Abstract
NPS6 is one of the nonribosomal peptide synthetase (NRPS) family members. The roles of NPS6 in ascomycetes are well known, but its roles in Fusarium oxysporum are unidentified. We investigated its function in the growth, morphology, stress sensitivity, allelochemical secretion, and pathogenesis in F. oxysporum (FoNPS6). The partial deletion of FoNPS6 orthologs (ΔFON-NPS6) resulted in hypersensitivity to H2O2 and KO2, iron depletion, and reduced virulence. Full virulence was restored by complementation. ΔFON-NPS6 not only inhibited spore formation but also displayed hyphal growth patterns that differed significantly from the wild-type strain. Plant leaching released allelochemicals, which FON-NPS6 broke down. All of these findings show that FoNPS6 quantitatively increases F. oxysporum’s pathogenicity.
1. Introduction
Fusarium oxysporum, a globally distributed soil-borne pathogen, is notorious for causing a variety of wilt diseases in economically significant crops. This filamentous fungus is highly diverse, with specific forms (forma specialis) adapting to infect a broad spectrum of host plants, including the well-known Fusarium wilt in watermelon, caused by Fusarium oxysporum f. sp. Niveum [1]. The pathogenicity of F. oxysporum is largely attributed to its ability to produce a wide range of bioactive secondary metabolites, which are crucial for the successful colonization and infection of its host plants. A key component in the pathogenic arsenal of F. oxysporum and other related species is the production of nonribosomal peptides (NRPs), which are synthesized by nonribosomal peptide synthetases (NRPSs) [2]. These enzymes play a critical role in the biosynthesis of many virulence factors, including phytotoxins that promote disease progression.
NRPSs are a group of enzymes characterized by their intricate structure, primarily enabling the production of small peptides without relying on ribosomal machinery for protein synthesis. Fungal NRPS-derived compounds are critical in mediating plant–microbe interactions. Research has established that NRPS metabolites produced by phytopathogenic ascomycetes function as phytotoxins. These toxins, specifically AM toxin and HC toxin synthesized by the apple pathotype of Alternaria alternata, are crucial contributors to the pathogenicity of the fungus. Both AM and HC toxins exhibit high toxicity not only to the host organisms but also to the producing fungi themselves [1,2,3]. A total of 12 genes encoding NRPS were identified in the Cochliobolus heterostrophus genome. NPS6 was found to be virulent only to maize when only a single deletion was made [2]. In numerous instances, various pathogen species have adopted shared pathogenic strategies, leading to a phenomenon where pathogenic gene function is maintained through vertical inheritance and exposure to common host defense selection factors during pathogenesis in the same or related hosts. NPS6 has been confirmed to be conserved across filamentous ascomycetes, similar to observations in C.heterostrophus. The disruption of NPS6 orthologs in Cochliobolus miyabeanus, a pathogen of rice; Fusarium graminearum, which infects wheat, maize, and barley; and Alternaria brassicicola, a pathogen affecting cruciferous plants led to reduced virulence toward its specific hosts as well as heightened susceptibility to reactive oxygen species and iron-deficient conditions. NPS6 was demonstrated to be responsible for extracellular siderophore biosynthesis [3].
Achromobactin, a siderophore produced by Erwinia chrysanthemi, has been shown to contribute to the pathogen’s virulence [4]. Glycophilic bacteria produced by the closely related apple disease Erwinia amylovora causes a successful infection with desferrioxamine, which is mandatory for apple owners [5]. In contrast, siderophiles do not appear to be necessary for virulence in the crown gall pathogen Agrobacterium tumefaciens [6] or another closely related species, Erwinia carotovora subsp carotovora [7]. This suggests that siderophores have distinct roles in plant–microbe interactions in various pathosystems. NPS6 has been identified as a conserved factor essential for the virulence of the plant pathogen Fusarium graminearum [8], and as there is not yet a report about the NPS6 gene in Fusarium oxysporum, its function should be further determined.
Phytochemicals can be released into the environment through various processes, such as plant leaching, root exudation, volatilization, and the decomposition of plant residues [9,10]. Current research indicates that allelochemicals, such as phenolic acid (PA), can modify cell membrane permeability and inhibit the function of H+-ATPase enzymes, which subsequently causes damage to both DNA and proteins. In more severe scenarios, this disruption can stimulate the formation of lipid peroxidation signaling molecules. The cumulative impact of these alterations can result in extensive cellular injury, potentially compromising cellular integrity and, in extreme cases, inducing cell death. This sequence of events highlights the complex biochemical interactions by which allelochemicals exert their toxic effects on cellular structures and functions [11]. Another adverse effect of allelochemicals on plants is that they tend to alter the abundance and community structure of rhizobial flora, disrupting the balance of the microbial community [12,13]. Since NPS6 has been confirmed to have a relation to the virulence of F. graminearum [14], we speculate that NPS6 might affect the allelochemical secretion of F. graminearum. However, whether NPS6 has the same role in plants has not been determined before.
Therefore, we aim to identify the NPS6 gene in F. oxysporum, clarify the role of the NPS6 gene in the pathogenic behavior of F. oxysporum, and then verify the effects of NPS6 in F. oxysporum on allelochemical secretion in plants.
2. Materials and Methods
2.1. Fungal Isolates, Plant Materials, and Growth Conditions
F. oxysporum f. sp. niveum race 1 was obtained from Jiangsu Academy of Agricultural Sciences; it was isolated from watermelon which had been infected with Fusarium wilt disease and was stored at −80 °C with 50% glycerol as a microconidial suspension. Genomic DNA was extracted from the Fusarium oxysporum strain using the E.Z.N.A.™ Fungus DNA Kit (Omega Bio-Tek, Norcross, GA, USA) following the manufacturer’s instructions for fungal DNA isolation. PCR amplification was performed using Phusion High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA). The pathotype of the isolate was confirmed periodically by plant infection assays. Unless mentioned otherwise, it was grown on CM at 24 °C under continuous fluorescent light. The minimal medium (MM) was complete medium (CM) without yeast extract, acid-hydrolyzed casein, and enzymically hydrolyzed casein.
