Effect of Buah Merah (Pandanus conoideus Lamk.) Extract Supplementation on the Density and Apoptosis of Photoreceptor and Retinal Ganglion Cells in a Diabetic Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Experimental Setting
2.2. Experimental Animals (Pre-Treatment Phase)
2.3. Induction of Diabetes (Treatment Initiation Phase)
2.4. Preparation of Pandanus Conoideus (Buah Merah) Extract
2.5. Administration of Buah Merah Extract (During-Treatment Phase)
2.6. Sample Termination and Tissue Collection (Post-Treatment Phase)
2.7. Histopathological and Immunohistochemical Evaluation
2.8. Statistical Analysis
3. Results
3.1. Blood Sugar Profiles in Experimental Animals
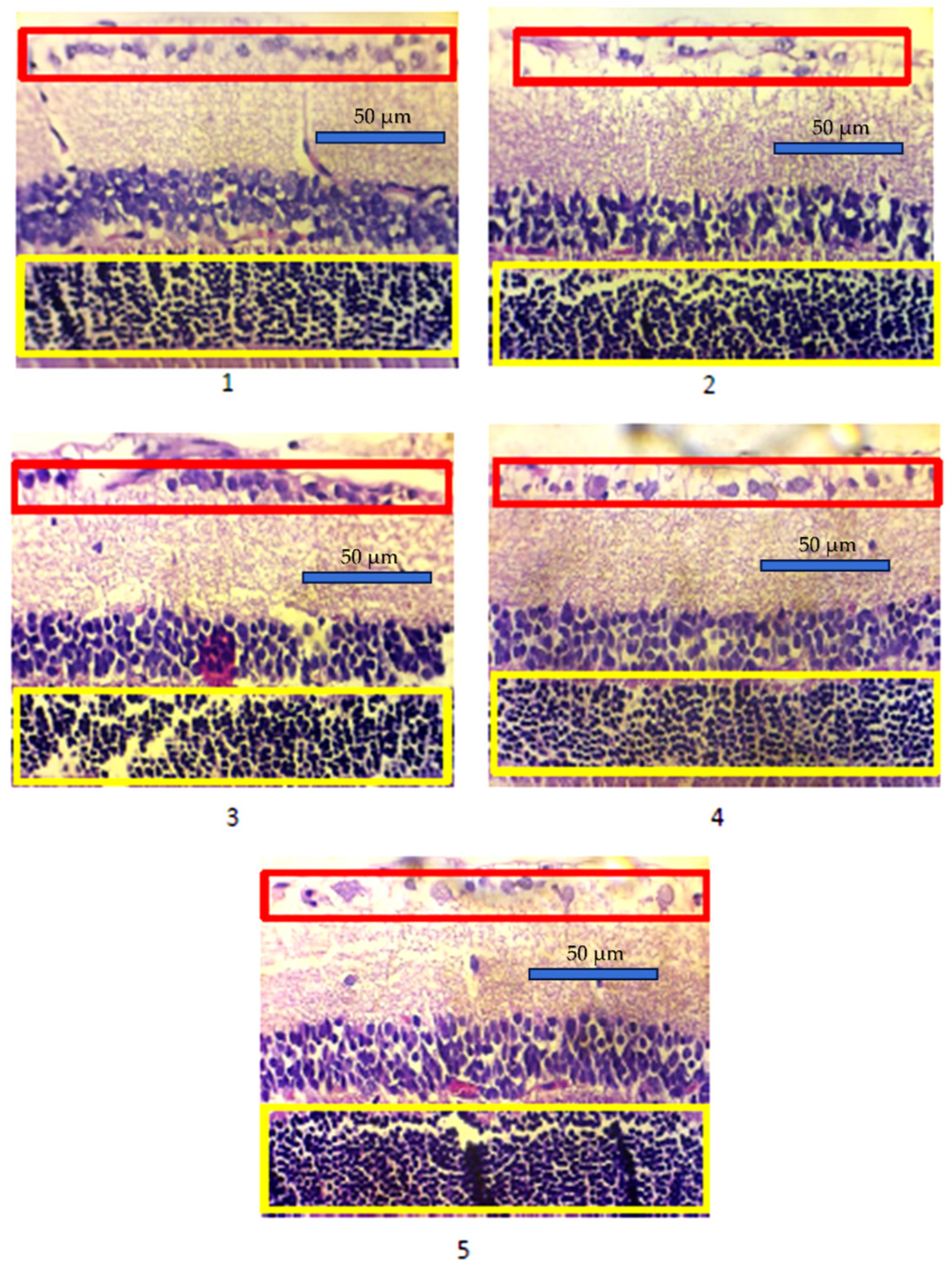
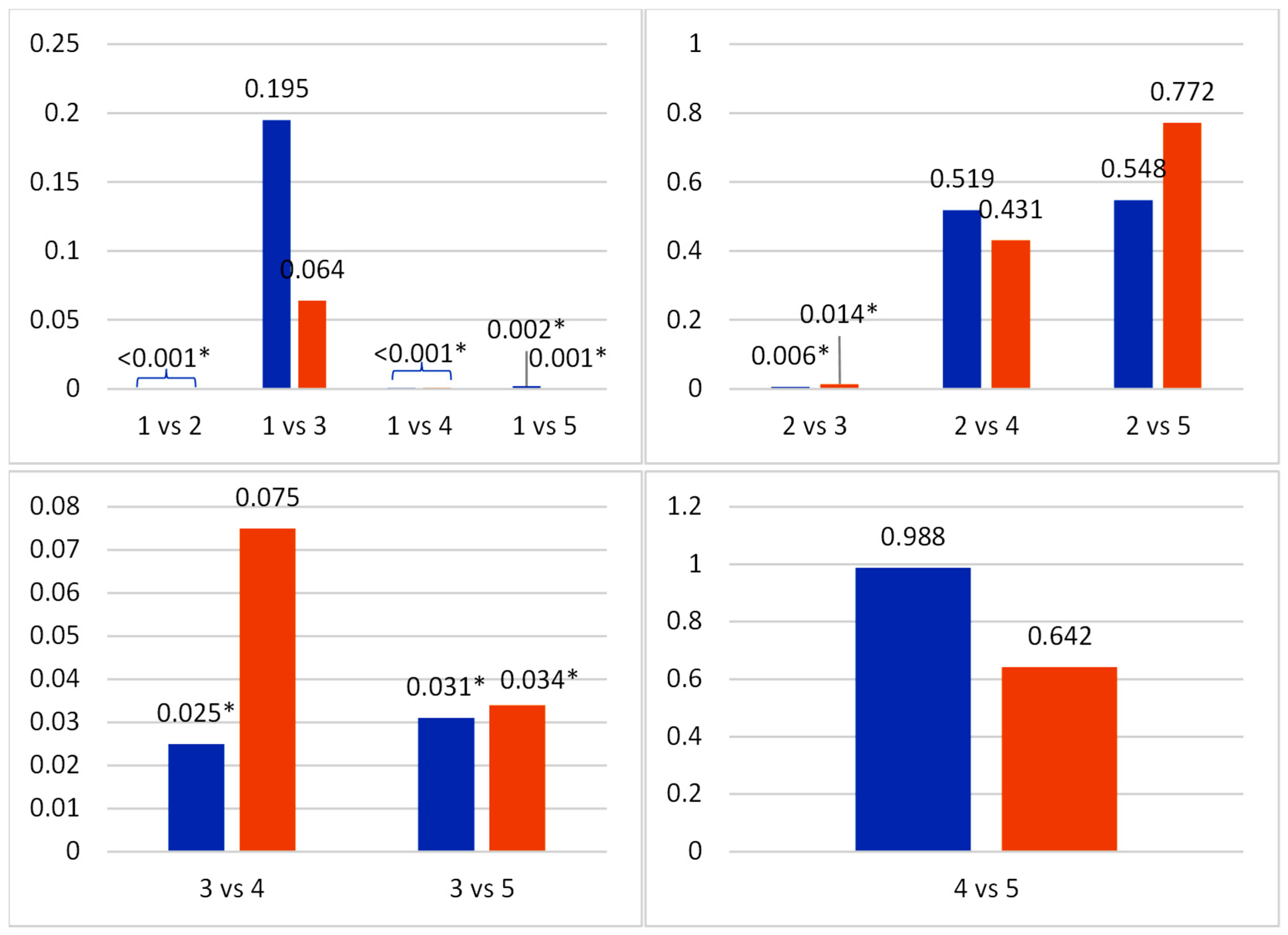
3.2. Photoreceptor Cell Density and Retinal Ganglion Cell Density in Experimental Animals
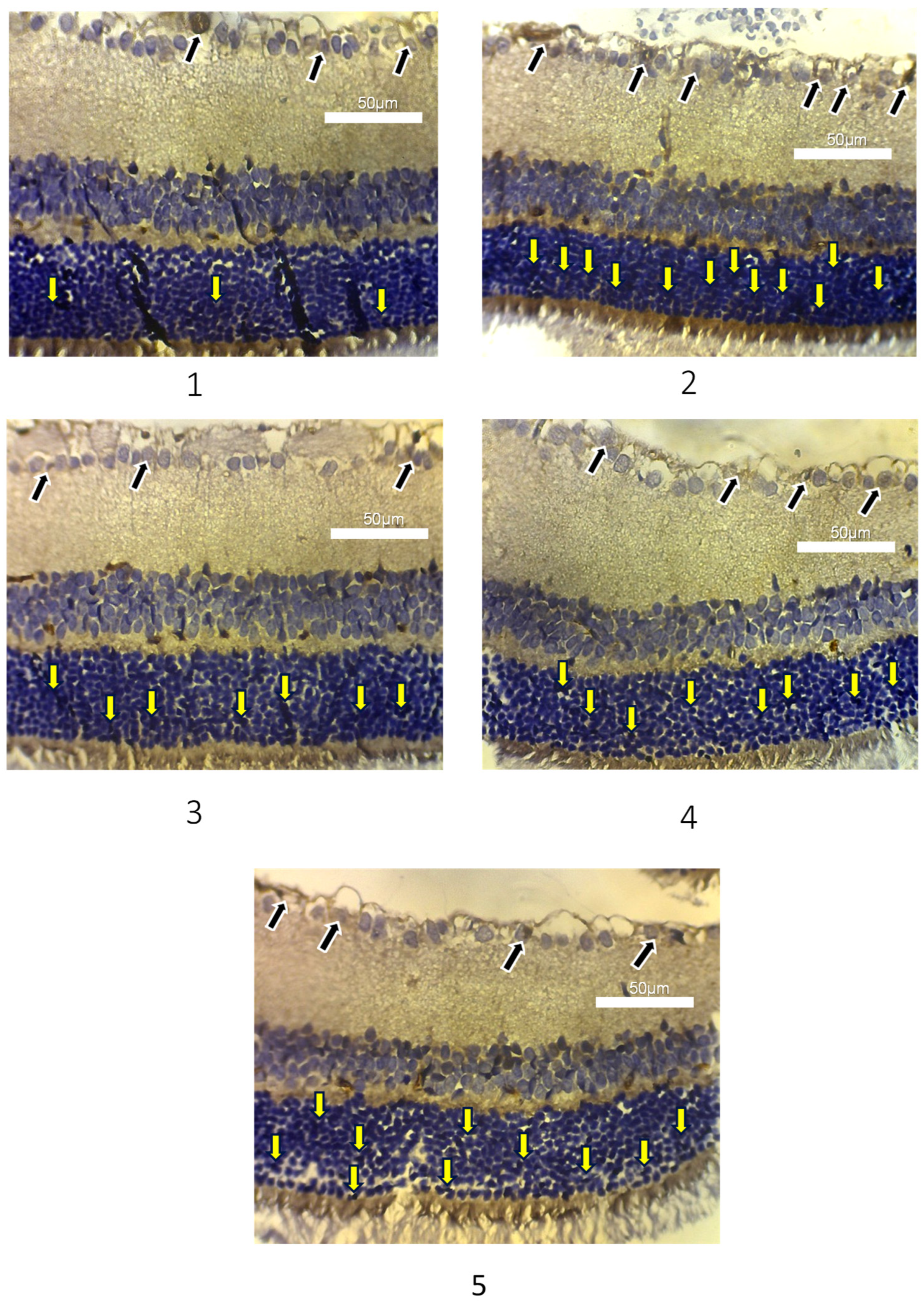
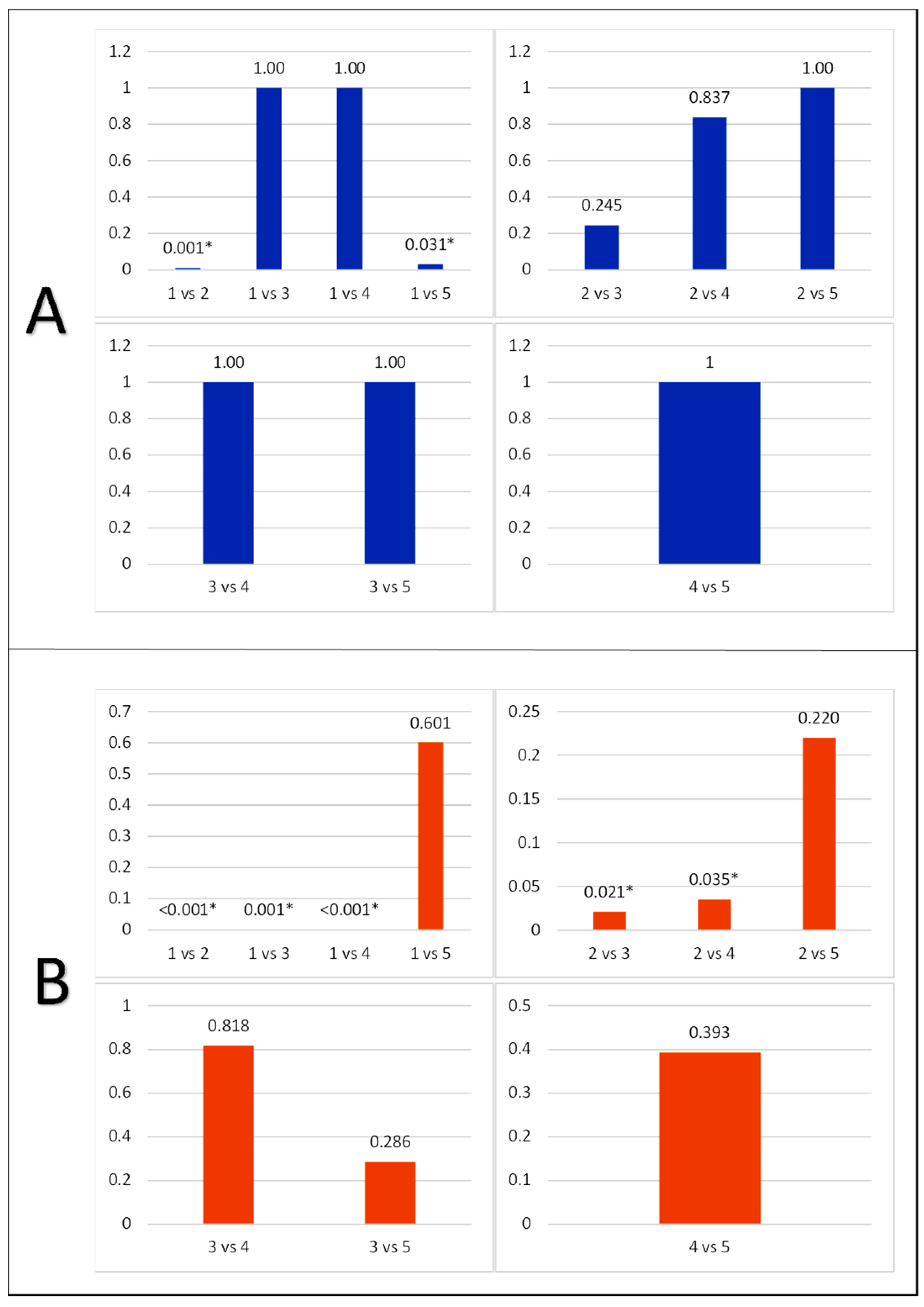
3.3. Apoptosis of Photoreceptor Cells and Ganglion Cells in Experimental Animals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasongko, M.B.; Widyaputri, F.; Agni, A.N.; Wardhana, F.S.; Kotha, S.; Gupta, P.; Widayanti, T.W.; Haryanto, S.; Widyaningrum, R.; Wong, T.Y.; et al. Prevalence of Diabetic Retinopathy and Blindness in Indonesian Adults With Type 2 Diabetes. Am. J. Ophthalmol. 2017, 181, 79–87. [Google Scholar] [CrossRef]
- Lechner, J.; O’LEary, O.E.; Stitt, A.W. The pathology associated with diabetic retinopathy. Vision Res. 2017, 139, 7–14. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-Vellosillo, M. Update on the effects of antioxidants on diabetic retinopathy: In vitro experiments, animal studies and clinical trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of Retinal Cell Death in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [CrossRef]
