Abstract
Staphylococcus spp. skin colonization is involved in the pathogenesis of atopic dermatitis (AD). While coagulase-positive Staphylococcus aureus strains are known to worsen symptoms, the role of coagulase-negative staphylococci (CoNS) remains controversial. Further research is needed to clarify the pathogenicity of CoNS in AD patients. A study involving 329 children with AD (mean age: 4.89 years) assessed the frequency of staphylococcal colonization on affected skin, along with the toxin-producing properties and antibiotic resistance of isolated strains. Mild AD: Predominantly colonized by CoNS (especially S. epidermidis). Moderate/Severe AD: Showed a significant increase in S. aureus colonization. CoNS (including S. epidermidis) could produce enterotoxins (A, B, C) and toxic shock syndrome toxin-1 (TSST-1), though less frequently than S. aureus strains. In severe AD, the number of toxin-producing CoNS strains (especially enterotoxin A producers) was higher than in mild AD, and the number of non-toxin-producing strains was lower. CoNS exhibited higher resistance rates than S. aureus. Methicillin-resistant S. epidermidis (MRSE): 23.4%. Methicillin-resistant S. aureus (MRSA): 1.27%. CoNS may contribute to AD pathogenesis through toxin production (exacerbating inflammation) and antibiotic resistance (limiting treatment options). Severe AD may involve a synergistic effect between S. aureus and toxin-producing CoNS.
1. Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disorder with a complex, heterogeneous etiology, involving impaired skin barrier function, intradermal and systemic T-lymphocyte activation, and increased susceptibility to cutaneous infections [1]. Typically, skin of AD patients is colonized by Staphylococcus aureus, a coagulase-positive staphylococcus (CoPS) [2,3]. S. aureus exacerbates AD through expression of virulence factors that trigger disease flares [4], contribution to chronic relapsing course of the disease, and potential resistance to anti-inflammatory corticosteroid treatment [5]. Factors promoting S. aureus colonization in AD include Th2/Th17 cytokines overexpression, dysregulation of antimicrobial peptides (e.g., HNP1 and β-defensins), microbial dysbiosis, and skin barrier defects [6]. Bacterial toxins further perpetuate inflammation by altering interleukin secretion, creating a self-sustaining inflammatory cycle [7]. CoNS such as Staphylococcus epidermidis also colonize patients with AD, but the role of these bacteria in the disease remains poorly understood [8].
S. epidermidis is a key component of the normal skin microbiota. During AD flares, S. epidermidis colonization increases compared to other commensals [9,10], suggesting a potential compensatory role in suppressing S. aureus overgrowth. S. epidermidis can modulate host immune responses and prevent pathogenic microorganism invasion by stimulating keratinocytes to produce endogenous antimicrobial peptides and by independently secreting bacteriocins with a potent antimicrobial activity [11]. In murine AD models, S. epidermidis-derived vesicles downregulate pro-inflammatory genes (TNFα, IL1β, IL6, IL8 and iNOS), upregulate human β-defensins 2 and 3, and enhance resistance to S. aureus colonization [12]. Additionally, S. epidermidis produces specific compounds such as serine proteases and phenol-soluble modulins that inhibit biofilm formation and restrict S. aureus colony growth [13]. These observations support the potential protective role of CoNS in AD [14].
Despite its beneficial roles, S. epidermidis possesses virulence factors that may exacerbate AD. It has the ability to produce enterotoxins that function as superantigens [15]. Pathogenicity islands, containing genes for staphylococcal enterotoxin B (SEB), have been identified in S. epidermidis phages [16]. Staphylococcal superantigens can stimulate Th2 lymphocytes to produce interleukin (IL)-31, which suppress filaggrin expression, increase pro-inflammatory cytokines production, activate basophils, and induce intense pruritus [17,18]. Staphylococcal enterotoxins may also act as allergens [19]. Clinical studies have shown positive correlations between S. epidermidis colonization density and serum anti-SEB IgE levels [20]. Nevertheless, no studies have directly confirmed enterotoxin production by CoNS strains isolated from AD patients’ skin. The potential adverse effects of S. epidermidis and other CoNS on the course of AD can be aggravated by their multi-drug-resistant properties [21,22]. Our study aims to provide a more detailed investigation of these potentially harmful characteristics of CoNS and compare them with those of S. aureus strains in a pediatric cohort of AD patients.
2. Materials and Methods
We conducted an observational cross-sectional study at the outpatient department of the University Children’s Clinical Hospital, First Moscow State Medical University. Potential participants were initially identified by pediatricians, with subsequent evaluation by allergists and a dermatologists to confirm AD diagnosis using the Hanifin and Rajka criteria and assess eligibility.
The study included children aged 2 to 18 years with either recently or previously diagnosed AD, regardless of disease duration, severity, or comorbid allergic conditions. Key inclusion criteria required visible AD lesions in both antecubital fossae. Patients were divided into three groups (mild AD, moderate AD, and severe AD) based on their SCORAD (SCORing Atopic Dermatitis) index, a standardized clinical tool used to objectively measure the severity of AD by evaluating three key aspects: extent, intensity, and subjective symptoms [23]. Group definitions were mild AD (SCORAD ≤ 25), moderate AD (25–50), and severe AD (≥50). Exclusion criteria comprised the following: age < 2 years; active skin infection; immunodeficiency disorders, recent (within 1 month) use of immunosuppressants (including oral corticosteroids), systemic/topical antibiotics, topical anti-inflammatory medications, or moisturizers.
Trained allergist/dermatologists obtained bilateral antecubital fossa swabs for Staphylococcus spp. identification. Samples were processed using MicroScan WalkAway plus System (Beckman-Coulter, Inc., Brea, CA, USA) for microb ial identification and antibiotic susceptibility testing.
