Too Late to Reverse: An Atypical Postpartum Case of Acute Necrotizing Pancreatitis with Refractory ARDS Despite ECMO Support
Abstract
1. Introduction
1.1. Incidence and Etiology
1.2. Pathophysiology
1.3. Particularity of the Case
2. Case Presentation
2.1. Patient History, Onset of the Disease and Clinical Findings
2.2. Admission
2.3. Evolution
2.4. ICU Admission and Management
2.5. Advanced Supportive Therapy
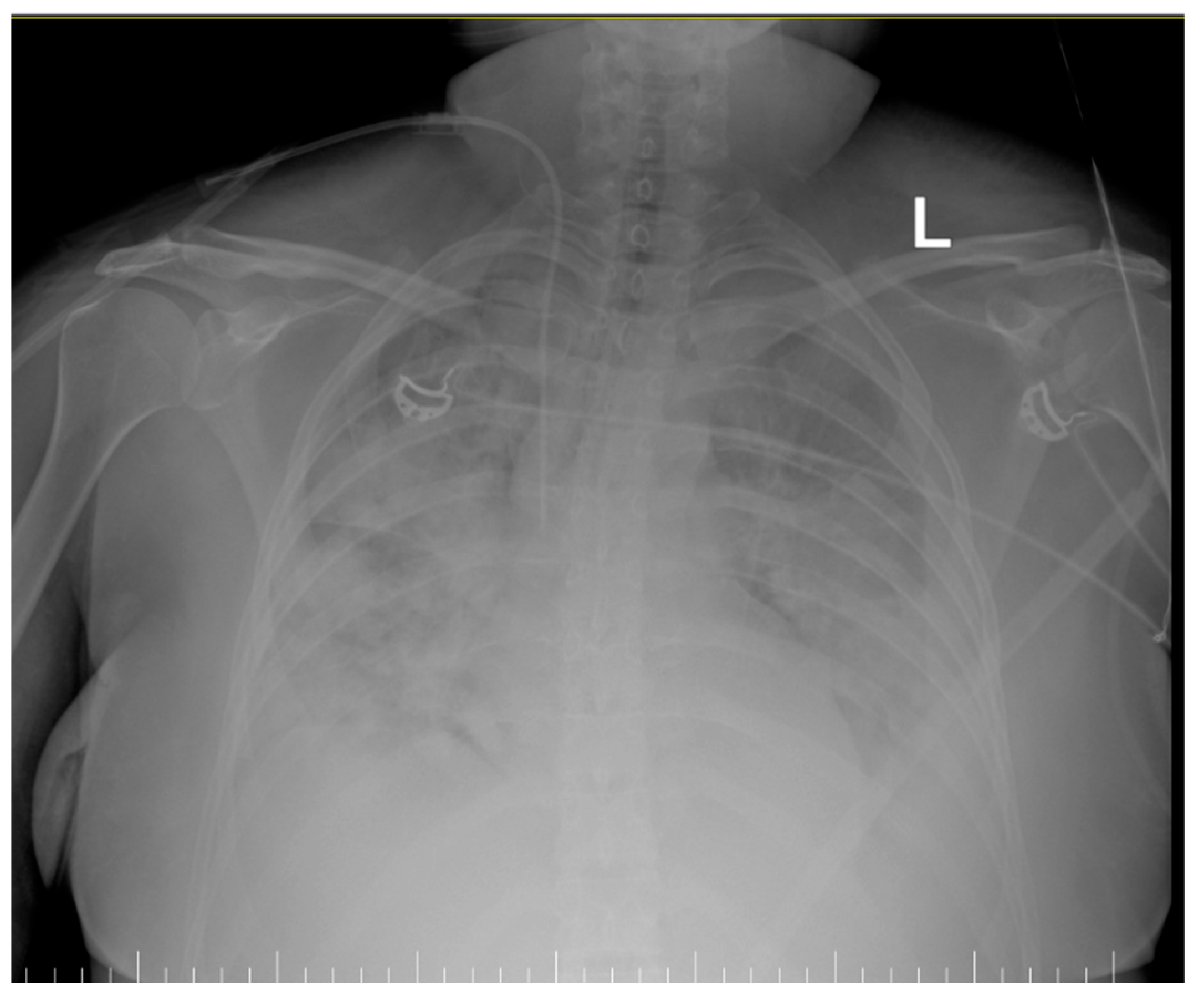
- Inhomogeneous veiling of the entire left lung field and, respectively, ½ of the right lung field—foci of pulmonary condensation and atelectasis. Bilateral pleural effusion. ECMO cannula at the level of the right internal jugular vein.
