Abstract
Chronic pain is a maladaptive neurological disease that remains a major global healthcare problem. Voltage-gated sodium channels (Navs) are major drivers of the excitability of sensory neurons, and the Nav subtype 1.7 (Nav1.7) has been shown to be critical for the transmission of pain-related signaling. This is highlighted by demonstrations that gain-of-function mutations in the Nav1.7 gene SCN9A result in various pain pathologies, whereas loss-of-function mutations cause complete insensitivity to pain. A substantial body of evidence demonstrates that chronic neuropathy and inflammation result in an upregulation of Nav1.7, suggesting that this channel contributes to pain transmission and sensation. As such, Nav1.7 is an attractive human-validated target for the treatment of pain. Nonetheless, a lack of subtype selectivity, insufficient efficacy, and adverse reactions are some of the issues that have hindered Nav1.7-targeted drug development. This review summarizes the pain behavior profiles mediated by Nav1.7 reported in multiple preclinical models, outlining the current knowledge of the biophysical, physiological, and distribution properties required for a Nav1.7 inhibitor to produce analgesia.
1. Introduction
Pain is a leading global healthcare problem, with chronic pain, in particular, being a major concern, affecting 1 in 10 adults annually. Treatments for chronic pain usually involve a variety of approaches, including, but not limited to, pharmacological interventions, physical exercise or therapy, and psychological support [1]. Although many types of pain are well managed with current medications, the development of new pharmacological agents remains an urgent need. For some chronic pain conditions, such as neuropathic pain, cancer, or chemotherapy-induced pain, the available drugs have partial or limited efficacy and, in some cases, can provoke addiction and abuse [2,3]. The widespread use of opioid-derived painkillers is one of the primary causes of the worldwide opioid crisis, which causes thousands of deaths in many countries and represents a major health and social challenge in modern society [4].
The sensation of pain originates in the peripheral nervous system (PNS) through the activation of nociceptors, which are remarkably heterogeneous in nature, encompassing various types of receptors and ion channels [5]. Among them, transient receptor potential (TRP) channels are widely expressed in sensory neurons and mediate the transduction of noxious (thermal, electrical, and mechanical) stimuli into electric impulses. Pain-associated TRP channels include members of the subfamilies ankyrin (A), vanilloid (V), melastatin (M), and canonical (C), and their roles in physiological and pathological conditions, including pain, have been expertly reviewed [6]. The nociceptive signals travel from the sensory neurons of the dorsal root ganglia (DRG) to neurons in the dorsal horn of the spinal cord, where they are finally transmitted to the brain to be processed [7]. The transmission of pain signals is mediated by the activity of ion channels present in the membranes of the neurons responsible for the generation and propagation of the action potentials. In particular, the voltage-gated sodium channels (Navs) are fundamental players in this process, triggering and modulating the amplitude, duration, and frequency of action potentials [8] (Figure 1).
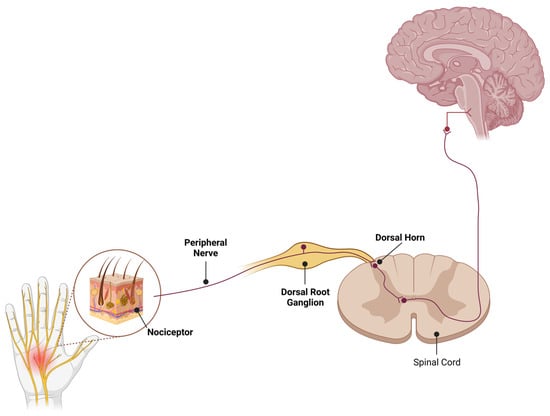
Figure 1.
Schematic diagram of pain pathways where high Nav1.7 expression has been reported. Nav1.7 display robust expression in peripheral sensory neurons of all sizes. In addition, expression of Nav1.7 is detected in both central and peripheral projections of DRG neurons along with the central terminals of sensory neurons in the laminae I and II of the dorsal horn. Illustrations created in BioRender (www.biorender.com).
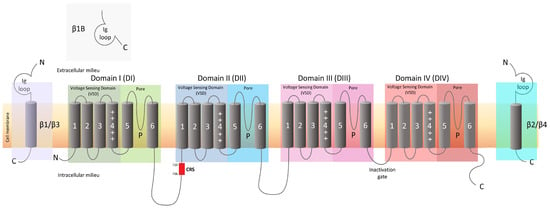
Navs are involved in the control of many fundamental physiological processes in various tissues of the human body. The Nav family 1 consists of nine members, each represented by pore-forming α subunits, which, in mammals, are numerically named from Nav1.1 to 1.9 and are encoded by the genes SCN1A, SCN2A, SCN3A, SCN4A, SCN5A, SCN8A, SCN9A, SCN10A, and SCN11A, respectively [9]. Although these isoforms share a common overall structural motif, each Nav subtype has a different function and exhibits different expression profiles [10]. Nav1.1, Nav1.2, Nav1.3, and Nav1.6 are mainly expressed in the central nervous system (CNS) [11]; Nav1.7, Nav1.8, and Nav1.9 are present in the PNS; Nav1.4 is the skeletal muscle sodium channel; and Nav1.5 is the predominant cardiac myocyte channel [12,13,14].
For decades, the pharmaceutical industry has pursued drugs modulating Nav channel activity in the search for effective painkillers. Unfortunately, these attempts have often resulted in drugs with an insufficient therapeutic index (i.e., the efficacy–safety ratio). The ideal analgesic should be able to reduce the pain sensation at the PNS level without having adverse effects on the CNS and without interfering with other organs’ functionality. Nav1.7, Nav1.8, and Nav1.9, expressed in nociceptors, are among the most studied targets for pain treatment [15,16]. Nav1.7, in particular, is responsible for the initiation of the action potential in pain responses, and, due to this critical role, its dysfunction is associated with several pain related disorders. Genetic studies have shown that mutations in the SCN9A gene, which encodes the Nav1.7 protein, result in familial pain disorders [17]. Mutations in the SCN9A gene can be categorized as gain-of-function mutations (increased Nav1.7 activity), causing paroxysmal extreme pain disorder (PEPD) and erythromelalgia (EM); or loss-of-function mutations (reduced Nav1.7 activity), causing congenital insensitivity to pain (CIP) [18]. An important characteristic of the patients suffering from CIP is that even in the total absence of Nav1.7 activity, they do not exhibit any cognitive, cardiac, motor, or sensory deficits, supporting Nav1.7 as a valid—and indeed attractive—target for the development of drugs against pain. Interestingly, rodent models of neuropathic pain showed increased Nav1.7 activity, further supporting the importance of Nav1.7 as a pain target [19,20,21]. These findings have fueled a race to develop a myriad of molecules targeting Nav1.7 for pain applications.
3. Preclinical Discovery of Analgesic Compounds
3.1. Animal Models of Pain
Animal models are essential for understanding disease mechanisms and testing novel therapies, particularly in pain research, due to the practical and ethical concerns associated with human experimentation. Overall, analgesic drugs are effective in animal models, indicating that similar nociceptive machinery is present. Pain models can be broadly categorized into inflammatory (e.g., CFA, carrageenan, and formalin test), neuropathic (e.g., spinal nerve ligation (SNL), chronic constriction injury (CCI), spared nerve injury (SNI)), arthritic (e.g., monoiodoacetate (MIA)-induced arthritis model), chemotherapy-induced peripheral neuropathy (CIPN), and postoperative pain models (e.g., plantar incision model) (Figure 3).
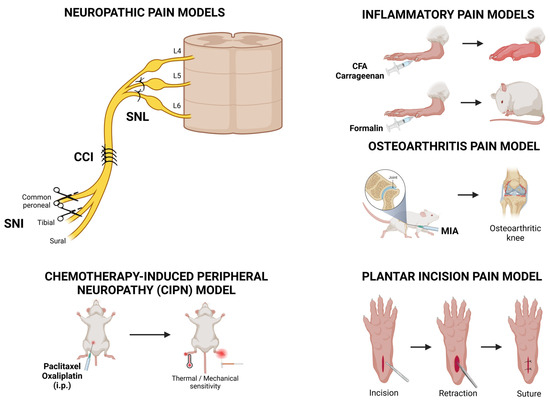
Figure 3.
Overview of various pre-clinical pain models used in Nav1.7 research. Among the neuropathic pain models, the ligation of the distal dorsal root ganglion (DRG) is used to induce the spinal nerve ligation (SNL) model. The chronic constriction injury (CCI) model is established by ligating the main trunk of the sciatic nerve, whereas the spared nerve injury (SNI) model ligates the tibial nerve and the common peroneal nerve, leaving the sural nerve intact. Chemotherapy-induced peripheral neuropathy (CIPN) models are obtained following the intraperitoneal administration of chemotherapeutic drugs, resulting in the development of thermal and mechanical sensitivity. Inflammatory pain models involve the intraplantar injection of complete Freund’s adjuvant (CFA), carrageenan, or formalin. Osteoarthritis pain models are obtained with an injection of monoiodoacetate (MIA) to the knee joint, leading to pathological changes that resemble human osteoarthritis. Finally, the plantar incision pain model has been developed as a preclinical tool to identify the molecular, cellular, and physiological mechanisms that underlie postoperative pain. Illustrations created in BioRender.
3.1.1. Complete Freund’s Adjuvant (CFA) and Carrageenan-Induced Pain Models
Inflammatory pain models rely on the administration of a chemical inflammogen or causing tissue damage via mechanical or thermal means. CFA and carrageenan are two of the most commonly used agents [37,38]. CFA is a suspension of heat-killed Mycobacterium butyricum or Mycobacterium tuberculosum, whereas the carrageenan are sulfated polysaccharides extracted from seaweed. Injection of these compounds into the paw of rodents induces edema and thermal and mechanical hyperalgesia. This pain hypersensitivity develops within 30 min post injection, peaks between 2 h and 1 day, and can persist for at least 7 days [39,40]. Inflammatory pain results from the sensitization of primary afferent nociceptive fibers innervating the inflamed tissue. This phenomenon is mediated by a complex series of events, involving the activation of multiple signal transduction pathways and the modulation of neuronal function and activity, resulting in lowered activation thresholds and increase evoked afferent activity [41,42]. In particular, the modulation of Nav1.7 channels has been shown to have a prominent role in inflammatory pain.
3.1.2. Formalin Test
The formalin test has been widely used as a valuable tool for rapidly screening the efficacy of potential analgesic drugs [43,44]. Intraplantar injection of formalin elicits a spontaneous biphasic nocifensive behavior that involves the licking, flinching, and biting of the injected paw. The phase 1 (acute phase) is characterized by intense pain believed to result from the direct chemical stimulation of nociceptors [45]. This is followed by an interphase, a period of reduced pain, and finally by a final phase, during which pain resumes (second phase). This second phase is thought to reflect ongoing inflammation and sensitization of the dorsal horn in the spinal cord, although this view was recently contested by Hoffmann and collaborators [46]. A great advantage of the formalin test is that it is relatively simple to perform as it does not require complex surgical procedures or specialized equipment. Furthermore, positive behavioral responses are obvious and easily quantifiable.