- da Silva, S.B.; Costa, J.P.; Pintado, M.E.; de Carvalho Ferreira, D.; Sarmento, B. Antioxidants in the Prevention and Treatment of Diabetic Retinopathy—A Review. J. Diabetes Metab. 2010, 1, 1000111. [Google Scholar] [CrossRef]
- Vieira, L.M.C.; Silva, N.F.A.; dos Santos, A.M.D.; dos Anjos, R.S.; Pinto, L.A.P.A.; Vicente, A.R.; Borges, B.I.C.C.J.; Ferreira, J.P.T.; Amado, D.M.; da Cunha, J.P.P.B. Retinal ganglion cell layer analysis by optical coherence tomography in toxic and nutritional optic neuropathy. J. Neuro-Ophthalmology 2015, 35, 242–245. [Google Scholar] [CrossRef]
- Ruamviboonsuk, V.; Grzybowski, A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. J. Clin. Med. 2022, 11, 6490. [Google Scholar] [CrossRef] [PubMed]
- Chiew, Y.; Tan, S.M.Q.; Ahmad, B.; Khor, S.E.; Kadir, K.A. Tocotrienol-rich vitamin e from palm oil (Tocovid) and its effects in diabetes and diabetic retinopathy: A pilot phase ii clinical trial. Asian J. Ophthalmol. 2021, 17, 375–399. [Google Scholar] [CrossRef]
- Oshitari, T. Neurovascular Cell Death and Therapeutic Strategies for Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 12919. [Google Scholar] [CrossRef]
- Putri, K.; Darsono, L.; Mandalas, H. Anti-inflammatory properties of mangosteen peel extract on the mice gingival inflammation healing process. Padjadjaran J. Dent. 2017, 29, 190–195. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef] [PubMed]
