Simple Rules and Assessment of Different NEoplasias in the adneXa Model. J. Ultrasound Med. 2022, 41, 471–482. [Google Scholar]
- Virgilio, B.A.; Blasis, I.D.E.; Sladkevicius, P.; Moro, F.; Zannoni, G.F.; Arciuolo, D.; Mascilini, F.; Ciccarone, F.; Timmerman, D.; Kaijser, J.; et al. Imaging in gynecological disease (16): Clinical and ultrasound characteristics of serous cystadenofibromas in adnexa. Ultrasound Obstet. Gynecol. 2019, 54, 823–830. [Google Scholar] [CrossRef]
- Goldstein, S.R.; Timor-Tritsch, I.E.; Monteagudo, A.; Monda, S.; Popiolek, D. Cystadenofibromas: Can transvaginal ultrasound appearance reduce some surgical interventions? J. Clin. Ultrasound. 2015, 43, 393–396. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Errasti, T.; Mínguez, J.A.; Galán, M.J.; García-Manero, M.; Ceamanos, C. Sonographic features of ovarian cystadenofibromas: Spectrum of findings. J. Ultrasound Med. 2001, 20, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Li, B.; Gu, C. Outcomes of fertility-sparing surgery for stage II and III serous borderline ovarian tumors. J. Int. Med. Res. 2019, 47, 4895–4903. [Google Scholar] [CrossRef] [Green Version]
- Ludovisi, M.; Foo, X.; Mainenti, S.; Testa, A.C.; Arora, R.; Jurkovic, D. Ultrasound diagnosis of serous surface papillary borderline ovarian tumor: A case series with a review of the literature. J. Clin. Ultrasound 2015, 43, 573–577. [Google Scholar] [CrossRef]