3.1.3. Chronic Constriction Injury (CCI) Model
The CCI model is a widely used model of neuropathic pain that mimics the lesions of nerve fibers located at the surface of the peripheral nerves and reproduces the main sensory symptoms associated with neuropathic pain in humans [47]. In this model, ligatures loosely tied around the sciatic nerve induce long-lasting chemical and thermal hypersensitivity, cold and mechanical allodynia, and spontaneous pain [48,49].
3.1.4. Spared Nerve Injury (SNI) Model
The SNI involves ligating and severing two terminal branches of the sciatic nerve, the common peroneal and tibial nerves, while leaving the sural nerve intact [50]. This procedure leads to the development of mechanical and thermal hypersensitivity in the area innervated by the sural nerve only on the ipsilateral side, but not the contralateral side. Mechanical hypersensitivity is observed within 3 days post-surgery, peaks around 14 days post-surgery, and lasts for at least 4 weeks [51,52]. Originally described in rats, the SNI model has since been adapted and validated in mice [53]. One major implication of performing SNI studies in mice versus rats is the origin of sciatic never fibers. In rats, the majority of sciatic nerve fibers originate from the L4 and L5 DRG [54]. However, in mice, the functional and anatomical equivalents of the rat L4 and L5 DRG are instead the L3 and L4 DRG [55]. This distinction was further demonstrated in the SNI model, where ATF3 immunoreactivity, a marker of axotomized neurons, was significantly increased in the L3 and L4 DRG 7 days after surgery. In contrast, the percentage of ATF3-positivity in the L5 DRG was similar to that of sham animals [56].
3.1.5. Spinal Nerve Ligation (SNL) Model
The SNL, which involves the ligation and transection of the L5 spinal nerve, is one of the most widely used models of neuropathic pain in rodents [57]. This procedure results in spontaneous ectopic discharges in afferent A-fibers (Aβ and Aδ), which are believed to be a key driver of the abnormal sensations observed in this model [58]. The spinal nerve transection of the fifth lumbar segment model is a slight modification of that described originally by Kim and Chung [57]. In this model, two ligatures, approximately 5 mm apart, are placed in the L5 spinal nerve, followed by a transection between the ligatures [59]. In both models, animals develop long-lasting spontaneous pain, hyperalgesia, and allodynia in the ipsilateral hind paw within a few days after surgery [57,59,60].
3.1.6. Spinal Cord Injury (SCI) Models
In clinical settings, SCI is an exceptionally heterogeneous condition, and as such, several animal models have been developed with variable locations, extents, and means to induce injury [61]. Blunt direct trauma by contusion that mimics clinical traumatic injuries, ischemic lesions, and complete or partial spinal cord transection are some of the methods of inducing SCI in preclinical studies, and they are associated with the development of mechanical allodynia and thermal hyperalgesia following model induction [62,63,64,65,66].
3.1.7. Chemotherapy-Induced Peripheral Neuropathy (CIPN) Models
Paclitaxel (Taxol®, Princeton, NJ, USA) is a chemotherapeutic agent commonly used in the treatment of proliferative breast, ovary, and lung cancers [67]. It acts by stabilizing microtubule scaffolding within treated cells, thus blocking the mitotic spindle dynamics required for cell division. This results in cell cycle arrest and apoptosis of the rapidly dividing cancer cells [68]. However, known side effects of paclitaxel treatment are its pathological effects on sensory neurons of the peripheral nervous system, a condition known as CIPN [69]. Clinically, CIPN manifests as chronic pain, often localized in the hands and feet [70]. In the rodent model, the chemotherapy agent is typically administered through intravenous or intraperitoneal injections four times, separately, at doses that range from 2 to 8 mg/kg, administered every other day, which is thought to mimic one cycle of chemotherapy treatment in humans [71]. The pathophysiological processes induced by paclitaxel include inflammation, oxidative stress, loss of primarily epidermal nerve fibers, and alterations to the mitochondrial function and excitability of peripheral neurons.
3.1.8. Plantar Incision Model of Postoperative Pain
Postoperative pain is the most common form of acute pain, and a major concern is that following this phase, a significant number of patients will develop chronic pain that will severely impact their quality of life [72]. Rat and mouse models of acute incisional pain have been developed as preclinical tools to identify the molecular, cellular, and physiological mechanisms that underlie postoperative pain [73,74,75]. The hind paw incision model in rodents causes reversible edema around the wound site and is characterized by non-evoked pain behavior, as well as mechanical and heat hyperalgesia, that lasts for several days [74]. It is an established and reliable model of postoperative pain that greatly simulates the clinical features observed in patients. Indeed, the main advantage of the plantar incision model is that the pain following the skin and muscle incision more closely mimics the inflammatory pain process in patients than other models such as the CFA- or carrageenan-induced models.
3.1.9. Osteoarthritis (OA) Models
Osteoarthritis is a disabling, degenerative disorder marked by progressive joint failure, which is associated with cartilage breakdown and a loss of the extracellular matrix, which, under physiological conditions, provides the compressive resilience essential for joint function [76]. Surgical and chemical induction in mice are the two most common preclinical models of OA, and, similar to the pathogenesis in humans, both methods exhibit articular cartilage erosion or loss and OA-related pain [77]. Injection of sodium iodoacetate (MIA model) or CFA into the knee joint are the two most commonly used models of OA and are associated with the progressive degradation of the cartilage and subchondral bone lesions in the knee [78,79,80]. At the molecular level, these models involve the up-regulation of the neuronal damage marker cAMP-dependent transcription factor ATF-3 in the peripheral nerves that innervate the knee joint, a reduction in intra-epidermal nerve fiber density, and alterations in neuroimmune cells within the spinal cord [81,82]. The MIA model, in particular, presents robust mechanical and cold allodynia, along with a significant decrease in hind limb weight-bearing on the ipsilateral side two weeks post-induction [80,83,84].
3.2. Mechanical and Thermal Evoked Tests
Pain tests are classified based on nociception modalities that can be of mechanical (i.e., von Frey, mechanical conflict) or thermal (i.e., Hargreaves assay, hot and cold plate, temperature place preference) nature [85,86]. Mechanical allodynia refers to the perception of pain in response to non-painful stimuli such as light touch or pressure, and it is a crucial indicator of neuropathic pain development. One of the most widely used methods of assessing mechanical allodynia is the von Frey filament test, which involves applying a series of calibrated filaments to the animal’s paw to determine the pain sensitivity threshold, i.e., the point at which the animal responds to the mechanical stimulus [87]. Traditionally, standard Von Frey hairs are employed for this test; however, more recently, electronic von Frey algometers have emerged as the preferred choice due to their higher reliability, precise control over the applied force, and consistent rate of stimulus application, which allows for standardized testing conditions across experiments and consistency [88].
Thermal nociception is another critical method for assessing pain in preclinical research. This test involves applying a radiant heat source to the animal’s paw, triggering a withdrawal reflex. The latency period between the heat stimulus application and the withdrawal response is then measured, providing a reliable metric for heat sensitivity. This test demonstrates a remarkable ability to differentiate between diverse analgesic agents and is highly reliable in evaluating heat hyperalgesia in animal models [37]. These behavioral tests allow researchers to quantitatively evaluate abnormal pain sensations that develop as complications in pre-clinical pain models, and they are routinely used to investigate the potential analgesic effects of a myriad of compounds.
Experimental models of pain sensitivity include testing threshold responses to high-intensity stimuli such as heat. Acute thermal pain is commonly assessed by the hot-plate and tail-flick tests, where the latency of the animal’s response to the stimulus is recorded as the dependent variable [89]. In the hot-plate test, animals are injected with test compounds or controls before being placed on a hot plate. The latency to respond to the heat stimulus is measured as the time it takes for the animal to react by jumping or licking the affected paw. The hot-plate test is a rapid, inexpensive, reliable, and reproducible assessment that measures the supraspinal responses to nociception without causing inflammation [89]. The temperature is typically set at 52 or 55 °C, which allows for the observing of baseline response latencies of approximately 10 s for paw licking or other observable responses. In the past decade, a modified hot-plate test has been developed, where the temperature is gradually increased from non-noxious to noxious levels. Such dynamic hot plates provide precise control over temperature ramps, allowing for the differentiation between thermal allodynia and thermal hyperalgesia [90].
The tail-flick test is widely used to assess acute nociception, involving the application of a high-intensity thermal stimulus to the tail of a mouse or a rat. The time from onset of stimulation to the animal’s rapid tail flick or withdrawal from the heat source is recorded as the primary outcome measure [89]. One advantage of this test is its reduced dependency on the animals’ motor coordination compared to the hot-plate test. Unlike the hot-plate test, where the endpoint consists of a complex behavior such as paw licking or jumping, this test induces a simple, spinally mediated flexion reflex response. This is an important distinction as it highlights the different levels of nociceptive processing assessed by the two tests. The hot-plate test primarily evaluates supraspinal responses, whereas the tail-flick test measures spinal nociceptive reflexes.
Tamoxifen-inducible Nav1.7 knockout (KO) mice (Nav1.7 cKO) display decreased SCN9A mRNA expression in tissues that normally express Nav1.7, including trigeminal ganglia and DRG neurons and sympathetic ganglia. These Nav1.7 conditional KO mice, as well as mice with global Nav1.7 KO (Nav1.7 gKO), show prolonged response latencies to noxious heat in the hot-plate test [91,92]. These findings are further corroborated by studies using animals with Nav1.7 deletions in specific subsets of neuronal populations. For instance, transgenic Nav1.7Wnt1 mice, generated by using the Wnt1 promoter to drive Cre expression in neuronal crest derivatives (including autonomic and sensory neurons), demonstrated attenuated responses in the hot-plate test [93]. Similarly, the use of Advillin (Nav1.7Advill) and Wnt1 (Nav1.7Wnt1) promoters for the generation of conditional Nav1.7 KO strains allowed for the specific deletion of the channel in DRG and sensory and sympathetic neurons, respectively [35]. The same study also indicates that Nav1.7 KO animals with specific deletions in the DRG (Nav1.7Advill) or in sensory neurons driven by the Nav1.8 promoter (Nav1.7 Nav1.8) have normal hotplate pain behavior [93]. Interestingly, performing similar studies using a heat ramp paradigm suggests that different heat stimulus intensities require distinct neuronal subpopulations. When temperature is increased at rate of 0.6 °C/s, a significant increase in response threshold is observed not only for Nav1.7Wnt1 mice but also Nav1.7Nav1.8, Nav1.7Advill when compared to littermate controls [94]. However, when a heat ramp of 2 °C/s was applied to the plantar surface of the hind paw, only Nav1.7Advill and Nav1.7Wnt1 displayed increased withdrawal latency. The requirement of Nav1.7 channels in Nav1.8-positive sensory neurons under certain conditions was further validated by Hockley and collaborators, who demonstrated that Nav1.7Nav1.8 mice displayed an increased latency and thermal threshold in the ramping hot-plate test [95].
