Right Colectomy with Complete Mesocolic Excision and Intracorporeal Anastomosis: A Monocentric, Single-Surgeon Comparison of Dexter, DaVinci and Laparoscopic Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Variables and Definitions
2.3. Surgical Technique
2.4. Statistical Analysis
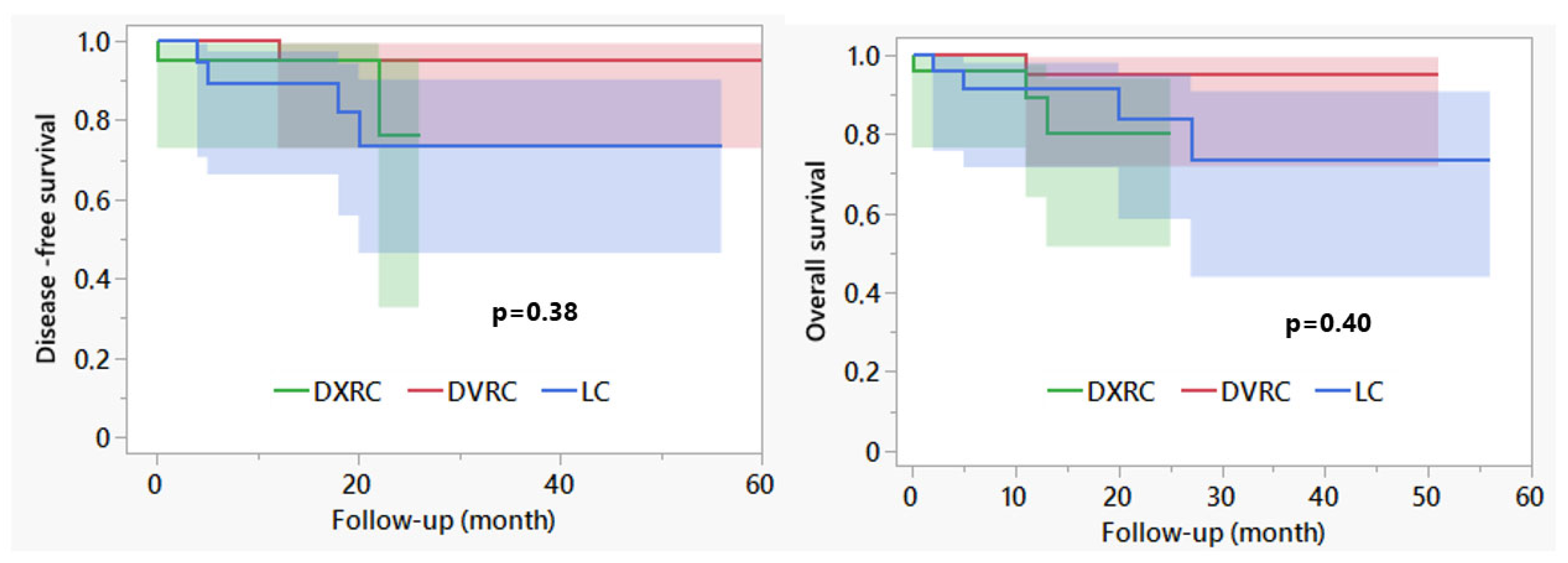
3. Results
| DXRC | DVRC | LRC | p-Value | |
|---|---|---|---|---|
| N | 25 | 25 | 25 | |
| Age | 67.1 ± 13.3 | 67.0 ± 14.7 | 68.1 ± 11.5 | 0.95 |
| Female gender | 13 (52.0) | 9 (36.0) | 13 (52.0) | 0.42 |
| BMI | 26.3 ± 4.3 | 27.1 ± 4.2 | 25.3 ± 4.2 | 0.34 |
| Pre-existing diseases | ||||
| Cardiovascular | 8 (32) | 9 (36) | 7 (28) | 0.83 |
| Arterial hypertension | 11 (44) | 6 (24) | 14 (56) | 0.07 |
| Pulmonary | 0 | 3 (12) | 2 (8) | 0.22 |
| Metabolic | 4 (16) | 5 (20) | 5 (20) | 0.92 |
| Renal | 1 (4) | 0 | 1 (4) | 0.60 |
| ASA ≥ 3 | 6 (24.0) | 7 (28.0) | 3 (12.0) | 0.36 |
| Previous abdominal surgery | 8 (32) | 11 (44) | 7 (28) | 0.47 |
| Tumor stage (UICC) | ||||
| HGIEN | 3 (12) | 2 (8) | 1 (4) | |
| I | 5 (20) | 4 (16) | 4 (16) | |
| II | 8 (32) | 9 (36) | 12 (48) | |
| III | 5 (20) | 7 (28) | 4 (16) | |
| IV | 4 (16) | 3 (12) | 4 (16) | 0.93 |
| MMR deficiency | 10 (40) | 8 (32) | 6 (24) | 0.46 |
| DXRC | DVRC | LRC | p-Value | |
|---|---|---|---|---|
| Type of anastomosis | ||||
| Extracorporeal hand-sewn | 0 | 1 (4) | 11 (44) | |
| Intracorporeal stapled | 20 (80) | 18 (72) | 14 (56) | |
| Intracorporeal hand-sewn | 5 (20) | 6 (24) | 0 | <0.01 |
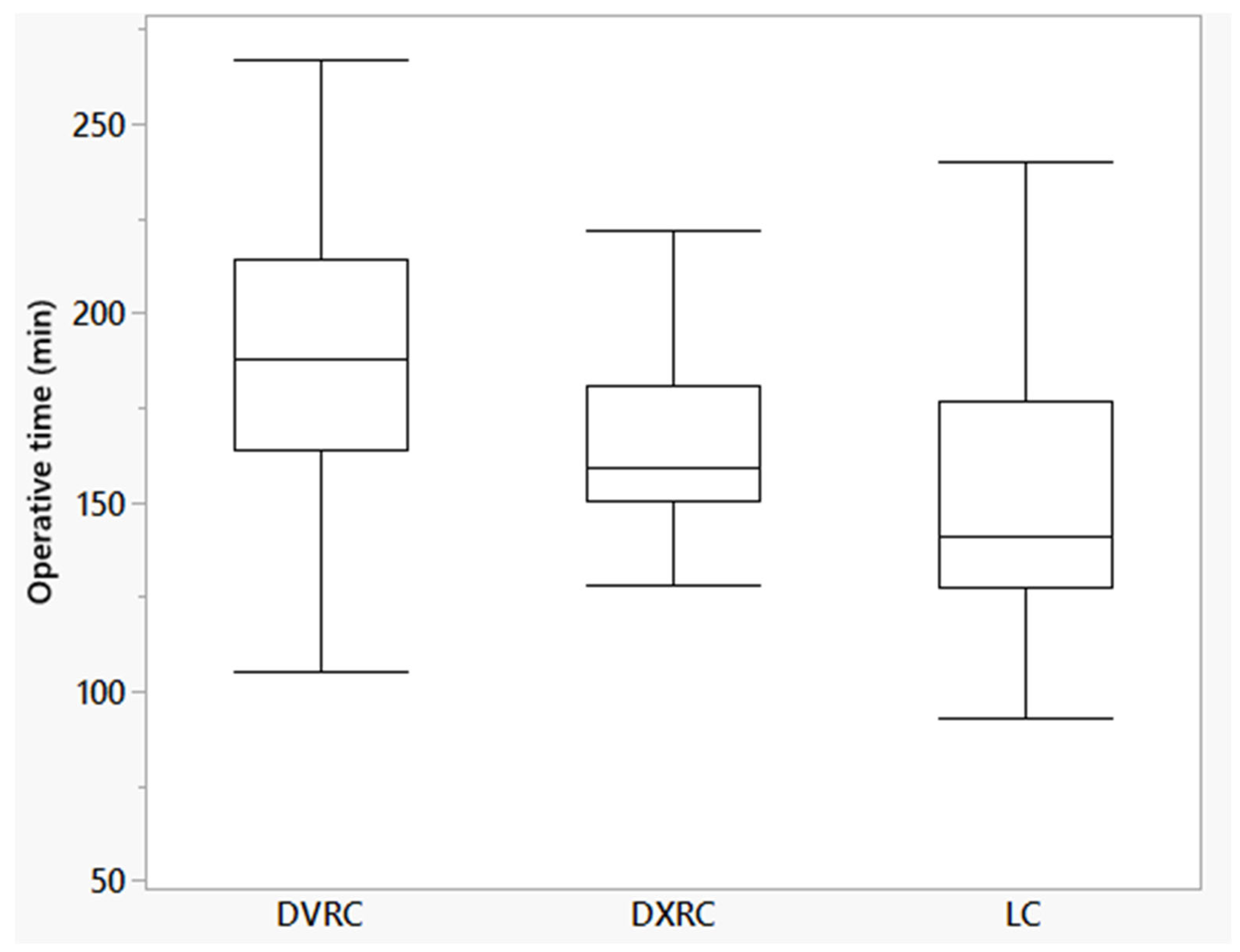
| Operative time (min [95% confidence interval]) | 164.8 (151.7–178.0) | 190.5 (177.3–203.7) | 152.6 (139.4–165-7) | <0.01 |
| Initial docking (min) | 5.6 ± 2.5 | 9.6 ± 3.4 | n/a | <0.01 |
| TAP-block | 13 (52.0) | 7 (28.0) | 5 (20.0) | 0.04 |
| Height of staples (mm) | ||||
| 1.5–2.5 (blue) | 22 (88) | 11 (44) | 18 (72) | |
| 3.0–4.0 (purple) | 3 (12) | 10 (40) | 7 (28) | |
| 1.8–3.0 (gold) | 0 | 1 (4) | 0 | |
| 4.3 (green) | 0 | 3 (12) | 0 | 0.02 |