Isolated staphylococcal strains were placed on a liquid nutrient medium—Casman’s salt composition medium with our modifications. The acidic casein hydrolysate (Difco) was replaced with an enzymatic casein hydrolysate from the Gamaleya Research Centre of Epidemiology and Microbiology, Moscow, Russian Federation, and 1% BHI was added. Subsequent cultivation was performed on a rotary shaker at 210 rpm for 24 h at 37 °C. For cultivation, 50 mL tubes were used, into which 4.5 mL of culture medium was added. Bacterial cells were removed by centrifugation 9218× g for 15 min (centrifuge Janetzki K-24, fixed-angle rotor 6 × 35 mL) and obtained supernatant was heated for 30 min at 100 °C. Detection of staphylococcal enterotoxin C (SEC) was conducted using a double diffusion method in a gel with monospecific serum to the SEC [24]. Detection of staphylococcal enterotoxin A (SEA), and staphylococcal enterotoxin B (SEB) was determined with an enzyme-linked immunoassay test kit with a sensitivity of 2.0 ng/mL for SEA and 1.0 ng/mL for SEB [25,26]. Toxic shock syndrome toxin 1 (TSST-1) was determined by using an enzyme immunoassay kit with a sensitivity of 10.0 ng/mL [27].
We evaluated colonization patterns (CoNS vs. CoPS), toxin production profiles, and antibiotic resistance rates with comparative analysis across AD severity groups.
3. Results
3.1. Microbial Isolation Patterns
Our study included 329 children with AD (median age 4.89 years, IQR 2–18). Staphylococcus spp. colonization was identified in 244 participants (74.4%), yielding 300 isolated staphylococcal strains:
- ○
- S. aureus: 160 strains (53.3%).
- ○
- CoNS: 140 strains (46.6%):
- ▪
- S. epidermidis: 87 (29.0%);
- ▪
- S. haemolyticus: 22 (7.3%);
- ▪
- S. hominis: 15 (5.0%);
- ▪
- S. capitis: 7 (2.3%);
- ▪
- S. warneri: 5 (1.7%);
- ▪
- S. cohnii: 2 (0.7%);
- ▪
- Single isolates of S. simulans and S. saprophyticus.
We detected poly-staphylococcal colonization in 52 patients (15.8%), with the following associations:
- ○
- Most frequent: S. aureus + S. epidermidis (n = 29);
- ○
- Moderate frequency: S. epidermidis + S. haemolyticus (n = 5), S. aureus + S. haemolyticus (n = 4), S. epidermidis + S. hominis (n = 4);
- ○
- Rare associations (n = 1 each): S. aureus + S. saprophyticus/capitis, S. epidermidis + S. warneri/cohnii/capitis, S. haemolyticus + S. capitis;
- ○
- Triple colonization: S. aureus + S. epidermidis + S. haemolyticus/hominis.
No staphylococci were isolated from 85 AD cases (25.8%).
Mean SCORAD index in patients with S. aureus skin colonization was 54.0 ± 4.9, with CoNS skin colonization was 41.4 ± 6.3, and with no staphylococcal growth was 43.2 ± 6.4. In patients with S. aureus + CoNS co-colonization, the mean SCORAD index was 51.7 ± 4.6. Statistical comparisons revealed significantly higher SCORAD among patients with S. aureus skin colonization compared with CoNS skin colonization or no staphylococcal growth (p = 0.0066 and p = 0.011).
Among patients with mild AD, CoNS represented the predominant skin colonizers (p < 0.05). In moderate AD cases, we observed comparable detection rates of CoNS and S. aureus (p < 0.05). The colonization pattern shifted markedly in severe AD, where S. aureus became the predominant species (p < 0.05). Statistical analysis revealed two significant trends in the severe AD subgroup: a progressive decline in CoNS detection alongside a concurrent increase in S. aureus colonization (both p < 0.05). Patients showing no staphylococcal growth were predominantly diagnosed with mild AD (Table 1).

Table 1.
Demographics of AD patients and Staphylococcus spp. skin colonization.
3.2. Toxin-Producing Properties of CoNS vs. CoPS
The toxin-producing properties of 83 staphylococcal strains were investigated, including 32 CoPS (S. aureus) and 51 CoNS. The CoNS group comprised 30 S. epidermidis, 8 S. haemolyticus, 7 S. hominis, 3 S. warneri, 2 S. capitis, and 1 S. simulans strain. All strains exhibited the ability to produce at least one toxin, with many strains producing multiple toxins simultaneously.
Among S. aureus strains, 78.1% (25/32) produced more than one toxin: 18.75% (6/32) synthesized two toxins, 46.9% (15/32) three, and 12.5% (4/32) expressed all four tested toxins. Similarly, 47.0% (24/51) of CoNS strains produced several toxins: 9.8% (5/51) produced two, 31.4% (16/51) three, and 5.8% (3/51) all four toxins. Non-toxigenic strains were observed in 9.0% (3/32) of S. aureus and 15.6% (8/51) of CoNS isolates, with no statistically significant difference (p > 0.05).