Cold sensitivity can be determined by a variety of behavioral methods. One of the simplest methods is the acetone evaporation test, which involves dabbing or spraying acetone on the mouse paw and measuring the time the mouse spends flicking the paw [96]. The acetone evaporation produces a cold stimulus that is typically non-nociceptive in naïve animals but induces cold allodynia in certain pain models [96]. In addition, cold plates may also be used to assess nociceptive responses that include paw withdrawal latency at a specific temperature, the number of flinches within a defined timeframe, or the aversive reaction to a cooling ramp that determines the cold response threshold [97,98]. This test is widely used in both inflammatory and nerve-injury models, where it has been demonstrated that the withdrawal threshold for temperatures of 15 °C, a non-painful temperature in naïve rats, is significantly reduced [99].
Cold-plate tests indicate that behavioral responses to noxious cold are Nav1.7-independent. This is based on observations that deletion of Nav1.7 in the DRG, Nav1.8-positive sensory neurons or in autonomic and sensory neurons has no effect on aversion to extreme cold [93]. These results are further corroborated by evidence that neither Nav1.7Nav1.8 mice nor the application of the selective Nav1.7 antagonist PF-5198007 (another variation of PF-04856264) affects cold-evoked afferent firing [95]. Interestingly, responses to noxious cooling triggered by acetone application appear to depend on Nav1.7 expression in Nav1.8-negative sensory neurons [93]. This is based on observations that the behavioral responses in Nav1.7Nav1.8 KO mice were not significantly different from littermate controls, whereas deletion of Nav1.7 in the DRG, or in autonomic and sensory neurons in the Nav1.7Advill and Nav1.7Wnt1 KO mice, respectively, had no effect on the behavioral responses induced by acetone [93].
Of note, the use of behavioral tests, such as responses to von Frey filaments or temperature gradients, rely on reflexive responses to painful stimuli, which are not necessarily representative of the clinical condition and potentially limit the translation of their findings. More recently, alternative options to reflective response testing have emerged. These include the assessment of facial expression changes using the mouse grimace scale, condition and temperature place preference tests, and evaluations of balance, coordination, and walking gait [100]. While stimulus-free pain response quantification more closely mimics clinical settings, these tests have not been widely adopted due to challenges that range from cost to difficulty of implementation and require further refinement and validation in animal models. Nonetheless, these methods offer a new avenue for objective preclinical pain assessment that does not rely on the application of noxious stimuli and that better resembles the symptomatology of pain observed in clinical settings.
9. Challenges and Future Direction
As summarized in Table 1, studies across numerous pre-clinical models have validated Nav1.7 as a promising target for therapeutic intervention and provided valuable insights into its role in pain transmission and underlying biology (Table 1). However, despite robust pre-clinical evidence demonstrating the efficacy of Nav1.7 blockers in reducing pain, its clinical translation has yielded rather disappointing results. This emphasizes the need for more innovative and refined approaches, as discussed by Eagles and cols [29].

Table 1.
Summary of test compounds and genetic models, demonstrating the role of Nav1.7 in pain models.
Patients with chronic pain exhibit significant heterogeneity in pathophysiology, etiology, and clinical presentation. This is in contrast with the majority of pain models employed in pre-clinical studies, which fail to capture the heterogenous disease mechanisms observed in patients [166,167]. This poor correlation between preclinical test subjects and the clinical patient population likely contributes to the translational challenges associated with the development of novel analgesics, i.e., not only those targeting Nav1.7 but also a broader range of analgesic compounds. For instance, most preclinical studies are performed in young, healthy, male rodents of a genetically similar background. In contrast, neuropathic pain in clinical settings is most commonly found in middle-aged or elderly patients with heterogeneous genetic profiles [168]. Notably, a study comparing paclitaxel-induced neuropathy in CD1 mice of different ages found that juvenile and aged mice exhibited more severe mechanical allodynia and thermal hyperalgesia compared to young adult mice [169]. Moreover, different mouse strains showed variable sensitivity to pain in different behavioral assays, further highlighting the limitations of using a single-rodent model/mouse strain to test candidate compounds in preclinical studies [170]. Sex differences in pain are also increasingly recognized as critical factors in understanding chronicity, severity, and response to analgesics. Given that a significant proportion of chronic pain patients are female, there is a growing interest in examining these differences, which may have profound implications for the translational efficacy of new therapies [171,172]. Finally, differences in pain processing and assessment between rodents and humans present challenges for extrapolating preclinical findings. To mitigate this issue, the use of larger animal models and human tissues in these preclinical studies is recommended in order to bridge this knowledge gap, as reviewed and discussed by Sadler and colleagues [173]. Taken together, it has increasingly been accepted that a broader range of validated and long-lasting pain assays is essential to fully determine the efficacy of a therapeutic drug. Incorporating operant and non-invasive protocols for assessing analgesia in pre-clinical settings may also improve the translation of animal-based observations into effective human therapeutics.
A key element in drug discovery is delineating the relationship between the in vitro potency, the pharmacokinetic properties of a compound, its drug exposure at site of action, target engagement, the desired pharmacological response, and overall efficacy, all while monitoring for signs of toxicity and performing safety assessments [174,175,176]. For drugs targeting Nav1.7, an additional requirement may include the ability to cross the blood–brain barrier and the blood–nerve barrier as Nav1.7 is expressed in the central terminal of DRG neurons in the dorsal horn, which lies behind the blood–spinal cord barrier [177]. As such, targeting Nav1.7 activity in the dorsal horn might be necessary to effectively inhibit pain signaling. These considerations are particularly important considering the failure in translating the pre-clinical success of Nav1.7 into clinical efficacy. To date, despite demonstrating effectiveness in some animal models, the selective small-molecule Nav1.7 inhibitors tested in clinical trials have failed to meet the defined efficacy criteria. Uncertainty remains around the exact level of the Nav1.7 blockade necessary to achieve analgesia [27]. A further look into its properties identified that it was unable to achieve sufficient coverage of the Nav1.7 IC50 to effectively engage the target in vivo in both rodents and non-human primates [111,178].
Nav1.7 selective inhibitors may impact the autonomic nervous system owing to the expression of Nav1.7 in sympathetic tissues. mRNA for Nav1.7 channel has been reported in 82% of the sympathetic neurons isolated from the stellate ganglion [179,180]. A previous report described the presence of Nav1.7 mRNA in the postganglionic parasympathetic nerves regulating human and guinea-pig airway smooth muscle, while no Nav1.7 mRNA was observed in the same nerves in mice [181]. Consequently, Nav1.7 blockers could inhibit the sympathetic nerve-mediated adrenergic contraction of blood vessels and abolish parasympathetic nerve-mediated cholinergic contractions of the airway smooth muscle, suggesting that Nav1.7 blockers could have profound effects on the sympathetic and parasympathetic systems [180,181]. This is corroborated by observations that patients with hereditary erythromelalgia experience flushing, which may be related to altered sympathetic neurons activation and vasodilation [182]. In addition, hypotensive effects following intravenous administration of Nav1.7 inhibitors have been reported in rodents, non-human primates, and humans [183,184,185]. Telemetry data showed that ST-2560 (a selective inhibitor of Nav1.7 with IC50 of 39 nM) induced a dose-dependent reduction in systolic and diastolic blood pressure in conscious, free-moving cynomolgus monkeys. This effect was transient, with blood pressure gradually returning to baseline levels within 5 hours post-dosing [185]. Similarly, in the clinical testing of MK-2075, another small-molecule selective Nav1.7 inhibitor, orthostatic hypotension linked to Nav1.7-dependent cardiac autonomic dysfunction was observed in patients administered with either 30 mg by intravenous infusion over 8 h or 8 mg over 2 h [186]. These findings highlight the necessity of characterizing the effects of potential Nav1.7-targeting candidates on the autonomic nervous system and cardiovascular endpoints. This is particularly important for molecules capable of achieving high levels of target engagement as acute inhibition of Nav1.7 could also lead to autonomic side effects, including reduced heart rate variability and impaired spontaneous baroreceptor sensitivity.
Side effects due to off-target binding to other Nav subtypes are another major concern when developing compounds targeting Nav1.7. One possible solution is the generation of antibodies against unique epitopes of Nav1.7. Although promising, this strategy faces several challenges that must be addressed in order to succeed. Navs are considered difficult targets for antibody development due to (i) the high degree of similarity in amino acid sequence and tertiary structures among Nav1.x isoforms; (ii) their numerous transmembrane segments; (iii) the short extracellular domains; and (iv) the different steric conformations they adopt depending on their kinetic state (i.e., closed, active/open, inactivated) [187]. Compared to small molecules, antibodies have the significant advantage of recognizing highly specific amino acid sequences. When this sequence is unique to a particular isoform, antibodies can achieve exceptional target specificity. However, while some monoclonal antibodies against Nav1.7 have been developed (e.g., SVmab), they lack functional activity, despite successfully binding to the channel. Better success was achieved by Martina and collaborators, who developed a novel antigen design and presentation strategy, resulting in the generation of a single-domain antibody (VHH DI-D) with analgesic effect in animal pain models, including the OD1 and formalin pain models in mice and the Hargraves and spare nerve injury pain models in rats [110]. The epitope targeted by VHH DI-D is located on the third extracellular loop of domain DIII and is unique to Nav1.7. Unlike small molecules, the mode of action of VHH DI-D does not involve preventing the movement of the voltage sensor upon changes in membrane voltage (as spider toxins do) or obstructing the conductive pathway (as marine toxins do). Instead, it slows the deactivation process of the channel, thus decreasing the firing capability of sensory neurons. This mechanism is probably a key factor in its analgesic efficacy in rat and mouse preclinical pain models [110].
Another promising strategy consists of developing molecules that specifically modulate Nav1.7 activity by interacting with the intracellular proteins regulating its membrane trafficking. One such molecule was developed by the company Regulonix and called compound 194. Derived from a class of benzoyl piperidyl benzimidazole, compound 194 inhibits the addition of SUMO onto the Nav1.7-interacting cytosolic CRMP2. This mechanism effectively blocks Nav1.7 function by preventing its trafficking to the cell surface [128]. As discussed previously in this review, compound 194 displayed robust antinociceptive properties in multiple preclinical models of acute and chronic pain when administered not only intrathecally but also via the oral route, which is very relevant when considering its potential clinical translation [124,125,126,127,128,134,135].
Agents targeting other ion channels are subject to active drug discovery and development efforts. A significant breakthrough was recently achieved when the U.S. Food and Drug Administration approved suzetrigine (VX-548), a first-in-class Nav1.8 inhibitor, for the treatment of moderate-to-severe acute pain [188]. Interest in Nav1.9 modulators has grown since the discovery that mutations in its gene are associated with painful neuropathies [189]. ANP-230, which inhibits Nav1.7, Nav1.8, and Nav1.9 with similar potencies, is currently undergoing clinical trials in Japan (jRCT2061200046). Additionally, calcium, potassium, and acid-sensing ion channels are also being targeted in chronic pain control [190,191,192]. Considering their role in conveying thermal, mechanical, and chemical stimuli signals in afferent sensory neurons, it is not surprising that TRP channels are also being targeted for pain relief. Clinical trials are currently underway for agents against TRPA-1, TRPV-1, TRPV-3, and TRPM-8 [193,194,195]. Nevertheless, adverse effects and, in some cases, lack of therapeutic efficacy in clinical trials hamper the development of compounds targeting TRP channels, highlighting the similar challenges faced in the discovery of Nav1.7 inhibitors, as previously discussed in this review.