- Quasi-complete resorption of pulmonary condensation foci and, respectively, reduction in pleural effusion bilaterally. Lungs with increased transparency. ECMO cannula at the level of the right internal jugular vein.
3. Discussions
3.1. Pathophysiology
3.2. Management
3.3. Implications for Future Practice
3.4. Other Cases of Post-Partum Pancreatitis and ARDS: Data from the Literature
| Study | Maternal Age | Timing Postpartum | Type of Birth | Etiology | Complications | Interventions | Outcomes |
|---|---|---|---|---|---|---|---|
| Toth et al. [39] | 27-year-old | 2 days postpartum | C-section | Idiopathic | - | Conservative treatment | Discharged on the 7th day |
| Kim et al. [40] | 35-year-old | 3 days postpartum | C-section | Gallstones | intra-abdominal fluid collections and gastric bleeding | percutaneous drainage, endoscopic hemostasis, angiographic embolization | Discharged on the 31st day |
| Hofstrand et al. [20] | 19-year-old | 3 months postpartum | Not mentioned | Gallbladder “sludge” | - | Conservative | Discharged on the 6th day |
| Cao et al. [41] | 26-year-old | 35 weeks of pregnancy | Emergency C-section | Hypertriglyceridemia | - | Conservative treatment, insulin infusion, heparin, plasmapheresis and CRRT | Discharged on the 11th day |
| Shiddapur et al. [43] | 25-year-old | 30 weeks of pregnancy | Emergency C-section | Pregnancy-induced hypertension complicated with eclampsia | moderate ascites | Conservative treatment and paracentesis | Not mentioned |
| Lee et al. [44] | 38-year-old | 1st day after delivery | Vaginal delivery | Hypercalcemia caused by primary hyperparatiroidism | bilateral hydronephrosis caused by renal stones | Conservative treatment, CT-guided percutaneous drainage | Right parathyroidectomy with good recovery at 1 year follow-up |
| Jallouli et al. [45] | 19-year-old | 4 weeks postpartum | Vaginal delivery | Idiopathic | - | Conservative management | Discharged on the 14th day |
| Poo et al. [46] | 30-year-old | 3 days postpartum | Emergency C-section | insulin-dependent diabetes mellitius from pancreatic islet cell destruction | diabetic ketoacidosis | Conservative treatment + CRRT | long-term insulin requirement |
| Amir et al. [30] | 32-year-old | 10 days postpartum | Vaginal delivery | Idiopathic | - | Conservative treatment | Discharged on the 19th day, good recovery at one month follow-up |
| Dale et al. [47] | 31-year-old | 32 weeks of pregnancy | C-section | Hypercalcemia caused by primary hyperparatiroidism | - | Conservative management | Discharged on 7th day, referred for parathyroid adenoma resection |
3.5. Key Learning Points
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABPP | acute biliary pancreatitis related to pregnancy |
| AJG | American Journal of Gastroenterology |
| AKI | acute kidney failure |
| ALT | alanine aminotransferase |
| AP | acute pancreatitis |
| APACHE | acute physiology and chronic health evaluation |
| ARDS | acute respiratory distress syndrome |
| AST | aspartate aminotransferase |
| BP | blood pressure |
| CRP | C-reactive protein |
| CRRT | continuous renal replacement therapy |
| CT | computed tomography |
| ECMO | extracorporeal membrane oxygenation |
| FiO2 | fraction of inspired Oxygen |
| HELLP syndrome | hemolysis, elevated liver enzymes, and low platelet count |
