Pregnancy Under Pressure: Oxidative Stress as a Common Thread in Maternal Disorders
Abstract
1. Introduction
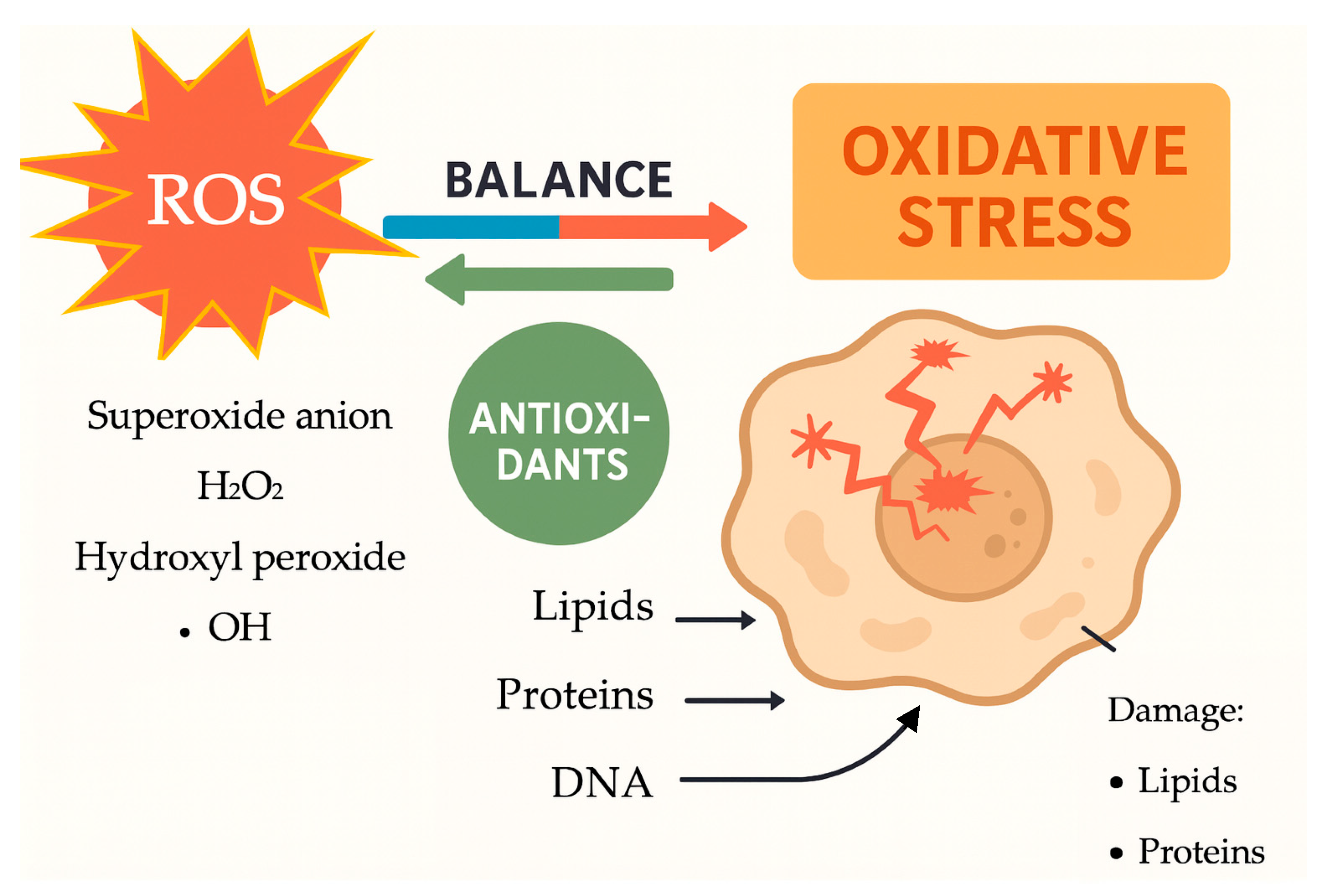
- ROS target polyunsaturated fatty acids in cellular membranes through lipid peroxidation, generating lipid radicals and end-products like malondialdehyde (MDA) that damage membrane integrity, increase cell permeability, and promote inflammation. In pregnancy, increased lipid peroxidation signifies oxidative stress, linked to conditions such as PE and FGR;
- Proteins can be altered by ROS, affecting their structure and function. This can lead to a loss of enzyme activity, disrupted signaling, and impaired cellular processes. For instance, oxidative damage to antioxidant enzymes like superoxide dismutase (SOD) and catalase can reduce the cell’s ability to neutralize ROS, starting a self-perpetuating cycle of oxidative damage;
- ROS can cause oxidative damage to DNA, leading to strand breaks, base modifications, and cross-linking, which can result in mutations and impaired cellular function. This DNA damage, caused by ROS, contributes to placental aging during pregnancy and has been linked to complications like RPL.