- Landolfo, C.; Valentin, L.; Franchi, D.; Van Holsbeke, C.; Fruscio, R.; Froyman, W.; Sladkevicius, P.; Kaijser, J.; Ameye, L.; Bourne, T.; et al. Differences in ultrasound features of papillations in unilocular-solid adnexal cysts: A retrospective international multicenter study. Ultrasound Obstet. Gynecol. 2018, 52, 269–278. [Google Scholar] [CrossRef]
- Fagotti, A.; Ludovisi, M.; De Blasis, I.; Virgilio, B.; Di Legge, A.; Mascilini, F.; Moruzzi, M.; Giansiracusa, C.; Fanfani, F.; Tropeano, G.; et al. The sonographic prediction of invasive carcinoma in unilocular-solid ovarian cysts in premenopausal patients: A pilot study. Hum. Reprod. 2012, 27, 2676–2683. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, D.; Moerman, P.; Vergote, I. Meigs’ syndrome with elevated serum CA 125 levels: Two case reports and review of the literature. Gynecol. Oncol. 1995, 59, 405–408. [Google Scholar] [CrossRef]
- Hiett, A.K.; Sonek, J.D.; Guy, M.; Reid, T.J. Performance of IOTA Simple Rules, Simple Rules risk assessment, ADNEX model and O-RADS in differentiating between benign and malignant adnexal lesions in North American women. Ultrasound Obstet. Gynecol. 2022, 59, 668–676. [Google Scholar]