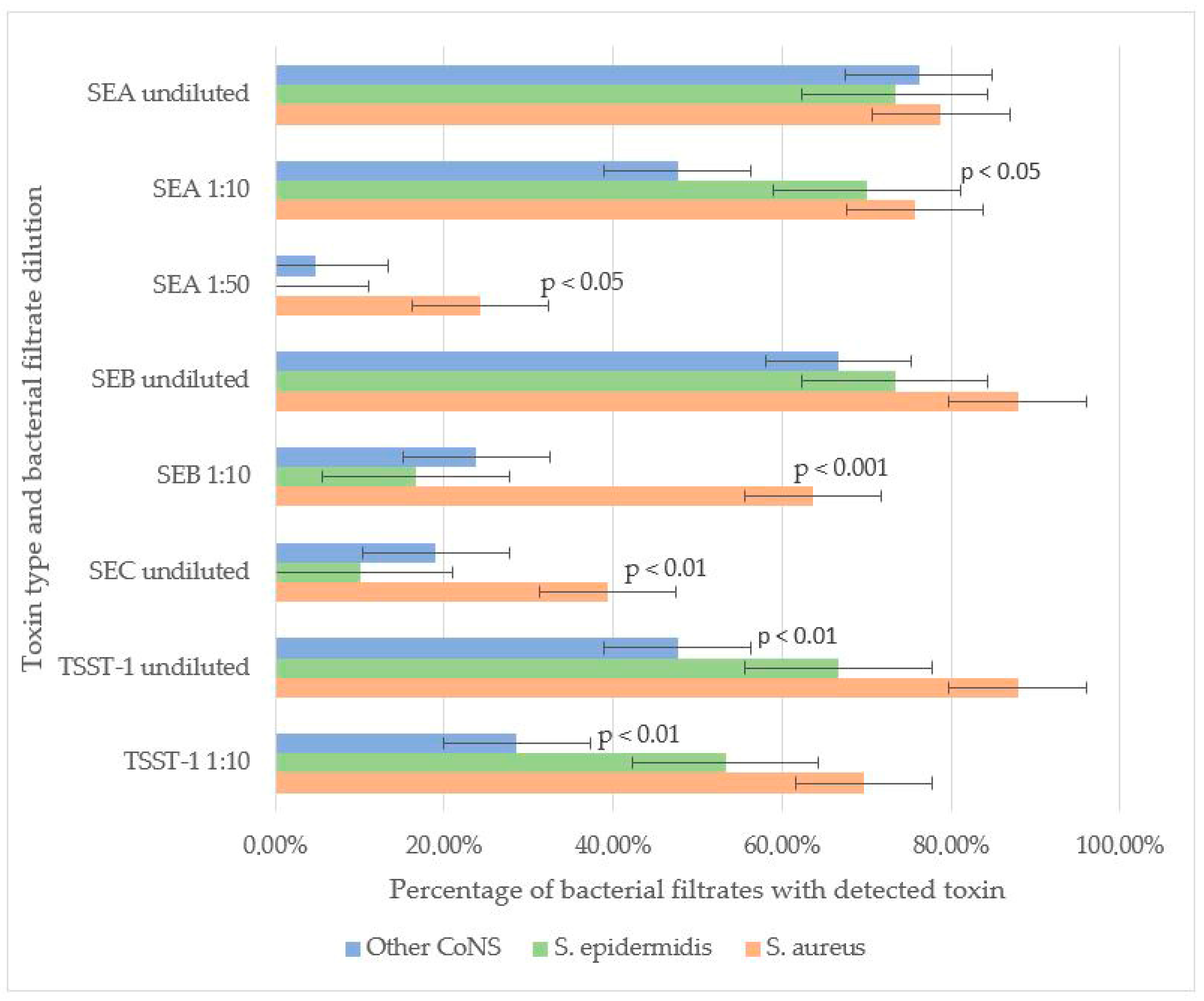
SEB and TSST-1 were the most frequently detected toxins in staphylococcal filtrates. In undiluted samples, SEB was identified in 87.9% of S. aureus, 73.3% of S. epidermidis, and 66.7% of other CoNS strains, with no significant difference in detection rates. However, at a 1:10 dilution, SEB was significantly more prevalent in S. aureus (63.6%) compared to S. epidermidis (16.6%) and other CoNS (23.8%) (p < 0.001). These results suggest that, under laboratory conditions, S. aureus may produce higher amounts of SEB compared to other staphylococcal strains.
For TSST-1, no significant difference was observed between S. aureus (87.9% undiluted, 69.7% at 1:10) and S. epidermidis (66.7% undiluted, 53.3% at 1:10), indicating comparable production levels. However, other CoNS strains exhibited significantly lower TSST-1 detection rates (47.61% undiluted, 28.57% at 1:10) compared to S. aureus (p < 0.01). Neither SEB nor TSST-1 was detectable in the filtrates of staphylococci strains at a 1:50 dilution.
SEA was the second most prevalent toxin. No significant differences were observed in undiluted filtrates among S. aureus (78.8%), S. epidermidis (73.3%), and other CoNS (76.2%). At a 1:10 dilution, SEA remained highly detectable in S. aureus (75.6%) and S. epidermidis (70.0%), but its prevalence dropped in other CoNS (47.6%, p < 0.05 vs. S. aureus). Notably, in S. aureus filtrates SEA remained detectable even at 1:50 dilution (24.2%), whereas in S. epidermidis filtrates SEA was not detected at this dilution. In a single S. simulans strain filtrate (4.7% of other CoNS), SEA was detected at 1:50, suggesting that some CoNS strains can generate SEA in quantities comparable to S. aureus.
SEC was the least frequently detected toxin. It was more prevalent in S. aureus (39.4%) than in S. epidermidis (10.0%, p < 0.01) or other CoNS (19.0%). SEC detection in diluted filtrates was not performed (Figure 1).
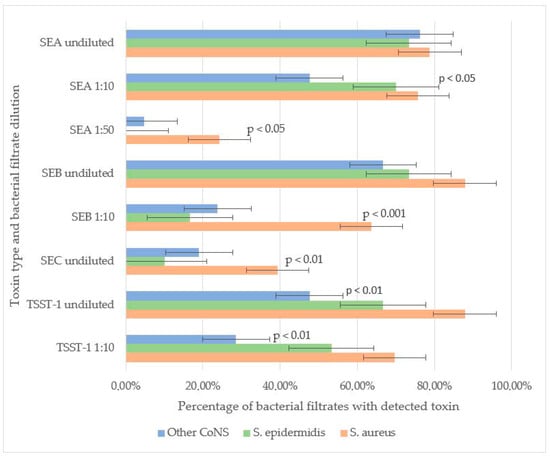
Figure 1.
Toxin-producing properties of Staphylococcus spp. SEA was detected at similar frequencies in undiluted bacterial filtrates among all staphylococci. In the 1:10 dilution, SEA was detected at similar frequencies in S. aureus and S. epidermidis strains, and less frequently among other CoNS species (p < 0.05). In the 1:50 dilution, SEA was detected more frequently in S. aureus strains compared to CoNS, including S. epidermidis (p < 0.05). SEB was detected at similar frequencies in undiluted filtrates. In the 1:10 dilution, it was detected more frequently in S. aureus strains compared to CoNS, including S. epidermidis (p < 0.001). SEC was detected more frequently in S. aureus filtrates compared to S. epidermidis filtrates (p < 0.01). TSST-1 was detected at similar frequencies in undiluted filtrates and in the 1:10 dilution of both S. aureus and S. epidermidis. However, other CoNS strains exhibited significantly lower TSST-1 detection rates (in both undiluted and 1:10 filtrates) compared to S. aureus (p < 0.01).