2. Materials and Methods
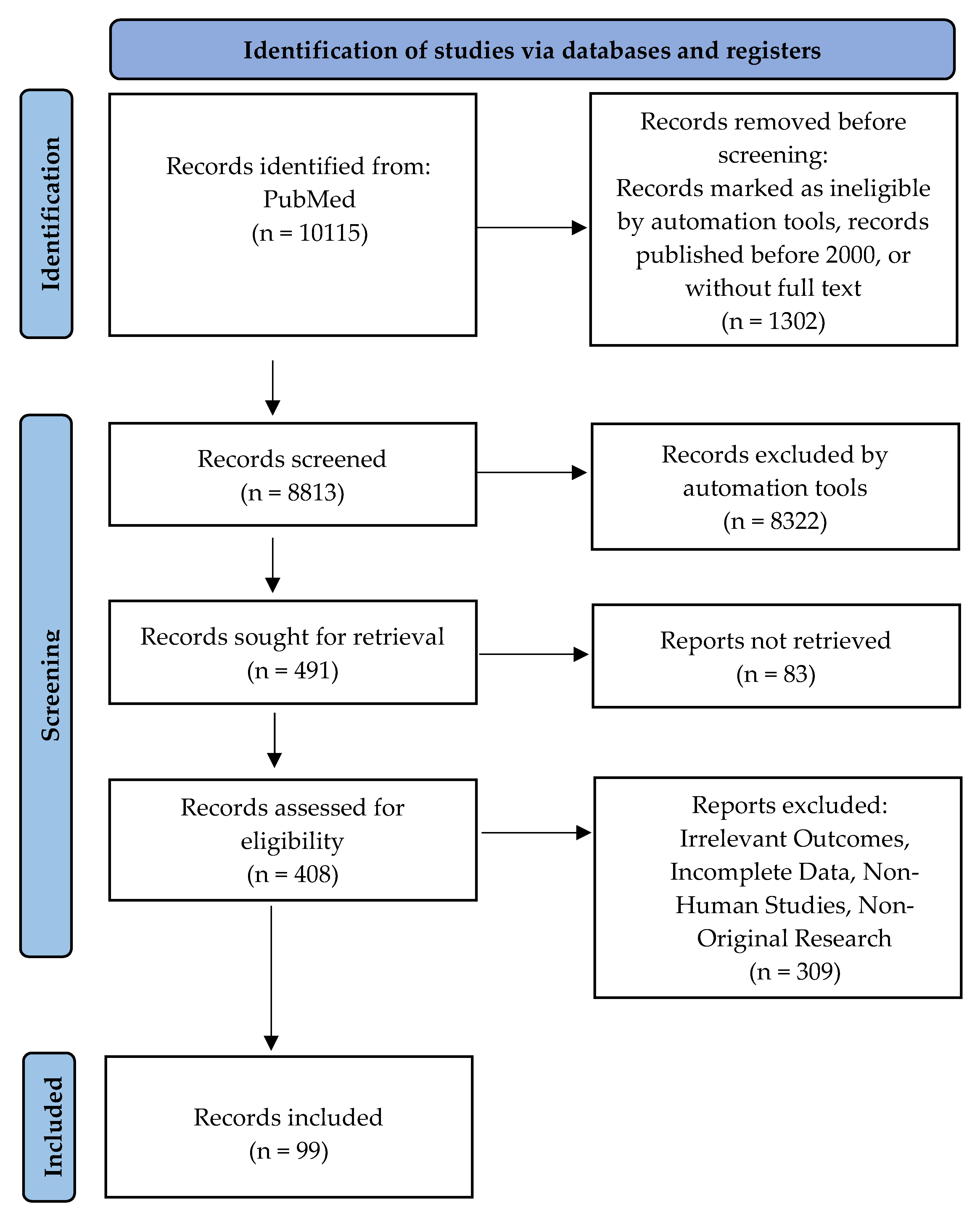
2.1. Search Strategy
2.2. Inclusion Criteria and Exclusion Criteria
- Research Type: Original studies, clinical trials, and systematic reviews in peer-reviewed journals;
- Population: Research on human participants, particularly pregnant women with complications related to oxidative stress, including preeclampsia, gestational diabetes, fetal growth restriction, or recurrent pregnancy loss;
- Content: Research on oxidative stress biomarkers, mechanisms, and antioxidant therapies, as well as clinical outcomes related to oxidative stress during pregnancy;
- Publication Date: Studies published within the past 25 years (2000–2025).Research studies were eliminated based on specific criteria:
- Irrelevant Outcomes: Articles that focus on oxidative stress in non-pregnancy settings or in populations other than pregnant women;
- Incomplete Data: Studies that lack sufficient information on oxidative stress markers, clinical outcomes, or intervention details;
- Non-Human Studies: Research conducted exclusively on animals or in vitro, without direct relevance to human clinical applications;
- Non-Original Research: We excluded editorials, opinion pieces, and case reports.
