Abstract
Ductal carcinoma in situ (DCIS) of male breast is a rare lesion, often associated with invasive carcinoma. When the in situ component is present in pure form, histological grade is usually low or intermediate. Imaging is difficult as gynaecomastia is often present and can mask underlying findings. We report a rare case of pure high-grade DCIS in a young male patient, with associated intraductal papilloma and atypical ductal hyperplasia. Digital breast tomosynthesis (DBT) showed an area of architectural distortion at the union of outer quadrants of the left breast without gynaecomastia. Triple assessment suggested performing a nipple-sparing mastectomy, which revealed the presence of a focal area of high-grade DCIS of 2 mm. DCIS, even of high grade, is difficult to detect with mammography and even more rare, especially when associated with other proliferative lesions. DBT with 2D synthetic reconstruction is useful as the imaging step of a triple assessment and it should be performed in both symptomatic and asymptomatic high-risk men to differentiate between malignant and benign lesions. We propose a diagnostic model to early detect breast cancer in men, optimizing resources according to efficiency, effectiveness and economy, and look forward to radiomics as a powerful tool to help radiologists.
1. Introduction
Male breast cancer (MBC) is a rare entity representing less than 1% of all male malignancies and less than 1% of breast cancers. The annual incidence of MBC in Europe is approximately 1/100,000 men. Ductal carcinoma in situ (DCIS) of the male represents approximately 0.1% of all breast cancers and less than 0.1% of all cancers in men [1,2,3].
The most relevant risk factor for MBC is the increasing age, probably related to testicular malfunction and increased levels of estrogen, so that the age of onset varied between 60 and 70 years with an average age of 67, 5 to 10 years older than women at the time of diagnosis [1,4]. Other risk factors include family history (positive in 15 to 20% of men), mutations of breast cancer related genes (BRCA2 > BRCA1), Cowden and Klinefelter syndromes, alcohol consumption, and liver disease. Although claims have been made to the contrary, there is no proven direct link between gynaecomastia and MBC [1]. Since it is difficult to diagnose MBC at an early stage, it could be useful to define a specified protocol according to the literature and future perspectives. In this paper, we present a rare case of pure high-grade DCIS of the male breast in a young patient. Furthermore, starting from this experience, we reviewed the literature to define the correct use of breast imaging according to the criteria of efficiency, effectiveness and economy, presenting a diagnostic algorithm and also showing the possibilities of radiomics.
2. Case Report
A 43-year-old Caucasian male reported a 1-month history of spontaneous clear left side nipple discharge with a recent appearance of a homolateral painless breast swelling. There was no history of bloody discharge. Past medical history was pertinent for obesity class I (BMI: 33.3) and bilateral hypoacusia for otosclerosis. There was no family history for breast or ovarian cancer. His social history indicated no use of alcohol, but previous use (twelve years ago) of tobacco products.
On physical examination, he was an overweight Caucasian male with symmetrical breasts. On palpation, there was a bilateral pseudogynaecomastia with a smooth, ill-defined left breast thickening, especially at the union of the outer quadrants. With applied pressure, a minimal clear stream of discharge fluid was elicited from the left nipple and was felt to be localized to a single duct.
Digital breast tomosynthesis (DBT) with synthesized reconstructed 2D images (s2D) was performed in medio-lateral-oblique (MLO) projections for each breast and in both cranio-caudal (CC) and latero-medial (LM) projections for the left breast. The s2D images showed a regular appearance of the breast buttons without gynaecomastia, and an area of asymmetrical density at the union of outer quadrants of the left breast that was better identified at the DBT images as an area of architectural distortion with scattered peripheral punctate calcifications, sparing the nipple-areolar complex. (Figure 1).
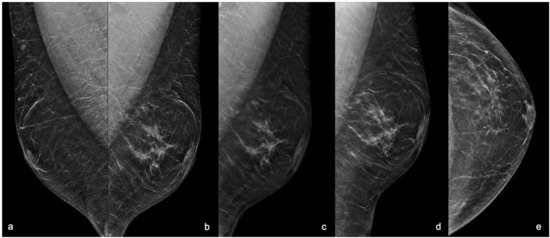
Figure 1.
Mammography with s2D and DBT projections. (a) Right MLO s2D showing normal appearance of male breast with false gynaecomastia. (b) Left MLO s2D; (c) Left MLO DBT; (d) Left LM s2D; (e) Left CC s2D. 2D mammograms showed an area of asymmetric density at the union of outer quadrants of the left breast with regular breast buttons in absence of gynaecomastia. DBT showing the area of architectural distortion at the union of outer quadrants of the left breast with rare punctate microcalcifications and small peripheral pseudo-nodular opacities referred to focal ductal ectasia, sparing the nipple-areolar complex. MLO: medio-lateral-oblique projection; LM: latero-medial projection; CC: cranio-caudal projection; s2D: 2D synthetic reconstruction; DBT: digital breast tomosynthesis.
A breast ultrasound (US), performed on the same day, showed in correspondence of the mammographic findings, the presence of an ill-defined, hypoechoic area of acoustic shadowing with peripheral anechoic lacunae and a close small focal ductal ectasia. (Figure 2)
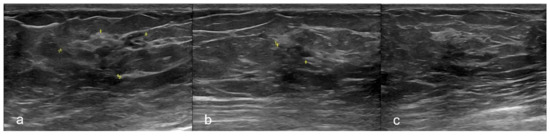
Figure 2.
Ultrasonography. Breast ultrasound (US) in different projections showing an ill-defined, hypoechoic area of acoustic shadowing with peripheral anechoic lacunae at the union of outer quadrants of the left breast. (a) The yellow mark showed the whole area. (b) The yellow mark showed the small area of architectural distortion with acoustic shadowing. (c) US-guided Fine-Needle Aspiration Cytology (FNAC) of the lesion.
According to Breast Imaging Reporting and Data System (BI-RADS) [5], these findings were classified as category 4b.
An US-guided Fine-Needle Aspiration Cytology (US-FNAC) was performed. Our laboratory received 4 microscope slides fixed in alcohol and coloured with Papanicolaou stain. Microscopic examination showed atypical ductal cells with poor myoepithelium arguing for several types of proliferative non-malignant lesions but also DCIS. According to the IAC Yokohama System for Reporting Breast Fine-Needle Aspiration Biopsy Cytopathology (1st Edition, 2020) [6], these findings were classified as category C4 (Figure 3). Since the Rapid On-Site Evaluation (ROSE) [7] already showed this suspicious atypia, we contextually performed an US-guided biopsy (US-CNB) with a 16 Gauge (G) semi-automated core biopsy needle. Our laboratory received two specimens of breast tissue of 1.5 cm of maximum dimension who were submitted for routine processing. Microscopic examination showed breast parenchyma with fibrosis with a focal area of atypical ductal hyperplasia (ADH), p63 positive and with focal positivity for CK5/6. According to Guidelines for non-operative diagnostic procedures and reporting in breast cancer screening [8], these findings were classified as category B3 (Figure 4).