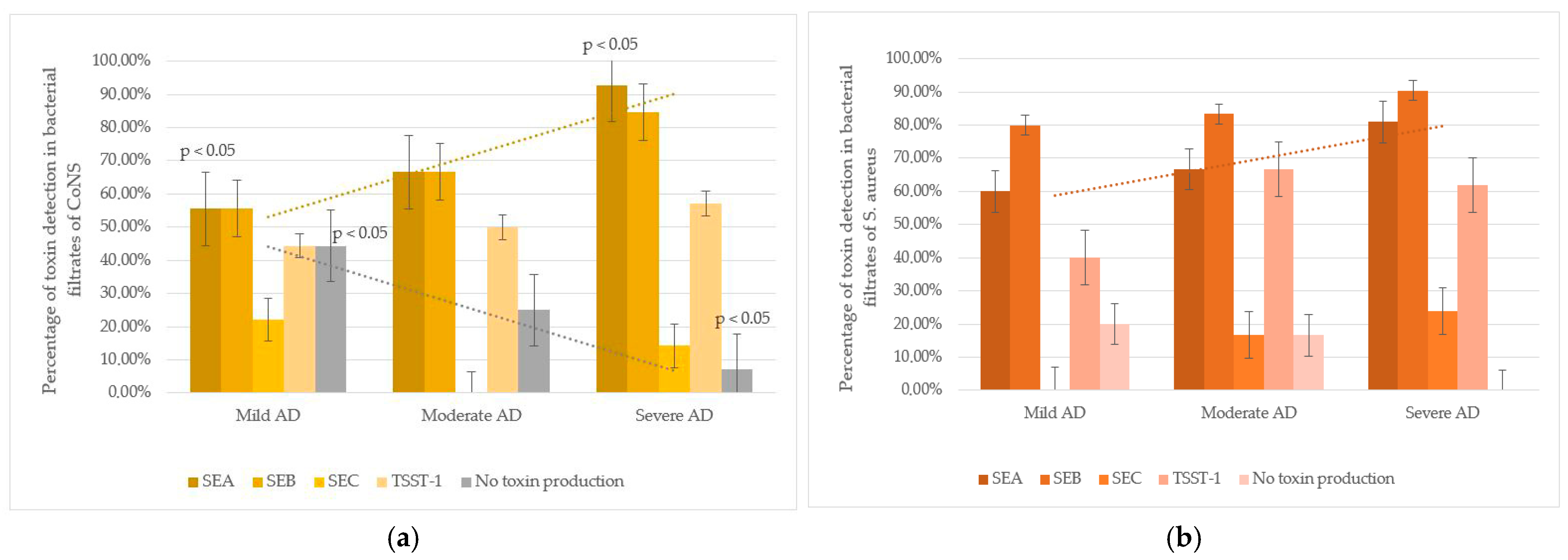
An intriguing trend was observed in CoNS strains: the frequency of SEA detection in culture filtrates was higher in severe AD subgroup compared to mild AD. In mild AD, 53.8% (7/13) of CoNS strains produced SEA; in moderate AD, 61.1% (11/18); and in severe AD, 90.0% (18/20). The difference between mild and severe AD cases was statistically significant (p < 0.05). Additionally, non-toxigenic CoNS strains, in whose culture filtrates none of the tested toxins were detected, were more frequent in mild AD (44.4%, 4/9) than in moderate (25.0%, 3/12) or severe AD (7.1%, 1/14), with a significant difference between mild and severe cases (p < 0.05). These findings suggest that severe AD is associated with higher colonization by SEA-producing CoNS and lower colonization by non-toxigenic strains (Figure 2).
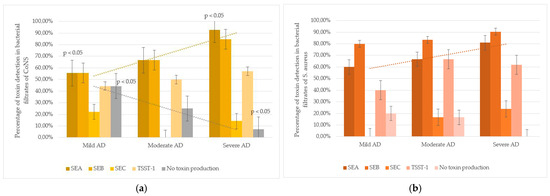
Figure 2.
Staphylococcus spp. toxins production and AD severity: (a) CoNS strains (including S. epidermidis): toxin-producing properties were studied in 51 strains (mild AD—13, moderate AD—18, severe AD—20). In mild AD, CoNS produced SEA less often compared to severe AD group (p < 0.05). The number of non-toxin-producing strains was significantly higher among mild AD compared to severe AD (p < 0.05). (b) S. aureus strains: toxin-producing properties were studied in 32 strains (mild AD—5, moderate AD—6, severe AD—21). The strains had pronounced toxic properties, regardless of the severity of the disease.
3.3. Antibiotic Susceptibility of CoNS vs. CoPS
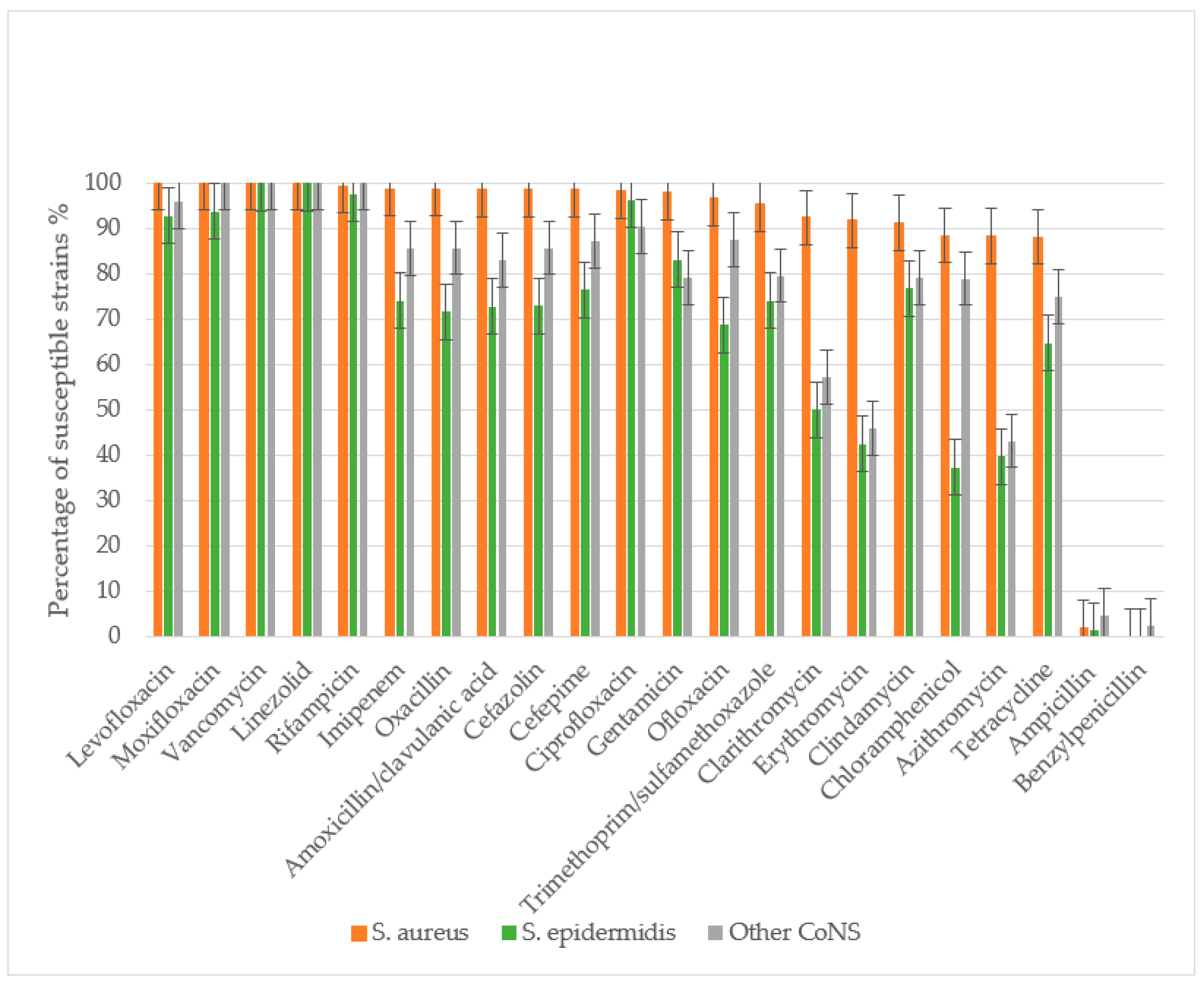
The antibacterial susceptibility of 160 S. aureus strains, 87 S. epidermidis strains, and 53 other CoNS strains (excluding S. epidermidis) was evaluated. The study assessed sensitivity to the following antibacterial agents: β-lactam antibiotics including penicillins (benzylpenicillin, ampicillin, amoxicillin-clavulanate, oxacillin), cephalosporins (cefazolin, cefepime), and carbapenems (imipenem); macrolides (azithromycin, clarithromycin, erythromycin); fluoroquinolones (ciprofloxacin, ofloxacin, levofloxacin, moxifloxacin); glycopeptides (vancomycin); lincosamides (clindamycin); rifamycins (rifampicin); tetracyclines (tetracycline); amphenicols (chloramphenicol); sulfonamides with trimethoprim (co-trimoxazole); aminoglycosides (gentamicin); and oxazolidinones (linezolid) (Figure 3).
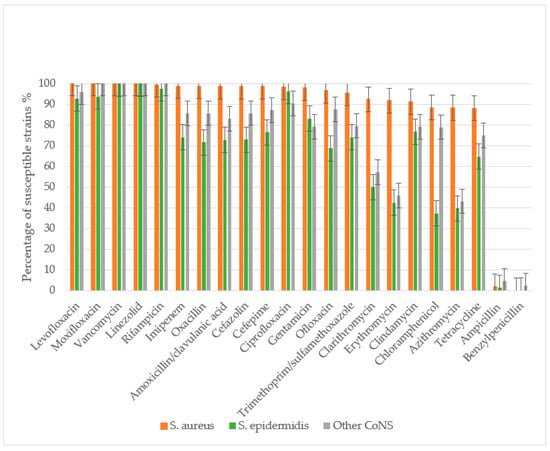
Figure 3.