| HLAP | hyperlipidemia acute pancreatitis |
| HR | heart rate |
| ICU | intensive care unit |
| IL | interleukin |
| LDH | lactate dehydrogenase |
| MAP | mean arterial pressure |
| MODS | multiorgan dysfunction syndrome |
| PaO2 | partial pressure of Oxygen in arterial blood |
| PaCO2 | partial pressure of Carbon Dioxide in arterial blood |
| PCT | procalcitonin |
| PEEP | positive end expiratory pressure |
| RR | respiratory rate |
| RRT | renal replacement therapy |
| RUQ US | right upper quadrant ultrasound |
| SaO2 | oxygen saturation of arterial blood |
| SIRS | systemic inflammatory response syndrome |
| SOFA | sequential organ failure assessment |
| TNF | tumor necrosis factor |
| VA-ECMO | venoarterial extracorporeal membrane oxygenation |
| VV-ECMO | venovenous extracorporeal membrane oxygenation |
References
- Maringhini, A.; Rossi, M.; Patti, R.; Maringhini, M.; Vassallo, V. Acute Pancreatitis during and after Pregnancy: A Review. J. Clin. Med. 2024, 13, 2028. [Google Scholar] [CrossRef]
- Hot, S.; Eğin, S.; Gökçek, B.; Yeşiltaş, M.; Karakaş, D.Ö. Acute Biliary Pancreatitis during Pregnancy and in the Post-Delivery Period. Turk. J. Trauma Emerg. Surg. TJTES 2019, 25, 253–258. [Google Scholar] [CrossRef]
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.A.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef]
- Valverde-López, F.; Martínez-Cara, J.G.; Redondo-Cerezo, E. Pancreatitis Aguda. Med. Clín. 2022, 158, 556–563. [Google Scholar] [CrossRef]
- Hong, W.; Pan, J.; Goyal, H.; Zippi, M. Editorial: Acute Pancreatitis Infection: Epidemiology, Prevention, Clinical Characteristics, Treatment, and Prediction. Front. Cell. Infect. Microbiol. 2023, 13, 1175195. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Liti, B.; Barrett, C.; Thompson, P.D.; Fernandez, A.B. Prevention and Management of Hypertriglyceridemia-Induced Acute Pancreatitis During Pregnancy: A Systematic Review. Am. J. Med. 2022, 135, 709–714. [Google Scholar] [CrossRef]
- Kolber, W.; Dumnicka, P.; Maraj, M.; Kuśnierz-Cabala, B.; Ceranowicz, P.; Pędziwiatr, M.; Maziarz, B.; Mazur-Laskowska, M.; Kuźniewski, M.; Sporek, M.; et al. Does the Automatic Measurement of Interleukin 6 Allow for Prediction of Complications during the First 48 h of Acute Pancreatitis? Int. J. Mol. Sci. 2018, 19, 1820. [Google Scholar] [CrossRef]
- Jain, S.; Midha, S.; Mahapatra, S.J.; Gupta, S.; Sharma, M.K.; Nayak, B.; Jacob, T.G.; Shalimar; Garg, P.K. Interleukin-6 Significantly Improves Predictive Value of Systemic Inflammatory Response Syndrome for Predicting Severe Acute Pancreatitis. Pancreatology 2018, 18, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, A.; Grama, A.; Colceriu, M.-C.; Benţa, G.; Popoviciu, M.-S.; Pop, T.L. Role of Interleukin 6 in Acute Pancreatitis: A Possible Marker for Disease Prognosis. Int. J. Mol. Sci. 2024, 25, 8283. [Google Scholar] [CrossRef] [PubMed]
- Sherer, M.L.; Posillico, C.K.; Schwarz, J.M. The Psychoneuroimmunology of Pregnancy. Front. Neuroendocrinol. 2018, 51, 25–35. [Google Scholar] [CrossRef]
- Morton, A.; Teasdale, S. Physiological Changes in Pregnancy and Their Influence on the Endocrine Investigation. Clin. Endocrinol. 2022, 96, 3–11. [Google Scholar] [CrossRef]