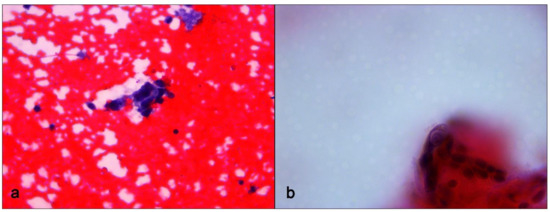
Figure 3.
Fine-Needle Aspiration Cytology sampling. (a) Small cluster of ductal cells showing loose aggregation, irregular chromatin pattern and nuclear polymorphism. Papanicolaou stain, ×400 magnification. (b) Small cluster of ductal cells showing cellular details. Papanicolaou stain, ×600 magnification.
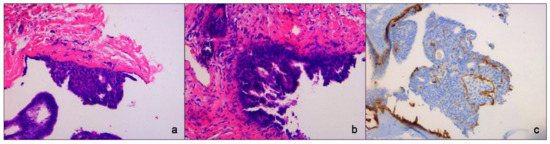
Figure 4.
Core-Needle Biopsy sampling. (a,b) Atypical Ductal Hyperplasia (ADH) with true papillary projections. Hematoxylin eosin stain, ×200 magnification. (c) Immunohistochemistry for p63 showing myoepithelial component, ×200 magnification.
All the findings of cytology and histology in association with the radiological assessment underlined the importance of the triple test [9] and recommended us to perform surgical excision of the suspected area. Therefore, after discussing with the multidisciplinary team (MDT), according to the patient’s consent, a left subcutaneous nipple-sparing mastectomy was performed without sentinel lymph node biopsy (Figure 5).
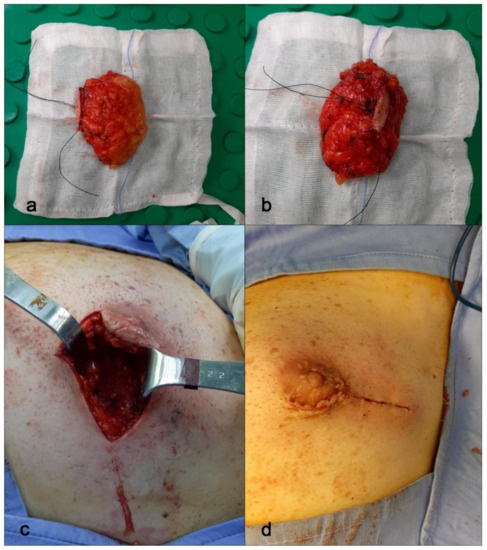
Figure 5.
Nipple-sparing mastectomy. (a,b) Breast tissue samples from mastectomy surgery. Here, the entire breast sample. (c,d) Periareolar incision with lateral extension and breast reconstruction.
Our laboratory received a specimen designated left mastectomy, weighing 90 g (Figure 5). It comprised oriented fibro-fatty tissue, 8 × 4.5 × 4 cm in aggregate, which on gross examination revealed an area of small cystic formations and a whitish lesion of 0.2 cm on which random samplings were performed. Microscopic examination showed an intraductal papilloma (IP) with ADH and a single focus of DCIS solid-cribriform type, respectively. Nuclear grade was 3 (p63+, CK5/6−) with negative surgical margins. No invasive cancer was present (Figure 6). The specimen was sent for immunohistochemical examination of estrogen (ER) and progesterone (PR) receptor. The tissue was positive for both ER (with 70% of nuclei staining with strong intensity) and PR (80% of nuclei staining strongly) (Figure 6). Venipuncture samples were subsequently sent to an external laboratory for genetic analysis, the results of which were negative for BRCA1 and 2, and other mutations (Table 1).
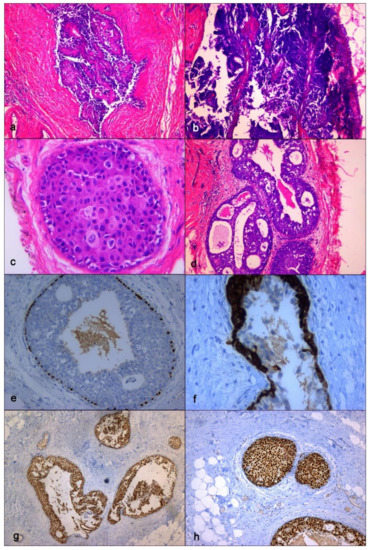
Figure 6.
Definitive histopathological specimen. (a) Intraductal papillary lesion with epithelial atypia, hematoxylin eosin stain, ×50 magnification; (b) Papillary lesion representative of epithelial atypia at higher magnification, hematoxylin eosin stain, x100 magnification; (c) High grade ductal carcinoma in situ (DCIS), hematoxylin eosin stain, ×400 magnification; (d) High grade DCIS, solid-cribriform type, hematoxylin eosin stain, ×400 magnification; (e) Immunohistochemstry (IHC). Study of p63 expression showing immunoreactivity of myoepithelial cell along the basement membrane, ×200 magnification; (f) IHC. Study of cytokeratin 5/6 immunoreactivity showing Atypical Ductal Hyperplasia (AD), ×400 magnification; (g) IHC. Study of estrogen receptor (ER) immunoreactivity with 70% of nuclei staining with strong intensity, ×50 magnification; (h) IHC. Study of progesterone receptor (PR) immunoreactivity with 80% of nuclei staining with strong intensity, ×100 magnification.

Table 1.
Genetic Analysis. Panel of genes.
According to these surprising results, after a clinically and radiologically evaluation of the left axillary lymph nodes, the MDT decided not to perform axillary dissection and to administer tamoxifen as approved adjuvant hormone treatment of men with ER-positive early stage breast cancer [10].
The patient was noted to be doing well 6 months post-operatively and is still on follow-up at the time of writing.
3. Discussion
If male breast cancer (MBC) is a rare disease, atypical ductal hyperplasia (ADH), intraductal papilloma (IP) and a pure high-grade ductal carcinoma in situ (DCIS), with no associated gynaecomastia and significant risk factors, are even more rare, especially if they occur concomitantly [11,12,13].
Despite its rarity, the incidence of MBC has increased by 20–25% in the past few decades and continues to rise [14]. Nevertheless, clinical research is limited and most available data come from observational retrospective studies [14,15,16,17].
Presenting symptoms are similar to female breast cancer (FBC). Often, MBC presents as a nodule found on self-examination (91.5%). The main presenting symptom is a painless retro-areolar mass (50–95%). Other clinical signs either isolated or in association with the breast mass are mastodynia, breast discharge (1–12%), inflammatory signs, ulceration (4–17%) or symptoms related to a metastatic disease (3–12%) [18]. Nipple abnormalities are early and more frequent than in women (40–50%) because of the low volume of breast tissue in men and the central location of some tumours. Paget’s disease complicates 1.45% of male breast cancers versus 0.68% in women [19].
Unlike women, a definition for men at “high risk” for breast cancer has not yet been established. One review paper considered men at “high risk” if they had a lifetime risk greater than the male average risk [20]. As reported in literature, we can consider in this category all the men with a known BRCA genetic mutation, Ashkenazi Jewish ancestry, a strong family history, or a personal history of breast cancer [20,21].