Soils collected from Nanjing, Jiangsu Province, China, were sterilized by triple autoclaving (121 °C for 1 h over three consecutive days) with the following chemical properties: organic matter content: 51.23 mg/g; total nitrogen: 3.13 mg/g; total phosphorus: 2.97 mg/g; pH: 6.8. Watermelon seeds (Sumi No. 5) were purchased from the Jiangsu Academy of Agricultural Sciences, China. This cultivar has no resistance to Fusarium wilt.
2.2. PCR Cloning of the FONPS6 Gene
Genomic DNA from the F. oxysporum strain was isolated using the E.Z.N.A.™ Fungus DNA Kit (Omega Bio-Tek, Norcross, GA, USA) in accordance with the manufacturer’s protocol. PCR amplification was carried out using Phusion DNA polymerase (NEB) following the provided instructions. Using the specified primer pair, orthologs of the F. oxysporum FONPS6 genes were successfully amplified via PCR from the NPS6 genes of F. graminearum and A. brassicicola. This approach enables the comparative analysis of gene function and conservation across these different fungal species, providing insights into the role of NPS6 orthologs in pathogenicity and potential evolutionary relationships among the phytopathogens (NPF: 5′-ATGGGAGACGTTCAGTCGTC-3′; NPR: 5′-CTAGATCAAATCGTTAGGGTT-3′). PCR products of approximately 6kb were inserted into the pGEM-T Easy vector (Promega, Madison, WI, USA) for sequencing.
2.3. The Targeted Disruption of the FONPS6 Gene
Following two rounds of PCR amplification, a chimeric fragment was generated, consisting of a portion of the FONPS6 gene fused with the HYG segment [15] using a Phusion DNA polymerase (NEB). This process facilitated the construction of a recombinant DNA segment essential for further analysis and experimentation. The HYG gene is responsible for encoding a phosphatase that confers resistance to chlorothalonil. This gene operates under the regulatory influence of the trpC promoter and terminator derived from Aspergill us niger. In the first round, HYG fragment was amplified from pUCATPH [16] with the primers M13R and M13F, respectively. The 5′NPS6 fragment was amplified by PCR with the primers FP1(5′-GCGTTATGACGTACCTGTTCTTCG-3′) and M13 RRP1(5′-TCCTGTGTGAAATTGTTATCCGCTCTCGCCACCGATACCAAGACT-3′), and the 3′NPS6 fragment was amplified with M13 FFP2 (5′-GTCGTGACTGGGAAAACCCTGGCGCAGATTGGTATTGAGGCTGACGAG-3′) and RP2 (5′-CTCGTCAGCCTCAATACCAATCTG-3′) from the genomic DNA of F. oxysporum. In this study, the sequences highlighted in primers M13RP1 and M13FFP2 represent oligonucleotides that are completely complementary to the sequences of M13R and M13F primers. During the second PCR round, the 5′NPS6::HYG disruption fragment was created by directly fusing the 5′NPS6 and HYG segments without the need for additional primers. Subsequently, the HYG fragment was combined with the 3′NPS6 segment to produce the HYG::3′NPS6 disruption fragment. The resulting fragments, each at a concentration of 10 µg/mL, were then mixed and directly introduced into the protoplasts of the wild-type strain using CaCl2 and polyethylene glycol [17]. Transformants were selected on CM agar supplemented with 200 µg/mL hygromycin (Roche Applied Science, Penzberg, Germany) and screened for successful integration. The deletion of FONPS6 in the transformants was verified through PCR using M13R and M13F primers. Transformants with positive PCR results for the remaining three primers were used as controls, while the PCR results for M13R and M13F were negative.
2.4. Complementation
The F. oxysporum FONPS6 ORF (~5 kb) with 5′ and 3′ flanking sequences (each ~2 kb) was PCR-amplified with the primer pair FP1/RP2. Approximately 5 µg of the PCR product and 10 µg of plasmid pII99, containing the nptII gene [18,19], were used to transform strain ΔFON-NPS6. Secondary screening of the transformants was performed on CM without salts [20] containing 400 µg/mL of G418. G418-resistant transformants were then selected and tested for hygromycin B sensitivity. Through a polymerase chain reaction (PCR) analysis, it was confirmed that the FONPS6 fragment successfully substituted the hygB gene within the 418R hygBS transformant.
2.5. Characterization of Growth
To evaluate the growth characteristics of the mycelium, 8 mm diameter plugs were cut from the periphery of fungal colonies of both the WT and ΔFON-NPS6 strains on CM agar. These plugs were subsequently positioned at the center of two distinct media: CM and MM. After incubating the cultures for 72 h at 25 °C in light, the diameters were measured. The data were collected from two independent experiments, each comprising three replicates. Morphology of strains was analyzed with scanning electron microscopy. The germination of asexual spores (conidia) was evaluated by preparing a suspension of the spores from both the WT strain and ΔFON-NPS6, cultured on CM, at a spore suspension concentration of 105/mL. Subsequently, 300 µL of the suspension was transferred into 1.5 mL test tubes and incubated at 30 °C for 1, 2, or 3 h. A drop of the spore suspension was then placed onto a well of a glass slide, and the samples were observed under a bright-field microscope. The number of germinated spores was counted for each sample, with a total of 50 spores being counted per sample.
2.6. Stress Sensitivity Assays
Cultivation of the WT and ΔFON-NPS6 strains was carried out on solid minimal medium including stressors as well as without them. An 8 mm mycelial plug, taken from the edge of fungal colonies grown on MM agar that does not contain FeSO4 (the sole iron source in MM, rendering it iron-deficient), was placed in the center of the medium. To assess the susceptibility of the FON-NPS6 strain, three or four independent transformants were tested under each condition, with triplicate experiments being carried out for each. All stress assay chemicals were obtained from Sigma. Two oxidants, H2O2 (3%) and the superoxide generator KO2, were used at final concentrations of 20 mM and 40 mM, respectively. Iron-depleted conditions were induced by using the iron chelator 2DP at a final concentration of 400 µM. Additionally, WT and ΔFON-NPS6 strains were grown on MM, iron-deficient MM, and MM supplemented with 1 mM ferric citrate, ferrous ammonium sulfate, or ammonium ferric sulfate for 3 days. Five replicates per strain were prepared for each condition, and three independent ΔFON-NPS6 strains were analyzed. Colony diameters were measured, and data were evaluated using one-way ANOVA.