| Conversion to open | 0 | 0 | 1 (4) | 0.88 |
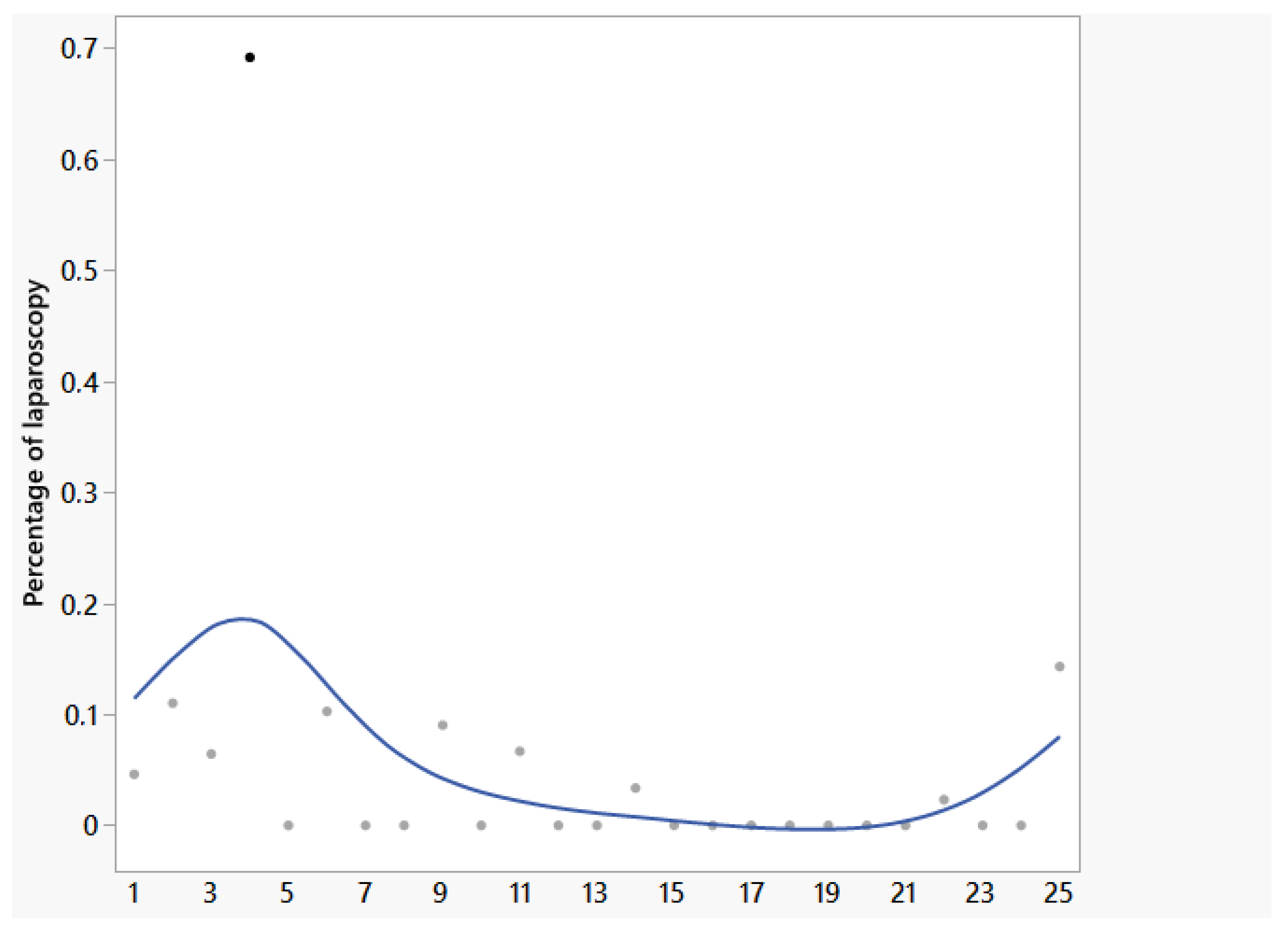
| Percentage of laparoscopy (%) | 0 (0–69) | 0 | 100 | - |
| Harvested lymph nodes | 22 (10–60) | 28 (12–46) | 26 (10–47) | 0.18 |
| CME fulfilled | 24 (96) | 22 (88) | 22 (88) | 0.53 |
| Pathological classification Benz-type 0 if CME fulfilled | 24 (100) | 20 (90.9) | 21 (95.5) | 0.32 |
| Intra-abdominal drainage | 1 (4) | 16 (64) | 6 (24) | <0.01 |
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists Physical Status System |
| BMI | Body Mass Index |
| CD | Clavien–Dindo Classification |
| CME | Complete mesocolic excision |
| HGIEN | High-grade intraepithelial neoplasia |
| MMR | Mismatch repair |
| UICC | Union Internationale Contre le Cancer |
References
- Alkatout, I.; Mechler, U.; Mettler, L.; Pape, J.; Maass, N.; Biebl, M.; Gitas, G.; Laganà, A.S.; Freytag, D. The Development of Laparoscopy-A Historical Overview. Front. Surg. 2021, 8, 799442. [Google Scholar] [CrossRef]
- Kelley, W.E. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. J. Soc. Laparoendosc. Surg. 2008, 12, 351–357. [Google Scholar]
- Buunen, M.; Veldkamp, R.; Hop, W.C.J.; Kuhry, E.; Jeekel, J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; Morino, M.; et al. Survival after laparoscopic surgery versus open surgery for colon cancer: Long-term outcome of a randomised clinical trial. Lancet Oncol. 2009, 10, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.H.; Francis, E.A.; Wharton, R.; Blazeby, J.M.; Quirke, P.; West, N.P.; Dutton, S.J. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J. Clin. Oncol. 2014, 32, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.G.; Guillou, P.J.; Thorpe, H.; Quirke, P.; Copeland, J.; Smith, A.M.H.; Heath, R.M.; Brown, J.M. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J. Clin. Oncol. 2007, 25, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Dohrn, N.; Klein, M.F.; Gögenur, I. Robotic versus laparoscopic right colectomy for colon cancer: A nationwide cohort study. Int. J. Colorectal Dis. 2021, 36, 2147–2158. [Google Scholar] [CrossRef]