Several genes have been reported to be mutated, such as BRCA2 (4–16%), BRCA1 (0–4%), PTEN (phosphatase and tensin homolog), p53, CHEK2 (checkpoint kinase 2), PALB2 (partner and localizer of BRCA2) and CYP17A1 (cytochrome P450 family 17 subfamily A member 1) [22]. Therefore, the American Society of Clinical Oncology (ASCO) recommends that genetic counselling and testing regardless of family history should be offered to all men with breast cancer [4]. Additional risk factors include obesity, testicular abnormalities or pituitary adenomas that led to hormonal imbalance (increased level of estrogen), hepatic disease (cirrhosis), hormonal exogenous estrogens therapies, race (black men) and radiation exposure.
In our case, the patient did not show any of the previous described risk factors, except for a class I obesity according to BMI Index. Furthermore, no gynaecomastia was found and even if there is no proven direct link of its influence on MBC [1], it could be considered an important limitation on breast imaging due to its aspects that could mask underlying lesions. In fact, we can identified three patterns of gynaecomastia seen at mammography (MX): nodular, dendritic, and diffuse glandular [23]. While gynaecomastia is central and most commonly bilateral, MBC can occasionally develop in the sub-areolar region, but more often, it tends to spare this region resulting in an eccentric unilateral non-tender mass. Nipple retraction is seen due to the late diagnosis that led also to skin thickening and axillary adenopathy [24].
In our patient, the lesion was found to be an eccentric area of architectural distortion extending to the union of outer quadrants, with no relationship with the nipple-areolar complex. It was an ill-defined tender mass that could be underrated in the absence of other clinical findings as the nipple discharge described by the patient himself. The absence of other clinical relevant findings on palpation is due to the early onset of the pathology and the intraductal development of the cancer that was detected prior to progression to invasion.
The median duration of symptoms for pure DCIS is 2 months, whereas for patients with DCIS and associated invasive carcinoma it is 6 months [25]. In our case, the patient had breast pain for 1-month with nipple discharge but the pathological analysis of breast tissue also showed an area of focal ductal ectasia with a papilloma, near the DCIS, which were probably responsible for these symptoms.
The vast majority of male breast cancers are invasive ductal carcinomas. Invasive lobular carcinoma is rare (1%), presumably due to the lack of lobular development in the male breast [1]. Lobular carcinoma in situ has been reported but usually in association with an invasive lobular carcinoma [26]. If male DCIS represents approximately 10% of all male breast cancers, pure DCIS only accounts for 5% of these cases, while the remaining DCIS cases are associated with invasive carcinoma [1]. Pure DCIS is present in the 20% of female breast cancers [27]. This difference seems to be related to the increased awareness and regular breast screening in female patients, which leads to earlier detection and overall better prognosis.
In our case, the patient is 43 years old. He was younger than the average age of onset (67 years) as described in literature [1,4,19]. MBC seems to present from 5 to 10 years later than in women, probably due to several aspects such as a delay between onset of symptoms and timing of diagnosis (from 6 to 35 months) [19] and a general diagnosis at an advanced stage, several years after the real onset of the pathology. The late diagnosis itself could be the result of the absence of a screening programme, the lack of symptoms in the early stages and the ignorance or denial of the disease, considered as strictly feminine by men and extremely rare by the practitioners [19].
As described clearly by Foerster et al., 81.5% of male breast cancers are diagnosed at tumour stage II while 56.8% have lymph node involvement at the time of diagnosis [28].
Data from the National Institute of Surveillance, Epidemiology and End Results (SEER) show that, in United States, early forms of MBC increased from 1973 to 2012 for both men and women, but if this increase is more significant for women, men were more likely than women at stage III and IV (24.9% vs. 17.2%) [19].
The histological patterns seen in female DCIS are also seen in males but with varying frequency. Hittmair et al. [25] looked at 84 cases of pure DCIS of the male breast and found that the most common histological subtype was papillary carcinoma (74%) with a superimposed cribriform pattern. Pure DCIS cases in this series were found to be of either low or intermediate grades. To our knowledge, only a few cases of pure high-grade DCIS have been described, more recently in association with gynaecomastia and more typical risk factors [3]. There were no cases of pure high-grade DCIS in association with IP and ADH as seen in our patient.
Our diagnosis showed the importance of triple assessment or triple test. It consists of clinical assessment, radiologic assessment (mammography and/or ultrasound) and tissue biopsy (fine-needle aspiration cytology and/or core biopsy) [9]. In their review, Irwig et al. [29] showed that when any component is positive, the triple test has a sensitivity of 99.6% (95–100%) and a specificity of 62%. Similarly, the sensitivity of FNAC is consistently high (92%). The relatively low specificity can now be overwhelmed by core biopsy or other interventional procedures such as vacuum-assisted breast biopsy (VABB), performed under the guide of ultrasound, mammography or tomosynthesis (tomobiopsy). Nevertheless, the low volume of male breast makes it difficult to perform these types of procedures.
In our case, the complexity of the area in which we found both proliferative lesions and carcinoma, determined the different results of cytology and histology that, in accordance with clinical and DBT findings, suggested that we perform surgery. In fact, the presence of the asymmetry at standard 2D mammograms with the absence of a high PPV finding of the US could have been misinterpreted as a dendritic gynaecomastia. By using DBT, the identification of the architectural distortion, similar to the findings of a FBC, increased the PPV of both techniques, leading us to perform biopsy and then surgery.
The role of imaging in MBC is controversial. Because of its limited volume, the male breast is not as easy to examine with standard imaging techniques as for women, especially mammography. Furthermore, the superimposed presence of gynaecomastia could mask the lesion or other relevant findings such as architectural distortions or microcalcifications, not clearly evaluable with ultrasounds.
Physical examination is very sensitive (85–100%) for the detection of cancer but lacks specificity (PPV of 19.2%). Mammography showed the highest sensitivity (94.7%, NPV of 99.7%) and ultrasound is the most specific (95.3%) for detection of malignancy [30]. Nevertheless, if, as Evans et al. [31] suggested, the routine use of mammography could be an adjunct to physical examination in distinguishing benign and malignant processes, Adibelli et al. [32] proposed a diagnostic algorithm in which ultrasonography may be used to evaluate palpable abnormalities as the first diagnostic tool of choice. In a review of the literature, the discordance between different algorithms proposing the use of mammography [30,33,34,35,36], underlined the lack of specific data concerning the use of imaging in this specific clinical setting. In most cases diagnosis is made by triple assessment (clinical assessment, mammography or ultrasonography, and core biopsy) and ultrasound-guided core biopsy is preferred because it can enable a definitive diagnosis of invasive breast cancer [37].