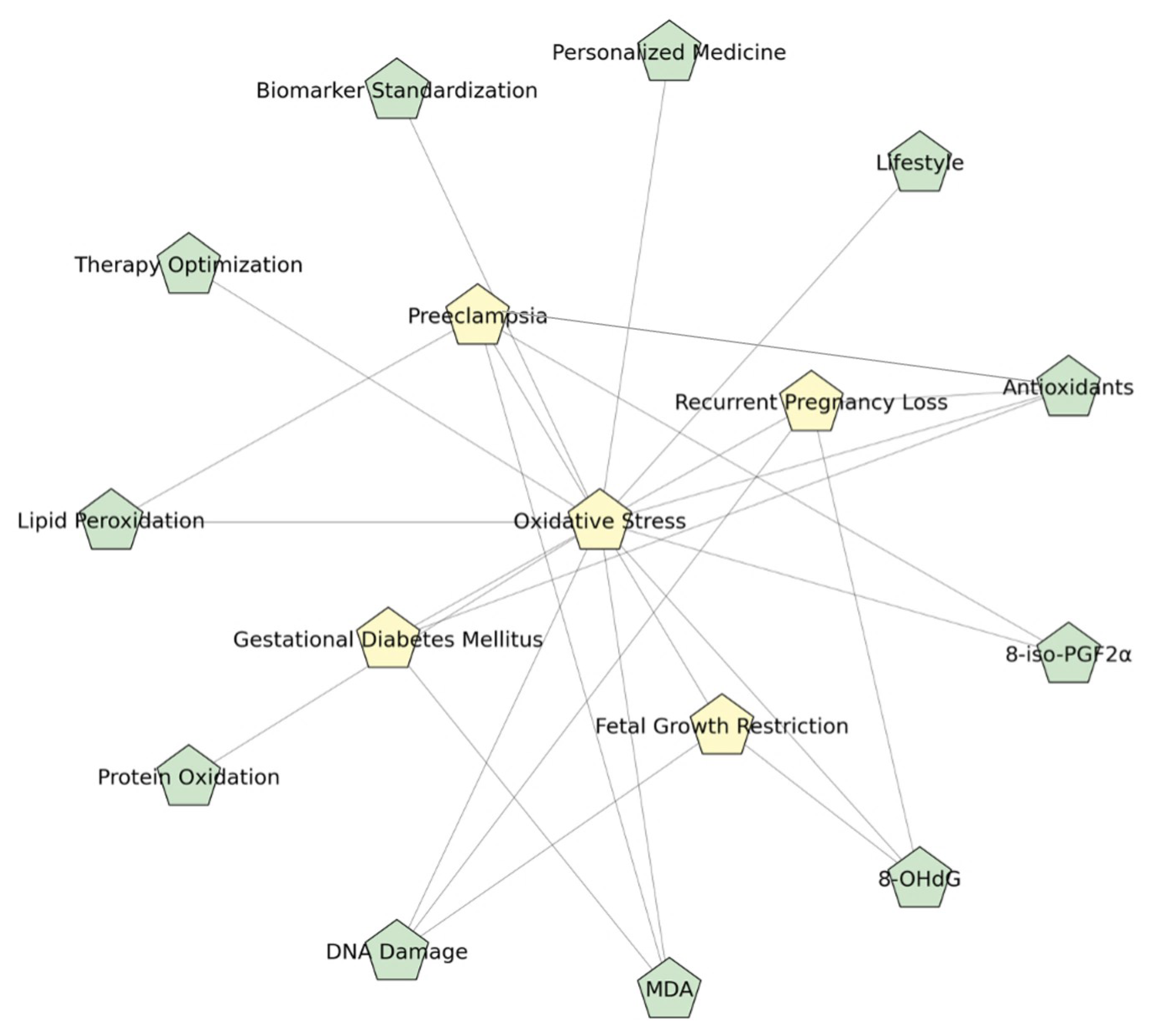
3. Understanding Oxidative Stress Mechanisms During Pregnancy
3.1. The Connection Between Lipid Peroxidation and Pregnancy-Related Hypertension
3.2. Advances in Clinical Insights on Angiogenic Imbalance and Oxidative Biomarkers in Preeclampsia
3.3. The Relationship Between 8-iso-Prostaglandin F2α and Preeclampsia
3.4. Lifestyle Factors Influencing Oxidative Stress During Pregnancy
3.5. Additional Pathways Associated with Oxidative Stress
4. Pregnancy Complications Associated with Oxidative Stress
4.1. Preeclampsia
4.2. Gestational Diabetes Mellitus
4.3. Fetal Growth Restriction
4.4. Recurrent Pregnancy Loss
5. Interventions for Antioxidant Support During Pregnancy
5.1. Commonly Studied Antioxidants
5.2. Evidence-Based Clinical Studies on Antioxidant Therapy
6. Recognizing Research Gaps and the Importance of Standardizing Biomarkers
6.1. Standardizing Oxidative Stress Biomarkers for Improved Clinical Diagnosis
6.2. The Significance of Tailored Antioxidant Treatment
6.3. Fine-Tuning the Dose and Timing of Antioxidant Treatment
6.4. Future Research Recommendations
- Standardization of biomarker quantification is critical for ensuring reproducibility and clinical utility. Implementing uniform protocols for assessing oxidative stress biomarkers such as MDA, 8-OHdG, and AOPPs will facilitate more accurate inter-study comparisons, establish definitive oxidative stress thresholds, and improve patient stratification for targeted antioxidant therapies;
- Small sample sizes and heterogeneity in study methodologies predominantly constrain existing literature on antioxidant therapy in pregnancy. To establish robust evidence for efficacy, large-scale randomized controlled trials (RCTs) adopting standardized protocols and evaluating clinical endpoints—such as reductions in PE, GDM, FGR, and RPL—are essential;
- Tailoring antioxidant interventions based on biomarker profiles and genetic predispositions has the potential to enhance therapeutic efficacy. Future research should focus on identifying prognostic biomarkers and genetic markers capable of predicting antioxidant requirements, thereby facilitating a precision medicine approach to optimize pregnancy outcomes.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D.A. Comprehensive Review of Free Radicals, Oxidative Stress, and Antioxidants: Overview, Clinical Applications, Global Perspectives, Future Directions, and Mechanisms of Antioxidant Activity of Flavonoid Compounds. J. Chem. 2024, 2024, 5594386. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility—A clinician’s perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khoo, M.I.; Ismail, E.H.E.; Hussain, N.H.N.; Zin, A.A.M.; Noordin, L.; Abdullah, S.; Mahdy, Z.A.; Lah, N. Oxidative stress biomarkers in pregnancy: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 93. [Google Scholar] [CrossRef] [PubMed]
- Boldeanu, L.; Dijmărescu, A.L.; Radu, M.; Siloşi, C.A.; Popescu-Drigă, M.V.; Poenariu, I.S.; Siloşi, I.; Boldeanu, M.V.; Novac, M.B.; Novac, L.V. The role of mediating factors involved in angiogenesis during implantation. Rom. J. Morphol. Embryol. Rev. Roum. De Morphol. Et Embryol. 2020, 61, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, Y.; Yang, Y.; Du, Z.; Fan, Y.; Zhao, Y.; Yuan, S. Oxidative stress on vessels at the maternal-fetal interface for female reproductive system disorders: Update. Front. Endocrinol. 2023, 14, 1118121. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Lorzadeh, N.; Kazemirad, Y.; Kazemirad, N. Investigating the preventive effect of vitamins C and E on preeclampsia in nulliparous pregnant women. J. Perinat. Med. 2020, 48, 625–629. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Sultana, Z.; Qiao, Y.; Maiti, K.; Smith, R. Involvement of oxidative stress in placental dysfunction, the pathophysiology of fetal death and pregnancy disorders. Reproduction 2023, 166, R25–R38. [Google Scholar] [CrossRef]
- Zejnullahu, V.A.; Zejnullahu, V.A.; Kosumi, E. The role of oxidative stress in patients with recurrent pregnancy loss: A review. Reprod. Health 2021, 18, 207. [Google Scholar] [CrossRef]