- Miller, P.E.; Dao, H.; Paluvoi, N.; Bailey, M.; Margolin, D.; Shah, N.; Vargas, H.D. Comparison of 30-Day Postoperative Outcomes after Laparoscopic vs Robotic Colectomy. J. Am. Coll. Surg. 2016, 223, 369–373. [Google Scholar] [CrossRef]
- Clarke, E.M.; Rahme, J.; Larach, T.; Rajkomar, A.; Jain, A.; Hiscock, R.; Warrier, S.; Smart, P. Robotic versus laparoscopic right hemicolectomy: A retrospective cohort study of the Binational Colorectal Cancer Database. J. Robotic Surg. 2022, 16, 927–933. [Google Scholar] [CrossRef]
- Aiolfi, A.; Bona, D.; Rausa, E.; Manara, M.; Biondi, A.; Basile, F.; Campanelli, G.; Kelly, M.E.; Bonitta, G.; Bonavina, L. Effect of complete mesocolic excision (cme) on long-term survival after right colectomy for cancer: Multivariate meta-analysis and restricted mean survival time estimation. Langenbecks Arch. Surg. 2024, 409, 80. [Google Scholar] [CrossRef]
- Creavin, B.; Balasubramanian, I.; Common, M.; McCarrick, C.; El Masry, S.; Carton, E.; Faul, E. Intracorporeal vs extracorporeal anastomosis following neoplastic right hemicolectomy resection: A systematic review and meta-analysis of randomized control trials. Int. J. Colorectal Dis. 2021, 36, 645–656. [Google Scholar] [CrossRef]
- Brown, R.F.; Cleary, R.K. Intracorporeal anastomosis versus extracorporeal anastomosis for minimally invasive colectomy. J. Gastrointest. Oncol. 2020, 11, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Abou-Khalil, M.; Liberman, S.; Boutros, M.; Fried, G.M.; Feldman, L.S. Incidence of incisional hernia in the specimen extraction site for laparoscopic colorectal surgery: Systematic review and meta-analysis. Surg. Endosc. 2017, 31, 5083–5093. [Google Scholar] [CrossRef] [PubMed]