- Shukla, U.V.; Tripathy, K. Diabetic Retinopathy; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Domènech, E.B.; Marfany, G. The relevance of oxidative stress in the pathogenesis and therapy of retinal dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef]
- Xiong, R.; Yuan, Y.; Zhu, Z.; Wu, Y.; Ha, J.; Han, X.; Wang, W.; He, M. Micronutrients and Diabetic Retinopathy: Evidence From The National Health and Nutrition Examination Survey and a Meta-analysis. Am. J. Ophthalmol. 2022, 238, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Bonomini, M.; Bartolini, D.; Zatini, L.; Reboldi, G.; Marcantonini, G.; Gentile, G.; Sirolli, V.; Di Pietro, N. Vitamin E (Alpha-Tocopherol) Metabolism and Nutrition in Chronic Kidney Disease. Antioxidants 2022, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Murtiningrum, M.; Sarungallo, Z.L.; Mawikere, N.L. The exploration and diversity of red fruit (Pandanus conoideus L.) from Papua based on its physical characteristics and chemical composition. Biodiversitas J. Biol. Divers. 2011, 13, 124–129. [Google Scholar] [CrossRef]
- Wismandanu, O.; Maulidya, I.; Indariani, S.; Batubara, I. Acute toxicity of red fruits (Pandanus conoideus Lamk) oil and the hepatic enzyme level in rat. J. Phytopharm. 2016, 5, 176–181. [Google Scholar] [CrossRef]
- Khairani, A.C.; Wijayanti, T.; Widodo, G.P. Antihyperglycemic Activity of Red Fruit Oil (Pandanus conoideus Lam) on Improving Kidney Function in STZ-NA-Induced Nephropathy Rats. J. Farm. Dan Ilmu Kefarmasian Indones. 2023, 10, 173–183. [Google Scholar] [CrossRef]