10. Conclusions
In summary, the available genetic and pharmacological evidence strongly supports the development of new molecules targeting Nav1.7 for various—though not all—types of pain in which this channel plays a role. However, there is a pressing need for a robust translational workflow that incorporates in vivo characterization across multiple models, reflecting the heterogenicity of pain presentation observed in the clinic. In addition, it is increasingly evident that achieving high specificity for Nav1.7 alone is insufficient for the development of a successful analgesic. Novel mechanisms of action must be explored, and pharmacokinetic, pharmacodynamic, and toxicity profiles should be carefully evaluated early in the drug development process. This comprehensive approach is essential to overcoming the challenges of translating preclinical findings into clinically effective therapies targeting this channel for pain management in humans.
Author Contributions
Conceptualization, A.Y., U.B. and M.M.; writing—original draft preparation, A.Y., U.B. and M.M.; writing—review and editing, A.Y., U.B., M.M. and M.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CCI | Chronic constriction injury |
| Cdk5 | Cyclin-dependent kinase 5 |
| CFA | Complete Freund’s adjuvant |
| CIP | Congenital insensitivity to pain |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| CNS | Central nervous system |
| CRMP2 | Collapsin response mediator protein 2 |
| DRG | Dorsal root ganglia |
| EM | Erythromelalgia |
| ERK1/2 | Extracellular signal-related kinase 1/2 |
| MIA | Monoiodoacetate arthritis |
| MAPK | Mitogen-activated protein kinase |
| NAD | Nicotinamide adenine dinucleotide |
| Nav1.7 | Voltage-gated sodium channel subtype 1.7 |
| Navs | Voltage-gated sodium channels |
| OA | Osteoarthritis |
| OD1 | First α toxin from Odonthobuthus doriae scorpion |
| PEPD | Paroxysmal extreme pain disorder |
| PNS | Peripheral nervous system |
| SCI | Spinal cord injury |
| SGK1 | Serum glucocorticoid-regulated kinase 1 |
| SNI | Spared nerve injury |
| SNL | Spinal nerve ligation |
| SUMO | Small ubiquitin-like modifier |
| TrkA | Tropomyosin receptor kinase A |
| TRP | Transient receptor potential |
| VSD | Voltage-sensing domain |
References
- Rice, A.S.C.; Smith, B.H.; Blyth, F.M. Pain and the global burden of disease. Pain 2016, 157, 791–796. [Google Scholar] [CrossRef]
- Marshall, B.; Bland, M.K.; Hulla, R.; Gatchel, R.J. Considerations in addressing the opioid epidemic and chronic pain within the USA. Pain Manag. 2019, 9, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Banderali, U.; Moreno, M.; Martina, M. The elusive Nav1.7: From pain to cancer. Curr. Top. Membr. 2023, 92, 47–69. [Google Scholar] [CrossRef]
- Humphreys, K.; Shover, C.L.; Andrews, C.M.; Bohnert, A.S.B.; Brandeau, M.L.; Caulkins, J.P.; Chen, J.H.; Cuéllar, M.F.; Hurd, Y.L.; Juurlink, D.N.; et al. Responding to the opioid crisis in North America and beyond: Recommendations of the Stanford-Lancet Commission. Lancet 2022, 399, 555–604. [Google Scholar] [CrossRef]
- Woller, S.A.; Eddinger, K.A.; Corr, M.; Yaksh, T.L. An overview of pathways encoding nociception. Clin. Exp. Rheumatol. 2017, 35, 40–46. [Google Scholar] [PubMed]
- Koivisto, A.P.; Voets, T.; Iadarola, M.J.; Szallasi, A. Targeting TRP channels for pain relief: A review of current evidence from bench to bedside. Curr. Opin. Pharmacol. 2024, 75, 102447. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Garraway, S.M. A review of dorsal root ganglia and primary sensory neuron plasticity mediating inflammatory and chronic neuropathic pain. Neurobiol. Pain 2024, 15, 100151. [Google Scholar] [CrossRef]
- Kanellopoulos, A.H.; Matsuyama, A. Voltage-gated sodium channels and pain-related disorders. Clin. Sci. 2016, 130, 2257–2265. [Google Scholar] [CrossRef]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The Na(V)1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 1, 49–62. [Google Scholar] [CrossRef]
- Savio-Galimberti, E.; Gollob, M.H.; Darbar, D. Voltage-gated sodium channels: Biophysics, pharmacology, and related channelopathies. Front. Pharmacol. 2012, 3, 124. [Google Scholar] [CrossRef]
- Awad, S.S.; Lightowlers, R.N.; Young, C.; Chrzanowska-Lightowlers, Z.M.; Lomo, T.; Slater, C.R. Sodium channel mRNAs at the neuromuscular junction: Distinct patterns of accumulation and effects of muscle activity. J. Neurosci. 2001, 21, 8456–8463. [Google Scholar] [CrossRef]
- Ogata, N.; Ohishi, Y. Molecular diversity of structure and function of the voltage-gated Na+ channels. Jpn. J. Pharmacol. 2002, 88, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.G.; Westenbroek, R.E.; Maass, A.H.; Lange, V.; Renner, A.; Wischmeyer, E.; Bonz, A.; Muck, J.; Ertl, G.; Catterall, W.A.; et al. Distribution and function of sodium channel subtypes in human atrial myocardium. J. Mol. Cell Cardiol. 2013, 61, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Leo, S.; D’Hooge, R.; Meert, T. Exploring the role of nociceptor-specific sodium channels in pain transmission using Nav1.8 and Nav1.9 knockout mice. Behav. Brain Res. 2010, 208, 149–157. [Google Scholar] [CrossRef] [PubMed]
- McDermott, L.A.; Weir, G.A.; Themistocleous, A.C.; Segerdahl, A.R.; Blesneac, I.; Baskozos, G.; Clark, A.J.; Millar, V.; Peck, L.J.; Ebner, D.; et al. Defining the Functional Role of NaV1.7 in Human Nociception. Neuron 2019, 101, 905–919. [Google Scholar] [CrossRef]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006, 444, 894–898. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. Sodium channels in normal and pathological pain. Annu. Rev. Neurosci. 2010, 33, 325–347. [Google Scholar] [CrossRef]
- Hong, S.; Morrow, T.J.; Paulson, P.E.; Isom, L.L.; Wiley, J.W. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J. Biol. Chem. 2004, 279, 29341–29350. [Google Scholar] [CrossRef]
- Mukai, M.; Sakuma, Y.; Suzuki, M.; Orita, S.; Yamauchi, K.; Inoue, G.; Aoki, Y.; Ishikawa, T.; Miyagi, M.; Kamoda, H.; et al. Evaluation of behavior and expression of NaV1.7 in dorsal root ganglia after sciatic nerve compression and application of nucleus pulposus in rats. Eur. Spine J. 2014, 23, 463–468. [Google Scholar] [CrossRef]
- Laedermann, C.J.; Cachemaille, M.; Kirschmann, G.; Pertin, M.; Gosselin, R.D.; Chang, I.; Albesa, M.; Towne, C.; Schneider, B.L.; Kellenberger, S.; et al. Dysregulation of voltage-gated sodium channels by ubiquitin ligase NEDD4-2 in neuropathic pain. J. Clin. Investig. 2013, 123, 3002–3013. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-gated sodium channels at 60: Structure, function and pathophysiology. J. Physiol. 2012, 590, 2577–2589. [Google Scholar] [CrossRef] [PubMed]
- Bagal, S.K.; Chapman, M.L.; Marron, B.E.; Prime, R.; Storer, R.I.; Swain, N.A. Recent progress in sodium channel modulators for pain. Bioorg. Med. Chem. Lett. 2014, 24, 3690–3699. [Google Scholar] [CrossRef]
- Deuis, J.R.; Wingerd, J.S.; Winter, Z.; Durek, T.; Dekan, Z.; Sousa, S.R.; Zimmermann, K.; Hoffmann, T.; Weidner, C.; Nassar, M.A.; et al. Analgesic Effects of GpTx-1, PF-04856264 and CNV1014802 in a Mouse Model of NaV1.7-Mediated Pain. Toxins 2016, 8, 78. [Google Scholar] [CrossRef]
- Mueller, A.; Starobova, H.; Morgan, M.; Dekan, Z.; Cheneval, O.; Schroeder, C.I.; Alewood, P.F.; Deuis, J.R.; Vetter, I. Antiallodynic effects of the selective NaV1.7 inhibitor Pn3a in a mouse model of acute postsurgical pain: Evidence for analgesic synergy with opioids and baclofen. Pain 2019, 160, 1766–1780. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, J.M.; Palmer, J.; Morisset, V.; Giblin, G.M.; Obermann, M.; Ettlin, D.A.; Cruccu, G.; Bendtsen, L.; Estacion, M.; Derjean, D.; et al. Safety and efficacy of a Nav1.7 selective sodium channel blocker in patients with trigeminal neuralgia: A double-blind, placebo-controlled, randomised withdrawal phase 2a trial. Lancet Neurol. 2017, 16, 291–300. [Google Scholar] [CrossRef]
- McDonnell, A.; Collins, S.; Ali, Z.; Iavarone, L.; Surujbally, R.; Kirby, S.; Butt, R.P. Efficacy of the Nav1.7 blocker PF-05089771 in a randomised, placebo-controlled, double-blind clinical study in subjects with painful diabetic peripheral neuropathy. Pain 2018, 159, 1465–1476. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Yarov-Yarovoy, V. Towards Structure-Guided Development of Pain Therapeutics Targeting Voltage-Gated Sodium Channels. Front. Pharmacol. 2022, 13, 842032. [Google Scholar] [CrossRef]
- Eagles, D.A.; Chow, C.Y.; King, G.F. Fifteen years of NaV 1.7 channels as an analgesic target: Why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy? Br. J. Pharmacol. 2022, 179, 3592–3611. [Google Scholar] [CrossRef]
- Neff, R.A.; Wickenden, A.D. Selective Targeting of Nav1.7 with Engineered Spider Venom-Based Peptides. Channels 2021, 15, 179–193. [Google Scholar] [CrossRef]
- Ouyang, X.; Su, M.; Xue, D.; Dong, L.; Niu, H.; Li, W.; Liu, Y.; Wang, K.; Shao, L. Design, synthesis, and biological evaluation of acyl sulfonamide derivatives with spiro cycles as NaV1.7 inhibitors for antinociception. Bioorg. Med. Chem. 2023, 86, 117290. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, X.; Li, L.; Huang, Y.; Cui, C.; Hu, Q.; Xu, H.; Yin, B.; Chen, X.; Zhao, D.; et al. Recent advances in small molecule Nav 1.7 inhibitors for cancer pain management. Bioorg. Chem. 2024, 150, 107605. [Google Scholar] [CrossRef] [PubMed]