- Rassie, K.; Giri, R.; Joham, A.E.; Mousa, A.; Teede, H. Prolactin in Relation to Gestational Diabetes and Metabolic Risk in Pregnancy and Postpartum: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 1069625. [Google Scholar] [CrossRef]
- Calomino, N.; Poto, G.E.; Carbone, L.; Bagnacci, G.; Piccioni, S.; Andreucci, E.; Nenci, L.; Marano, L.; Verre, L.; Petrioli, R.; et al. Neuroendocrine Tumors’ Patients Treated with Somatostatin Analogue Could Complicate with Emergency Cholecystectomy. Ann. Ital. Chir. 2023, 94, 518–522. [Google Scholar]
- Shah, J.; Rana, S.S. Acute respiratory distress syndrome in acute pancreatitis. Indian J. Gastroenterol. 2020, 39, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, B.-L.; Fodor, R.Ș. Endocrine Disorders in Critically Ill Patients—The Smooth Criminal? J. Crit. Care Med. 2024, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Langouche, L.; Téblick, A.; Gunst, J.; Van den Berghe, G. The Hypothalamus-Pituitary-Adrenocortical Response to Critical Illness: A Concept in Need of Revision. Endocr. Rev. 2023, 44, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Meduri, G.U.; Annane, D.; Chrousos, G.P.; Marik, P.E.; Sinclair, S.E. Activation and Regulation of Systemic Inflammation in ARDS: Rationale for Prolonged Glucocorticoid Therapy. Chest 2009, 136, 1631–1643. [Google Scholar] [CrossRef]
- Zhou, M.-T.; Chen, C.-S.; Chen, B.-C.; Zhang, Q.-Y.; Andersson, R. Acute Lung Injury and ARDS in Acute Pancreatitis: Mechanisms and Potential Intervention. World J. Gastroenterol. 2010, 16, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.D. Gallstone Disease and Pancreatitis in Pregnancy. Gastroenterol. Clin. N. Am. 1992, 21, 803–815. [Google Scholar] [CrossRef]
- Hofstrand, R.; Singhal, M.; Doad, J.; Watts, R. Postpartum Idiopathic Pancreatitis Complicated by Acute Necrotizing Pancreatitis. Cureus 2023, 15, e34002. [Google Scholar] [CrossRef]
- Hu, X.; Han, Z.; Zhou, R.; Su, W.; Gong, L.; Yang, Z.; Song, X.; Zhang, S.; Shu, H.; Wu, D. Altered Gut Microbiota in the Early Stage of Acute Pancreatitis Were Related to the Occurrence of Acute Respiratory Distress Syndrome. Front. Cell. Infect. Microbiol. 2023, 13, 1127369. [Google Scholar] [CrossRef]
- Schmandt, M.; Glowka, T.R.; Kreyer, S.; Muders, T.; Muenster, S.; Theuerkauf, N.U.; Kalff, J.C.; Putensen, C.; Schewe, J.-C.; Ehrentraut, S.F. Secondary ARDS Following Acute Pancreatitis: Is Extracorporeal Membrane Oxygenation Feasible or Futile? J. Clin. Med. 2021, 10, 1000. [Google Scholar] [CrossRef]
- Beger, H.G.; Rau, B.M. Severe Acute Pancreatitis: Clinical Course and Management. World J. Gastroenterol. WJG 2007, 13, 5043–5051. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.R.; Chen, L.; Amato, M.B.P.; Brochard, L.J. Fifty Years of Research in ARDS. Respiratory Mechanics in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 196, 822–833. [Google Scholar] [CrossRef]
- Harpa, M.M.; Oltean, S.F.; Al Hussein, H.; Anitei, D.E.; Puscas, I.A.; Bănceu, C.M.; Veres, M.; Opriș, D.R.; Balau, R.A.; Suciu, H. Successful Treatment of Unilateral Pulmonary Edema as Minimally Invasive Mitral Valve Surgery Complication—Case Presentation. J. Clin. Med. 2024, 13, 7654. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Pozzi, M.; Siegenthaler, N.; Brunner, M.-E.; Tassaux, D.; Obadia, J.-F.; Bendjelid, K.; Giraud, R. Veno-Venous Extracorporeal Membrane Oxygenation: Cannulation Techniques. J. Thorac. Dis. 2016, 8, 3762–3773. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Sommer, W.; Tudorache, I.; Avsar, M.; Siemeni, T.; Salman, J.; Puntigam, J.; Optenhoefel, J.; Greer, M.; Welte, T.; et al. Veno-Veno-Arterial Extracorporeal Membrane Oxygenation for Respiratory Failure with Severe Haemodynamic Impairment: Technique and Early Outcomes. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 761–767. [Google Scholar] [CrossRef]