- Handayani, M.D.N.; Soekarno, P.A.; Wanandi, S.I. Red fruit oil supplementation fails to prevent oxidative stress in rats. Universa Med. 2013, 32, 86–91. [Google Scholar]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.A.; Rad, B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef]
- Ottomana, A.M.; Presta, M.; O’lEary, A.; Sullivan, M.; Pisa, E.; Laviola, G.; Glennon, J.C.; Zoratto, F.; Slattery, D.A.; Macrì, S. A systematic review of preclinical studies exploring the role of insulin signalling in executive function and memory. Neurosci. Biobehav. Rev. 2023, 155, 105435. [Google Scholar] [CrossRef]
- Ichsan, A.M.; Bukhari, A.; Lallo, S.; Miskad, U.A.; Dzuhry, A.A.; Islam, I.C.; Muhiddin, H.S. Effect of retinol and α-tocopherol supplementation on photoreceptor and retinal ganglion cell apoptosis in diabetic rats model. Int. J. Retin. Vitr. 2022, 8, 40. [Google Scholar] [CrossRef]
- Houde, C.; Banks, K.G.; Coulombe, N.; Rasper, D.; Grimm, E.; Roy, S.; Simpson, E.M.; Nicholson, D.W. Caspase-7 expanded function and intrinsic expression level underlies strain-specific brain phenotype of Caspase-3-null mice. J. Neurosci. 2004, 24, 9977–9984. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Adeosun, A.M.; Akinloye, O.A. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar]
- ZNazarian-Samani, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Medicinal Plants with Multiple Effects on Diabetes Mellitus and Its Complications: A Systematic Review. Curr. Diab. Rep. 2018, 18, 72. [Google Scholar] [CrossRef]
- Febriyanti, R. Pengaruh Pemberian Ekstrak Buah Merah (Pandanus conoideus) Terhadap Kadar Glukosa Darah Tikus Putih (Rattus norvegicus) Diabetik. J. Peternak. 2011, 8, 21–26. [Google Scholar]