- Dormer, A.; Narayanan, M.; Schentag, J.; Achinko, D.; Norman, E.; Kerrigan, J.; Jay, G.; Heydorn, W. A Review of the Therapeutic Targeting of SCN9A and Nav1.7 for Pain Relief in Current Human Clinical Trials. J. Pain Res. 2023, 16, 1487–1498. [Google Scholar] [CrossRef]
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017, 16, 810. [Google Scholar] [CrossRef]
- Minett, M.S.; Falk, S.; Santana-Varela, S.; Bogdanov, Y.D.; Nassar, M.A.; Heegaard, A.M.; Wood, J.N. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. 2014, 6, 301–312. [Google Scholar] [CrossRef]
- Wheeler, D.W.; Lee, M.C.; Harrison, E.K.; Menon, D.K.; Woods, C.G. Case Report: Neuropathic pain in a patient with congenital insensitivity to pain. F1000Research 2014, 3, 135. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- McCarson, K.E.; Fehrenbacher, J.C. Models of Inflammation: Carrageenan- or Complete Freund’s Adjuvant (CFA)-Induced Edema and Hypersensitivity in the Rat. Curr. Protoc. 2021, 1, e202. [Google Scholar] [CrossRef]
- Eijkelkamp, N.; Heijnen, C.J.; Carbajal, A.G.; Willemen, H.L.; Wang, H.; Minett, M.S.; Wood, J.N.; Schedlowski, M.; Dantzer, R.; Kelley, K.W.; et al. G protein-coupled receptor kinase 6 acts as a critical regulator of cytokine-induced hyperalgesia by promoting phosphatidylinositol 3-kinase and inhibiting p38 signaling. Mol. Med. 2012, 18, 556–564. [Google Scholar] [CrossRef]
- Liang, L.; Fan, L.; Tao, B.; Yaster, M.; Tao, Y.X. Protein kinase B/Akt is required for complete Freund’s adjuvant-induced upregulation of Nav1.7 and Nav1.8 in primary sensory neurons. J. Pain 2013, 14, 638–647. [Google Scholar] [CrossRef]
- Marchand, F.; Perretti, M.; McMahon, S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 2005, 6, 521–532. [Google Scholar] [CrossRef]
- Dunham, J.P.; Kelly, S.; Donaldson, L.F. Inflammation reduces mechanical thresholds in a population of transient receptor potential channel A1-expressing nociceptors in the rat. Eur. J. Neurosci. 2008, 27, 3151–3160. [Google Scholar] [CrossRef] [PubMed]
- Tjølsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.M.; Cater, H.L.; Thakur, M.; Wells, S.; McMahon, S.B. A refinement to the formalin test in mice. F1000Research 2019, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Hoffmann, T.; Klemm, F.; IKichko, T.; Sauer, S.K.; Kistner, K.; Riedl, B.; Raboisson, P.; Luo, L.; Babes, A.; Kocher, L.; et al. The formalin test does not probe inflammatory pain but excitotoxicity in rodent skin. Physiol. Rep. 2022, 10, e15194. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Austin, P.J.; Wu, A.; Moalem-Taylor, G. Chronic constriction of the sciatic nerve and pain hypersensitivity testing in rats. J. Vis. Exp. 2012, 61, 3393. [Google Scholar] [CrossRef]
- Medeiros, P.; Dos Santos, I.R.; Júnior, I.M.; Palazzo, E.; da Silva, J.A.; Machado, H.R.; Ferreira, S.H.; Maione, S.; Coimbra, N.C.; de Freitas, R.L. An Adapted Chronic Constriction Injury of the Sciatic Nerve Produces Sensory, Affective, and Cognitive Impairments: A Peripheral Mononeuropathy Model for the Study of Comorbid Neuropsychiatric Disorders Associated with Neuropathic Pain in Rats. Pain Med. 2021, 22, 338–351. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Cai, W.; Zhao, Q.; Shao, J.; Zhang, J.; Li, L.; Ren, X.; Su, S.; Bai, Q.; Li, M.; Chen, X.; et al. MicroRNA-182 Alleviates Neuropathic Pain by Regulating Nav1.7 Following Spared Nerve Injury in Rats. Sci. Rep. 2018, 8, 16750. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xu, X.; Chen, Y.; Lin, C.; Lin, F.; Liu, R. Effects of High-Voltage Pulsed Radiofrequency on the Ultrastructure and Nav1.7 Level of the Dorsal Root Ganglion in Rats with Spared Nerve Injury. Neuromodulation 2022, 25, 980–988. [Google Scholar] [CrossRef]
- Bourquin, A.F.; Süveges, M.; Pertin, M.; Gilliard, N.; Sardy, S.; Davison, A.C.; Spahn, D.R.; Decosterd, I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 2006, 122, 14.e1–14.e14. [Google Scholar] [CrossRef]
- Swett, J.E.; Torigoe, Y.; Elie, V.R.; Bourassa, C.M.; Miller, P.G. Sensory neurons of the rat sciatic nerve. Exp. Neurol. 1991, 114, 82–103. [Google Scholar] [CrossRef]
- Rigaud, M.; Gemes, G.; Barabas, M.E.; Chernoff, D.I.; Abram, S.E.; Stucky, C.L.; Hogan, Q.H. Species and strain differences in rodent sciatic nerve anatomy: Implications for studies of neuropathic pain. Pain 2008, 136, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Laedermann, C.J.; Pertin, M.; Suter, M.R.; Decosterd, I. Voltage-gated sodium channel expression in mouse DRG after SNI leads to re-evaluation of projections of injured fibers. Mol. Pain 2014, 10, 19. [Google Scholar] [CrossRef]
- Ho Kim, S.; Mo Chung, J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Eschenfelder, S.; Blenk, K.H.; Jänig, W.; Häbler, H. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain 2000, 84, 309–318. [Google Scholar] [CrossRef]
- Mabuchi, T.; Matsumura, S.; Okuda-Ashitaka, E.; Kitano, T.; Kojima, H.; Nagano, T.; Minami, T.; Ito, S. Attenuation of neuropathic pain by the nociceptin/orphanin FQ antagonist JTC-801 is mediated by inhibition of nitric oxide production. Eur. J. Neurosci. 2003, 7, 1384–1392. [Google Scholar] [CrossRef]
- Clifford, J.L.; Mares, A.; Hansen, J.; Averitt, D.L. Preemptive perineural bupivacaine attenuates the maintenance of mechanical and cold allodynia in a rat spinal nerve ligation model. BMC Anesthesiol. 2015, 15, 135. [Google Scholar] [CrossRef]
- Widerström-Noga, E. Neuropathic Pain and Spinal Cord Injury: Management, Phenotypes, and Biomarkers. Drugs 2023, 83, 1001–1025. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.J.; Xu, J.X.; Aldskogius, H.; Seiger, Å.; Wiesenfeld-Hallin, Z. Allodynia-like effects in rat after ischaemic spinal cord injury photochemically induced by laser irradiation. Pain 1991, 45, 175–185. [Google Scholar] [CrossRef]
- Scheff, S.W.; Rabchevsky, A.G.; Fugaccia, I.; Main, J.A.; Lumpp, J.E., Jr. Experimental modeling of spinal cord injury: Characterization of a force-defined injury device. J. Neurotrauma 2003, 20, 179–193. [Google Scholar] [CrossRef]
- Yoon, Y.W.; Dong, H.; Arends, J.J.; Jacquin, M.F. Mechanical and cold allodynia in a rat spinal cord contusion model. Somatosens. Mot. Res. 2004, 21, 25–31. [Google Scholar] [CrossRef]
- Masri, R.; Keller, A. Chronic pain following spinal cord injury. Adv. Exp. Med. Biol. 2012, 760, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.L.; Minhas, N.K.; Jutzeler, C.R.; Erskine, E.L.; Liu, L.J.; Ramer, M.S. Neuropathic pain following traumatic spinal cord injury: Models, measurement, and mechanisms. J. Neurosci. Res. 2017, 95, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alkahtani, H.M.; AlQuadeib, B.T. Paclitaxel. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 44, pp. 205–238. [Google Scholar] [CrossRef]
- Bates, D.; Eastman, A. Microtubule destabilising agents: Far more than just antimitotic anticancer drugs. Br. J. Clin. Pharmacol. 2017, 83, 255–268. [Google Scholar] [CrossRef]
- Staff, N.P.; Fehrenbacher, J.C.; Caillaud, M.; Damaj, M.I.; Segal, R.A.; Rieger, S. Pathogenesis of paclitaxel-induced peripheral neuropathy: A current review of in vitro and in vivo findings using rodent and human model systems. Exp. Neurol. 2020, 324, 113121. [Google Scholar] [CrossRef]
- Mahon, S.M.; Carr, E. Peripheral Neuropathy: Common Side Effect. Clin. J. Oncol. Nurs. 2021, 25. [Google Scholar] [CrossRef]
- Pennypacker, S.D.; Fonseca, M.M.; Morgan, J.W.; Dougherty, P.M.; Cubillos-Ruiz, J.R.; Strowd, R.E.; Romero-Sandoval, E.A. Methods and protocols for chemotherapy-induced peripheral neuropathy (CIPN) mouse models using paclitaxel. Methods Cell Biol. 2022, 168, 277–298. [Google Scholar] [CrossRef]
- Fregoso, G.; Wang, A.; Tseng, K.; Wang, J. Transition from Acute to Chronic Pain: Evaluating Risk for Chronic Postsurgical Pain. Pain. Physician 2019, 22, 479–488. [Google Scholar] [PubMed]
- Brennan, T.J.; Vandermeulen, E.P.; Gebhart, G.F. Characterization of a rat model of incisional pain. Pain 1996, 64, 493–502. [Google Scholar] [CrossRef]
- Pogatzki, E.M.; Raja, S.N. A mouse model of incisional pain. Anesthesiology 2003, 99, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Cowie, A.M.; Stucky, C.L. A Mouse Model of Postoperative Pain. Bio-Protocol 2019, 9, e3140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Ren, K.; Dubner, R. Osteoarthritis pain mechanisms: Basic studies in animal models. Osteoarthr. Cartil. 2013, 21, 1308–1315. [Google Scholar] [CrossRef]
- Zaki, S.; Blaker, C.L.; Little, C.B. OA foundations—Experimental models of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 357–380. [Google Scholar] [CrossRef]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef]
- Wilson, A.W.; Medhurst, S.J.; Dixon, C.I.; Bontoft, N.C.; Winyard, L.A.; Brackenborough, K.T.; De Alba, J.; Clarke, C.J.; Gunthorpe, M.J.; Hicks, G.A.; et al. An animal model of chronic inflammatory pain: Pharmacological and temporal differentiation from acute models. Eur. J. Pain 2006, 10, 537–549. [Google Scholar] [CrossRef]
- Pitcher, T.; Sousa-Valente, J.; Malcangio, M. The Monoiodoacetate Model of Osteoarthritis Pain in the Mouse. J. Vis. Exp. 2016, 111, 53746. [Google Scholar] [CrossRef]