- Benfield, R.D.; Newton, E.R.; Tanner, C.J.; Heitkemper, M.M. Cortisol as a Biomarker of Stress in Term Human Labor: Physiological and Methodological Issues. Biol. Res. Nurs. 2014, 16, 64–71. [Google Scholar] [CrossRef]
- Schwertner, H.A.; Torres, L.; Jackson, W.G.; Maldonado, H.A.; Whitson, J.D.; Troxler, R.G. Cortisol and the Hypercholesterolemia of Pregnancy and Labor. Atherosclerosis 1987, 67, 237–244. [Google Scholar] [CrossRef]
- Amir, W.; Nawaz, M.; Ahmed, Z. A Rare Case of Acute Idiopathic Pancreatitis in Third Trimester Which Aggravated in Early Postpartum Period. Cureus 2020, 12, e7348. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, S.H.; Choi, H.W.; Song, D.J.; Jeong, C.Y.; Lee, D.H.; Whang, I.S. Acute Idiopathic Pancreatitis in Pregnancy: A Case Study. World J. Gastroenterol. 2014, 20, 16364–16367. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.; Perelman, A.; Birk, J.W. Acute Management of Pancreatitis: The Key to Best Outcomes. Postgrad. Med. J. 2019, 95, 328–333. [Google Scholar] [CrossRef]
- Sarr, M.G. 2012 Revision of the Atlanta Classification of Acute Pancreatitis. Pol. Arch. Intern. Med. 2013, 123, 118–124. [Google Scholar] [CrossRef]
- Hartmann, J.; Werge, M.; Schmidt, P.N.; Hansen, E.F.; Pedersen, U.G.; Kristiansen, K.T.; Gluud, L.L.; Novovic, S. Modified Marshall Score Predicts Mortality in Patients With Walled-off Pancreatic Necrosis Treated in an Intensive Care Unit. Pancreas 2019, 48, e68–e70. [Google Scholar] [CrossRef]
- Valentin, C.; Le Cosquer, G.; Tuyeras, G.; Culetto, A.; Barange, K.; Hervieu, P.E.; Carrère, N.; Muscari, F.; Mokrane, F.; Otal, P.; et al. Step-up Approach for the Treatment of Infected Necrotising Pancreatitis: Real Life Data from a Single-Centre Experience with Long-Term Follow-Up. BMC Gastroenterol. 2024, 24, 213. [Google Scholar] [CrossRef]
- Wiley, M.B.; Mehrotra, K.; Bauer, J.; Yazici, C.; Bialkowska, A.B.; Jung, B. Acute Pancreatitis: Current Clinical Approaches, Molecular Pathophysiology, and Potential Therapeutics. Pancreas 2023, 52, e335. [Google Scholar] [CrossRef]
- Grigorescu, B.-L.; Săplăcan, I.; Bordea, I.R.; Petrisor, M.; Coman, O.; Puiac, C.I.; Toncean, A.; Fodor, R.S. Endogenous Carboxyhemoglobin Level Variation in COVID-19 and Bacterial Sepsis: A Novel Approach? Microorganisms 2022, 10, 305. [Google Scholar] [CrossRef]
- Grigorescu, B.-L.; Coman, O.; Văsieșiu, A.M.; Bacârea, A.; Petrișor, M.; Săplăcan, I.; Fodor, R.Ș. Is Carboxyhaemoglobin an Effective Bedside Prognostic Tool for Sepsis and Septic Shock Patients? J. Crit. Care Med. 2023, 9, 239–251. [Google Scholar] [CrossRef]