Staphylococcus spp. antibiotic susceptibility in descending order for S. aureus. S. aureus demonstrated absolute sensitivity to vancomycin, linezolid, and later-generation fluoroquinolones (levofloxacin and moxifloxacin), with a low MRSA prevalence of 1.27% as determined by oxacillin resistance. S. epidermidis showed significantly more resistant properties to most antibiotics, including β-lactams, macrolides, cephalosporins, tetracyclines, aminoglycosides, lincosamides, and fluoroquinolones (p < 0.05). The incidence of MRSE was 28.4%.
All S. aureus and S. epidermidis strains demonstrated complete resistance to benzylpenicillin, with only minimal sensitivity retained to ampicillin. Resistance rates to ampicillin were 97.9% for S. aureus, 98.9% for S. epidermidis, and 95.9% for other CoNS. S. aureus maintained high sensitivity to amoxicillin/clavulanate, with only 1.38% of strains showing resistance. The prevalence of MRSA was low at 1.27%. In contrast, S. epidermidis and other CoNS exhibited significantly higher resistance to amoxicillin/clavulanate (27.2% and 17.03%, respectively), with methicillin-resistant strains reaching 28.4% (MRSE) and 14.2% (p < 0.01 compared to S. aureus).
S. aureus showed high susceptibility to cephalosporins, with resistance to both cefazolin and cefepime observed in only 1.27% of strains. Significantly higher resistance rates were noted in CoNS: 27.0% (S. epidermidis) and 14.3% (other CoNS) to cefazolin, and 23.5% and 12.8% to cefepime, respectively.
CoNS demonstrated substantially greater resistance to macrolides than S. aureus (p < 0.01). Resistance rates among S. epidermidis ranged from 50% (clarithromycin) to 60.3% (azithromycin), while other CoNS showed 42.8–56.8% resistance across macrolides. In contrast, S. aureus resistance remained low (8.16–11.62%), though still higher than its resistance to β-lactam/β-lactamase inhibitor combinations and cephalosporins.
For fluoroquinolones, S. aureus maintained high susceptibility, with only 1.86–3% resistance to second-generation agents (ofloxacin, ciprofloxacin) and complete sensitivity to third- and fourth-generation fluoroquinolones (levofloxacin, moxifloxacin). CoNS showed significantly higher resistance, particularly S. epidermidis (31.25% to ofloxacin). Resistance to newer fluoroquinolones was lower (3.71–7.32% in S. epidermidis, 4.17–12.5% in other CoNS), with all CoNS remaining susceptible to moxifloxacin.
All staphylococcal strains retained sensitivity to reserve antibiotics vancomycin and linezolid. Resistance to rifampicin and imipenem was rare in S. aureus (0.63–1.26%) but more prevalent in CoNS (25.97% of S. epidermidis and 14.59% of other CoNS to imipenem). While 2.46% of S. epidermidis showed rifampicin resistance, other CoNS maintained complete susceptibility.