- Catanzarite, T.; Tan-Kim, J.; Whitcomb, E.L.; Menefee, S. Ergonomics in Surgery: A Review. Female Pelvic Med. Reconstr. Surg. 2018, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di Lascia, A.; Tartaglia, N.; Petruzzelli, F.; Pacilli, M.; Maddalena, F.; Fersini, A.; Pavone, G.; Vovola, F.; Ambrosi, A. Right hemicolectomy: Laparoscopic versus robotic approach. Ann. Ital. Chir. 2020, 91, 478–485. [Google Scholar]
- Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Fiasconaro, M.; Patil, S.; Widmar, M.; Wei, I.H.; Pappou, E.P.; Smith, J.J.; Nash, G.M.; et al. Comparison of Robotic, Laparoscopic, and Open Resections of Nonmetastatic Colon Cancer. Dis. Colon Rectum 2023, 66, 1347–1358. [Google Scholar] [CrossRef]
- Meyer, J.; Meyer, E.; Meurette, G.; Liot, E.; Toso, C.; Ris, F. Robotic versus laparoscopic right hemicolectomy: A systematic review of the evidence. J. Robot. Surg. 2024, 18, 116. [Google Scholar] [CrossRef]
- Marchegiani, F.; Siragusa, L.; Zadoroznyj, A.; Laterza, V.; Mangana, O.; Schena, C.; Ammendola, M.; Memeo, R.; Bianchi, P.; Spinoglio, G.; et al. New Robotic Platforms in General Surgery: What’s the Current Clinical Scenario? Medicina 2023, 59, 1264. [Google Scholar] [CrossRef]
- Hahnloser, D.; Rrupa, D.; Grass, F. Feasibility of on-demand robotics in colorectal surgery: First cases. Surg. Endosc. 2023, 37, 8594–8600. [Google Scholar] [CrossRef]
- Thillou, D.; Robin, H.; Ricolleau, C.; Benali, N.A.; Forgues, A.; Emeriau, D.; Mignot, H.; Hugues, G. Robot-assisted Radical Prostatectomy with the Dexter Robotic System: Initial Experience and Insights into On-demand Robotics. Eur. Urol. 2024, 85, 185–189. [Google Scholar] [CrossRef]
- Böhlen, D.; Gerber, R. First Ever Radical Prostatectomy Performed with the New Dexter Robotic System™. Eur. Urol. 2023, 83, 479–480. [Google Scholar] [CrossRef]
- Alkatout, I.; O’Sullivan, O.; Peters, G.; Maass, N. Expanding Robotic-Assisted Surgery in Gynecology Using the Potential of an Advanced Robotic System. Medicina 2024, 60, 53. [Google Scholar] [CrossRef]