- Lai, H.; Lyu, G.; Kang, Z.; Li, L.; Zhang, Y.; Huang, Y. Comparison of O-RADS, GI-RADS, and ADNEX for Diagnosis of Adnexal Masses: An External Validation Study Conducted by Junior Sonologists. J. Ultrasound Med. 2021, 41, 1497–1507. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Hsu, T.-F.; Chan, I.-S.; Liu, C.-H.; Chao, W.-T.; Shih, Y.-C.; Jiang, L.-Y.; Chang, Y.-H.; Wang, P.-H.; Chen, Y.-J. Comparison of the O-RADS and ADNEX models regarding malignancy rate and validity in evaluating adnexal lesions. Eur. Radiol. 2022, 32, 7854–7864. [Google Scholar] [CrossRef]
- Basha, M.A.A.; Metwally, M.I.; Gamil, S.A.; Khater, H.M.; Aly, S.A.; El Sammak, A.A.; Zaitoun, M.M.A.; Khattab, E.M.; Azmy, T.M.; Alayouty, N.A.; et al. Comparison of O-RADS, GI-RADS, and IOTA simple rules regarding malignancy rate, validity, and reliability for diagnosis of adnexal masses. Eur. Radiol. 2021, 31, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.T.; Wang, Y.Q.; Xiang, Z.S.; Du, Z.S.; Huang, S.X.; Chen, Y.J.; Tang, L.N. Efficacy of IOTA simple rules, O-RADS, and CA125 to distinguish benign and malignant adnexal masses. J. Ovarian Res. 2022, 15, 15. [Google Scholar] [CrossRef] [PubMed]

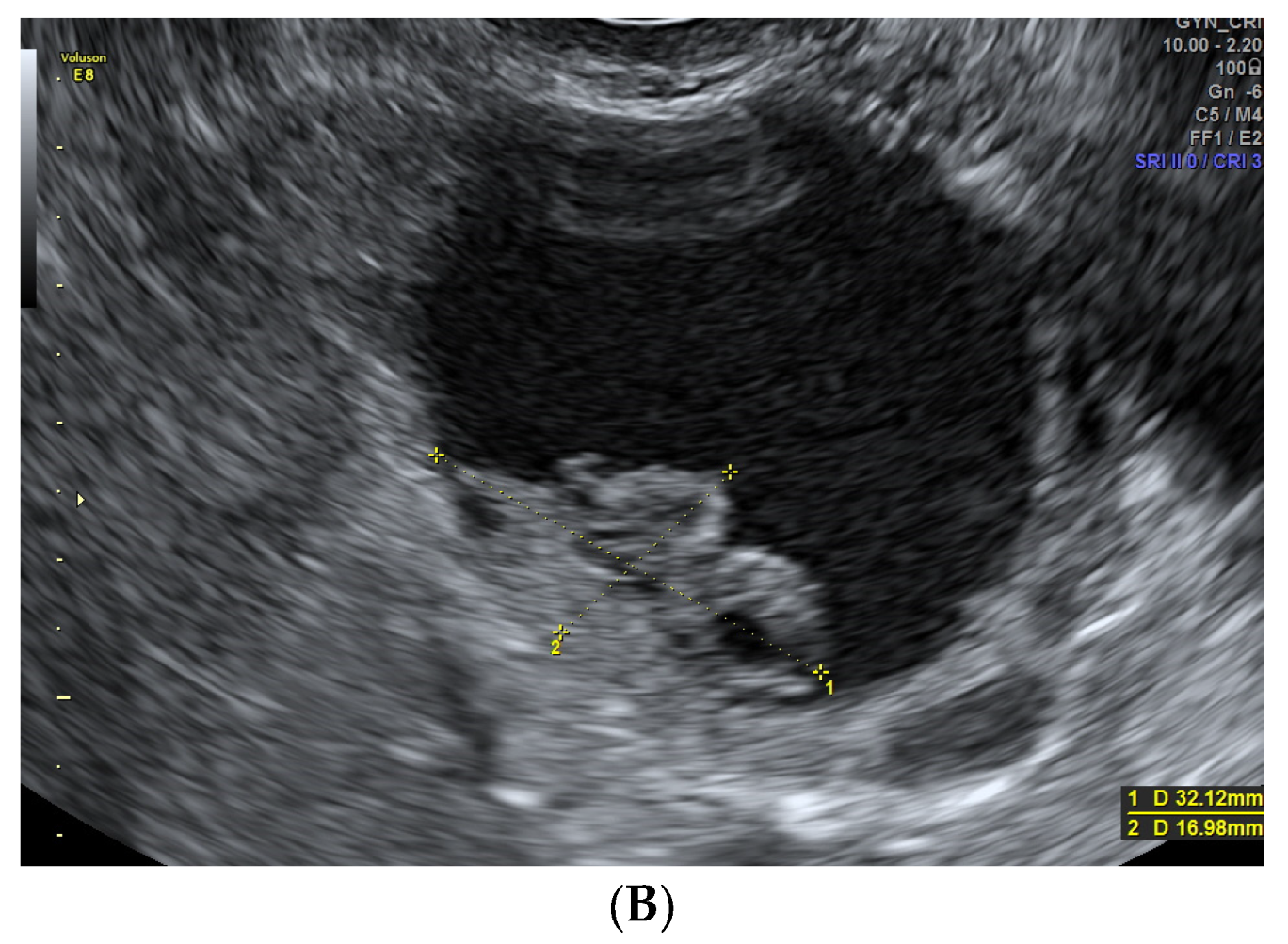