- Ivanavicius, S.P.; Ball, A.D.; Heapy, C.G.; Westwood, R.F.; Murray, F.; Read, S.J. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: Increased expression of ATF-3 and pharmacological characterisation. Pain 2007, 128, 272–282. [Google Scholar] [CrossRef]
- Im, H.J.; Kim, J.S.; Li, X.; Kotwal, N.; Sumner, D.R.; van Wijnen, A.J.; Davis, F.J.; Yan, D.; Levine, B.; Henry, J.L.; et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010, 10, 2995–3005. [Google Scholar] [CrossRef]
- Fu, W.; Vasylyev, D.; Bi, Y.; Zhang, M.; Sun, G.; Khleborodova, A.; Huang, G.; Zhao, L.; Zhou, R.; Li, Y.; et al. Nav1.7 as a chondrocyte regulator and therapeutic target for osteoarthritis. Nature 2024, 625, 557–565. [Google Scholar] [CrossRef]
- Strickland, I.T.; Martindale, J.C.; Woodhams, P.L.; Reeve, A.J.; Chessell, I.P.; McQueen, D.S. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur. J. Pain 2008, 12, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.A.; Zahra, F.T.; Martin, L.J. IUPHAR review: Navigating the role of preclinical models in pain research. Pharmacol. Res. 2024, 200, 107073. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.D.; Parekh, A.; Pancholi, Y.N. Evaluating pain behaviours: Widely used mechanical and thermal methods in rodents. Behav. Brain Res. 2023, 446, 114417. [Google Scholar] [CrossRef] [PubMed]
- Bradman, M.J.; Ferrini, F.; Salio, C.; Merighi, A. Practical mechanical threshold estimation in rodents using von Frey hairs/Semmes-Weinstein monofilaments: Towards a rational method. J. Neurosci. Methods 2015, 255, 92–103. [Google Scholar] [CrossRef]
- Campana, G.; Rimondini, R. Mechanical Nociception in Mice and Rats: Measurement with Automated von Frey Equipment. Methods Mol. Biol. 2021, 2201, 195–198. [Google Scholar] [CrossRef]
- Bannon, A.W.; Malmberg, A.B. Models of nociception: Hot-plate, tail-flick, and formalin tests in rodents. Curr. Protoc. Neurosci. 2007, 41, 8.9.1–8.9.16. [Google Scholar] [CrossRef]
- Yalcin, I.; Charlet, A.; Freund-Mercier, M.J.; Barrot, M.; Poisbeau, P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J. Pain 2009, 10, 767–773. [Google Scholar] [CrossRef]
- Shields, S.D.; Deng, L.; Reese, R.M.; Dourado, M.; Tao, J.; Foreman, O.; Chang, J.H.; Hackos, D.H. Insensitivity to Pain upon Adult-Onset Deletion of Nav1.7 or Its Blockade with Selective Inhibitors. J. Neurosci. 2018, 38, 10180–10201. [Google Scholar] [CrossRef]
- Gingras, J.; Smith, S.; Matson, D.J.; Johnson, D.; Nye, K.; Couture, L.; Feric, E.; Yin, R.; Moyer, B.D.; Peterson, M.L.; et al. Global Nav1.7 knockout mice recapitulate the phenotype of human congenital indifference to pain. PLoS ONE 2014, 9, e105895. [Google Scholar] [CrossRef] [PubMed]
- Minett, M.S.; Nassar, M.A.; Clark, A.K.; Passmore, G.; Dickenson, A.H.; Wang, F.; Malcangio, M.; Wood, J.N. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat. Commun. 2012, 3, 791. [Google Scholar] [CrossRef] [PubMed]
- Minett, M.S.; Eijkelkamp, N.; Wood, J.N. Significant determinants of mouse pain behaviour. PLoS ONE 2014, 9, e104458. [Google Scholar] [CrossRef]
- Hockley, J.R.; González-Cano, R.; McMurray, S.; Tejada-Giraldez, M.A.; McGuire, C.; Torres, A.; Wilbrey, A.L.; Cibert-Goton, V.; Nieto, F.R.; Pitcher, T.; et al. Visceral and somatic pain modalities reveal NaV 1.7-independent visceral nociceptive pathways. J. Physiol. 2017, 595, 2661–2679. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Wook, Y.Y.; Sik, N.H.; Ho, K.S.; Mo, C.J. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994, 59, 369–376. [Google Scholar] [CrossRef]
- Jasmin, L.; Kohan, L.; Franssen, M.; Janni, G.; Goff, J.R. The cold plate as a test of nociceptive behaviors: Description and application to the study of chronic neuropathic and inflammatory pain models. Pain 1998, 75, 367–382. [Google Scholar] [CrossRef]
- Brenner, D.S.; Golden, J.P.; Gereau, R.W., 4th. A novel behavioral assay for measuring cold sensation in mice. PLoS ONE 2012, 7, e39765. [Google Scholar] [CrossRef]
- Allchorne, A.J.; Broom, D.C.; Woolf, C.J. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol. Pain 2005, 1, 36. [Google Scholar] [CrossRef]
- Huerta, M.Á.; Cisneros, E.; Alique, M.; Roza, C. Strategies for measuring non-evoked pain in preclinical models of neuropathic pain: Systematic review. Neurosci. Biobehav. Rev. 2024, 163, 105761. [Google Scholar] [CrossRef]
- Djouhri, L.; Koutsikou, S.; Fang, X.; McMullan, S.; Lawson, S.N. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J. Neurosci. 2006, 26, 1281–1292. [Google Scholar] [CrossRef]
- Matson, D.J.; Hamamoto, D.T.; Bregman, H.; Cooke, M.; DiMauro, E.F.; Huang, L.; Johnson, D.; Li, X.; McDermott, J.; Morgan, C.; et al. Inhibition of Inactive States of Tetrodotoxin-Sensitive Sodium Channels Reduces Spontaneous Firing of C-Fiber Nociceptors and Produces Analgesia in Formalin and Complete Freund’s Adjuvant Models of Pain. PLoS ONE 2015, 10, e0138140. [Google Scholar] [CrossRef]
- Black, J.A.; Liu, S.; Tanaka, M.; Cummins, T.R.; Waxman, S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004, 108, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J., 3rd; England, J.D.; Soignier, R.D.; Nolan, P.; Minor, L.D.; Liu, Z.P.; Levinson, S.R.; Paul, D. Ibuprofen blocks changes in Na v 1.7 and 1.8 sodium channels associated with complete Freund’s adjuvant-induced inflammation in rat. J. Pain 2004, 5, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.A.; Stirling, L.C.; Forlani, G.; Baker, M.D.; Matthews, E.A.; Dickenson, A.H.; Wood, J.N. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA 2004, 101, 12706–12711. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, D.C.; Levinson, S.R.; Peters, M.C.; Koszowski, A.G.; Tzabazis, A.Z.; Gilly, W.F.; Wilson, S.P. Decrease in inflammatory hyperalgesia by herpes vector-mediated knockdown of Nav1.7 sodium channels in primary afferents. Hum. Gene Ther. 2005, 16, 271–277. [Google Scholar] [CrossRef]
- Moreno, A.M.; Alemán, F.; Catroli, G.F.; Hunt, M.; Hu, M.; Dailamy, A.; Pla, A.; Woller, S.A.; Palmer, N.; Parekh, U.; et al. Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice. Sci. Transl. Med. 2021, 13, eaay9056. [Google Scholar] [CrossRef]
- Li, S.; Jin, Y.; Li, M.; Yu, H. NAN-190, a 5-HT1A antagonist, alleviates inflammatory pain by targeting Nav1.7 sodium channels. Life Sci. 2023, 319, 121520. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, R.; Zhang, M.; Chen, D.; Zhang, Q.; Zhang, N.; Shi, Y.; Hu, X.; Li, N.; Fang, Q. NaV1.7 Channel Blocker [Ala5, Phe6, Leu26, Arg28]GpTx-1 Attenuates CFA-induced Inflammatory Hypersensitivity in Rats via Endogenous Enkephalin Mechanism. J. Pain 2023, 24, 840–859. [Google Scholar] [CrossRef]
- Martina, M.; Banderali, U.; Yogi, A.; Arbabi Ghahroudi, M.; Liu, H.; Sulea, T.; Durocher, Y.; Hussack, G.; van Faassen, H.; Chakravarty, B.; et al. A Novel Antigen Design Strategy to Isolate Single-Domain Antibodies that Target Human Nav1.7 and Reduce Pain in Animal Models. Adv. Sci. 2024, 11, e2405432. [Google Scholar] [CrossRef]
- Bankar, G.; Goodchild, S.J.; Howard, S.; Nelkenbrecher, K.; Waldbrook, M.; Dourado, M.; Shuart, N.G.; Lin, S.; Young, C.; Xie, Z.; et al. Selective NaV1.7 Antagonists with Long Residence Time Show Improved Efficacy against Inflammatory and Neuropathic Pain. Cell Rep. 2018, 24, 3133–3145. [Google Scholar] [CrossRef]
- Han, P.; Liu, S.; Zhang, M.; Zhao, J.; Wang, Y.; Wu, G.; Mi, W. Inhibition of Spinal Interlukin-33/ST2 Signaling and Downstream ERK and JNK Pathways in Electroacupuncture Analgesia in Formalin Mice. PLoS ONE 2015, 10, e0129576. [Google Scholar] [CrossRef] [PubMed]
- Stamboulian, S.; Choi, J.S.; Ahn, H.S.; Chang, Y.W.; Tyrrell, L.; Black, J.A.; Waxman, S.G.; Dib-Hajj, S.D. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J. Neurosci. 2010, 30, 1637–1647. [Google Scholar] [CrossRef]
- Zhao, Z.; Pan, T.; Chen, S.; Harvey, P.J.; Zhang, J.; Li, X.; Yang, M.; Huang, L.; Wang, S.; Craik, D.J.; et al. Design, synthesis, and mechanism of action of novel μ-conotoxin KIIIA analogues for inhibition of the voltage-gated sodium channel Nav1.7. J. Biol. Chem. 2023, 299, 103068. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Ou-Yang, X.S.; Zhou, P.; Dong, L.Y.; Shao, L.M.; Wang, K.W.; Liu, Y.N. Inhibition of TTX-S Na+ currents by a novel blocker QLS-278 for antinociception. J. Pharmacol. Exp. Ther. 2024, 392, 100030. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Maier, C.; Attal, N.; Binder, A.; Bouhassira, D.; Cruccu, G.; Finnerup, N.B.; Haanpää, M.; Hansson, P.; Hüllemann, P.; et al. Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain 2017, 158, 261–272. [Google Scholar] [CrossRef]
- Wei, Z.; Fei, Y.; Su, W.; Chen, G. Emerging Role of Schwann Cells in Neuropathic Pain: Receptors, Glial Mediators and Myelination. Front. Cell Neurosci. 2019, 13, 116. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef]
- Boakye, P.A.; Tang, S.J.; Smith, P.A. Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β. Front. Pain Res. 2021, 2, 698157. [Google Scholar] [CrossRef]
- Li, M.; Zhang, S.J.; Yang, L.; Fang, X.L.; Hu, H.F.; Zhao, M.Y.; Li, L.; Guo, Y.Y.; Shao, J.P. Voltage-gated sodium channel 1.7 expression decreases in dorsal root ganglia in a spinal nerve ligation neuropathic pain model. Kaohsiung J. Med. Sci. 2019, 35, 493–500. [Google Scholar] [CrossRef]