The American College of Radiology Appropriateness Criteria Committee recently recommended criteria for imaging the breasts in symptomatic men [38]. The panel recommends mammography or digital breast tomosynthesis (DBT) in men aged 25 and older if there are symptoms or if physical examination suggests BC. Other studies also show the usefulness of mammography in asymptomatic men at high risk for developing BC [39]. The role of DBT in female breast cancer diagnosis is well known. The addition of DBT to standard 2D mammography reduces recall rates and improves cancer detection rates of screening mammography compared with standard 2D digital mammography alone [40,41]. DBT improves the visualization of findings that may be subtle or occult on 2D, reducing the superimposition of fibroglandular tissue, especially in dense breast and for architectural distortions [42,43,44]. In fact, the cancer detection rate of DBT in mammographically occult architectural distortions has been reported to be 21.1% [43] and 47.2% [45]. Furthermore, the substantial equivalence of standard 2D images (2DFF) with 2D synthetic reconstruction (s2D) [46], obtained after performing DBT, addresses the issues related with increasing dose in the combined use of 2DFF with DBT, allowing the use of the s2D images rather than standard 2D views. All these aspects suggested the use of DBT also in male breast imaging. Increasing conspicuousness of findings makes early detection of cancer before its progression to invasive forms possible, especially in cases like our one, in which the lesion presented as an area of architectural distortion, and in case of superimposed gynaecomastia that could mask underlying pathological findings, as happens in the case of dense breast tissue in women [47] (Figure 7).
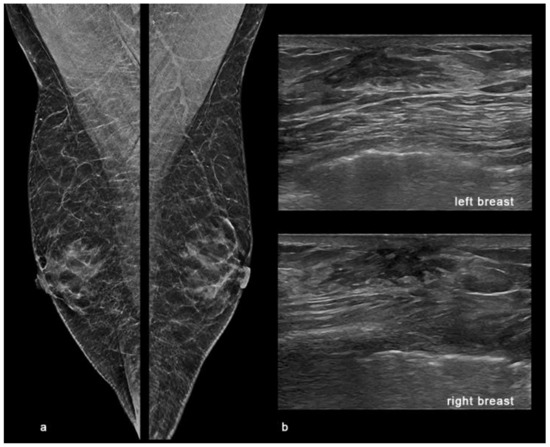
Figure 7.
Gynaecomastia. Digital breast tomosynthesis (DBT) and ultrasound (US) showing bilateral gynaecomastia in a 74-year-old male observed at our department with tender lumps in both breasts and mastodynia at clinical examination. (a) The synthetic 2D reconstructed medio-lateral-oblique images (s2D-MLO) showed a symmetric increased flame-shaped sub-areolar density in both breasts. (b) US showed a retro-areolar, triangular, hypoechoic mass in both breasts.
The diagnostic impact of magnetic resonance imaging (MRI) for the male breast is not sufficiently examined. In small studies, the MRI features of benign breast diseases and breast cancers in male patients seemed to be comparable to those seen in female patients, and therefore its diagnostic use should be limited to corresponding applications [48].
To our knowledge, there are no cases of male DCIS presenting as architectural distortion described in most of the literature about MBC [23,49,50,51,52,53,54,55].
According to this, in mammography, MBC usually occurs as a round, oval or irregular high-density sub-areolar mass with circumscribed, indistinct, speculative or micro-lobulated margins [36] (Figure 8). Microcalcifications are rare and observed in only 13% to 30% of cases [56], often with benign or nonspecific appearance [3]. In women, architectural distortion is the third most common mammographic appearance of non-palpable breast cancer, representing nearly 6% of abnormalities detected on screening mammography [56]. According to BI-RADS System [5], it was described as an appearance in which “the normal architecture of the breast is distorted with no definite mass visible”, including spiculations radiating from a point and focal retraction or distortion at the edge of the parenchyma. Chopier et al. [57] suggested classifying all the architectural distortions as BI-RADS 4, except in case of a known scar. The PPV for malignancy of an architectural distortion detected on mammography is 74.5% [58] and on diagnostic DBT, it is malignant in a third of cases. The presence of an ultrasound correlate reinforced the association with malignancy [59].
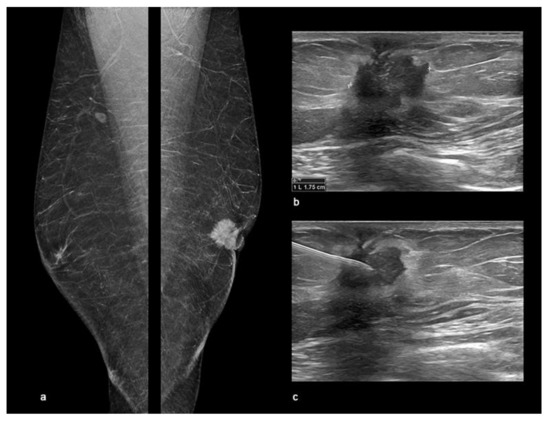
Figure 8.
Male Breast Cancer. Digital breast tomosynthesis (DBT) and ultrasound (US) showing an invasive ductal carcinoma of the left breast in a 58-year-old male observed at our department with a palpable retro-areolar mass and nipple retraction at clinical examination. (a) The synthetic 2D reconstructed medio-lateral-oblique images (s2D-MLO) showed a sub-areolar dense mass with spiculated margins and skin retraction. (b) US showed a left retro-areolar, hypoechoic mass with indistinct border and spiculated margins of 1.75 cm. (c) US-guided Fine-Needle Aspiration Cytology (FNAC) resulting C5 according to the the IAC Yokohama System for Reporting Breast Fine-Needle Aspiration Biopsy Cytopathology (1st Edition, 2020).
DCIS most commonly presents typically with pleomorphic, linear, or linear branching microcalcifications, but architectural distortion has been described as occurring in up to 2–10% of women. Although the most common features of DCIS on ultrasound are hypoechoic masses and microcalcifications, architectural distortion may be seen in up to 4% of cases [56]. Marino et al. [39] found that, of 163 men who underwent screening mammography, 6 were assessed BI-RADS 3 for focal asymmetry and/or architectural distortion but none of them showed malignant findings at follow-up or biopsy (dendritic gynaecomastia, post-surgical distortion). In their review, AlSharif et al. [60] found a single case of focal asymmetry that showed segmental homogenous non-mass like enhancement at magnetic resonance imaging (MRI). Instead, Isley et al. [61] described a case of male DCIS presenting as microcalcifications alone in absence of homolateral gynaecomastia.