- Yeh, P.-T.; Yang, C.-M.; Huang, J.-S.; Chien, C.-T.; Yang, C.-H.; Chiang, Y.-H.; Shih, Y.-F. Vitreous Levels of Reactive Oxygen Species in Proliferative Diabetic Retinopathy. Ophthalmology 2008, 115, 734–738. [Google Scholar] [CrossRef]
- Fathalipour, M.; Fathalipour, H.; Safa, O.; Nowrouzi-Sohrabi, P.; Mirkhani, H.; Hassanipour, S. The therapeutic role of carotenoids in diabetic retinopathy: A systematic review. Diabetes Metab. Syndr. Obes. 2020, 13, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Tih, F. Aktivitas sitotoksik minyak buah merah (Pandanus conoideus Lam.) dan inhibisi ekspresi gen siklooksigenase-2 (COX-2) pada sel raji. J. Med. Health 2016, 1, 214–223. [Google Scholar]
- Agustina, W.; Widjiati, W.; Hayati, A. Effects of Red Fruit (Pandanus conoideus Lam) Oil on Malondialdehyde Level and Spermatozoa Quality in Mice (Mus musculus) Exposed to Monosodium Glutamate. Folia Medica Indones. 2018, 54, 84–88. [Google Scholar] [CrossRef]
- Kaur, N. Role of Nicotinamide in Streptozotocin Induced Diabetes in Animal Models. J. Endocrinol. Thyroid Res. 2017, 2, 555577. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The benefits and risks of certain dietary carotenoids that exhibit both anti-and pro-oxidative mechanisms—A comprehensive review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- de Oliveira, B.F.; Costa, D.C.; Nogueira-Machado, J.A.; Chaves, M.M. β-Carotene, α-tocopherol and ascorbic acid: Differential profile of antioxidant, inflammatory status and regulation of gene expression in human mononuclear cells of diabetic donors. Diabetes. Metab. Res. Rev. 2013, 29, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Kalariya, N.M.; Ramana, K.V.; Srivastava, S.K.; van Kuijk, F.J. Carotenoid Derived Aldehydes—Induced Oxidative Stress Causes Apoptotic Cell Death in Human Retinal Pigment Epithelial Cells. Exp Eye Res. 2008, 86, 70–80. [Google Scholar] [CrossRef]