| Total (n: 187) | Benign (n: 134, 71.7%) | Malignant (n: 53, 28.3%) | p Value | |
|---|---|---|---|---|
| Age (years) | 49.6 (±15.9) | 47.2 (±15.7) | 55.6 (±14.9) | (1) 0.001 |
| Menopause: | (2) <0.001 | |||
| - Yes | 85 (45.5%) | 49 (36.6%) | 36 (67.9%) | |
| - No | 102 (54.5%) | 85 (63.4%) | 17 (32.1%) | |
| Parity: | (2) 0.371 | |||
| - Nuliparous | 91 (48.7%) | 63 (47.0%) | 28 (52.8%) | |
| - Parous | 96 (51.3%) | 71 (53.0%) | 25 (47.2%) | |
| BMI (kg/m) | 26.4 (±5.4) (n: 80) | 27.1 (±5.3) (n: 52) | 25.2 (±5.2) (n: 28) | (3) 0.130 |
| Clinic: | (2) 0.114 | |||
| - Asymptomatic | 71 (38.0%) | 56 (41.8%) | 15 (28.3%) | |
| - Digestive | 73 (39.0%) | 53 (39.5%) | 20 (37.7%) | |
| - Bleeding | 16 (8.6%) | 10 (7.5%) | 6 (11.3%) | |
| - Other | 27 (14.4%) | 15 (11.2%) | 12 (22.7%) |
| Total (n: 187) | Benign (n: 134) | Malignant (n: 53) | p Value | |
|---|---|---|---|---|
| Surgical approach: | (2) <0.001 | |||
| - Laparoscopic | 125 (66.8%) | 108 (80.6%) | 17 (32.1%) | |
| - Laparotomy | 62 (33.2%) | 26 (19.4%) | 36 (67.9%) | |
| Surgical procedure: | (2) <0.001 | |||
| - Double adnexectomy | 54 (28.9%) | 39 (29.1%) | 15 (28.3%) | |
| - Hysterectomy and double adnexectomy | 34 (18.2%) | 11 (8.2%) | 23 (43.4%) | |
| - Cystectomy/ooforectomy/unilateral adnexectomy | 99 (52.9%) | 84 (62.7%) | 15 (28.3%) | |