Early diagnosis of MBC is fundamental to optimise therapeutic options and improve the survival rate. Although DCIS is extremely rare, it is an entity to be considered. Thirty to fifty percent of all male and female patients with DCIS develop invasive cancer in the following 10–20 years [60]. The American Cancer Society estimated that about 2650 new cases of invasive breast cancer will be diagnosed in the United States for 2021 and about 530 men will die (20%). For men, the lifetime risk of getting breast cancer is 1 in 833 and black people have a worse prognosis than white [62]. In Italy, the lifetime risk is 1 in 629 while the standardized incidence rates per 100,000 are 1.9, 1.5 and 1.6 for Northern, Central and Southern Italy, respectively [63]. The comparison of overall prognosis for male and female breast cancer patients is controversial. Studies reporting a poor prognosis for men have suggested that male breast anatomy may provide fewer barriers to metastasis or that more aggressive tumour biology may be the basis for survival variation [64]. However, others have found that, once separated by lymph node stage or involvement, the prognosis is the same. The reported 5-year overall survival rate for male breast carcinoma varies considerably from 40% to 80% with a specific survival rate of 89% and 72% at 5 years and 10 years respectively [19]. As most breast cancers in men are estrogen-receptor positive, tamoxifen is generally the standard adjuvant therapy [10,65,66,67,68,69]. Anti-androgen therapies may be an alternative and complementary treatment option for patients with triple-negative breast cancer with limited therapeutic possibilities [49]. Surgical decisions for a male DCIS are not easy and should be tailored. A modified nipple-sparing mastectomy could be useful in cases such as ours in which there was no involvement of the nipple-areolar complex. Sentinel node biopsy is suggested but not mandatory and it could spare axillary dissection in early stages MBC [70]. Since the disease is usually more locally advanced in men than in women, adjuvant loco-regional radiotherapy was more frequently administered to men than to women [71]. Adjuvant chemotherapy is generally considered a medium to high risk for BC. There are no prospective studies of endocrine neoadjuvant therapy and only a few reports of neoadjuvant chemotherapy [72].
Recent studies have explored the possible use of radiomics in breast disease, including distinguishing benign and malignant lesions [73], depicting the biology of tumours, differentiating between early and advanced stages, predicting cancer response to treatment and determining the risk of recurrence [74,75,76].
Radiomics is a rapidly evolving field of research concerned with the extraction of quantitative data within medical images, using advanced mathematical algorithms. Radiomic features, as they are defined, capture tissue and lesion characteristics such as heterogeneity and shape and may, alone or in combination with demographic, histologic, genomic, or proteomic data, be used for clinical problem solving [77].
Huang et al. [78] were the first to develop a model based on a radiomics analysis with traditional imaging features in mammography to distinguish male benign and malignant breast lesions. They selected five major features, including asymmetry, lesions located in non-retro-areola region, lesion eccentricity, skin thickening and calcification to differentiate MBC. Finally, they developed and validated an imaging-radiomics nomogram for clinicians to determine breast cancer risk for every male patient, recommending biopsy or surgery for all patients at high risk. In our case, there was at least three features suggesting cancer (asymmetry, eccentricity, and non-retro-areola region) and we think that radiomics could have been and will be a useful tool to help in the diagnosis of MBC even if further studies are necessary to validate its use.
4. Conclusions
The understanding of biology, clinical presentation and imaging features, genetics, and management of MBC is evolving but there still remains a large knowledge gap due to the rarity of this disease. Furthermore, there are no standardized protocols for male breast imaging and radiologists are generally less familiar with breast disease in male patients than female patients. Therefore, the knowledge of clinical and imaging presentation of DCIS is fundamental for the radiologists and clinicians involved.
Our case report is a unique mammographic and clinical presentation of a pure high-grade DCIS in a young male, associated with IP and ADH, without gynaecomastia or significant risk factors.
Atypical ductal hyperplasia (ADH) significantly increases the risk of breast cancer in women. However, little is known about the implications of ADH in men. In their study, Coopey et al. [11] showed no evolution to BC and this suggested that ADH in men does not pose the same risk as ADH in women.
Furthermore, our patient showed a clear, not bloody, nipple discharge, probably related to the focal ductal ectasia with the papilloma. This experience confirmed that a nipple discharge of any kind should be always considered suspicious for BC [79] and it could be the only clinical sign of carcinoma in situ.
In conclusion, although knowledge about FBC can inform MBC diagnosis and treatment, molecular and clinic-pathologic features differ between two genres. There is an unmet need for research and diagnostic-therapeutic options for this disease. Since mammography and ultrasonography are useful to diagnose a carcinoma in initial stages, avoiding unnecessary biopsies, we propose a single simple algorithm to help radiologists who interface male breast disease in both symptomatic and asymptomatic patients at high risk elder than 25 years old. In our diagram (Figure 9), we suggest to perform physical examination with at least a single mammography MLO projection for both breast, better if in digital breast tomosynthesis (DBT) with synthetic 2D reconstruction (s2D). In case of doubtful findings on both clinical and imaging first examination, other mammographic views could be performed (cranio-caudal, medio-lateral, magnification or spot-views).
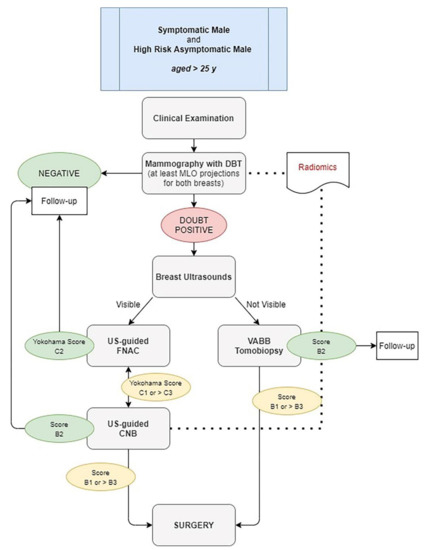
Figure 9.
Diagnostic Algorithm for both symptomatic and high-risk asymptomatic male aged > 25 years. DBT: digital breast tomosynthesis. US: ultrasound. VABB: vacuum-assisted breast biopsy. FNAC: fine-needle aspiration cytology. CNB: core-needle biopsy.
Ultrasonography could be an adjunct to mammography, especially to perform US-guided cytology. This type of triple test could avoid unnecessary biopsy and surgical procedures. Therefore, in case of suspicious findings (BI-RADS > 3 or IAC Yokohama System > C3), a core or vacuum-assisted breast biopsy should be performed before surgery.
However, since the radiologists’ experience affected the process of discriminating benign and malignant male breast lesions, the potential support of radiomics could enhance clinical decision-making. In fact, imaging features are often assessed visually and described qualitatively. This could lead to a large variability while it would certainly be more appropriate to objectively and reproducibly quantify these aspects. The use of a standardized imaging-radiomics nomogram, based on the evaluation of well-determined features, could help radiologists to determine breast cancer risk for every male patient, recommending biopsy or surgery when at high risk.
Evidence regarding the cost-effectiveness of healthcare technology is increasingly required to inform the decision on whether to fund and implement new treatment or diagnostic tools [80]. Many decision-making bodies require interventions to be assessed using cost per quality-adjusted-life-years (QALYs) [81], a single summary measure combining life expectancy with individuals’ relative preferences for health states in terms of quality of life [82]. Nevertheless, evaluating the true cost-effectiveness of breast cancer prevention will remain problematic [83]. Female breast cancer screening is affected by many challenges related to the assessment of its harms and benefits, such as anxiety or reassurance, the long-term implications of breast surgery, the improvement of therapies versus the advantages of early detection, over diagnosis versus undervaluation. Male breast cancer is a rare pathology but it could be an interesting field of research for both clinical and economical aspects. To analyse the costs of the diagnostic-therapeutic care pathway for each patient, using the micro-costing “bottom-up” approach [84], could be useful to gather all the needed information to assess better qualitative and quantitative performance in according to the best practice in clinical governance.