- Alkatout, I.; Becker, T.; Nuhn, P.; Pochhammer, J.; Peters, G.; Donald, K.M.; Mettler, L.; Ackermann, J. The first robotic-assisted hysterectomy below the bikini line with the Dexter robotic system™. Facts Views Vis. ObGyn 2024, 16, 87–91. [Google Scholar] [CrossRef]
- Mignot, H.; Diack, B.; Capitaine, J.; Emeriau, D. Retrospective evaluation of a single surgeon’s experience in robot-assisted inguinal repair with the Dexter System™ during the learning curve. Int. J. Abdom. Wall Hernia Surg. 2024, 7, 75–82. [Google Scholar] [CrossRef]
- Gantner, L.; Mignot, H.; Pochhammer, J.; Grieder, F.; Breitenstein, S. Robotic minimally invasive inguinal hernia repair with the Dexter robotic system™: A prospective multicenter clinical investigation. Surg. Endosc. 2024, 38, 7647–7655. [Google Scholar] [CrossRef] [PubMed]
- Conrad, P.V.; Mehdorn, A.-S.; Alkatout, I.; Becker, T.; Beckmann, J.H.; Pochhammer, J. The Combination of Laparoscopic and Robotic Surgery: First Experience with the Dexter Robotic System™ in Visceral Surgery. Life 2024, 14, 874. [Google Scholar] [CrossRef] [PubMed]
- Strey, C.W.; Wullstein, C.; Adamina, M.; Agha, A.; Aselmann, H.; Becker, T.; Grützmann, R.; Kneist, W.; Maak, M.; Mann, B.; et al. Laparoscopic right hemicolectomy with CME: Standardization using the “critical view” concept. Surg. Endosc. 2018, 32, 5021–5030. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Horan, T.C.; Gaynes, R.P.; Martone, W.J.; Jarvis, W.R.; Emori, T.G. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Infect. Control Hosp. Epidemiol. 1992, 13, 606–608. [Google Scholar] [CrossRef]
- Benz, S. Adoption of standardized approach to right hemicolectomy with complete mesocolic excision using the critical view concept and open-book model for robotic surgery—A video vignette. Colorectal Dis. 2021, 23, 2216–2217. [Google Scholar] [CrossRef]
- Benz, S.; Tannapfel, A.; Tam, Y.; Grünenwald, A.; Vollmer, S.; Stricker, I. Proposal of a new classification system for complete mesocolic excison in right-sided colon cancer. Tech. Coloproctol. 2019, 23, 251–257. [Google Scholar] [CrossRef]