| Laterality: | (2) 0.238 | |||
| - Right | 78 (41.7%) | 61 (45.5%) | 17 (32.1%) | |
| - Left | 59 (31.6%) | 39 (29.1%) | 20 (37.7%) | |
| - Bilateral | 50 (26.7%) | 34 (25.4%) | 16 (30.2%) | |
| CA125 (IU/mL) | 226.3 (±1181.0) | 36.0 (±84.2) | 707.5 (±2154.5) | (1) <0.001 |
| Total | Total | ||
|---|---|---|---|
| BENIGN | 134 | MALIGNANT | 53 |
| Mature cystic teratoma | 29 | Ovarian Serous carcinoma | 19 |
| Endometriosis | 24 | Clear cell carcinoma | 9 |
| Fibroma/Fibrotecoma | 13 | Serous Borderline carcinoma | 8 |
| Serous Cistoadenoma | 19 | Mucinous Borderline carcinoma | 5 |
| Mucinous Cistoadenoma | 12 | Endometrioid carcinoma | 4 |
| Cistoadenofibroma | 10 | Neuroendocrine carcinoma | 1 |
| Brenner Tumor | 5 | Stromal hyperplasia | 1 |
| Hidrosalpinx | 6 | Steroid cells carcinoma | 1 |
| PID | 4 | Disgerminoma | 1 |
| Functional cyst | 2 | Struma ovarii | 1 |
| Paraovarian cyst | 4 | Sex cord tumor | 1 |
| Hiperthecosis | 1 | ||
| Twisted cyst | 5 |
| Total (n: 187) | Benign (n: 134) | Malignant (n: 53) | p Value | |
|---|---|---|---|---|
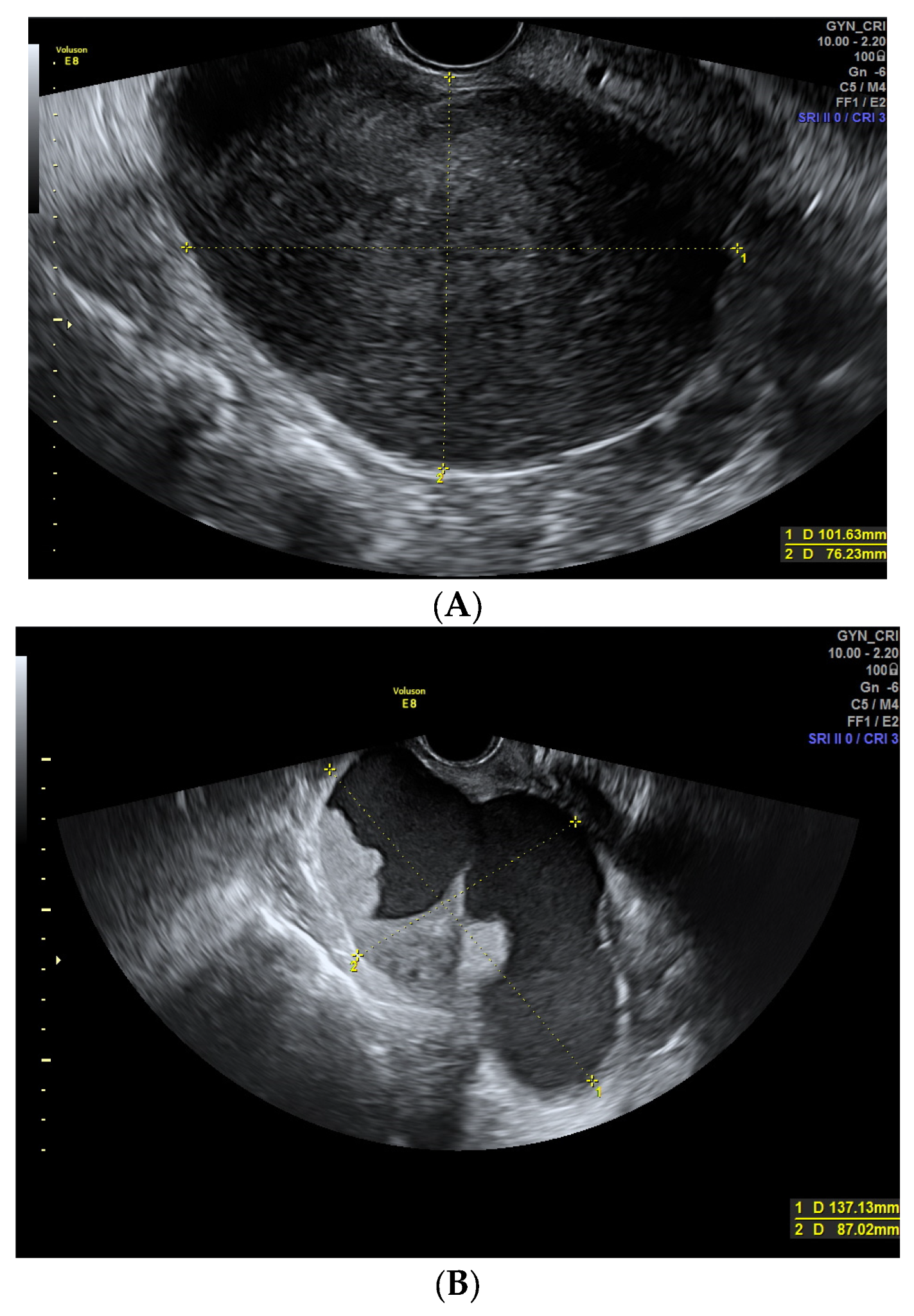
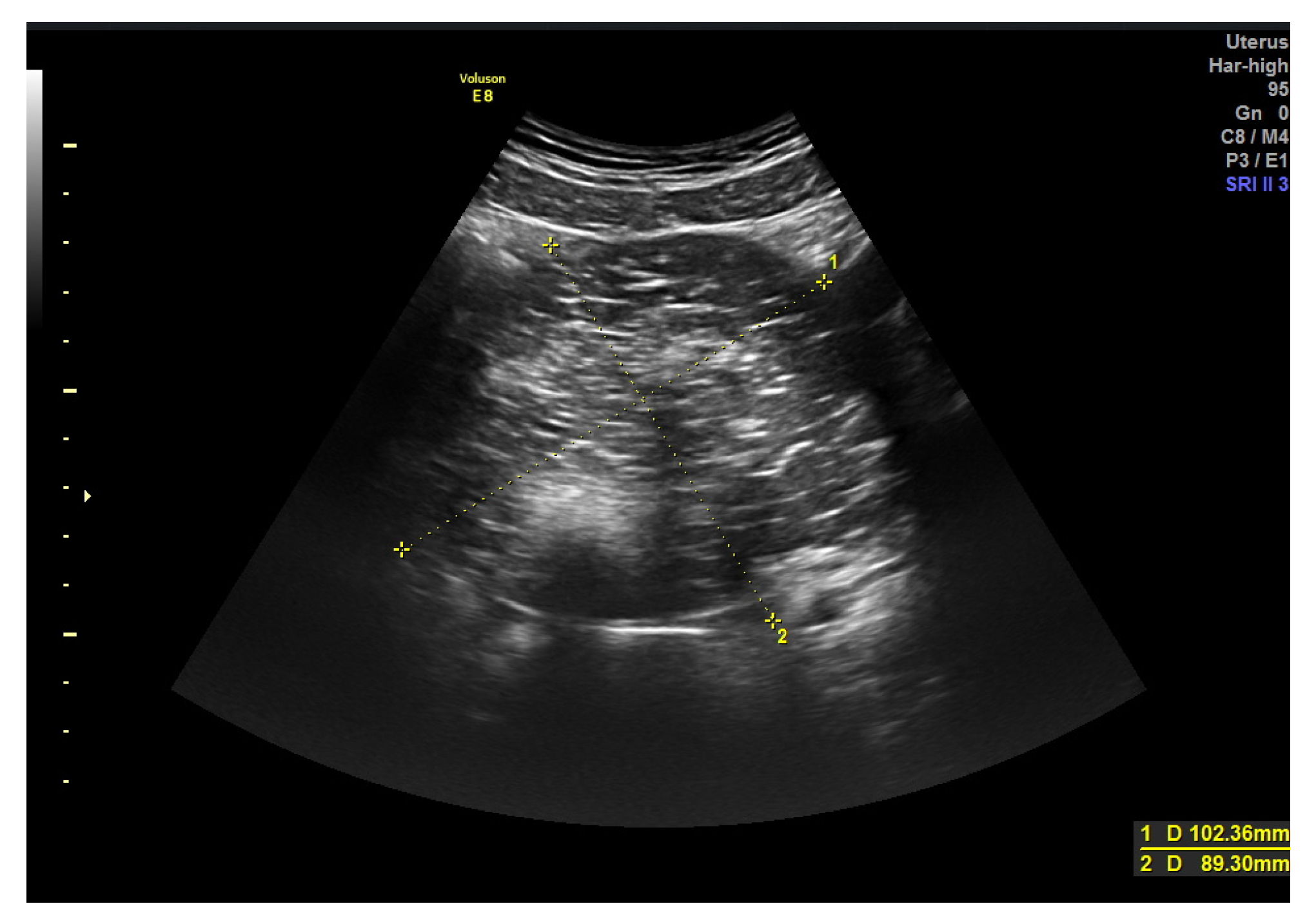
| Largest size (mm) | 88.4 (±47.3) | 83.1 (±45.9) | 101.9 (±49.1) | (1) 0.128 |
| Contour: | (2) <0.001 | |||
| - Regular | 148 (79.1%) | 122 (91.0%) | 26 (49.1%) | |