- Guerby, P.; Vidal, F.; Garoby-Salom, S.; Vayssiere, C.; Salvayre, R.; Parant, O.; Negre-Salvayre, A. Oxidative stress and preeclampsia: A review. Gynecol. Obs. Fertil. 2015, 43, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Bozkaya, V.; Oskovi-Kaplan, Z.A.; Erel, O.; Keskin, L.H. Anemia in pregnancy: It’s effect on oxidative stress and cardiac parameters. J. Matern. Fetal Neonatal Med. 2021, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Toboła, K.; Pietryga, M.; Dydowicz, P.; Szukalska, M.; Brązert, J.; Florek, E. Association of Oxidative Stress on Pregnancy. Oxid. Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Jaleel, A.; Tamimi, W.; Al Kadri, H.M. Role of oxidative stress in the pathogenesis of preeclampsia. Arch. Gynecol. Obstet. 2010, 282, 469–474. [Google Scholar] [CrossRef]
- Drejza, M.A.; Rylewicz, K.; Majcherek, E.; Gross-Tyrkin, K.; Mizgier, M.; Plagens-Rotman, K.; Wójcik, M.; Panecka-Mysza, K.; Pisarska-Krawczyk, M.; Kędzia, W.; et al. Markers of Oxidative Stress in Obstetrics and Gynaecology-A Systematic Literature Review. Antioxidants 2022, 11, 1477. [Google Scholar] [CrossRef]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, J.; Yang, L.; Feng, X.; Yuan, X. Oxidative stress and its role in recurrent pregnancy loss: Mechanisms and implications. J. Mol. Histol. 2024, 56, 55. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; González, M.C.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Tasta, O.; Swiader, A.; Grazide, M.H.; Rouahi, M.; Parant, O.; Vayssière, C.; Bujold, E.; Salvayre, R.; Guerby, P.; Negre-Salvayre, A. A role for 4-hydroxy-2-nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction. Free Radic. Biol. Med. 2021, 164, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Phoswa, W.N.; Khaliq, O.P. The Role of Oxidative Stress in Hypertensive Disorders of Pregnancy (Preeclampsia, Gestational Hypertension) and Metabolic Disorder of Pregnancy (Gestational Diabetes Mellitus). Oxid. Med. Cell. Longev. 2021, 2021, 5581570. [Google Scholar] [CrossRef]
- Chapple, S.J.; Cheng, X.; Mann, G.E. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013, 1, 319–331. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; de Oliveira, A.C.M.; Goulart, M.O.F. Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia. Oxid. Med. Cell. Longev. 2019, 2019, 8238727. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. Inflammasome activity regulation by PUFA metabolites. Front. Immunol. 2024, 15, 1452749. [Google Scholar] [CrossRef]
- Hung, T.H.; Burton, G.J. Hypoxia and reoxygenation: A possible mechanism for placental oxidative stress in preeclampsia. Taiwan. J. Obstet. Gynecol. 2006, 45, 189–200. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Zhang, L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef]
- Petkova-Parlapanska, K.; Kostadinova-Slavova, D.; Angelova, M.; Sadi, J.A.-D.R.; Georgieva, E.; Goycheva, P.; Karamalakova, Y.; Nikolova, G. Oxidative Stress and Antioxidant Status in Pregnant Women with Gestational Diabetes Mellitus and Late-Onset Complication of Pre-Eclampsia. Int. J. Mol. Sci. 2025, 26, 3605. [Google Scholar] [CrossRef]
- Taravati, A.; Tohidi, F. Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan. J. Obstet. Gynecol. 2018, 57, 779–790. [Google Scholar] [CrossRef]
- Onovughakpo-Sakpa, E.O.; Onyeneke, C.E.; Ayinbuomwan, E.; Atoe, K. Antioxidant and Malondialdehyde Status in Preeclampsia. Niger. J. Exp. Clin. Biosci. 2021, 9, 110–116. [Google Scholar] [CrossRef]
- Dijmărescu, A.L.; Tănase, F.; Novac, M.B.; Siminel, M.A.; Rotaru, I.; Caragea, D.C.; Manolea, M.M.; Văduva, C.C.; Boldeanu, M.V.; Boldeanu, L. Longitudinal 8-Epi-Prostaglandin F2-Alpha and Angiogenic Profile Mediator Evaluation during Pregnancy in Women with Suspected or Confirmed Pre-eclampsia. Biomedicines 2024, 12, 433. [Google Scholar] [CrossRef]
- Boldeanu, L.; Văduva, C.C.; Caragea, D.C.; Novac, M.B.; Manasia, M.; Siloși, I.; Manolea, M.M.; Boldeanu, M.V.; Dijmărescu, A.L. Association between Serum 8-Iso-Prostaglandin F2α as an Oxidative Stress Marker and Immunological Markers in a Cohort of Preeclampsia Patients. Life 2023, 13, 2242. [Google Scholar] [CrossRef] [PubMed]
- Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients 2022, 14, 5246. [Google Scholar] [CrossRef]
- Morales, E.; García-Serna, A.M.; Larqué, E.; Sánchez-Campillo, M.; Serrano-Munera, A.; Martinez-Graciá, C.; Santaella-Pascual, M.; Suárez-Martínez, C.; Vioque, J.; Noguera-Velasco, J.A.; et al. Dietary Patterns in Pregnancy and Biomarkers of Oxidative Stress in Mothers and Offspring: The NELA Birth Cohort. Front. Nutr. 2022, 9, 869357. [Google Scholar] [CrossRef] [PubMed]