2.7. HPLC Analysis of Phenolic Acids
The rhizobial chamber was divided into a central zone (30 mm wide) and left and right zones (30 mm wide) separated by a 0.22 m acetocellulose membrane, with the central zone being the side with a rough surface intended to prevent the movement of roots and microorganisms to the other side. In each zone of the rhizobial chamber, a piece of nylon cloth was placed 1 mm from the membrane, and in three spaces in the zone, including the space between the film and the first nylon cloth, pre-prepared sterilized soil was placed, and these samples between the membrane and the nylon cloth were used for the adsorption of exudates migrating from the central zone through the membrane as the watermelon root system was kept within the central zone.
Plants thinned to four were sprayed with a spore suspension (7.2 × 105 CFUs/g) of dry soil inoculated with WT or FON-NPS6 strains. Plants were ensured to be grown under greenhouse conditions with temperatures ranging from 20 to 30 degrees Celsius during the day and 20 to 25 degrees Celsius at night. Forty-five days after sowing into rhizobial boxes, the soil between the film and nylon cloth was collected and brought back to air dry.
Allelochemicals were extracted following the method outlined by Martens [21]. The extracts were analyzed with an Agilent 1200 semipreparative HPLC system (Santa Clara, CA, USA). Phenolic acids were separated using a synthetic solvent gradient elution method under certain assay conditions. The gradient program was designed as follows: 0–27 min, 100–90% solvent A, 0–10% solvent B; 27–42 min, 90–85% solvent A, 10–15% solvent B; 42–50 min, 85–70% solvent A, 15–30% solvent B; 50–60 min, 70–50% solvent A, 30–50% solvent B; 60–70 min, 50–0% solvent A, 50–100% solvent B. Standards for 4-hydroxybenzoic acid, vanillic acid, ferulic acid, benzoic acid, 3-phenylpropanoic acid, and cinnamic acid were sourced from Sigma-Aldrich (Steinem, Germany), and stock solutions were created in methanol. The HPLC chromatogram for PAs was created following the previously outlined method with an injection volume of 5 μL.
2.8. Virulence Assays
To assess the virulence of WT, ΔFON-NPS6, and complementary strains, spores were resuspended in sterile distilled water supplemented with 0.02% (v/v) Tween 20, with a final spore concentration of 1 × 107 CFU/mL. Three-week-old watermelon plants (ten per assay) were transplanted into pots with a volume of 640 cm3. The plants were placed in a mist chamber for 24 h, followed by incubation in a growth chamber with a 16 h light/8 h dark cycle at 24 °C. Virulence was assessed 5 days post-inoculation using the method outlined by Pritsch et al. [22]. Fusarium wilt symptoms in each plant were assessed using a 0–5 scale as follows: 0 means the plant remained entirely healthy; 1 means less than 10% of the leaves showed wilting; 2 means 11–20% of the leaves wilted; 3 means 21–50% of the leaves wilted; and 4 means 50–100% of the leaves were affected.
The disease index was calculated using the following formula:
Disease Index = Σ (level value × plant number)/(total plant number × 4) × 100%
Leaf point inoculation was conducted following a modified version of the method by Rahman et al. [23]. In brief, 5 μL droplets of spore suspension (105/mL) in water were applied to the leaves of 4-week-old watermelon plants (five plants per assay).
Control plants received the same volume of water without fungal spores. Inoculation was followed by a 7-day incubation period in a growth chamber. Symptoms were visually assessed. Additionally, each virulence assay was conducted a minimum of three times for every pathosystem. Watermelon roots were placed in Erlenmeyer flasks filled with a microspore suspension at a concentration of 5 × 106 µg/mL. The flasks were then incubated in a growth chamber at a speed of 80 rpm for 24 h to evaluate the roots’ penetration capabilities. After incubation, the samples were examined using scanning electron microscopy and analyzed following the methodology outlined by Sanderson et al. [24].
3. Results
3.1. Mutagenesis of FoNPS6, a Gene Encoding Nonribosomal Peptide Synthetase 6 in F. oxysporum
The NPS6 gene (FoNPS6) was cloned from F. oxysporum f. sp. niveum race 1(WT). The gene contains a 6272 bp open reading frame (ORF). A BLAST search against the complete genome database of F. oxysporum using the amino acid sequence of F. graminearum NPS6 identified a single potential ortholog, FOXG_09785.2, which encodes a hypothetical protein consisting of 2053 amino acids. The predicted FoNPS6 protein has a conserved domain architecture including a phosphopantetheine attachment site, a condensation domain, and an amino acid adenylation domain. The predicted protein exhibited a high degree of identity to previously characterized fungal NPS6 (e.g., F. graminearum, 91%; Gibberella zeae PH-1, 81%; and Aspergillus brassicicola, 76%). NPS6 has been identified to contribute to the virulence of F. graminearum [25]. Given the significant sequence conservation between F. oxysporum and F. graminearum, as indicated by sequence homology, this gene plays a crucial role. Therefore, a further investigation into the function of FoNPS6 in F. oxysporum was conducted.
3.2. Deletion of FoNPS6 Affected Multiple Growth-Related Characteristics
The role of FONPS6 in F. oxysporum infection was investigated by altering the 5193 bp FONPS6 genomic sequence (FON-NPS6), and finally, a partial FONPS6 knockout was obtained (Figure 1A). Growth characteristics of ΔFON-NPS6 on two different solid media, including complete medium (CM) [25] and minimal medium (MM) were compared with WT strains. ΔFON-NPS6 strains showed reduction in radial growth relative to these WT strains (Figure 1B) on MM and CM. The surface topography of the hyphae from the WT and FON-NPS6 strains was visualized using scanning electron microscopy, which showed that the surface of the WT mycelium was visibly smoother than the surface of the FON-NPS6 mycelium (Figure 1C,D). Conidia of WT and FON-NPS6 strains were taken out and inoculated into liquid MM at different times for microscopic examination. Differences in shoot elongation, septum formation, and mycelial meristem were found between the two strains.