Our protocol could be an example of a male breast cancer prevention model, optimizing the use of resources in according to the criteria of “3Es”: efficiency, effectiveness and economy.
Author Contributions
Conceptualization, D.U.T.; methodology and investigation, D.U.T. and L.M.; resources and data curation: D.U.T.; writing-original draft preparation, D.U.T.; writing-review and editing, D.U.T., L.M., A.G. and F.P.; supervision and project administration, A.G. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Study was conducted according to the guidelines of the Declaration of Helsinki. Institutional Review Board is not applicable considering the retrospective nature of the study.
Informed Consent Statement
Informed consent was obtained from the patient(s) to publish this paper, before any radiological exam either mammography or US and before any interventional procedure.
Data Availability Statement
The reported data come from saniarp.it, the Caserta LHA reporting database and from the register of our daily activities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fentiman, I.S.; Fourquet, A.; Hortobagyi, G.N. Male breast cancer. Lancet 2006, 367, 595–604. [Google Scholar] [CrossRef]
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef]
- Brents, M.; Hancock, J. Ductal Carcinoma In situ of the Male Breast. Breast Care 2016, 11, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, K.J.; Winer, E.P. Male breast cancer: Risk factors, biology, diagnosis, treatment, and survivorship. Ann. Oncol. 2013, 24, 1434–1443. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Field, A.S.; Raymond, W.A.; Rickard, M.; Arnold, L.; Brachtel, E.F.; Chaiwun, B.; Chen, L.; Di Bonito, L.; Kurtycz, D.F.I.; Lee, A.H.S.; et al. The International Academy of Cytology Yokohama System for Reporting Breast Fine-Needle Aspiration Biopsy Cytopathology. Acta Cytol. 2019, 63, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Field, A.S.; Raymond, W.A.; Rickard, M.; Schmitt, F. Breast fine needle aspiration biopsy cytology: The potential impact of the International Academy of Cytology Yokohama System for Reporting Breast Fine Needle Aspiration Biopsy Cytopathology and the use of rapid on-site evaluation. J. Am. Soc. Cytopathol. 2020, 9, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.S.; Carder, P.; Deb, R.; Ellis, I.O.; Howe, M.; Jenkins, J.A.; Pinder, S.E. Guidelines for Non-Operative Diagnostic Procedures and Reporting in Breast Cancer Screening; The Royal College of Pathologists Publications G-150: London, UK, 2021. [Google Scholar]
- Nofal, M.N.; Yousef, A.J. The diagnosis of male breast cancer. Neth. J. Med. 2019, 77, 356–359. [Google Scholar]
- Khan, N.A.J.; Tirona, M. An updated review of epidemiology, risk factors, and management of male breast cancer. Med. Oncol. 2021, 38, 39. [Google Scholar] [CrossRef]
- Coopey, S.B.; Kartal, K.; Li, C.; Yala, A.; Barzilay, R.; Faulkner, H.R.; King, T.A.; Acevedo, F.; Garber, J.E.; Guidi, A.J.; et al. Atypical ductal hyperplasia in men with gynecomastia: What is their breast cancer risk? Breast Cancer Res. Treat. 2019, 175, 1–4. [Google Scholar] [CrossRef]
- Vagios, I.; Nonni, A.; Liakea, A.; Constantinidou, A.; Kontos, M. Intraductal papilloma of the male breast: A case report and review of the literature. J. Surg. Case. Rep. 2019, 2019, rjz023. [Google Scholar] [CrossRef]
- Bharti, S.; Bharti, J.N.; Vishnoi, J.R.; Soudamini, A.B. A rare case of intraductal papilloma with atypical ductal hyperplasia in a male breast: A pathological diagnosis. J. Fam. Community Med. 2020, 27, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Cohen, D.S.; Buzdar, A.U.; Perkins, G.; Hortobagyi, G.N. Breast carcinoma in men: A population-based study. Cancer 2004, 101, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Speirs, V.; Shaaban, A.M. The rising incidence of male breast cancer. Breast Cancer Res. Treat 2009, 115, 429–430. [Google Scholar] [CrossRef]
- White, J.; Kearins, O.; Dodwell, D.; Horgan, K.; Hanby, A.M.; Speirs, V. Male breast carcinoma: Increased awareness needed. Breast Cancer Res. 2011, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Bartlett, J.M.S.; Slaets, L.; van Deurzen, C.H.M.; van Leeuwen-Stok, E.; Porter, P.; Linderholm, B.; Hedenfalk, I.; Schröder, C.; Martens, J.; et al. Characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann. Oncol. 2018, 29, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Bohli, M.; Tebra Mrad, S.; Zrafi, I.; Bouaouina, N. Cancer du sein chez l’homme: Quelles particularités? Cancer/Radiothérapie 2017, 21, 701. [Google Scholar] [CrossRef]
- Methamem, M.; Ghadhab, I.; Hidar, S.; Briki, R. Breast cancer in men: A serie of 45 cases and literature review. Pan Afr. Med. J. 2020, 36, 183. [Google Scholar] [CrossRef]
- Gao, Y.; Heller, S.L.; Moy, L. Male Breast Cancer in the Age of Genetic Testing: An Opportunity for Early Detection, Tailored Therapy, and Surveillance. Radiographics 2018, 38, 1289–1311. [Google Scholar] [CrossRef]
- Woods, R.W.; Salkowski, L.R.; Elezaby, M.; Burnside, E.S.; Strigel, R.M.; Fowler, A.M. Image-based screening for men at high risk for breast cancer: Benefits and drawbacks. Clin. Imaging 2020, 60, 84–89. [Google Scholar] [CrossRef]
- Biganzoli, L.; Calabrese, M.; Conte, B.; Cortesi, L.; Criscitiello, C.; Del Mastro, L.; Fiorentino, A.; Levaggi, A.; Montemurro, F.; Marchiò, C.; et al. Linee Guida AIOM-Neoplasie Della Mammella; Edizione: Treviso, Italy, 2020. [Google Scholar]
- Nguyen, C.; Kettler, M.D.; Swirskt, M.E.; Miller, V.I.; Scott, C.; Krause, R.; Hadro, J.A. Male Breast Disease: Pictorial Review with Radiologic-Pathologic Correlation. RadioGraphics 2013, 33, 763–779. [Google Scholar] [CrossRef]
- Mathew, J.; Perkins, G.H.; Stephens, T.; Middleton, L.P.; Yang, W.T. Primary breast cancer in men: Clinical, imaging, and pathologic findings in 57 patiens. AJR Am. J. Roentgenol. 2008, 191, 1631–1639. [Google Scholar] [CrossRef]
- Hittmair, A.P.; Lininger, R.A.; Tavassoli, F. A: Ductal carcinoma in situ (DCIS) in the male breast. A morphologic study of 84 cases of pure DCIS and 30 cases of DCIS associated with invasive carcinoma—A preliminary report. Cancer 1998, 83, 2139–2149. [Google Scholar] [CrossRef]