- Fortis, M.F.; Fraga, L.R.; Boquett, J.A.; Kowalski, T.W.; Dutra, C.G.; Gonçalves, R.O.; Vianna, F.S.L.; Schüler-Faccini, L.; Sanseverino, M.T.V. Angiogenesis and oxidative stress-related gene variants in recurrent pregnancy loss. Reprod. Fertil. Dev. 2018, 30, 498–506. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R. Aerobic exercise training during pregnancy increases antioxidant status in nulliparous women: Secondary analysis of a controlled clinical trial. Endocrinol. Nutr. (Engl. Ed.) 2013, 60, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Chatzakis, C.; Sotiriadis, A.; Fatouros, I.G.; Jamurtas, A.Z.; Deli, C.K.; Papagianni, M.; Dinas, K.; Mastorakos, G. The Effect of Physical Exercise on Oxidation Capacity and Utero-Placental Circulation in Pregnancies with Gestational Diabetes Mellitus and Uncomplicated Pregnancies, a Pilot Study. Diagnostics 2022, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shen, J.; Fang, R.; Huang, H.; Lai, Y.; Hu, Y.; Zheng, J. The impact of environmental and dietary exposure on gestational diabetes mellitus: A comprehensive review emphasizing the role of oxidative stress. Front. Endocrinol. 2025, 16, 1393883. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Wiktor, H.; Kankofer, M.; Schmerold, I.; Dadak, A.; Lopucki, M.; Niedermüller, H. Oxidative DNA damage in placentas from normal and pre-eclamptic pregnancies. Virchows Arch. 2004, 445, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Pan, Q.; Chen, B.; Huang, Y.; Li, S.; Gou, C.; Gao, Y. Placental trophoblast aging in advanced maternal age is related to increased oxidative damage and decreased YAP. Front. Cell Dev. Biol. 2025, 13, 1479960. [Google Scholar] [CrossRef]
- Sedeek, M.; Gilbert, J.S.; LaMarca, B.B.; Sholook, M.; Chandler, D.L.; Wang, Y.; Granger, J.P. Role of Reactive Oxygen Species in Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats. Am. J. Hypertens. 2008, 21, 1152–1156. [Google Scholar] [CrossRef]
- Braekke, K.; Harsem, N.K.; Staff, A.C. Oxidative Stress and Antioxidant Status in Fetal Circulation in Preeclampsia. Pediatr. Res. 2006, 60, 560–564. [Google Scholar] [CrossRef]
- Endurance, E.O.; Akpe, C.I.; Adewole, A.S.; Onwuka, K.; Obiesi C. N Nze, P.O.; Ohiwerei, W.O. Predictive Role Of Oxidative Stress Biomarkers (Malondialdehyde, Glutathione, Catalase and Superoxide Dismutase) in Preeclamptic Pregnant Women In The Third Trimester Of Pregnancy. Int. J. Public Health 2025, 1, 221–231. [Google Scholar] [CrossRef]
- Ortega, M.A.; Garcia-Puente, L.M.; Fraile-Martinez, O.; Pekarek, T.; García-Montero, C.; Bujan, J.; Pekarek, L.; Barrena-Blázquez, S.; Gragera, R.; Rodríguez-Rojo, I.C.; et al. Oxidative Stress, Lipid Peroxidation and Ferroptosis Are Major Pathophysiological Signatures in the Placental Tissue of Women with Late-Onset Preeclampsia. Antioxidants 2024, 13, 591. [Google Scholar] [CrossRef]
- Dijmărescu, A.L.; Boldeanu, L.; Radu, M.; Rotaru, I.; Siminel, M.A.; Manolea, M.M.; Vrabie, S.C.; Novac, M.B.; Boldeanu, M.V.; Tănase, F. The potential value of diagnostic and predictive serum biomarkers for preeclampsia. Rom. J. Morphol. Embryol. = Rev. Roum. De Morphol. Et Embryol. 2021, 62, 981–989. [Google Scholar] [CrossRef]
- George, E.M.; Granger, J.P. Recent insights into the pathophysiology of preeclampsia. Expert. Rev. Obstet. Gynecol. 2010, 5, 557–566. [Google Scholar] [CrossRef]
- Shan, Y.; Guan, C.; Wang, J.; Qi, W.; Chen, A.; Liu, S. Impact of ferroptosis on preeclampsia: A review. Biomed. Pharmacother. 2023, 167, 115466. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Espino-y-Sosa, S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S.; Ruiz-Ramirez, E.; Velasco-Espin, M.; Cerda-Flores, P.; Ramirez-Gonzalez, A.; et al. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef]
- Cohen, J.M.; Beddaoui, M.; Kramer, M.S.; Platt, R.W.; Basso, O.; Kahn, S.R. Maternal Antioxidant Levels in Pregnancy and Risk of Preeclampsia and Small for Gestational Age Birth: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135192. [Google Scholar] [CrossRef]
- Yang, W.; Hu, Z.; Gu, W. Assessing the relationship between serum vitamin A, C, E, D, and B12 levels and preeclampsia. J. Matern. Fetal Neonatal Med. 2025, 38, 2466222. [Google Scholar] [CrossRef]