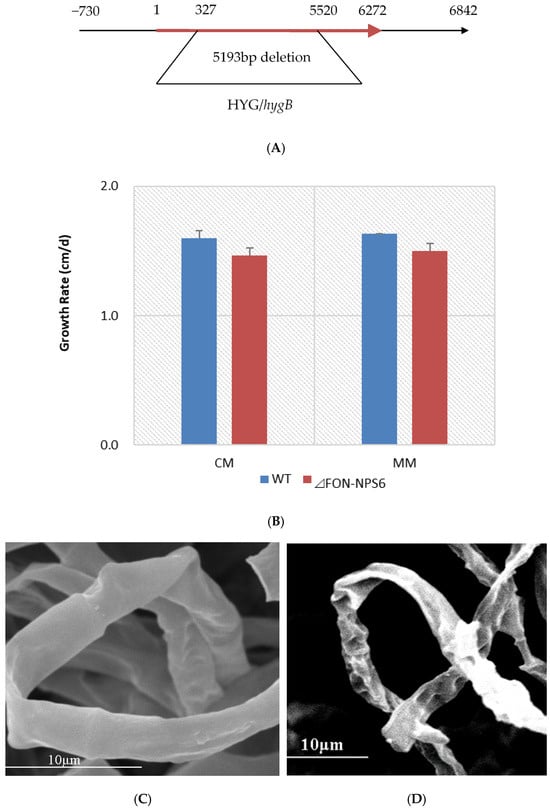
Figure 1.
Deletion of FoNPS6 affected multiple growth-related characteristics. (A) FoNPS6 was deleted with split marker constructs. Binding sites for primers used to confirm mutants are noted with asterisk. (B) Growth rate of average colony diameters of WT and ΔFON-NPS6 strains grown on MM or CM. Error bars indicate 95% confidence intervals. Statistically significant difference (p < 0.05) in growth was observed. (C,D) Scanning electron microscopy analysis of morphology of WT (C) and ΔFON-NPS6 (D) strains grown on MM. Surface of WT mycelium was smoother than that of ΔFON-NPS6 mycelium.
The germination rate of asexual spores of the WT strain was lower than that of the FON-NPS6 strain during the first 2 h, but the difference was not significant during the last 1 h (Table 1). These results demonstrate that FoNPS6 did not play an essential role in plant growth and conidiation in culture.

Table 1.
Percent F. oxysporum spore germination in vitro a.
3.3. Deletion of FoNPS6 Leads to Hypersensitivity to Superoxide and H2O2 and to Iron Depletion
To further investigate the function of FoNPS6, we evaluated the sensitivity of FOC-NPS6 strains to various stress conditions, including oxidative stress induced by superoxide and H2O2, as well as iron translocation and supplementation. The FOC-NPS6 strain exhibited heightened sensitivity to oxidative stress triggered by 40 mM KO2 and 20 mM H2O2 (Figure 2A,B), and also to iron deficiency, induced by the iron chelator 2,2′-dipyridyl (2DP) and MM lacking FeSO4, the sole iron source (Figure 2C,D). No differences in sensitivity to 1.0 mM of iron addition were detected between the WT and ΔFON-NPS6 strains regardless of whether ferric citrate, ferrous ammonium sulfate, or ammonium ferric sulfate was used (Figure 2D).
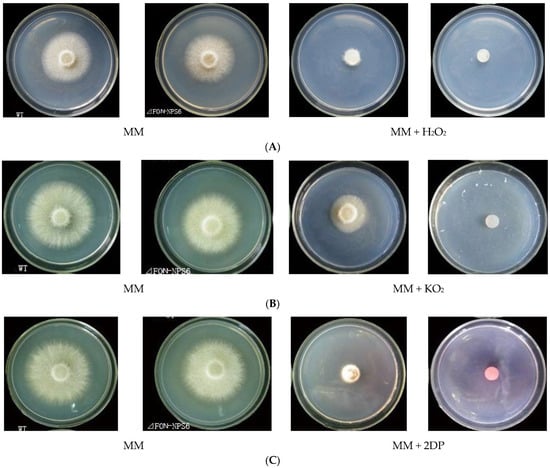
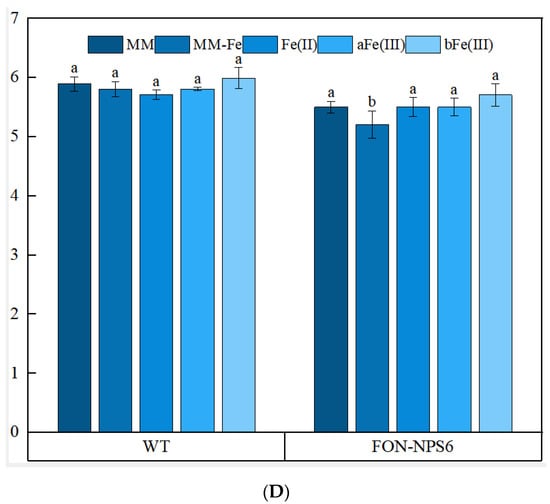
Figure 2.
The deletion of FoNPS6 leads to hypersensitivity to superoxide and H2O2 and to iron depletion. Different lowercase letters above the bars indicate significant differences between treatments (p ≤ 0.05). (A) The hypersensitivity of the ΔFON-NPS6 strain to 20 mM of H2O2. Three-day-old cultures of the WT (top row) and ΔFON-NPS6 (bottom row) strains. The growth of the ΔFON-NPS6 strain was completely inhibited on the MM, and the WT grew under the same conditions. (B) The hypersensitivity of the ΔFON-NPS6 strain to 40 mM of KO2. Three-day-old cultures of the WT (top row) and ΔFON-NPS6 (bottom row) strains. The growth of the ΔFON-NPS6 strain was completely inhibited on the MM, and the WT grew under the same conditions. (C) The hypersensitivity of the ΔFON-NPS6 strain to 400 μM of 2DP (iron chelator). Three-day-old cultures of the WT (top row) and ΔFON-NPS6 (bottom row) strains. The growth of the ΔFON-NPS6 strain was completely inhibited on the MM, and the WT grew under the same conditions. (D) The hypersensitivity of the ΔFON-NPS6 strain to iron deficiency (MM without FeSO4). The error bars indicate 95% confidence intervals. A statistically significant difference in size (p < 0.05) was observed. No differences in sensitivity to iron addition (1 mM of ferrous ammonium sulfate (Fe(Ⅱ)) or ammonium ferric sulfate (aFe(Ⅲ)) or ferric citrate (bFe(Ⅲ)) were detected between the WT and ΔFON-NPS6 strains. Different letters on the columns layer represent significant differences (p < 0.05).