- Accardi, G.; Di Majo, D.; Aiello, A. The Role of Natural Products in Immunopharmacology. Int. J. Mol. Sci. 2024, 25, 9256. [Google Scholar] [CrossRef]
| Blood Glucose Level (mg/dL) | ||||||
|---|---|---|---|---|---|---|
| Group | Pre-Alloxan (Mean ± SD) | Post-Alloxan (Mean ± SD) | p-Value | Post-Alloxan (Mean ± SD) | Pre-Termination (Mean ± SD) | p-Value |
| 1 | 103.50 ± 10.03 | N/A | - | N/A | 107.00 ± 3.90 | - |
| 2 | 109.50 ± 16.87 | 220.17 ± 68.64 | 0.038 | 220.17 ± 68.64 | 418.83 ± 144.51 | 0.012 |
| 3 | 106.83 ± 15.05 | 307.33 ± 97.66 | <0.001 | 307.33 ± 97.66 | 371.83 ± 61.40 | 0.031 |
| 4 | 113.50 ± 15.28 | 341.50 ± 128.10 | 0.010 | 341.50 ± 128.10 | 375.83 ± 66.79 | 0.182 |
| 5 | 106.40 ± 12.59 | 453.80 ± 65.40 | <0.001 | 453.80 ± 65.40 | 374.00 ± 51.16 | <0.001 |
| Group | Photoreceptor Cell Density (Mean ± SD) | p-Value | Retinal Ganglion Cell Density (Mean ± SD) | p-Value |
|---|---|---|---|---|
| 1 | 787.97 ± 18.77 | * 0.001 | 22.77 ± 2.63 | * 0.001 |
| 2 | 572.93 ± 45.40 | 13.20 ± 2.31 | ||
| 3 | 722.52 ± 147.56 | 18.73 ± 5.61 | ||
| 4 | 605.03 ± 77.46 | 14.86 ± 3.28 | ||
| 5 | 604.28 ± 75.23 | 13.84 ± 3.13 |
| Group | Caspase-3 Expression in Photoreceptor Cells (n) | p-Value |
|---|---|---|
| 1 | Negative: 5 | 0.020 |
| Low: 1 | ||
| High: 0 | ||
| 2 | Negative: 0 | |
| Low: 4 | ||
| High: 2 | ||
| 3 | Negative: 3 | |
| Low: 3 | ||
| High: 0 | ||
| 4 | Negative: 2 | |
| Low: 4 | ||
| High: 0 | ||