- Gunabalasingam, S.; De Almeida Lima Slizys, D.; Quotah, O.; Magee, L.; White, S.L.; Rigutto-Farebrother, J.; Poston, L.; Dalrymple, K.V.; Flynn, A.C. Micronutrient supplementation interventions in preconception and pregnant women at increased risk of developing pre-eclampsia: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2023, 77, 710–730. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Ushida, T.; Tano, S.; Matsuo, S.; Fuma, K.; Imai, K.; Kajiyama, H.; Kotani, T. Dietary supplements and prevention of preeclampsia. Hypertens. Res. 2025, 48, 1444–1457. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, E.L.S.S.; Fragoso, M.B.T.; de Oliveira, J.M.; Xavier, J.A.; Goulart, M.O.F.; de Oliveira, A.C.M. Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies. Antioxidants 2022, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Haidery, F.; Lambertini, L.; Tse, I.; Dodda, S.; Garcia-Ocaña, A.; Scott, D.K.; Baumel-Alterzon, S. NRF2 deficiency leads to inadequate beta cell adaptation during pregnancy and gestational diabetes. Redox Biol. 2025, 81, 103566. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Zhao, J.; Yang, L.; Lin, L. Oxidative stress and antioxidant status in women with gestational diabetes mellitus diagnosed by IADPSG criteria. Diabetes Res. Clin. Pract. 2015, 109, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Arribas, L.; Almansa, I.; Miranda, M.; Muriach, M.; Romero, F.J.; Villar, V.M. Serum Malondialdehyde Concentration and Glutathione Peroxidase Activity in a Longitudinal Study of Gestational Diabetes. PLoS ONE 2016, 11, e0155353. [Google Scholar] [CrossRef]
- Ruiz-Martínez, M.L.; Gómez-Díaz, R.A.; Valdez González, A.L.; Ángeles Mejía, S.; Mondragón González, R.; Díaz Flores, M.; Saldaña Espinoza, R.C.; Ramírez-García, L.A.; Díaz Velázquez, M.F.; Wacher, N.H. Association of Oxidative Stress Markers with Incident Hyperglycemia in Gestational Diabetes Mellitus in an Educational Intervention. Nutrients 2025, 17, 680. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Prasad, K.; Lemos, J.R.N.; Arevalo, G.; Hirani, K. Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management. Int. J. Mol. Sci. 2025, 26, 2320. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Z.; Ren, J.; Ma, L. Changes in Oxidative Stress Markers in Pregnant Women of Advanced Maternal Age with Gestational Diabetes and Their Predictive Value for Neurodevelopmental Impact. Diabetes Metab. Syndr. Obes. 2024, 17, 4003–4012. [Google Scholar] [CrossRef]
- Chatzakis, C.; Sotiriadis, A.; Tsakmaki, E.; Papagianni, M.; Paltoglou, G.; Dinas, K.; Mastorakos, G. The Effect of Dietary Supplements on Oxidative Stress in Pregnant Women with Gestational Diabetes Mellitus: A Network Meta-Analysis. Nutrients 2021, 13, 2284. [Google Scholar] [CrossRef]
- van der Pligt, P.; Wadley, G.D.; Lee, I.L.; Ebrahimi, S.; Spiteri, S.; Dennis, K.; Mason, S. Antioxidant Supplementation for Management of Gestational Diabetes Mellitus in Pregnancy: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Curr. Nutr. Rep. 2025, 14, 45. [Google Scholar] [CrossRef]
- Feenstra, M.E.; Bourgonje, M.F.; Bourgonje, A.R.; Schoots, M.H.; Hillebrands, J.-L.; Muller Kobold, A.C.; Prins, J.R.; van Goor, H.; Ganzevoort, W.; Gordijn, S.J. Systemic Oxidative Stress in Severe Early-Onset Fetal Growth Restriction Associates with Concomitant Pre-Eclampsia, Not with Severity of Fetal Growth Restriction. Antioxidants 2024, 13, 46. [Google Scholar] [CrossRef]
- Brooker, I.A.; Fisher, J.J.; Sutherland, J.M.; Pringle, K.G. Understanding the impact of placental oxidative and nitrative stress in pregnancies complicated by fetal growth restriction. Placenta 2024, 158, 318–328. [Google Scholar] [CrossRef]
- Tsikouras, P.; Antsaklis, P.; Nikolettos, K.; Kotanidou, S.; Kritsotaki, N.; Bothou, A.; Andreou, S.; Nalmpanti, T.; Chalkia, K.; Spanakis, V.; et al. Diagnosis, Prevention, and Management of Fetal Growth Restriction (FGR). J. Pers. Med. 2024, 14, 698. [Google Scholar] [CrossRef]
- Fujimaki, A.; Watanabe, K.; Mori, T.; Kimura, C.; Shinohara, K.; Wakatsuki, A. Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta 2011, 32, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Davenport, B.N.; Wilson, R.L.; Jones, H.N. Interventions for placental insufficiency and fetal growth restriction. Placenta 2022, 125, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.H.; Kim, Y.R.; Kim, N.; Jung, J.E.; Han, S.H.; Cho, H.Y. Effect of Endogenic and Exogenic Oxidative Stress Triggers on Adverse Pregnancy Outcomes: Preeclampsia, Fetal Growth Restriction, Gestational Diabetes Mellitus and Preterm Birth. Int. J. Mol. Sci. 2021, 22, 10122. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.R.; Miller, S.L.; Allison, B.J. The Use of Antioxidants for Cardiovascular Protection in Fetal Growth Restriction: A Systematic Review. Antioxidants 2024, 13, 1400. [Google Scholar] [CrossRef]