| - Irregular | 39 (20.9 %) | 12 (9.0%) | 27 (50.9%) | |
| Acoustic shadowing: | (2) <0.001 | |||
| - Yes | 109 (58.3%) | 97 (72.4%) | 12 (22.6%) | |
| - No | 78 (41.7%) | 37 (27.6%) | 41 (77.4%) | |
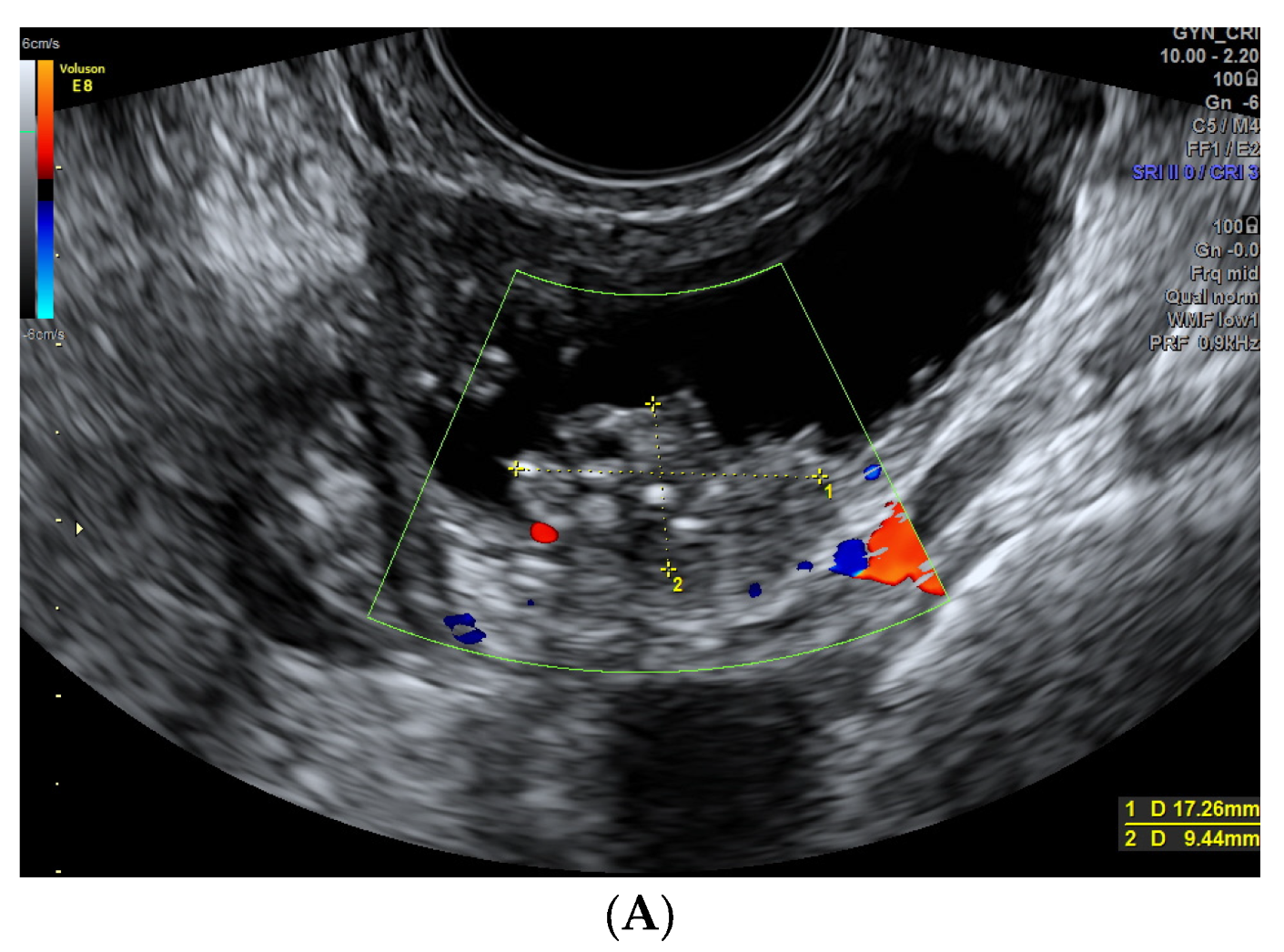
| Presence of solid areas: | (2) <0.001 | |||
| - Yes | 81 (43.3%) | 34 (25.4%) | 47 (88.7%) | |
| - No | 106 (56.7%) | 100 (74.6%) | 6 (11.3%) | |
| Size of solid areas (mm) | 51.1 (±39.1) (n: 81) | 47.9 (±35.2) (n: 34) | 53.4 (±41.8) (n: 47) | (3) 0.541 |
| Doppler of solid areas (Score color): | (2) <0.001 | |||
| - 1–2 | 42 (51.2%) | 28 (80.0%) | 14 (29.8%) | |
| - 3–4 | 40 (48.8%) | 7 (20.0%) | 33 (70.2%) | |
| Septum: | (2) 0.078 | |||
| - None | 118 (63.4%) | 86 (63.2%) | 32 (61.6%) | |
| - Thin | 47 (25.3%) | 37 (27.6%) | 10 (19.2%) | |
| - Thick/Irregular | 21 (11.3%) | 11 (8.2%) | 10 (19.2%) | |
| Doppler of septum (Score color): | (2) <0.001 | |||
| - 1–2 | 56 (77.8%) | 45 (90.0%) | 11 (47.8%) | |
| - 3–4 | 16 (22.2%) | 5 (10.0%) | 12 (52.2%) | |
| Number of locules: | (2) 0.288 | |||
| - 0 (solid mass) | 2 (1.1%) | 1 (0.7%) | 1 (1.9%) | |
| - 1 | 127 (67.9%) | 96 (71.6%) | 31 (58.5%) | |
| - 2–9 | 47 (25.1%) | 31 (23.1%) | 16 (30.2%) | |
| - ≥ 10 | 11 (5.9%) | 6 (4.5%) | 5 (9.4%) | |
| Number of papillae: | (2) <0.001 | |||