3.4. PA Degradation by ΔFON-NPS6
To assess whether FoNPS6 influenced the secretion of phenolic acids (PAs) by watermelon plants, a custom-made Rhizobox was employed to collect root exudates from the soil (Figure 3A). In the soil surrounding the wild-type (WT) plants, six specific PAs—4-hydroxybenzoic acid, vanillic acid, ferulic acid, benzoic acid, 3-phenylpropanoic acid, and cinnamic acid—were detected (Figure 3B). However, in the soil around the ΔFON-NPS6 mutants, only ferulic acid, benzoic acid, acrylic acid, and cinnamic acid were identified, with concentrations significantly lower than those found in the WT. Specifically, secretion levels were reduced by 66.2%, 99.8%, 80.4%, and 99.9%, respectively. Notably, hydroxybenzoic acid and vanillic acid were completely absent in the mutant exudates (Figure 3C). An analysis of the HPLC chromatograms showed that both the number and intensity of peaks in the exudates from the ΔFON-NPS6 treatment were markedly reduced compared to those from the WT treatment.
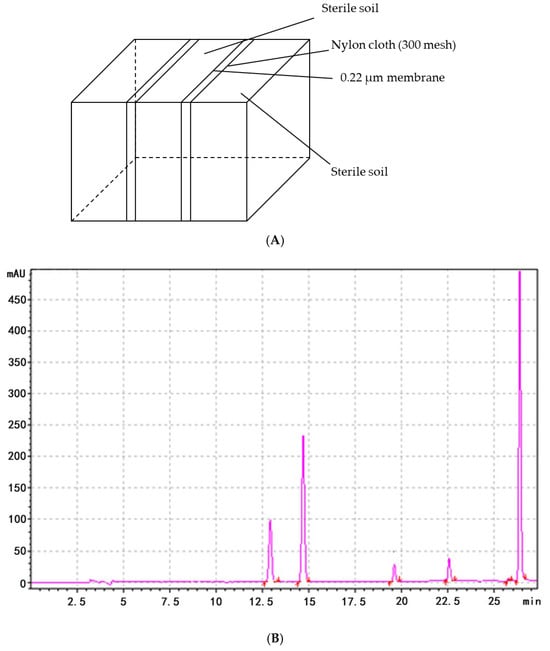
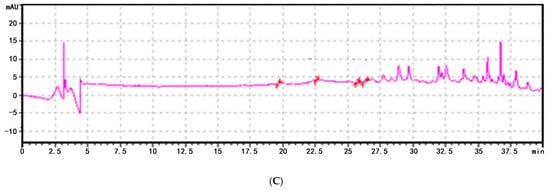
Figure 3.
FONPS6 affected PA secretion of plants. (A) Illustration of Rhizobox. (B) Chromatogram of HPLC of extraction from soil of cucumber in FOC strains. (C) Chromatogram of HPLC of extraction from soil of cucumber in ΔFON-NPS6 strains.
3.5. Mutant ΔFON-NPS6 Lacking FoNPS6 Gene Reduced Virulence, and Complementation Restored Full Virulence
To determine whether FoNPS6 inactivation impacted pathogenicity, virulence assays were quantified on watermelon plants inoculated with the WT, the mutant, and the complemented mutants (ΔFON-NPS6::FONPS6) (Figure 4A) in a separate experiment. After 30 days of watermelon growth, the WT treatment exhibited a Fusarium wilt disease index of 92%, while the ΔFON-NPS6+FONPS6 treatment showed an index of 90%. In contrast, the ΔFON-NPS6 treatment resulted in a significantly lower index of only 12.5% (Figure 4B). The mean vertical lengths of lesions induced by the WT strain and the ΔFON-NPS6 and ΔFON-NPS6::FONPS6 treatments demonstrated that the WT and ΔFON-NPS6::FONPS6 treatment lesion size were much longer (Figure 4C). So, the ΔFON-NPS6-complemented mutants had a restored full-disease phenotype that was not significantly different in disease progression from inoculations with the fully virulent WT. Disease progression was also significantly higher in the complement mutants and WT than in the FONPS6 knockout mutants (Figure 4D).
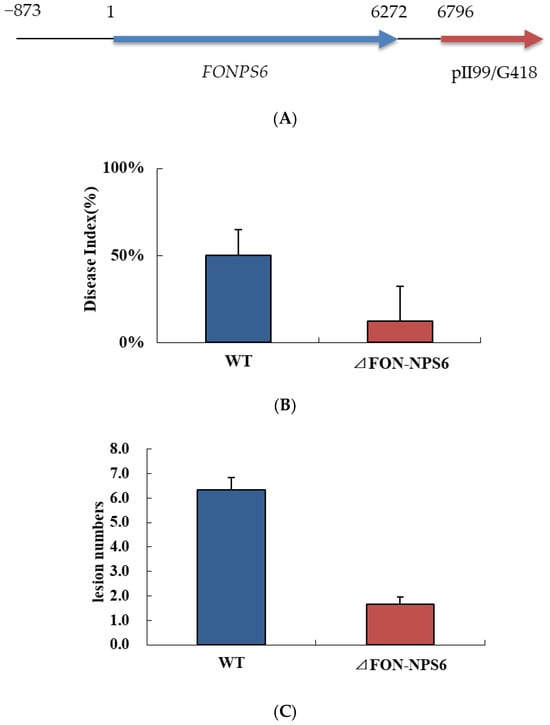

Figure 4.
ΔFON-NPS6 mutant lacking FoNPS6 gene reduced virulence. (A) ΔFON-NPS6 was complemented with split marker constructs. (B) Disease indices of watermelon inoculated with WT and ΔFON-NPS6 conidia (7.2 × 105 CFU per gram of dry soil). Error bars indicate 95% confidence intervals. Statistically significant difference in size (p < 0.05) was observed. (C) Average vertical lengths of lesions on watermelon leaves formed by WT and ΔFOC-NPS6 strains. Error bars indicate 95% confidence intervals. Statistically significant difference in size (p < 0.05) was observed. (D) Disease symptoms of plants grown using soil mix in plate are shown. Plants were inoculated with water (first), WT (second), ΔFON-NPS6 (third), and ΔFON-NPS6:: FONPS6 (fourth).