- Gündüz, R.; Ugur, M.G.; Bayramoglu Tepe, N.; Özcan, H.; Balat, Ö.; DemİR, S.; Taysi, S. Evaluation Of 8-Hydroxy-2-Deoxyguanosine And Malondialdehyde Levels In First-Trimester Miscarriage: A Prospective Cohort Study. Dicle Tıp Derg. 2020, 47, 74–81. [Google Scholar] [CrossRef]
- Abd El-Samee Mohammed, M.; Ahmed Sileem, S.; Abd El-Sabour Ahmed, F. ROLE OF ANTI-OXIDANT SUPPLEMENTATIONS IN RECURRENT EARLY PREGNANCY LOSS. Al-Azhar Med. J. 2020, 49, 923–930. [Google Scholar] [CrossRef]
- Davies, R.; Jayasena, C.N.; Rai, R.; Minhas, S. The Role of Seminal Oxidative Stress in Recurrent Pregnancy Loss. Antioxidants 2023, 12, 723. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chang, W.H.; Kuo, P.L.; Chen, H.C.; Chang, W.T.; Huang, P.C. Oxidative/nitrosative stress increased the risk of recurrent pregnancy loss-Taiwan Recurrent Pregnancy Loss and Environmental Study (TREPLES). Redox Biol. 2023, 68, 102940. [Google Scholar] [CrossRef]
- Câmara, F.E.A.; Sobral, D.d.S.; Neto, F.T.L.; Esteves, S.C.; Cavalcante, M.B. Seminal oxidative stress and recurrent pregnancy loss: A systematic review and meta-analysis. Reprod. Biomed. Online 2025, 51, 104873. [Google Scholar] [CrossRef]
- Khodaei, M.M.; Noori, Z.; Zare, F.; Meshkin, A. Ferroptosis and recurrent miscarriage: A critical review of pathophysiology and emerging therapeutic targets. Front. Cell Dev. Biol. 2025, 13, 1559300. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R.; Kusanovic, J.P.; Hassan, S.S. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011, 204, 503.e501–503.e512. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Appel, L.J.; Croft, K.D.; Miller, E.R., 3rd; Mori, T.A.; Puddey, I.B. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: Results of a randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 549–555. [Google Scholar] [CrossRef]
- Gallo, C.; Renzi, P.; Loizzo, S.; Loizzo, A.; Piacente, S.; Festa, M.; Caputo, M.; Tecce, M.F.; Capasso, A. Potential therapeutic effects of vitamin e and C on placental oxidative stress induced by nicotine: An in vitro evidence. Open Biochem. J. 2010, 4, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mesdaghinia, E.; Shahin, F.; Ghaderi, A.; Shahin, D.; Shariat, M.; Banafshe, H. The Effect of Selenium Supplementation on Clinical Outcomes, Metabolic Profiles, and Pulsatility Index of the Uterine Artery in High-Risk Mothers in Terms of Preeclampsia Screening with Quadruple Test: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial: Selenium and preeclampsia. Biol. Trace Elem. Res. 2023, 201, 567–576. [Google Scholar] [CrossRef]
- Biswas, K.; McLay, J.; Campbell, F. Selenium Supplementation in Pregnancy-Maternal and Newborn Outcomes. J. Nutr. Metab. 2022, 2022, 4715965. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Wilson, V.; Ramsay, M.M.; Symonds, M.E.; Pipkin, F.B. Reduced Selenium Concentrations and Glutathione Peroxidase Activity in Preeclamptic Pregnancies. Hypertension 2008, 52, 881–888. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ghanavati, M.; Ashtary-Larky, D.; Bagheri, R.; Rezaei Kelishadi, M.; Nazarian, B.; Nordvall, M.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2021, 10, 871. [Google Scholar] [CrossRef]
- Kaldygulova, L.; Ukybassova, T.; Aimagambetova, G.; Gaiday, A.; Tussupkaliyev, A. Biological Role of Folic Acid in Pregnancy and Possible Therapeutic Application for the Prevention of Preeclampsia. Biomedicines 2023, 11, 272. [Google Scholar] [CrossRef]
- Rumbold, A.; Duley, L.; Crowther, C.A.; Haslam, R.R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst. Rev. 2008, 2008, Cd004227. [Google Scholar] [CrossRef]
- Beazley, D.; Ahokas, R.; Livingston, J.; Griggs, M.; Sibai, B.M. Vitamin C and E supplementation in women at high risk for preeclampsia: A double-blind, placebo-controlled trial. Am. J. Obstet. Gynecol. 2005, 192, 520–521. [Google Scholar] [CrossRef]
- Alves, P.R.M.M.; Fragoso, M.B.T.; Tenório, M.C.S.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. The role played by oral antioxidant therapies in preventing and treating preeclampsia: An updated meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1277–1292. [Google Scholar] [CrossRef]
- Amin, A.F.; Shaaban, O.M.; Bediawy, M.A. N-acetyl cysteine for treatment of recurrent unexplained pregnancy loss. Reprod. Biomed. Online 2008, 17, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.; Obeagu, G. Antioxidant Supplementation and Prevention of Early Pregnancy Loss: A Narrative Review. Int. J. Curr. Res. Chem. Pharm. Sci. 2024, 11, 28–37. [Google Scholar]