- de Lange, G.; Davies, J.; Toso, C.; Meurette, G.; Ris, F.; Meyer, J. Complete mesocolic excision for right hemicolectomy: An updated systematic review and meta-analysis. Tech. Coloproctol. 2023, 27, 979–993. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, Z.F.; Carleton, J.D.; Agarwala, A. Robotic surgery: Current perceptions and the clinical evidence. Surg. Endosc. 2017, 31, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xiong, D.; Xu, M.; Fan, Q.; Zheng, H.; Shen, H.; Huang, B.; Wang, L.; Li, C.; Zhang, A.; et al. Robotic versus laparoscopic right hemicolectomy with complete mesocolic excision: A retrospective multicenter study with propensity score matching. Front. Oncol. 2023, 13, 1187476. [Google Scholar] [CrossRef] [PubMed]
- Merola, G.; Sciuto, A.; Pirozzi, F.; Andreuccetti, J.; Pignata, G.; Corcione, F.; Milone, M.; de Palma, G.D.; Castaldo, R.; Pecchia, L.; et al. Is robotic right colectomy economically sustainable? a multicentre retrospective comparative study and cost analysis. Surg. Endosc. 2020, 34, 4041–4047. [Google Scholar] [CrossRef] [PubMed]
- Negrut, R.L.; Cote, A.; Caus, V.A.; Maghiar, A.M. Systematic Review and Meta-Analysis of Laparoscopic versus Robotic-Assisted Surgery for Colon Cancer: Efficacy, Safety, and Outcomes-A Focus on Studies from 2020-2024. Cancers 2024, 16, 1552. [Google Scholar] [CrossRef]
- Kim, H.S.; Noh, G.T.; Chung, S.S.; Lee, R.-A. Long-term oncological outcomes of robotic versus laparoscopic approaches for right colon cancer: A systematic review and meta-analysis. Tech. Coloproctol. 2023, 27, 1183–1189. [Google Scholar] [CrossRef]
- Grass, F.; Hahnloser, D. On-demand robotics-The best of both worlds for robotic-assisted laparoscopic surgery. Surgery 2024, 176, 1534–1537. [Google Scholar] [CrossRef]
- Post, S.; Vilz, T. S3-Leitlinie “Perioperatives Management bei gastrointestinalen Tumoren (POMGAT)”. Chirurgie 2023, 94, 468. [Google Scholar] [CrossRef]