CoNS exhibited significantly greater resistance to most antibiotic classes (p < 0.05), including β-lactams, macrolides, cephalosporins, tetracyclines, aminoglycosides, lincosamides, and fluoroquinolones. No association was found between disease severity (mild, moderate, severe) and antibiotic resistance patterns for any staphylococcal species when tested against all studied antimicrobial agents.
4. Discussion
Our study revealed distinct patterns of staphylococcal colonization in AD patients. S. aureus colonization was significantly more prevalent in severe AD cases, while CoNS strains, particularly S. epidermidis, dominated in mild AD. Although CoNS strains were less frequently detected on the skin of severe AD patients, they retained the ability to produce toxins. For certain toxins (SEA), the detection frequency in culture filtrates of strains isolated from severe AD patients was higher than in strains from mild AD cases. This suggests that despite the lower isolation rate of CoNS in severe AD, they may play a significant role in sustaining inflammation through toxin production.
CoNS strains isolated from the skin of children with AD, regardless of disease severity, demonstrated the ability to produce enterotoxins in vitro. Most CoNS strains produced multiple enterotoxins simultaneously. The number of CoNS strains with no detectable toxins in their culture filtrates was slightly higher compared to S. aureus, though this difference was not statistically significant. SEB and TSST-1 were the most frequently detected toxins in culture filtrates of both S. aureus and S. epidermidis, occurring with equal frequency across strains. SEB was detected in 1:10 dilutions of bacterial filtrates from a larger proportion of S. aureus strains. The maximum dilution at which TSST-1 was detectable was 1:10 for both S. aureus and CoNS, with equal detection frequency in S. aureus and S. epidermidis, but less frequently in other CoNS species.
SEA was detected less frequently than SEB and TSST-1. In undiluted culture filtrates, it occurred equally in S. aureus and CoNS. At 1:10 dilution, it was less frequent in CoNS except S. epidermidis. At 1:50 dilution, SEA was detectable only in S. aureus filtrates and a single S. simulans strain. This indicates that in vitro, CoNS strains from AD patients generally produce less enterotoxin A than S. aureus, though certain strains produce quantities comparable to S. aureus. The proportion of CoNS strains with detectable SEA was statistically higher in the severe AD group. Conversely, the number of CoNS strains with no detectable toxins was significantly lower in severe AD.
SEC was the least frequently detected toxin in undiluted filtrates. It occurred significantly more often in S. aureus filtrates than in CoNS.
Our data align with previous studies confirming that enterotoxigenic potential of CoNS may influence the disease course. In a 2017 study by Hon, K. L et al. in a pediatric population, it was demonstrated that serum anti-SEB IgE levels were positively associated with S. aureus and/or S. epidermidis skin isolation and objective SCORAD, as well as with clinical signs and quality of life [20]. Toxin production is not the only mechanism negatively influencing AD course. While traditionally viewed as a natural antagonist of S. aureus, certain S. epidermidis strains appear less capable of inhibiting S. aureus virulence in AD patients [28]. Genomic analyses reveal strain-specific differences in histopathological potential, antibiotic resistance profiles (including methicillin resistance), and immunomodulatory capacity [29].
Phylogenetic studies show striking differences between staphylococcal species in AD: while S. aureus exhibits clonal expansion, S. epidermidis demonstrates remarkable phylogenetic diversity across all disease stages. Mild AD cases predominantly harbor clades A29 and A30 strains, contrasting with the A20 dominance in healthy adults [30]. Notably, AD skin often lacks protective CoNS strains (S. epidermidis and S. hominis) that produce anti-S. aureus antimicrobial peptides [29]. Instead, AD-derived S. epidermidis strains exhibit pathogenic potential through multiple mechanisms. S. epidermidis strains isolated from lesional AD skin exhibit distinct pathogenic properties that significantly alter epidermal structure and function. Unlike commensal strains from healthy skin, AD-associated preferentially activates STAT6 while suppressing the protective AhR/OVOL1 pathway, accompanied by significantly reduced indole production [31]. These changes lead to marked downregulation of key differentiation markers, including filaggrin and desmoglein-1, compromising epidermal barrier function. AD-derived S. epidermidis strains exhibit other proinflammatory properties. Production of cytotoxic phenol-soluble modulins that show a strong positive correlation with disease severity (r = 0.78, p < 0.001) [32]. Secretion of the cysteine protease EcpA, which effectively degrades both desmoglein-1 (by 62 ± 8%) and the antimicrobial peptide LL-37 in vitro, thereby impairing physical barrier function and promoting skin inflammation [33]. Generation of extracellular serine protease that triggers IL-13 activation (3.5-fold increase) and drives a Th2-polarized immune response, characteristic of AD pathogenesis [34]. These findings collectively demonstrate that specific S. epidermidis strains possess multiple virulence factors capable of exacerbating AD through both direct barrier disruption and immune modulation. Not all S. epidermidis strains can inhibit S. aureus biofilm formation. In some patients, these species coexist, forming mixed-species biofilms that enhance their survival and pathogenicity [35].
Our study revealed a high prevalence of antibiotic resistance among CoNS. A 2017 species-level analysis of AD flares demonstrated that MRSA was more common in severe cases, whereas MRSE predominated in milder forms [28]. Supporting this, a 2023 genome-wide association (GWA) study reported MRSA in 13.79% and MRSE in 39% of cases [16]. These rates are significantly higher than we observed in our study. S. epidermidis can function as a reservoir for methicillin resistance that can be passed on to other species, including S. aureus; through the action of the recombinase genes in SCCmec, the cassette can be excised from the genome and transferred between isolates and between species. Plasmid-mediated resistance to tetracycline, streptomycin, and erythromycin can also drive dissemination from S. epidermidis to other species [36]. Given the potential influence of CoNS on the inflammatory process in AD, particularly through toxin production, we cannot ignore its multidrug resistance when selecting therapy.