To investigate penetration by F. oxysporum into the surface of watermelon roots, scanning electron microscopy was used to analyze the roots inoculated with microconidia from both the WT and ΔFON-NPS6 strains. The results show the hyphae penetrating the root via gaps at the intersections of the epidermal cells (Figure 5A,B). In the WT, the frequency of penetration was higher than that in the ΔFON-NPS6 strain.
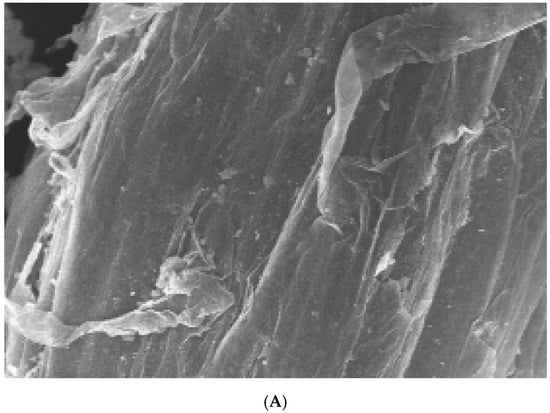
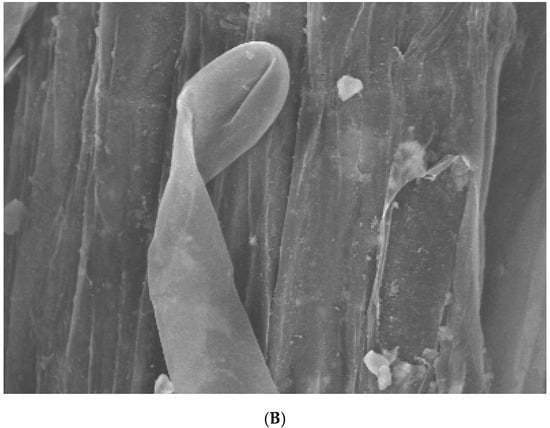
Figure 5.
(A,B) NPS6 contributes to penetration of watermelon roots. Scanning electron microscopy analysis of watermelon roots 24 h after inoculation with microconidia of F. oxysporum f. sp. niveum WT (A) or ΔFON-NPS6 (B) strain. (A) shows fungal hyphae entering root through openings at intercellular junctions. Arrows point to penetration events. (B) shows multiple hyphal fusion events indicated by ΔFON-NPS6.
4. Discussion
The NPS6 gene encodes a product recognized as an extracellular glycoconjugate, which acts as a conserved virulence factor in pathogenic ascomycetes, including the distantly related cereal pathogen F. graminearum [25]. However, although F. oxysporum has a close genetic relationship with F. graminearum, it does not belong to the ascomycete species. Therefore, whether this gene in F. oxysporum has the same or different role with those in F. graminearum and other ascomycetes species was studied in this study.
Here, we identified the F. oxysporum NPS6 gene by its homology to the NPS6 protein from F. graminearum, G. zeae PH-1, and A. brassicicola. These three proteins exhibit a high degree of amino acid sequence similarity and possess a shared domain architecture, including a phosphopantetheine attachment site, a condensation domain, and an amino acid adenylation domain. The presence of these three highly conserved genes in different species is a phenomenon that suggests either a common origin that may be recent or an evolutionarily conserved function that limits genetic diversity [26].
To dissect the function of FoNPS6 in F. oxysporum, we generated an independent F. oxysporum strain in which FONPS6 had been deleted (ΔFON-NPS6). Compared to the WT strain, the FON-NPS6 strain was observed to have a common characteristic phenotype similar to head growth failure and hypersensitivity to low iron. The ΔFON-NPS6 strain exhibits increased sensitivity to conditions of low iron availability, and its strong iron-chelating ability led us to hypothesize that FoNPS6 plays a role in plant colonization by providing the essential nutrient iron to the pathogen. Further characterization revealed that the ΔFON-NPS6 strain promotes the germination of asexual spores at first but is normal at last. These results indicate that FoNPS6 just affects the rate of germination of asexual spores and not the number [27].
The mutant of Xanthomonas oryzae pv. oryzae, known as the fur (ferric uptake regulator) variant, which exhibited an overproduction of extracellular siderophores, also demonstrated reduced virulence in rice and heightened sensitivity to H2O2 [28,29]. It has also been reported that the deletion of NPS6 reduces the pathogenicity of heterotrophic molds and Fusarium graminearum on maize and renders maize insensitive to H2O2 [30,31]. We found that FoN-NPS6 exhibits hypersensitivity to H2O2 and the superoxide generator KO2. Thus, the role of FoNPS6 in this characteristic function is similar to its role in Fusarium graminearum, as described above.
ΔFON-NPS6 significantly reduced virulence on watermelon. Furthermore, to test that the reduced levels of virulence in ΔFON-NPS6 was due to deletion of FoNPS6, ΔFON-NPS6 was complemented, and complementation restored the WT disease phenotype. Root colonization in hydroponic cultures was observed using the same pathogenic and non-pathogenic strains previously employed in the study by Voß et al. [32]. In the study, the pathogenic strain appeared to be a better and faster colonizer than the biocontrol strain. In our study, evidence suggests that the interaction of F. oxysporum with solid surfaces elicits a swift reaction, which requires FoNPS6. This may be one of the reasons why the FoNPS6 gene is associated with enhanced virulence. However, further studies are needed to draw conclusions about the role of NPS6-mediated iron metabolism in pathogenicity.
Overall, our findings are consistent with previous reports on F. graminearum [33], suggesting that NPS6 plays a widely conserved role in fungal pathogenicity. However, there is limited information regarding the mechanisms by which the NPS6 mutant degrades phenolic acids (PAs). This study demonstrated that in cultures inoculated with ΔFON-NPS6, the concentration of PAs was significantly lower compared to those inoculated with the wild-type strain. Allelochemicals, especially water-soluble, low-molecular-weight PAs, are known to exhibit fungitoxic effects against various fungi [34]. These compounds can have toxic effects on fungal species, either synergistically or even in a superimposed manner, resulting in a decrease in the microbial population in the area surrounding these toxins [35]. Notably, cinnamic acid and 4-hydroxybenzoic acid have been shown to enhance the growth status and infection probability of F. oxysporum f. sp. cucumerinum [36], whereas vanillin and 4-hydroxybenzoic acid promote Fusarium oxysporum infection in peanut plants [37]. Therefore, the observed decrease in the allelochemical content in soils treated with FON-NPS6 is likely due to the degradation of PAs by this strain. Additionally, pathogens with greater infective capacity tend to elicit a reduced secretion of plant allelochemicals. Nevertheless, for the optimal application of ΔFON-NPS6, further research is needed to investigate the processes that occur after allelochemicals are transported into the cells of ΔFON-NPS6 in future studies.