- Erhan, Y.; Zekioglu, O.; Erhan, Y. Invasive lobular carcinoma of the male breast. Can. J. Surg. 2006, 49, 365–366. [Google Scholar]
- Coroneos, C.J.; Hamm, C. Ductal carcinoma in situ in a 25-year-old man presenting with apparent unilateral gynecomastia. Curr. Oncol. 2010, 17, 133–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foerster, R.; Schroeder, L.; Foerster, F.; Wulff, V.; Schubotz, B.; Baaske, D.; Rudlowski, C. Metastatic male breast cancer: A retrospective cohort analysis. Breast Care 2014, 9, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Irwig, L.; Macaskill, P.; Houssami, N. Evidence relevant to the investigation of breast symptoms: The triple test. Breast 2002, 11, 2015–2220. [Google Scholar] [CrossRef]
- Muñoz Carrasco, R.; Alvarez Benito, M.; Muñoz Gomariz, E.; Raya Povedano, J.L.; Martínez Paredes, M. Mammography and ultrasound in the evaluation of male breast disease. Eur. Radiol. 2010, 20, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.F.; Anthony, T.; Turnage, R.H.; Schumpert, T.D.; Levy, K.R.; Amirkhan, R.H.; Campbell, T.J.; Lopez, J.; Appelbaum, A.H. The diagnostic accuracy of mammography in the evaluation of male breast disease. Am. J. Surg. 2001, 181, 96–100. [Google Scholar] [CrossRef]
- Adibelli, Z.H.; Oztekin, O.; Postaci, H.; Uslu, A. The Diagnostic Accuracy of Mammography and Ultrasound in the Evaluation of Male Breast Disease: A New Algorithm. Breast Care 2009, 4, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.; Raffetto, J.D.; Collure, D.W.; Hoover, E.L.; Doerr, R.J. Unilateral male breast masses: Cancer risk and their evaluation and management. Am. Surg. 1999, 65, 250–253. [Google Scholar]
- Hines, S.L.; Tan, W.; Larson, J.M.; Thompson, K.M.; Jorn, H.K.; Files, J.A. A practical approach to guide clinicians in the evaluation of male patients with breast masses. Geriatrics 2008, 63, 19–24. [Google Scholar] [PubMed]
- Munn, S. When should men undergo mammography? AJR Am. J. Roentgenol. 2002, 178, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Chau, A.; Jafarian, N.; Rosa, M. Male Breast: Clinical and Imaging Evaluations of Benign and Malignant Entities with Histologic Correlation. Am. J. Med. 2016, 129, 776–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, K.; Ames, V.; Wallis, M. The diagnostic value of clinical examination and imaging used as part of an age-related protocol when diagnosing male breast disease: An audit of 1141 cases from a single centre. Breast 2013, 22, 268–272. [Google Scholar] [CrossRef]
- Expert Panel on Breast Imaging; Niell, B.L.; Lourenco, A.P.; Moy, L.; Baron, P.; Didwania, A.D.; di Florio-Alexander, R.M.; Heller, S.L.; Holbrook, A.I.; Le-Petross, H.T.; et al. ACR Appropriateness Criteria® Evaluation of the Symptomatic Male Breast. J. Am. Coll. Radiol. JACR 2018, 15, S313–S320. [Google Scholar] [CrossRef]
- Marino, M.A.; Gucalp, A.; Leithner, D.; Keating, D.; Avendano, D.; Bernard-Davila, B.; Morris, E.A.; Pinker, K.; Jochelson, M.S. Mammographic screening in male patients at high risk for breast cancer: Is it worth it? Breast Cancer Res. Treat. 2019, 177, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, S.; Houssami, N.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): A prospective comparison study. Lancet Oncol. 2013, 14, 583–589. [Google Scholar] [CrossRef]
- Skaane, P.; Bandos, A.I.; Gullien, R.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Izadi, M.; Jebsen, I.N.; Jahr, G.; Krager, M.; et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013, 267, 47–56. [Google Scholar] [CrossRef]
- Ray, K.M.; Turner, E.; Sickles, E.A.; Joe, B.N. Suspicious findings at digital breast Tomosynthesis occult to conventional digital mammography: Imaging features and pathology findings. Breast J. 2015, 21, 538–542. [Google Scholar] [CrossRef]
- Partyka, L.; Lourenco, A.P.; Mainiero, M.B. Detection of mammographically occult architectural distortion on digital breast tomosynthesis screening: Initial clinical experience. AJR Am. J. Roentgenol. 2014, 203, 216–222. [Google Scholar] [CrossRef]
- Durand, M.A.; Wang, S.; Hooley, R.J.; Raghu, M.; Philpotts, L.E. Tomosynthesis-detected architectural distortion: Management algorithm with radiologic-pathologic correlation. Radiographics 2016, 36, 311–321. [Google Scholar] [CrossRef]
- Freer, P.E.; Niell, B.; Rafferty, E.A. Preoperative Tomosynthesis guided needle localization of mammographically and sonographically occult breast lesions. Radiology 2015, 275, 377–383. [Google Scholar] [CrossRef]
- Bernardi, D.; Macaskill, P.; Pellegrini, M.; Valentini, M.; Fantò, C.; Ostillio, L.; Tuttobene, P.; Luparia, A.; Houssami, N. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): A population-based prospective study. Lancet Oncol. 2016, 17, 1105–1113. [Google Scholar] [CrossRef]
- Sonnenblick, E.B.; Margolies, L.R.; Szabo, J.R.; Jacobs, L.M.; Patel, N.; Lee, K.A. Digital breast tomosynthesis of gynecomastia and associated findings-a pictorial review. Clin. Imaging. 2014, 38, 565–570. [Google Scholar] [CrossRef]
- Rudlowski, C. Male Breast Cancer. Breast Care 2008, 3, 183–189. [Google Scholar] [CrossRef]
- Héquet, D.; Mzoughi, S.; Rouzier, R.; Guccione, E. Androgen Receptors in Breast Cancer: Expression, Value and Therapeutic Perspectives. Bull Cancer 2017, 104, 363–369. [Google Scholar] [CrossRef]
- Appelbaum, A.H.; Evans, G.F.; Levy, K.R.; Amirkhan, R.H.; Schumpert, T.D. Mammographic appearances of male breast disease. Radiographics 1999, 19, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Günhan-Bilgen, I.; Bozkaya, H.; Ustün, E.; Memiş, A. Male breast disease: Clinical, mammographic, and ultrasonographic features. Eur. J. Radiol. 2002, 43, 246–255. [Google Scholar] [CrossRef]
- Yitta, S.; Singer, C.I.; Toth, H.B.; Mercado, C.L. Image presentation. Sonographic appearances of benign and malignant male breast disease with mammographic and pathologic correlation. J. Ultrasound Med. 2010, 29, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Şafak, K.Y. Mammography Findings of Male Breast Diseases. J. Breast Health 2015, 11, 106–110. [Google Scholar] [CrossRef][Green Version]