- Liu, C.; Cao, J.; Ren, X.; Zang, W. Nav1.7 protein and mRNA expression in the dorsal root ganglia of rats with chronic neuropathic pain. Neural Regen. Res. 2012, 7, 1540–1544. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Dong, W.; Zhang, L.; Yang, X. Activating Sirt1 by resveratrol suppresses Nav1.7 expression in DRG through miR-182 and alleviates neuropathic pain in rats. Channels 2020, 1, 69–78. [Google Scholar] [CrossRef]
- Luo, G.; Chen, L.; Easton, A.; Newton, A.; Bourin, C.; Shields, E.; Mosure, K.; Soars, M.G.; Knox, R.J.; Matchett, M.; et al. Discovery of Indole- and Indazole-acylsulfonamides as Potent and Selective NaV1.7 Inhibitors for the Treatment of Pain. J. Med. Chem. 2019, 62, 831–856. [Google Scholar] [CrossRef]
- Ghelardini, C.; Desaphy, J.F.; Muraglia, M.; Corbo, F.; Matucci, R.; Dipalma, A.; Bertucci, C.; Pistolozzi, M.; Nesi, M.; Norcini, M.; et al. Effects of a new potent analog of tocainide on hNav1.7 sodium channels and in vivo neuropathic pain models. Neuroscience 2010, 169, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stratton, H.J.; Lorca, S.A.; Grace, P.M.; Khanna, R. Small molecule targeting NaV1.7 via inhibition of the CRMP2-Ubc9 interaction reduces pain in chronic constriction injury (CCI) rats. Channels 2022, 16, 1–8. [Google Scholar] [CrossRef]
- Dustrude, E.T.; Wilson, S.M.; Ju, W.; Xiao, Y.; Khanna, R. CRMP2 protein SUMOylation modulates NaV1.7 channel trafficking. J. Biol. Chem. 2013, 288, 24316–24331. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Moutal, A.; Yu, J.; Chew, L.A.; Isensee, J.; Chawla, R.; Gomez, K.; Luo, S.; Zhou, Y.; Chefdeville, A.; et al. Selective targeting of NaV1.7 via inhibition of the CRMP2-Ubc9 interaction reduces pain in rodents. Sci. Transl. Med. 2021, 13, eabh1314. [Google Scholar] [CrossRef]
- Dustrude, E.T.; Moutal, A.; Yang, X.; Wang, Y.; Khanna, M.; Khanna, R. Hierarchical CRMP2 posttranslational modifications control NaV1.7 function. Proc. Natl. Acad. Sci. USA 2016, 113, E8443–E8452. [Google Scholar] [CrossRef]
- Yin, J.B.; Liu, H.X.; Dong, Q.Q.; Wu, H.H.; Liang, Z.W.; Fu, J.T.; Zhao, W.J.; Hu, H.Q.; Guo, H.W.; Zhang, T.; et al. Correlative increasing expressions of KIF5b and Nav1.7 in DRG neurons of rats under neuropathic pain conditions. Physiol. Behav. 2023, 263, 114115. [Google Scholar] [CrossRef]
- Berta, T.; Poirot, O.; Pertin, M.; Ji, R.R.; Kellenberger, S.; Decosterd, I. Transcriptional and functional profiles of voltage-gated Na(+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol. Cell Neurosci. 2008, 37, 196–208. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Zhang, Y.; Xun, X.; Tang, D.; Peng, D.; Yi, J.; Liu, Z.; Shi, X. Synthesis and analgesic effects of μ-TRTX-Hhn1b on models of inflammatory and neuropathic pain. Toxins 2014, 6, 2363–2378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shu, J.; Yang, H.; Hong, K.; Yang, X.; Guo, W.; Fang, J.; Li, F.; Liu, T.; Shan, Z.; et al. Nav1.7 Modulator Bearing a 3-Hydroxyindole Backbone Holds the Potential to Reverse Neuropathic Pain. ACS Chem. Neurosci. 2024, 15, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Moutal, A.; Dustrude, E.T.; Largent-Milnes, T.M.; Vanderah, T.W.; Khanna, M.; Khanna, R. Blocking CRMP2 SUMOylation reverses neuropathic pain. Mol. Psychiatry 2018, 23, 2119–2121. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.; Stratton, H.J.; Duran, P.; Loya, S.; Tang, C.; Calderon-Rivera, A.; François-Moutal, L.; Khanna, M.; Madura, C.L.; Luo, S.; et al. Identification and targeting of a unique NaV1.7 domain driving chronic pain. Proc. Natl. Acad. Sci. USA 2023, 120, e2217800120. [Google Scholar] [CrossRef]
- Fukuoka, T.; Noguchi, K. Contribution of the spared primary afferent neurons to the pathomechanisms of neuropathic pain. Mol. Neurobiol. 2002, 26, 57–67. [Google Scholar] [CrossRef]
- Shim, B.; Ringkamp, M.; Lambrinos, G.L.; Hartke, T.V.; Griffin, J.W.; Meyer, R.A. Activity-dependent slowing of conduction velocity in uninjured L4 C fibers increases after an L5 spinal nerve injury in the rat. Pain 2007, 128, 40–51. [Google Scholar] [CrossRef]
- Fukuoka, T.; Miyoshi, K.; Noguchi, K. De novo expression of Nav1.7 in injured putative proprioceptive afferents: Multiple tetrodotoxin-sensitive sodium channels are retained in the rat dorsal root after spinal nerve ligation. Neuroscience 2015, 284, 693–706. [Google Scholar] [CrossRef]
- Nassar, M.A.; Levato, A.; Stirling, L.C.; Wood, J.N. Neuropathic pain develops normally in mice lacking both Na(v)1.7 and Na(v)1.8. Mol. Pain 2005, 1, 24. [Google Scholar] [CrossRef]
- Grubinska, B.; Chen, L.; Alsaloum, M.; Rampal, N.; Matson, D.J.; Yang, C.; Taborn, K.; Zhang, M.; Youngblood, B.; Liu, D.; et al. Rat NaV1.7 loss-of-function genetic model: Deficient nociceptive and neuropathic pain behavior with retained olfactory function and intra-epidermal nerve fibers. Mol. Pain 2019, 15, 1744806919881846. [Google Scholar] [CrossRef]
- Hildebrand, M.E.; Smith, P.L.; Bladen, C.; Eduljee, C.; Xie, J.Y.; Chen, L.; Fee-Maki, M.; Doering, C.J.; Mezeyova, J.; Zhu, Y.; et al. A novel slow-inactivation-specific ion channel modulator attenuates neuropathic pain. Pain 2011, 152, 833–843. [Google Scholar] [CrossRef]
- Yang, S.W.; Ho, G.D.; Tulshian, D.; Bercovici, A.; Tan, Z.; Hanisak, J.; Brumfield, S.; Matasi, J.; Sun, X.; Sakwa, S.A.; et al. Bioavailable pyrrolo-benzo-1,4-diazines as Na(v)1.7 sodium channel blockers for the treatment of pain. Bioorg. Med. Chem. Lett. 2014, 24, 4958–4962. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Wu, W.; Li, Z.; Yang, G.; Qin, J.; Wang, Y.; Hu, Z.; Dong, H.; Hou, L.; Hu, G.; et al. Discovery of aminocyclohexene analogues as selective and orally bioavailable hNav1.7 inhibitors for analgesia. Bioorg. Med. Chem. Lett. 2017, 27, 4979–4984. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, L.; Zhu, F.; Xia, W.; Wen, T.; Xia, R.; Yu, X.; Xu, D.; Peng, C. Ectopic expression of Nav1.7 in spinal dorsal horn neurons induced by NGF contributes to neuropathic pain in a mouse spinal cord injury model. Front. Mol. Neurosci. 2023, 16, 1091096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dougherty, P.M. Enhanced excitability of primary sensory neurons and altered gene expression of neuronal ion channels in dorsal root ganglion in paclitaxel-induced peripheral neuropathy. Anesthesiology 2014, 120, 1463–1475. [Google Scholar] [CrossRef]
- Xia, Z.; Xiao, Y.; Wu, Y.; Zhao, B. Sodium channel Nav1.7 expression is upregulated in the dorsal root ganglia in a rat model of paclitaxel-induced peripheral neuropathy. SpringerPlus 2016, 5, 1738. [Google Scholar] [CrossRef]
- Li, Y.; North, R.Y.; Rhines, L.D.; Tatsui, C.E.; Rao, G.; Edwards, D.D.; Cassidy, R.M.; Harrison, D.S.; Johansson, C.A.; Zhang, H.; et al. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J. Neurosci. 2018, 38, 1124–1136. [Google Scholar] [CrossRef]
- Wang, G.J.; Zhang, X.; Huang, L.D.; Xiao, Y. Involvement of the Sodium Channel Nav1.7 in Paclitaxel-induced Peripheral Neuropathy through ERK1/2 Signaling in Rats. Curr. Neurovasc. Res. 2020, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.J.; Alsaloum, M.; Higerd, G.P.; Liu, S.; Zhao, P.; Dib-Hajj, F.B.; Waxman, S.G.; Dib-Hajj, S.D. Paclitaxel increases axonal localization and vesicular trafficking of Nav1.7. Brain 2021, 144, 1727–1737. [Google Scholar] [CrossRef]
- Chandra, S.; Wang, Z.; Tao, X.; Chen, O.; Luo, X.; Ji, R.R.; Bortsov, A.V. Computer-aided Discovery of a New Nav1.7 Inhibitor for Treatment of Pain and Itch. Anesthesiology 2020, 133, 611–627. [Google Scholar] [CrossRef]
- Misset, J.L.; Bleiberg, H.; Sutherland, W.; Bekradda, M.; Cvitkovic, E. Oxaliplatin clinical activity: A review. Crit. Rev. Oncol. Hematol. 2000, 35, 75–93. [Google Scholar] [CrossRef]
- Beijers, A.J.; Mols, F.; Vreugdenhil, G. A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support. Care Cancer 2014, 22, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Sereno, M.; Gutiérrez-Gutiérrez, G.; Rubio, J.M.; Apellániz-Ruiz, M.; Sánchez-Barroso, L.; Casado, E.; Falagan, S.; López-Gómez, M.; Merino, M.; Gómez-Raposo, C.; et al. Genetic polymorphisms of SCN9A are associated with oxaliplatin-induced neuropathy. BMC Cancer 2017, 17, 63. [Google Scholar] [CrossRef]
- Xu, L.J.; Wang, J.; Li, Y.D.; Shen, K.F.; He, X.H.; Wu, W.; Liu, C.C. Reduction of SIRT1-Mediated Epigenetic Upregulation of Nav1.7 Contributes to Oxaliplatin-Induced Neuropathic Pain. Pain Physician 2023, 26, E213–E222. [Google Scholar]
- Braden, K.; Stratton, H.J.; Salvemini, D.; Khanna, R. Small molecule targeting NaV1.7 via inhibition of the CRMP2-Ubc9 interaction reduces and prevents pain chronification in a mouse model of oxaliplatin-induced neuropathic pain. Neurobiol. Pain 2021, 11, 100082. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, X.; Tang, Q.; Deng, Y. Pain-related mediators underlie incision-induced mechanical nociception in the dorsal root ganglia. Neural Regen. Res. 2013, 8, 3325–3333. [Google Scholar] [CrossRef]
- Sun, J.; Li, N.; Duan, G.; Liu, Y.; Guo, S.; Wang, C.; Zhu, C.; Zhang, X. Increased Nav1.7 expression in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision in rats. Mol. Pain 2018, 14, 1744806918782323. [Google Scholar] [CrossRef]