5. Conclusions
The partial deletion of FoNPS6 orthologs led to a heightened sensitivity to oxidative stressors as well as to conditions of iron depletion. ΔFON-NPS6 could impair spore formation and exhibited hyphal growth patterns that markedly diverged from those of the wild-type strain. Interestingly, plants release allelochemicals through leaching, which FoNPS6 possesses the ability to degrade. Therefore, FoNPS6 quantitatively increases F. oxysporum pathogenicity.
6. Institutions
- The College of Land and Environment, Shenyang Agriculture University, Shenyang, Liaoning, China
- The National Engineering Research Center for Efficient Utilization of Soil and Fertilizer Resources, Shenyang, Liaoning, China
- The Northeast Key laboratory of conservation and improvement of cultivated land (Shenyang), Ministry of Agriculture and Rural Affairs, P.R., China
Author Contributions
Methodology, X.Y. and J.L.; Software, Y.G. and B.L.; Validation, Y.G.; Formal analysis, L.Z.; Investigation, Y.F.; Resources, H.Z.; Funding acquisition, X.Y. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Key Research Projects of the Liaoning Provincial Department of Education (JYTZD2023126), the National Natural Science Foundation of China (No. 41301255), Liaoning Province Science and Technology Plan Project (2023-BSBA-285) and Shenyang Agricultural University publicly recruits doctoral graduates for research start-up fund project (2022018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alam, K.M.; Alam, M.M.; Islam, M.M.; Momotaz, R. First Report on Fusarium oxysporum f. Sp. Niveum Causing Watermelon Fusarium Wilt in Bangladesh. Plant Dis. 2020, 104, 1859. [Google Scholar]
- Chen, L.-H.; Yang, S.L.; Chung, K.-R. Resistance to oxidative stress via regulating siderophore-mediated iron acquisition by the citrus fungal pathogen Alternaria alternata. Microbiology 2014, 160, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-R.; Shilts, T.; Li, W.; Timmer, L.W. Engineering a genetic transformation system for Colletotrichum acutatum, the causal fungus of lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol. Lett. 2002, 213, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Ma, X.; Wang, X.; Zhang, T.-A.; Hu, J.; Tsang, Y.F.; Gao, M.-T. Phenolic acids derived from rice straw generate peroxides which reduce the viability of Staphylococcus aureus cells in biofilm. Ind. Crops Prod. 2019, 140, 111561. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, T.; Zhu, W.; Li, L.; Fu, Q. A MADS-box transcription factor FoRlm1 regulates aerial hyphal growth, oxidative stress, cell wall biosynthesis and virulence in Fusarium oxysporum f. Sp. Cubense. Fungal Biol. 2020, 124, 183–193. [Google Scholar] [CrossRef]
- Fayyaz, A.; Robinson, G.; Chang, P.; Bekele, D.; Yimer, S.; Carrasquilla-Garcia, N.; Negash, K.; Surendrarao, A.; von Wettberg, E.J.B.; Kemal, S.-A.; et al. Hiding in plain sight: Genome-wide recombination and a dynamic accessory genome drive diversity in Fusarium oxysporum f.sp. ciceris. Proc. Natl. Acad. Sci. USA 2023, 120, e2220570120. [Google Scholar] [CrossRef]
- Gurdaswani, V.; Ghag, S.B.; Ganapathi, T.R. FocSge1 in Fusarium oxysporum f. Sp. Cubense race 1 is essential for full virulence. BMC Microbiol. 2020, 20, 255. [Google Scholar]
- Hua, D.; Duan, J.; Ma, M.; Li, Z.; Li, H. Reactive oxygen species induce cyanide-resistant respiration in potato infected by Erwinia Carotovora Subsp. Carotovora. J. Plant Physiol. 2020, 246–247, 153132. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, X.; Xia, J.; Wu, X. A Novel Strain of Fusarium oxysporum Virus 1 Isolated from Fusarium oxysporum f. Sp. Niveum Strain X-GS16 Influences Phenotypes of F. oxysporum Strain HB-TS-YT-1hyg. J. Fungi 2024, 10, 252. [Google Scholar] [CrossRef]
- Keinath, A.P.; Hassell, R.L. Suppression of Fusarium Wilt Caused by Fusarium oxysporum f. Sp. Niveum Race 2 on Grafted Triploid Watermelon. Plant Dis. 2014, 98, 1326–1332. [Google Scholar]
- Keinath, A.P.; Wechter, W.P.; Rutter, W. Cucurbit Rootstocks Resistant to Fusarium oxysporum f. Sp. Niveum Remain Resistant When Coinfected by Meloidogyne incognita in the Field. Plant Dis. 2019, 103, 1383–1390. [Google Scholar] [PubMed]
- Li, J.; Fokkens, L.; Rep, M. A single gene in Fusarium oxysporum limits host range. Mol. Plant Pathol. 2020, 22, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xie, L.; Wang, M.; Lin, Y.; Zhong, J.; Zhang, Y.; Zeng, J.; Kong, G.; Xi, P.; Li, H.; et al. FoQDE2-dependent milRNA promotes Fusarium oxysporum f. Sp. Cubense virulence by silencing a glycosyl hydrolase coding gene expression. PLOS Pathog. 2022, 18, e1010157. [Google Scholar]
- Lin, Q.; Wang, Y.; Yang, X.; Ruan, D.; Wang, S.; Wei, X.; Qiu, R. Effect of low-molecular-weight organic acids on hematite dissolution promoted by desferrioxamine B. Environ. Sci. Pollut. Res. 2017, 25, 163–173. [Google Scholar] [CrossRef]
- Lu, S.; Lyngholm, L.; Yang, G.; Bronson, C.; Yoder, O.C.; Turgeon, B.G. Tagged mutations at the Tox1 locus of Cochliobolus heterostrophus by restriction enzyme-mediated integration. Proc. Natl. Acad. Sci. USA 1994, 91, 12649–12653. [Google Scholar] [CrossRef]