- Hines, S.L.; Tan, W.W.; Yasrebi, M.; DePeri, E.R.; Perez, E.A. The role of mammography in male patients with breast symptoms. Mayo. Clin. Proc. 2007, 82, 297–300. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S. Ultrasonographic and Mammographic Findings of Male Breast Disease. J. Ultrasound Med. 2019, 38, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Dialani, V.; Slanetz, P.J.; Eisenberg, R.L. Architectural distortion of the breast. AJR Am. J. Roentgenol. 2013, 201, W662–W670. [Google Scholar] [CrossRef] [PubMed]
- Chopier, J.; Roedlich, M.N.; Mathelin, C. Imagerie mammaire du syndrome de masse, distorsion architecturale et asymétrie: Recommandations pour la pratique clinique [Breast imaging of mass, architectural distortion and asymmetry: Clinical practice guidelines]. J. Gynecol. Obs. Biol. Reprod. (Paris) 2015, 44, 947–959. [Google Scholar] [CrossRef]
- Bahl, M.; Baker, J.A.; Kinsey, E.N.; Ghate, S.V. Architectural Distortion on Mammography: Correlation with Pathologic Outcomes and Predictors of Malignancy. AJR Am. J. Roentgenol. 2015, 205, 1339–1345. [Google Scholar] [CrossRef]
- Pujara, A.C.; Hui, J.; Wang, L.C. Architectural distortion in the era of digital breast tomosynthesis: Outcomes and implications for management. Clin. Imaging 2019, 54, 133–137. [Google Scholar] [CrossRef]
- AlSharif, S.; Alshamrani, K.M.; Scaranelo, A.; Khoumais, N.; Subahi, A.; Mesurolle, B. Unusual Male Breast Lesions. J. Clin. Imaging. Sci. 2021, 11, 21. [Google Scholar] [CrossRef]
- Isley, L.M.; Leddy, R.J.; Rumboldt, T.; Bernard, J.M. Asymptomatic Incidental Ductal Carcinoma in situ in a Male Breast Presenting with Contralateral Gynecomastia. J. Clin. Imaging. Sci. 2012, 2, 9. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Male Breast Cancer Incidence and Mortality, United States—2013–2017; USCS Data Brief, No 19; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2020. [Google Scholar]
- AIRTUM Working Group; AIOM Working Group; PASSI Working Group; SIAPEC-IAP Working Group. I Numeri del Cancro in Italia, 9th ed.; Intermedia Editore: Brescia, Italy, 2019. [Google Scholar]
- Li, X.; Yang, J.; Krishnamurti, U.; Huo, L.; Ward, K.C.; O’Regan, R.; Peng, L. Hormone Receptor-Positive Breast Cancer Has a Worse Prognosis in Male Than in Female Patients. Clin. Breast Cancer 2017, 17, 356–366. [Google Scholar] [CrossRef]
- Johansson, I.; Killander, F.; Linderholm, B.; Hedenfalk, I. Molecular profiling of male breast cancer-Lost in translation? Int. J. Biochem. Cell Biol. 2014, 53, 526–535. [Google Scholar] [CrossRef]
- Meijer-van Gelder, M.E.; Look, M.P.; Bolt-de Vries, J.; Peters, H.A.; Klijn, J.G.; Foekens, J.A. Clinical relevance of biologic factors in male breast cancer. Breast Cancer Res. Treat. 2001, 68, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, L.; Barba, M.; Pizzuti, L.; Vici, P.; Sergi, D.; Di Benedetto, A.; Mottolese, M.; Speirs, V.; Santini, D.; De Maria, R.; et al. Androgen receptor and antiandrogen therapy in male breast cancer. Cancer Lett. 2015, 368, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Goss, P.E.; Reid, C.; Pintilie, M.; Lim, R.; Miller, N. Male breast carcinoma: A review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer 1999, 85, 629–639. [Google Scholar] [CrossRef]
- Ge, Y.; Sneige, N.; Eltorky, M.A.; Wang, Z.; Lin, E.; Gong, Y.; Guo, M. Immunohistochemical characterization of subtypes of male breast carcinoma. Breast Cancer Res. 2009, 11, R28. [Google Scholar] [CrossRef]
- Fentiman, I.S. Surgical options for male breast cancer. Breast Cancer Res. Treat. 2018, 172, 539–544. [Google Scholar] [CrossRef]
- Macdonald, G.; Paltiel, C.; Olivotto, I.A.; Tyldesley, S. A comparative analysis of radiotherapy use and patient outcome in males and females with breast cancer. Ann. Oncol. 2005, 16, 1442–1448. [Google Scholar] [CrossRef]
- Giordano, S.H.; Perkins, G.H.; Broglio, C.; Garcia, S.G.; Middleton, L.P.; Buzdar, A.U.; Hortobagyi, G.N. Adjuvant systemic therapy for male breast carcinoma. Cancer 2005, 104, 2359–2364. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Wang, X.; Yu, H.; Gao, Y.; Ren, Y.; Wang, G.; Zhou, X. Diagnostic Performance of Mammographic Texture Analysis in the Differential Diagnosis of Benign and Malignant Breast Tumors. Clin. Breast Cancer 2018, 18, e621-7. [Google Scholar] [CrossRef]
- Yip, S.S.; Aerts, H.J. Applications and limitations of radiomics. Phys Med Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Qu, J.; Zhang, R.; Zhou, X.; Li, L.; Sun, K.; Tang, Z.; Jiang, H.; Li, H.; et al. Radiomics of Multiparametric MRI for Pretreatment Prediction of Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Multicenter Study. Clin. Cancer Res. 2019, 25, 3538–3547. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Burnside, E.S.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Whitman, G.J.; Sutton, E.J.; Net, J.M.; et al. MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology 2016, 281, 382–391. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, Q.; Sun, Y.; Wang, Z.; Li, Q.; Wang, H.; Gu, Y. An Approach Based on Mammographic Imaging and Radiomics for Distinguishing Male Benign and Malignant Lesions: A Preliminary Study. Front Oncol. 2021, 10, 607235. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Carrasco, R.; Álvarez Benito, M.; del Rivin Campo, E. Value of mammography and breast ultrasound in male patients with nipple discharge. Eur. J. Radiol. 2013, 82, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- NICE. Guide to the Methods of Technology Appraisal; NICE: London, UK, 2013. [Google Scholar]
- Whitehead, S.J.; Ali, S. Health outcomes in economic evaluation: The QALY and utilities. Br. Med. Bull. 2010, 96, 5–21. [Google Scholar] [CrossRef]
- Bromley, H.L.; Petrie, D.; Mann, G.B.; Nickson, C.; Rea, D.; Roberts, T.E. Valuing the health states associated with breast cancer screening programmes: A systematic review of economic measures. Soc. Sci. Med. 2019, 228, 142–154. [Google Scholar] [CrossRef]
- Xu, X.; Grossetta Nardini, H.K.; Ruger, J.P. Micro-costing studies in the health and medical literature: Protocol for a systematic review. Syst. Rev. 2014, 3, 47. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).