- Barbosa Neto, J.O.; Garcia, J.B.S.; Cartágenes, M.D.S.S.; Amaral, A.G.; Onuchic, L.F.; Ashmawi, H.A. Influence of androgenic blockade with flutamide on pain behaviour and expression of the genes that encode the NaV1.7 and NaV1.8 voltage-dependent sodium channels in a rat model of postoperative pain. J. Transl. Med. 2019, 17, 287. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhang, J.; Hong, Y.S.; Li, N.B.; Liu, Y.; Zhang, M.; Wu, W.Y.; Zheng, H.; Lampert, A.; Zhang, X.W. NGF-Induced Nav1.7 Upregulation Contributes to Chronic Post-surgical Pain by Activating SGK1-Dependent Nedd4-2 Phosphorylation. Mol. Neurobiol. 2021, 58, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Xiang, G.; Zhang, X.; Yuan, R.; Zhan, H.; Qi, D. A single-nucleotide polymorphism in SCN9A may decrease postoperative pain sensitivity in the general population. Anesthesiology 2013, 118, 436–442. [Google Scholar] [CrossRef]
- Beckley, J.T.; Pajouhesh, H.; Luu, G.; Klas, S.; Delwig, A.; Monteleone, D.; Zhou, X.; Giuvelis, D.; Meng, I.D.; Yeomans, D.C.; et al. Antinociceptive properties of an isoform-selective inhibitor of Nav1.7 derived from saxitoxin in mouse models of pain. Pain 2021, 162, 1250–1261. [Google Scholar] [CrossRef]
- Staunton, C.A.; Lewis, R.; Barrett-Jolley, R. Ion channels and osteoarthritic pain: Potential for novel analgesics. Curr. Pain Headache Rep. 2013, 17, 378. [Google Scholar] [CrossRef]
- Reimann, F.; Cox, J.J.; Belfer, I.; Diatchenko, L.; Zaykin, D.V.; McHale, D.P.; Drenth, J.P.; Dai, F.; Wheeler, J.; Sanders, F.; et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc. Natl. Acad. Sci. USA 2010, 107, 5148–5153. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.; Dickenson, A.H. Osteoarthritis-dependent changes in antinociceptive action of Nav1.7 and Nav1.8 sodium channel blockers: An in vivo electrophysiological study in the rat. Neuroscience 2015, 295, 103–116. [Google Scholar] [CrossRef]
- Hestehave, S.; Allen, H.N.; Gomez, K.; Duran, P.; Calderon-Rivera, A.; Loya-López, S.; Rodríguez-Palma, E.J.; Khanna, R. Small molecule targeting NaV1.7 via inhibition of CRMP2-Ubc9 interaction reduces pain-related outcomes in a rodent osteoarthritic model. Pain 2024, 166, 99–111. [Google Scholar] [CrossRef]
- Abboud, C.; Duveau, A.; Bouali-Benazzouz, R.; Massé, K.; Mattar, J.; Brochoire, L.; Fossat, P.; Boué-Grabot, E.; Hleihel, W.; Landry, M. Animal models of pain: Diversity and benefits. J. Neurosci. Methods 2021, 348, 108997. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xie, Y.F.; Smith, R.; Ratté, S.; Prescott, S.A. Discordance between preclinical and clinical testing of Na V 1.7-selective inhibitors for pain. Pain 2025, 166, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, N.; Fahmideh, F.; Pascale, A.; Allegri, M.; Govoni, S. Neuropathic Pain in Aged People: An Unresolved Issue Open to Novel Drug Approaches, Focusing on Painful Diabetic Neuropathy. Curr. Neuropharmacol. 2024, 22, 53–64. [Google Scholar] [CrossRef]
- Ruiz-Medina, J.; Baulies, A.; Bura, S.A.; Valverde, O. Paclitaxel-induced neuropathic pain is age dependent and devolves on glial response. Eur. J. Pain 2013, 17, 75–85. [Google Scholar] [CrossRef]
- Mogil, J.S.; Wilson, S.G.; Bon, K.; Lee, S.E.; Chung, K.; Raber, P.; Pieper, J.O.; Hain, H.S.; Belknap, J.K.; Hubert, L.; et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception. Pain 1999, 80, 67–82. [Google Scholar] [CrossRef]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef]
- Shansky, R.M.; Murphy, A.Z. Considering sex as a biological variable will require a global shift in science culture. Nat. Neurosci. 2021, 24, 457–464. [Google Scholar] [CrossRef]
- Sadler, K.E.; Mogil, J.S.; Stucky, C.L. Innovations and advances in modelling and measuring pain in animals. Nat. Rev. Neurosci. 2022, 23, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Bohnert, T.; Damian-Iordache, V.; Gibson, C.; Hsu, C.P.; Krishnatry, A.S.; Liederer, B.M.; Lin, J.; Lu, Q.; Mettetal, J.T.; et al. Translational pharmacokinetic-pharmacodynamic analysis in the pharmaceutical industry: An IQ Consortium PK-PD Discussion Group perspective. Drug Discov. Today 2017, 22, 1447–1459. [Google Scholar] [CrossRef]
- Ballard, J.E.; Pall, P.S.; Vardigan, J.; Zhao, F.; Holahan, M.A.; Zhou, X.; Jochnowitz, N.; Kraus, R.L.; Klein, R.M.; Henze, D.A.; et al. Translational Pharmacokinetic-Pharmacodynamic Modeling of NaV1.7 Inhibitor MK-2075 to Inform Human Efficacious Dose. Front. Pharmacol. 2021, 12, 786078. [Google Scholar] [CrossRef]
- Jansson-Löfmark, R.; Hjorth, S.; Gabrielsson, J. Does In Vitro Potency Predict Clinically Efficacious Concentrations? Clin. Pharmacol. Ther. 2020, 108, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Frézel, N.; Dib-Hajj, S.D.; Waxman, S.G. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol. Pain 2012, 8, 82. [Google Scholar] [CrossRef]
- Kraus, R.L.; Zhao, F.; Pall, P.S.; Zhou, D.; Vardigan, J.D.; Danziger, A.; Li, Y.; Daley, C.; Ballard, J.E.; Clements, M.K.; et al. Na(v)1.7 target modulation and efficacy can be measured in nonhuman primate assays. Sci. Transl. Med. 2021, 13, eaay1050. [Google Scholar] [CrossRef] [PubMed]
- Morinville, A.; Fundin, B.; Meury, L.; Juréus, A.; Sandberg, K.; Krupp, J.; Ahmad, S.; O’Donnell, D. Distribution of the voltage-gated sodium channel Na(v)1.7 in the rat: Expression in the autonomic and endocrine systems. J. Comp. Neurol. 2007, 504, 680–689. [Google Scholar] [CrossRef]
- Kim, J.S.; Meeker, S.; Ru, F.; Tran, M.; Zabka, T.S.; Hackos, D.; Undem, B.J. Role of NaV1.7 in postganglionic sympathetic nerve function in human and guinea-pig arteries. J. Physiol. 2024, 602, 3505–3518. [Google Scholar] [CrossRef]
- Kollarik, M.; Ru, F.; Pavelkova, N.; Mulcahy, J.; Hunter, J.; Undem, B.J. Role of NaV 1.7 in action potential conduction along human bronchial vagal afferent C-fibres. Br. J. Pharmacol. 2022, 179, 242–251. [Google Scholar] [CrossRef]
- Rush, A.M.; Dib-Hajj, S.D.; Liu, S.; Cummins, T.R.; Black, J.A.; Waxman, S.G. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc. Natl. Acad. Sci. USA 2006, 103, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Dourado, M.; Reese, R.M.; Huang, K.; Shields, S.D.; Stark, K.L.; Maksymetz, J.; Lin, H.; Kaminker, J.S.; Jung, M.; et al. Nav1.7 is essential for nociceptor action potentials in the mouse in a manner independent of endogenous opioids. Neuron 2023, 111, 2642–2659. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Tagen, M.; Chang, J.H.; Boyce-Rustay, J.; Friesenhahn, M.; Hackos, D.H.; Hains, A.; Sutherlin, D.; Ward, M.; Cho, W. Safety, Tolerability, and Pharmacokinetics of GDC-0276, a Novel NaV1.7 Inhibitor, in a First-in-Human, Single- and Multiple-Dose Study in Healthy Volunteers. Clin. Drug Investig. 2019, 39, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, J.V.; Beckley, J.T.; Klas, S.D.; Odink, D.A.; Delwig, A.; Pajouhesh, H.; Monteleone, D.; Zhou, X.; Du Bois, J.; Yeomans, D.C.; et al. ST-2560, a selective inhibitor of the NaV1.7 sodium channel, affects nocifensive and cardiovascular reflexes in non-human primates. Br. J. Pharmacol. 2024, 181, 3160–3171. [Google Scholar] [CrossRef] [PubMed]
- Regan, C.P.; Morissette, P.; Kraus, R.L.; Wang, E.; Arrington, L.; Vavrek, M.; de Hoon, J.; Depre, M.; Lodeweyck, T.; Demeyer, I.; et al. Autonomic Dysfunction Linked to Inhibition of the Nav1.7 Sodium Channel. Circulation 2024, 149, 1394–1396. [Google Scholar] [CrossRef]
- Martina, M.; Perkins, M. Small molecules versus biologics: The quest for the ideal Nav1.7 inhibitor. Drug Target. Rev. 2015, 2, 34–37. [Google Scholar]
- Osteen, J.D.; Immani, S.; Tapley, T.L.; Indersmitten, T.; Hurst, N.W.; Healey, T.; Aertgeerts, K.; Negulescu, P.A.; Lechner, S.M. Pharmacology and Mechanism of Action of Suzetrigine, a Potent and Selective NaV1.8 Pain Signal Inhibitor for the Treatment of Moderate to Severe Pain. Pain Ther. 2025, 14, 655–674. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wen, J.; Yang, W.; Wang, C.; Gao, L.; Zheng, L.H.; Wang, T.; Ran, K.; Li, Y.; Li, X.; et al. Gain-of-function mutations in SCN11A cause familial episodic pain. Am. J. Hum. Genet. 2013, 93, 957–966. [Google Scholar] [CrossRef]
- Busserolles, J.; Tsantoulas, C.; Eschalier, A.; López García, J.A. Potassium channels in neuropathic pain: Advances, challenges, and emerging ideas. Pain 2016, 157 (Suppl. S1), S7–S14. [Google Scholar] [CrossRef]
- Patel, R.; Montagut-Bordas, C.; Dickenson, A.H. Calcium channel modulation as a target in chronic pain control. Br. J. Pharmacol. 2018, 175, 2173–2184. [Google Scholar] [CrossRef]
- Dulai, J.S.; Smith, E.S.J.; Rahman, T. Acid-sensing ion channel 3: An analgesic target. Channels 2021, 15, 94–127. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carvajal, A.; González-Muñiz, R.; Fernández-Ballester, G.; Ferrer-Montiel, A. Investigational drugs in early phase clinical trials targeting thermotransient receptor potential (thermoTRP) channels. Expert Opin. Investig. Drugs 2020, 29, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Jo, Y.Y.; Kim, Y.H.; Park, C.K. Current insights and therapeutic strategies for targeting TRPV1 in neuropathic pain management. Life Sci. 2024, 355, 122954. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).