- Tóth, K.; Császár, A.; Márton, S. Akut Hasnyálmirigy Gyulladás Császármetszést Követően. Orvosi. Hetilap. 2023, 164, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.H.; Shin, B.S.; Nam, J.Y.; Kang, E.A.; Kim, J.S.; Hwang, J.H.; Kim, J. A Case of Idiopathic Severe Acute Pancreatitis Following Cesarean Section Delivery. Korean J. Gastroenterol. 2016, 68, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Ni, X.; Gan, M.; Xie, B.; Xie, Y.; Wang, Q.; Meng, L.; He, C.; Chen, J.; Wang, X. Treatment and Diagnosis of Hyperlipidemia Acute Pancreatitis in Pregnancy Associated with Pre-pregnancy Obesity and Diabetes: A Case Report. Exp. Ther. Med. 2023, 26, 573. [Google Scholar] [CrossRef] [PubMed]
- Mądro, A. Pancreatitis in Pregnancy—Comprehensive Review. Int. J. Environ. Res. Public Health 2022, 19, 16179. [Google Scholar] [CrossRef]
- Shiddapur, G.; Agarwal, S.; Samal, A.; Sai Hareeswar, Y. An Unusual Cause of Pancreatitis: Eclampsia. Cureus 2024, 16, e71342. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chao, A.-S.; Chang, Y.-L.; Peng, H.-H.; Wang, T.-H.; Chao, A. Acute Pancreatitis Secondary to Primary Hyperparathyroidism in a Postpartum Patient: A Case Report and Literature Review. Taiwan. J. Obstet. Gynecol. 2014, 53, 252–255. [Google Scholar] [CrossRef]
- Jallouli, A.; Baba, H.; Zeroual, A.; Ramraoui, M.E.-S.; Elguazzar, A.; Lahkim, M.; Khader, A.E.; Barni, R.E. Pancréatite Aigüe Idiopathique Du Post-Partum: Difficultés Diagnostiques (à Propos d’un Cas). Pan. Afr. Med. J. 2022, 41, 48. [Google Scholar] [CrossRef]
- Poo, Z.X.; Sim, W.S.; Tan, L.K. Unexpected Case of Postnatal Pancreatitis: First Presentation of Autoimmune Diabetes. BMJ Case Rep. 2022, 15, e253133. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.G.; Holbrook, B.D.; Sobel, L.; Rappaport, V.J. Hyperparathyroidism in Pregnancy Leading to Pancreatitis and Preeclampsia with Severe Features. Case Rep. Obstet Gynecol. 2017, 2017, 6061313. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Prakash, B.; Perisetti, A.; Umapathy, C.; Gupta, V.; Collins, L.; Rawla, P.; Loganathan, P.; Dwivedi, A.; Dodoo, C.; et al. Predictors and outcomes of acute respiratory failure in hospitalised patients with acute pancreatitis. Frontline Gastroenterol. 2020, 12, 478–486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veres, M.; Flamind Oltean, S.; Pascanu, S.; Butiulca, M.; Branea, O.E.; Lazar, A.E.; Grigorescu, B.L. Too Late to Reverse: An Atypical Postpartum Case of Acute Necrotizing Pancreatitis with Refractory ARDS Despite ECMO Support. Life 2025, 15, 1347. https://doi.org/10.3390/life15091347
Veres M, Flamind Oltean S, Pascanu S, Butiulca M, Branea OE, Lazar AE, Grigorescu BL. Too Late to Reverse: An Atypical Postpartum Case of Acute Necrotizing Pancreatitis with Refractory ARDS Despite ECMO Support. Life. 2025; 15(9):1347. https://doi.org/10.3390/life15091347
Chicago/Turabian StyleVeres, Mihaly, Sanziana Flamind Oltean, Sorin Pascanu, Mihaela Butiulca, Oana Elena Branea, Alexandra Elena Lazar, and Bianca Liana Grigorescu. 2025. "Too Late to Reverse: An Atypical Postpartum Case of Acute Necrotizing Pancreatitis with Refractory ARDS Despite ECMO Support" Life 15, no. 9: 1347. https://doi.org/10.3390/life15091347
APA StyleVeres, M., Flamind Oltean, S., Pascanu, S., Butiulca, M., Branea, O. E., Lazar, A. E., & Grigorescu, B. L. (2025). Too Late to Reverse: An Atypical Postpartum Case of Acute Necrotizing Pancreatitis with Refractory ARDS Despite ECMO Support. Life, 15(9), 1347. https://doi.org/10.3390/life15091347