- Afrose, D.; Chen, H.; Ranashinghe, A.; Liu, C.-c.; Henessy, A.; Hansbro, P.M.; McClements, L. The diagnostic potential of oxidative stress biomarkers for preeclampsia: Systematic review and meta-analysis. Biol. Sex. Differ. 2022, 13, 26. [Google Scholar] [CrossRef]
- Mansoor, M.A.; Stea, T.H.; Slettan, A.; Perera, E.; Maddumage, R.; Kottahachchi, D.; Ali, D.S.; Cabo, R.; Blomhoff, R. Impact of single nucleotide polymorphisms (SNPs) in antioxidant-enzyme genes on the concentrations of folate, homocysteine and glutathione in plasma from healthy subjects after folic acid supplementation–a randomized controlled crossover trial. Genes. Nutr. 2025, 20, 1. [Google Scholar] [CrossRef]
- Goričar, K.; Debevec, T.; Dolžan, V.; Martin, A.; Pialoux, V.; Millet, G.P.; Osredkar, D. Antioxidant and neurodevelopmental gene polymorphisms in prematurely born individuals influence hypoxia-related oxidative stress. Sci. Rep. 2024, 14, 14956. [Google Scholar] [CrossRef]
- Hu, K.; Liu, X.; Bai, H.; Zhou, M.; Jiang, C.; Fan, P. Association of CYBA C242T and superoxide dismutase 2 A16V genetic variants with preeclampsia. Int. J. Gynecol. Obstet. 2022, 158, 597–604. [Google Scholar] [CrossRef]
- Longini, M.; Belvisi, E.; Proietti, F.; Bazzini, F.; Buonocore, G.; Perrone, S. Oxidative Stress Biomarkers: Establishment of Reference Values for Isoprostanes, AOPP, and NPBI in Cord Blood. Mediat. Inflamm. 2017, 2017, 1758432. [Google Scholar] [CrossRef] [PubMed]
- Graille, M.; Wild, P.; Sauvain, J.J.; Hemmendinger, M.; Guseva Canu, I.; Hopf, N.B. Urinary 8-OHdG as a Biomarker for Oxidative Stress: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3743. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Variations of malondialdehyde in pre-eclampsia. Hypertens. Pregnancy 2016, 35, 346–349. [Google Scholar] [CrossRef]
- Tsikas, D. GC–MS and GC–MS/MS measurement of malondialdehyde (MDA) in clinical studies: Pre-analytical and clinical considerations. J. Mass. Spectrom. Adv. Clin. Lab. 2023, 30, 10–24. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidants in Personalized Nutrition and Exercise. Adv. Nutr. 2018, 9, 813–823. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Gill, C.A.; Kurlak, L.O.; Seed, P.T.; Hesketh, J.E.; Méplan, C.; Schomburg, L.; Chappell, L.C.; Morgan, L.; Poston, L. Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks’ gestation in nulliparous women who subsequently develop preeclampsia. Free Radic. Biol. Med. 2015, 78, 147–155. [Google Scholar] [CrossRef]
- McDougall, A.R.; Dore, G.; Aboud, L.; Makama, M.; Nguyen, P.Y.; Mills, K.; Sanderson, B.; Hastie, R.; Ammerdorffer, A.; Vogel, J.P. The effect of selenium supplementation in pregnant women on maternal, fetal, and newborn outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 101160. [Google Scholar] [CrossRef] [PubMed]
- Cilar Budler, L.; Spevan, M.; Ivanisevic, K.; Budler, M. Evidence-Based Recommendations for Dietary Supplementation During Pregnancy: Is It Time for a Product Recall? Reprod. Female Child Health 2025, 4, e70011. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Di Fabrizio, C.; Giorgione, V.; Khalil, A.; Murdoch, C.E. Antioxidants in Pregnancy: Do We Really Need More Trials? Antioxidants 2022, 11, 812. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assani, A.-D.; Boldeanu, L.; Siloși, I.; Boldeanu, M.V.; Dijmărescu, A.L.; Assani, M.-Z.; Manolea, M.-M.; Văduva, C.-C. Pregnancy Under Pressure: Oxidative Stress as a Common Thread in Maternal Disorders. Life 2025, 15, 1348. https://doi.org/10.3390/life15091348
Assani A-D, Boldeanu L, Siloși I, Boldeanu MV, Dijmărescu AL, Assani M-Z, Manolea M-M, Văduva C-C. Pregnancy Under Pressure: Oxidative Stress as a Common Thread in Maternal Disorders. Life. 2025; 15(9):1348. https://doi.org/10.3390/life15091348
Chicago/Turabian StyleAssani, Alexandru-Dan, Lidia Boldeanu, Isabela Siloși, Mihail Virgil Boldeanu, Anda Lorena Dijmărescu, Mohamed-Zakaria Assani, Maria-Magdalena Manolea, and Constantin-Cristian Văduva. 2025. "Pregnancy Under Pressure: Oxidative Stress as a Common Thread in Maternal Disorders" Life 15, no. 9: 1348. https://doi.org/10.3390/life15091348
APA StyleAssani, A.-D., Boldeanu, L., Siloși, I., Boldeanu, M. V., Dijmărescu, A. L., Assani, M.-Z., Manolea, M.-M., & Văduva, C.-C. (2025). Pregnancy Under Pressure: Oxidative Stress as a Common Thread in Maternal Disorders. Life, 15(9), 1348. https://doi.org/10.3390/life15091348