| - 0 | 151 (80.7%) | 119 (88.8%) | 32 (60.4%) | |
| - 1 | 15 (8.0%) | 7 (5.2%) | 8 (15.1%) | |
| - >1 | 21 (11.2%) | 8 (6.0%) | 13 (24.5%) | |
| Size papillae (mm): | 23.1 (±17.7) (n: 36) | 13.7 (±7.9) (n: 15) | 29.8 (±19.8) (n: 21) | (3) 0.005 |
| Doppler of papillae (Score color): | (2) 0.071 | |||
| - 1–2 | 26 (70.3%) | 14 (87.5%) | 12 (57.1%) | |
| - 3–4 | 11 (29.7%) | 2 (12.5%) | 9 (42.9%) | |
| Ascites: | (2) 0.025 | |||
| - No-mild | 172 (92.0%) | 127 (94.8%) | 45 (84.9%) | |
| - Moderate-Severe | 15 (8.0%) | 7 (5.2%) | 8 (15.1%) |
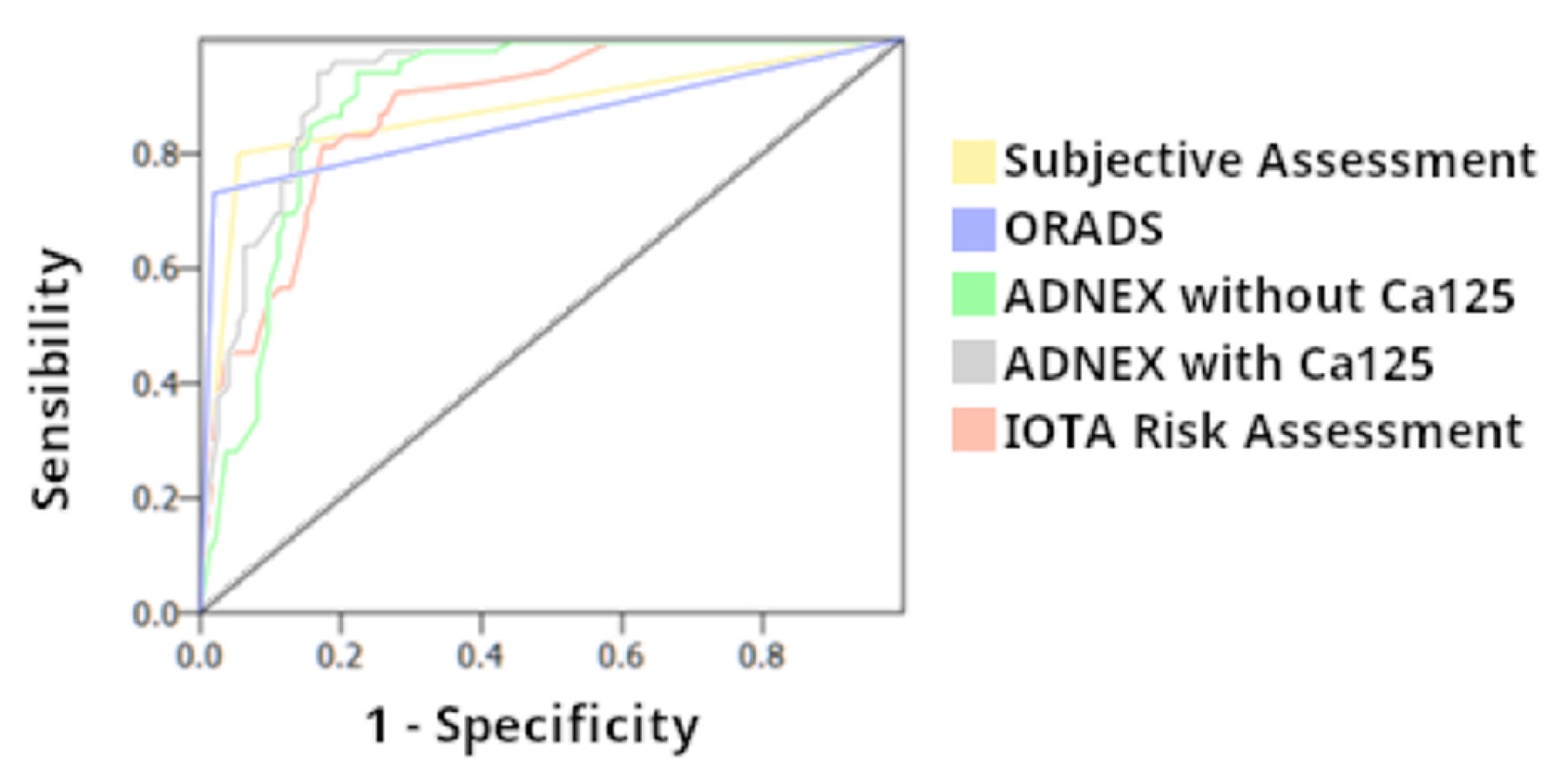
| Sensitivity (%) | Specifity (%) | PPV (%) | NPV (%) | AUC | OR | |
|---|---|---|---|---|---|---|
| Subjective assesment | 93.9 (87.4–100) | 80.2 (74.1–86.3) | 63.9 | 97.2 | 0.87 | 61.9 |
| Simple Rules Risk Assesment | 81.1 (71.6–90.6) | 82.1 (76.2–88.0) | 64.2 | 91.7 | 0.87 | 19.7 |
| ADNEX model with CA125 | 94.3 (88.2–100) | 82.8 (77.0–88.6) | 68.5 | 97.4 | 0.92 | 80.43 |
| ADNEX model without CA125 | 88.7 (80.7–96.7) | 77.6 (71.4–83.8) | 61.0 | 94.5 | 0.90 | 27.2 |
| O-RADS | 98.1 (94.5–100) | 73.1 (66.7–79.5) | 59.1 | 99.0 | 0.86 | 141.