| DXRC | DVRC | LRC | p-Value | |
|---|---|---|---|---|
| Length of stay | 4 (3–10) | 4 (3–48) | 5 (2–35) | 0.14 |
| Complications within 30 days | ||||
| Acute pancreatitis (CD II) | 0 | 0 | 1 (4) | |
| Intraluminal bleeding | 0 | 1 (4) | 0 | |
| Colonoscopy (IIIa) | 0 | |||
| Intraabdominal bleeding | 0 | 1 (4) | ||
| Re-laparoscopy (CD IIIb) | ||||
| Anastomotic leakage (CD IIIb) | 1 (4) | 1 (4) | 2 (8) | |
| Mortality due to cardiac arrest (CD V) | 1 (4) | 0 | 0 | |
| Surgical site infections | 1 (4) | 0 | 2 (8) | 0.35 |
| Follow-up | 8 (1–24) | 17 (1–51) | 19 (1–56) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pochhammer, J.; Franke, F.; Martin, M.; Beckmann, J.H.; Osmonov, D.; Alkatout, I.; Becker, T. Right Colectomy with Complete Mesocolic Excision and Intracorporeal Anastomosis: A Monocentric, Single-Surgeon Comparison of Dexter, DaVinci and Laparoscopic Approaches. Life 2025, 15, 1122. https://doi.org/10.3390/life15071122
Pochhammer J, Franke F, Martin M, Beckmann JH, Osmonov D, Alkatout I, Becker T. Right Colectomy with Complete Mesocolic Excision and Intracorporeal Anastomosis: A Monocentric, Single-Surgeon Comparison of Dexter, DaVinci and Laparoscopic Approaches. Life. 2025; 15(7):1122. https://doi.org/10.3390/life15071122
Chicago/Turabian StylePochhammer, Julius, Frederike Franke, Matthias Martin, Jan Henrik Beckmann, Daniar Osmonov, Ibrahim Alkatout, and Thomas Becker. 2025. "Right Colectomy with Complete Mesocolic Excision and Intracorporeal Anastomosis: A Monocentric, Single-Surgeon Comparison of Dexter, DaVinci and Laparoscopic Approaches" Life 15, no. 7: 1122. https://doi.org/10.3390/life15071122
APA StylePochhammer, J., Franke, F., Martin, M., Beckmann, J. H., Osmonov, D., Alkatout, I., & Becker, T. (2025). Right Colectomy with Complete Mesocolic Excision and Intracorporeal Anastomosis: A Monocentric, Single-Surgeon Comparison of Dexter, DaVinci and Laparoscopic Approaches. Life, 15(7), 1122. https://doi.org/10.3390/life15071122