- Lv, F.; Xu, Y.; Gabriel, D.W.; Wang, X.; Zhang, N.; Liang, W. Quantitative Proteomic Analysis Reveals Important Roles of the Acetylation of ER-Resident Molecular Chaperones for Conidiation in Fusarium oxysporum. Mol. Cell. Proteom. 2022, 21, 100231. [Google Scholar] [CrossRef]
- Minh, N.C.; Nguyen, V.H.; Schwarz, S.; Stevens, W.F.; Trung, T. Preparation of water soluble hydrochloric chitosan from low molecular weight chitosan in the solid state. Int. J. Biol. Macromol. 2018, 121, 718–726. [Google Scholar] [CrossRef]
- Murray, J.; Raid, R.N.; Miller, C. F First Report of Fusarium oxysporum f. Sp. Lactucae Causing Vascular Wilt of Lettuce in Florida. Plant Dis. 2020, 104, 3069. [Google Scholar]
- Oide, S.; Moeder, W.; Krasnoff, S.; Gibson, D.; Haas, H.; Yoshioka, K.; Turgeon, B.G. NPS6, Encoding a Nonribosomal Peptide Synthetase Involved in Siderophore-Mediated Iron Metabolism, Is a Conserved Virulence Determinant of Plant Pathogenic Ascomycetes. Plant Cell 2006, 18, 2836–2853. [Google Scholar] [CrossRef]
- Ospina-Giraldo, M.D.; Mullins, E.; Kang, S. Loss of function of the Fusarium oxysporum SNF1 gene reduces virulence on cabbage and Arabidopsis. Curr. Genet. 2003, 44, 49–57. [Google Scholar] [CrossRef]
- Pareja-Jaime, Y.; Martín-Urdíroz, M.; Roncero, M.I.G.; González-Reyes, J.A.; Roldán, M.D.C.R. Chitin synthase-deficient mutant of Fusarium oxysporum elicits tomato plant defence response and protects against wild-type infection. Mol. Plant Pathol. 2003, 11, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Pritsch, C.; Vance, C.P.; Bushnell, W.R.; Somers, D.; Hohn, T.M.; Muehlbauer, G.J. Systemic expression of defense response genes in wheat spikes as a response to Fusarium graminearum infection. Physiol. Mol. Plant Pathol. 2001, 58, 1–12. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Ahmad, K.; Siddiqui, Y. First Report of Fusarium Wilt Disease on Watermelon Caused by Fusarium oxysporum f. Sp. Niveum in Malaysia. Plant Dis. 2021, 105, 4169. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, K.E.; Srb, A.M. Heterokaryosis and parasexuality in the fungus ascochyta imperfecta. Am. J. Bot. 1965, 52, 72–81. [Google Scholar] [CrossRef]
- Sridhar, P.S.; Trofimova, D.; Subramaniam, R.; Fundora, D.G.-P.; Foroud, N.A.; Allingham, J.S.; Loewen, M.C. Ste2 receptor-mediated chemotropism of Fusarium graminearum contributes to its pathogenicity against wheat. Sci. Rep. 2020, 10, 10770. [Google Scholar] [CrossRef]
- Srinivas, C.; Nirmala Devi, D.; Narasimha Murthy, K. Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity—A review. Saudi J. Biol. Sci. 2019, 26, 1315–1324. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Zeller, K.A.; Sobieraj, J.H.; Nakhla, M.K. Genome Resources of Four Distinct Pathogenic Races Within Fusarium oxysporum f. Sp. Vasinfectum that Cause Vascular Wilt Disease of Cotton. Phytopathology® 2021, 111, 593–596. [Google Scholar] [CrossRef]
- Subramoni, S.; Sonti, R.V. Growth Deficiency of a Xanthomonas oryzae pv. Oryzae fur Mutant in Rice Leaves Is Rescued by Ascorbic Acid Supplementation. Mol. Plant-Microbe Interact.® 2005, 18, 644–651. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, P.; Lu, Z.; Qu, Y.; Huang, L.; Zheng, N.; Wang, Y.; Nie, H.; Jiang, Y.; Xu, L. The Photoreceptor Components FaWC1 and Fa WC2 of Fusarium asiaticum Cooperatively Regulate Light Responses but Play Independent Roles in Virulence Expression. Microorganisms 2020, 8, 365. [Google Scholar] [CrossRef]
- Jin, M.; Hu, S.; Wu, Q.; Feng, X.; Zhang, Y.; Jiang, Q.; Ma, J.; Qi, P.; Chen, G.; Jiang, Y.; et al. An Effector Protein of Fusarium Graminearum Targets Chloroplasts and Suppresses Cyclic Photosynthetic Electron Flow. Plant Physiol. 2024, 196, 2422–2436. [Google Scholar] [CrossRef]
- Vinchira-Villarraga, D.M.; Castellanos, L.; Moreno-Sarmiento, N.; Suarez-Moreno, Z.R.; Ramos, F.A. Antifungal activity of marine-derived Paenibacillus sp. PNM200 against Fusarium oxysporum f. Sp. Lycopersici, the causal agent of tomato vascular wilt. Biol. Control 2020, 154, 104501. [Google Scholar] [CrossRef]
- Voß, B.; Kirschhöfer, F.; Brenner-Weiß, G. Alternaria alternate uses two siderophore systems for iron acquisition. Sci. Rep. 2020, 10, 3587. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Calabria, J.; Chen, H.-W.; Somssich, M. The Arabidopsis thaliana–Fusarium oxysporum strain 5176 pathosystem: An overview. J. Exp. Bot. 2022, 73, 6052–6067. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Ming, Y. Characterization of the High-Quality Genome Sequence and Virulence Factors of Fusarium oxysporum f. Sp. Vasinfectum Race 7. J. Fungi 2024, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.F.; Zhou, Y.H.; Sun, Y.; Zou, L.Y.; Yu, J.Q. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot. 2006, 56, 255–262. [Google Scholar] [CrossRef]
- Zhao, S.; An, B.; Guo, Y.; Hou, X.; Luo, H.; He, C.; Wang, Q. Label free proteomics and systematic analysis of secretome reveals effector candidates regulated by SGE1 and FTF1 in the plant pathogen Fusarium oxysporum f. Sp. Cubense tropical race 4. BMC Genom. 2020, 21, 275. [Google Scholar] [CrossRef]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).