5. Conclusions
This study elucidates the complex dynamics of staphylococcal colonization, virulence, and antibiotic resistance in pediatric AD. S. epidermidis may play a more significant role in AD than previously thought. Critically, CoNS strains from severe AD demonstrate enhanced enterotoxigenic activity (notably SEA production). Our results emphasize the need for further research on staphylococcal dynamics in AD and the potential for targeted microbial interventions. Specifically, alongside conventional anti-inflammatory therapy, future treatments should consider agents that modulate bacterial colonization—suppressing pathogenic strains while restoring beneficial flora during both active disease and remission. This study advances our understanding of AD etiology and may guide novel therapeutic strategies.
This study has potential limitations. The culture-based approach used in this study cannot fully capture the heterogeneity of the skin commensal flora, as it primarily targets easily cultivable microorganisms (Staphylococcus spp.). This limits our ability to assess non-culturable or fastidious organisms within the community. DNA sequencing techniques are required for comprehensive identification of difficult-to-cultivate microorganisms. WgMLST of isolated Staphylococcus spp. genomes was not performed. Such analysis is essential to confirm the prevalence and genetic context of mobile elements encoding superantigens across staphylococcal species. Putative methicillin-resistant strains identified phenotypically were not validated via mecA gene detection. This study lacks a healthy control group for comparative analysis of microbial composition and toxin prevalence. Future work will include healthy controls, prioritizing cohabiting relatives of AD patients to account for shared environmental exposures.
Author Contributions
Conceptualization, A.K. and F.F.; methodology, A.K. and F.F.; software, K.G. and S.T.; validation, A.K., F.F. and D.Z.; formal analysis, S.T.; investigation, S.T.; resources, F.F.; data curation, K.G.; writing—original draft preparation, K.G.; writing—review and editing, E.R.; visualization, A.K.; supervision, L.K., O.O. and S.M.; project administration, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the I.M. Sechenov First Moscow State Medical University (Sechenov University) (protocol code 10–19 dated 17 July 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy reasons.
Acknowledgments
We thank Yulia Savvina for carrying out laboratory work on the isolation and identification of staphylococci strains, Joachim Fluhr (Charité—Universitätsmedizin Berlin) and Daniel Munblit (I.M. Sechenov First Moscow State Medical University) for comments on the manuscript, and Laura Hamilton for discussion and assistance in preparing the text of the manuscript.
Conflicts of Interest
Author Oksana Osipenko was employed by the company Family Medical Center LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Atopic dermatitis |
| CoNS | Coagulase-negative staphylococci |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MRSE | Methicillin-resistant Staphylococcus epidermidis |
| CoPS | Coagulase-positive staphylococci |
| SEA | Staphylococcal enterotoxin A |
| SEB | Staphylococcal enterotoxin B |
| SEC | Staphylococcal enterotoxin C |
| TSST-1 | Toxic shock syndrome toxin 1 |
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Bay, L.; Barnes, C.J.; Fritz, B.G.; Ravnborg, N.; Ruge, I.F.; Halling-Sønderby, A.-S.; Søeborg, S.R.; Langhoff, K.H.; Lex, C.; Hansen, A.J.; et al. Unique dermal bacterial signature differentiates atopic dermatitis skin from healthy. Msphere 2025, 10, e0015625. [Google Scholar] [CrossRef]
- Wang, Z.; Hülpüsch, C.; Traidl-Hoffmann, C.; Reiger, M.; Schloter, M. Understanding the role of Staphylococcus aureus in atopic dermatitis: Strain diversity, microevolution, and prophage influences. Front. Med. 2024, 11, 1480257. [Google Scholar] [CrossRef]
- Özdemir, E.; Öksüz, L. Effect of Staphylococcus aureus colonization and immune defects on the pathogenesis of atopic dermatitis. Arch. Microbiol. 2024, 206, 410. [Google Scholar] [CrossRef]
- Schachner, L.A.; Andriessen, A.; Gonzalez, M.E.; Lal, K.; Hebert, A.A.; Eichenfield, L.F.; Lio, P. A Consensus on Staphylococcus aureus Exacerbated Atopic Dermatitis and the Need for a Novel Treatment. J. Drugs Dermatol. JDD 2024, 23, 825–832. [Google Scholar] [CrossRef]
- Svitich, O.A.; Soboleva, V.A.; Abramova, N.D.; Gelezhe, K.A.; Kudryavtseva, A.V. Expression of HNP1 gene in children with atopic dermatitis. Vopr. Prakt. Pediatr. 2022, 17, 31–36. [Google Scholar] [CrossRef]
- Ogonowska, P.; Gilaberte, Y.; Barańska-Rybak, W.; Nakonieczna, J. Colon. with Staphylococcus aureus in Atopic Dermatitis Patients: Attempts to Reveal the Unknown. Front. Microbiol. 2021, 11, 567090. [Google Scholar] [CrossRef]
- Edslev, S.M.; Olesen, C.M.; Nørreslet, L.B.; Ingham, A.C.; Iversen, S.; Lilje, B.; Clausen, M.L.; Jensen, J.S.; Stegger, M.; Agner, T.; et al. Staphylococcal Communities on Skin Are Associated with Atopic Dermatitis and Disease Severity. Microorganisms 2021, 9, 432. [Google Scholar] [CrossRef]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; NISC Comparative Sequence Program; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Gelezhe, K.A.; Kudryavtseva, A.V.; Svitich, O.A. Dynamics of skin colonization by staphylococcus spp. in children and adolescents with atopic dermatitis. Pediatr. J. Named After G.N. Speransky 2019, 98, 88–93. [Google Scholar] [CrossRef]