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelayo, M.; Sancho-Sauco, J.; Sanchez-Zurdo, J.; Abarca-Martinez, L.; Borrero-Gonzalez, C.; Sainz-Bueno, J.A.; Alcazar, J.L.; Pelayo-Delgado, I. Ultrasound Features and Ultrasound Scores in the Differentiation between Benign and Malignant Adnexal Masses. Diagnostics 2023, 13, 2152. https://doi.org/10.3390/diagnostics13132152
Pelayo M, Sancho-Sauco J, Sanchez-Zurdo J, Abarca-Martinez L, Borrero-Gonzalez C, Sainz-Bueno JA, Alcazar JL, Pelayo-Delgado I. Ultrasound Features and Ultrasound Scores in the Differentiation between Benign and Malignant Adnexal Masses. Diagnostics. 2023; 13(13):2152. https://doi.org/10.3390/diagnostics13132152
Chicago/Turabian StylePelayo, Mar, Javier Sancho-Sauco, Javier Sanchez-Zurdo, Leopoldo Abarca-Martinez, Carlota Borrero-Gonzalez, Jose Antonio Sainz-Bueno, Juan Luis Alcazar, and Irene Pelayo-Delgado. 2023. "Ultrasound Features and Ultrasound Scores in the Differentiation between Benign and Malignant Adnexal Masses" Diagnostics 13, no. 13: 2152. https://doi.org/10.3390/diagnostics13132152
APA StylePelayo, M., Sancho-Sauco, J., Sanchez-Zurdo, J., Abarca-Martinez, L., Borrero-Gonzalez, C., Sainz-Bueno, J. A., Alcazar, J. L., & Pelayo-Delgado, I. (2023). Ultrasound Features and Ultrasound Scores in the Differentiation between Benign and Malignant Adnexal Masses. Diagnostics, 13(13), 2152. https://doi.org/10.3390/diagnostics13132152