- Guimarães, L.C.; Garcia, G.D.; Cavalcante, F.S.; Dias, G.M.; de Farias, F.M.; Saintive, S.; Abad, E.D.; Ferreira, D.C.; Dos Santos, K.R.N. Methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococcus produce antimicrobial substances against members of the skin microbiota in children with atopic dermatitis. FEMS Microbiol. Ecol. 2024, 100, fiae070. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Ring, S.; Jin, S.; Singh, S.; Mahnke, K. Extracellular Vesicles and Their Role in Skin Inflammatory Diseases: From Pathogenesis to Therapy. Int. J. Mol. Sci. 2025, 26, 3827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seiti Yamada Yoshikawa, F.; Feitosa de Lima, J.; Notomi Sato, M.; Álefe Leuzzi Ramos, Y.; Aoki, V.; Leao Orfali, R. Exploring the Role of Staphylococcus aureus Toxins in Atopic Dermatitis. Toxins 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Bier, K.; Schittek, B. Beneficial effects of coagulase-negative Staphylococci on Staphylococcus aureus skin colonization. Exp. Dermatol. 2021, 30, 1442–1452. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Gajewska, J.; Wiśniewski, P.; Zadernowska, A. Enterotoxigenic Potential of Coagulase-Negative Staphylococci from Ready-to-Eat Food. Pathogens 2020, 9, 734. [Google Scholar] [CrossRef]
- Saheb Kashaf, S.; Harkins, C.P.; Deming, C.; Joglekar, P.; Conlan, S.; Holmes, C.J.; NISC Comparative Sequencing Program; Almeida, A.; Finn, R.D.; Segre, J.A.; et al. Staphylococcal diversity in atopic dermatitis from an individual to a global scale. Cell Host Microbe 2023, 31, 578–592.e6. [Google Scholar] [CrossRef]
- Nakashima, C.; Otsuka, A.; Kabashima, K. Interleukin-31 and interleukin-31 receptor: New therapeutic targets for atopic dermatitis. Exp. Dermatol. 2018, 27, 327–331. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A.; Trento, E.; Toma, L.; Pimpinelli, F.; Capitanio, B.; et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef]
- Abdurrahman, G.; Schmiedeke, F.; Bachert, C.; Bröker, B.M.; Holtfreter, S. Allergy-A New Role for T Cell Superantigens of Staphylococcus aureus? Toxins 2020, 12, 176. [Google Scholar] [CrossRef]
- Hon, K.L.; Tsang, K.Y.; Kung, J.S.; Leung, T.F.; Lam, C.W.; Wong, C.K. Clinical Signs, Staphylococcus and Atopic Eczema-Related Seromarkers. Molecules 2017, 22, 291. [Google Scholar] [CrossRef]
- Fišarová, L.; Botka, T.; Du, X.; Mašlaňová, I.; Bárdy, P.; Pantůček, R.; Benešík, M.; Roudnický, P.; Winstel, V.; Larsen, J.; et al. Staphylococcus epidermidis Phages Transduce Antimicrobial Resistance Plasmids and Mobilize Chromosomal Islands. Msphere 2021, 6, e00223-21. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2019, 68, 309–315. [Google Scholar] [CrossRef]
- Stadler, J.F. Severity scoring of atopic dermatitis: The SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar] [CrossRef]
- Fluer, F.S.; Prokhorov, V.I.; Vesnina, A.F. Enzyme immunoassay system for detection of staphylococcal enterotoxin, type C. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 2002, 6, 65–68. [Google Scholar]
- Akatov, A.K.; Fluer, F.S.; Mikheeva, G.V.; Pavlova, I.P.; Chachanina, K.L.; Bobkova, E.V.; Melnikov, N.V. Enzyme immunoassay system for detection of staphylococcal enterotoxin, type A. Temporarily Pharm. Norms Regul. 1989, 89, 42–235. [Google Scholar]
- Akatov, A.K.; Fluer, F.S.; Mikheeva, G.V.; Chachanina, K.L. Enzyme immunoassay system for detection of staphylococcal enterotoxin, type B. Temporarily Pharm. Norms Regul. 1989, 89, 42–236. [Google Scholar]
- Fluer, F.S.; Pozhar, P.F.; Ratgauz, G.L.; Akatov, A.K. A rapid method of detecting the staphylococcal exotoxin of toxic shock. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 1990, 12, 70–73. [Google Scholar]
- Zhou, Y.; Xu, X.; Liu, Y.; Wang, A.; Luo, Y.; Liu, X.; Wang, X.; Li, W.; Yao, X. Heterogeneous Regulation of Staphylococcus aureus by Different Staphylococcus epidermidis agr Types in Atopic Dermatitis. J. Investig. Dermatol. 2023, 143, 2484–2493.e11. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic der-matitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Byrd, A.L.; Deming, C.; Cassidy, S.K.B.; Harrison, O.J.; Ng, W.I.; Conlan, S.; NISC Comparative Sequencing Program; Belkaid, Y.; Segre, J.A.; Kong, H.H. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 2017, 9, eaal4651. [Google Scholar] [CrossRef]
- Landemaine, L.; Da Costa, G.; Fissier, E.; Francis, C.; Morand, S.; Verbeke, J.; Michel, M.L.; Briandet, R.; Sokol, H.; Gueniche, A.; et al. Staphylococcus epidermidis isolates from atopic or healthy skin have opposite effect on skin cells: Potential implication of the AHR pathway modulation. Front. Immunol. 2023, 14, 1098160. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Bagood, M.D.; Enroth, T.J.; Bunch, Z.L.; Jiang, N.; Liu, E.; Almoughrabie, S.; Khalil, S.; Li, F.; Brinton, S.; et al. Staphylococcus epidermidis activates keratinocyte cytokine expression and promotes skin inflammation through the production of phenol-soluble modulins. Cell Rep. 2023, 42, 113024. [Google Scholar] [CrossRef]
- Cau, L.; Williams, M.R.; Butcher, A.M.; Nakatsuji, T.; Kavanaugh, J.S.; Cheng, J.Y.; Shafiq, F.; Higbee, K.; Hata, T.R.; Horswill, A.R.; et al. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 955–966.e16. [Google Scholar] [CrossRef] [PubMed]
- Abdurrahman, G.; Pospich, R.; Steil, L.; Gesell Salazar, M.; Izquierdo González, J.J.; Normann, N.; Mrochen, D.; Scharf, C.; Völker, U.; Werfel, T.; et al. The extracellular serine protease from Staphylococcus epidermidis elicits a type 2-biased immune response in atopic dermatitis patients. Front. Immunol. 2024, 15, 1352704. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.; Stevens, M.L.; Baatyrbek Kyzy, A.; Alarcon, R.; He, H.; Kroner, J.W.; Spagna, D.; Grashel, B.; Sidler, E.; Martin, L.J.; et al. Biofilm propensity of Staphylococcus aureus skin isolates is associated with increased atopic dermatitis severity and barrier dysfunction in the MPAACH pediatric cohort. Allergy 2021, 76, 302–313. [Google Scholar] [CrossRef]
- Burke, Ó.; Zeden, M.S.; O’Gara, J.P. The pathogenicity and virulence of the opportunistic pathogen Staphylococcus epidermidis. Virulence 2024, 15, 2359483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).