| 5 | Negative: 1 | |
| Low: 4 | ||
| High: 0 |
| Group | Caspase-3 Expression in Retinal Ganglion Cells (n) | p-Value |
|---|---|---|
| 1 | 23.50 ± 3.51 | 0.001 * |
| 2 | 46.33 ± 6.05 | |
| 3 | 37.50 ± 5.01 | |
| 4 | 38.33 ± 9.67 | |
| 5 | 41.60 ± 4.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichsan, A.M.; Salle, S.W.; Islam, I.C.; Lallo, S.; Zainuddin, A.A.; Mannyu, B.; Muhiddin, H.S. Effect of Buah Merah (Pandanus conoideus Lamk.) Extract Supplementation on the Density and Apoptosis of Photoreceptor and Retinal Ganglion Cells in a Diabetic Rat Model. Life 2025, 15, 1754. https://doi.org/10.3390/life15111754
Ichsan AM, Salle SW, Islam IC, Lallo S, Zainuddin AA, Mannyu B, Muhiddin HS. Effect of Buah Merah (Pandanus conoideus Lamk.) Extract Supplementation on the Density and Apoptosis of Photoreceptor and Retinal Ganglion Cells in a Diabetic Rat Model. Life. 2025; 15(11):1754. https://doi.org/10.3390/life15111754
Chicago/Turabian StyleIchsan, Andi Muhammad, Susan Waterina Salle, Itzar Chaidir Islam, Subehan Lallo, Andi Alfian Zainuddin, Budu Mannyu, and Habibah Setyawati Muhiddin. 2025. "Effect of Buah Merah (Pandanus conoideus Lamk.) Extract Supplementation on the Density and Apoptosis of Photoreceptor and Retinal Ganglion Cells in a Diabetic Rat Model" Life 15, no. 11: 1754. https://doi.org/10.3390/life15111754
APA StyleIchsan, A. M., Salle, S. W., Islam, I. C., Lallo, S., Zainuddin, A. A., Mannyu, B., & Muhiddin, H. S. (2025). Effect of Buah Merah (Pandanus conoideus Lamk.) Extract Supplementation on the Density and Apoptosis of Photoreceptor and Retinal Ganglion Cells in a Diabetic Rat Model. Life, 15(11), 1754. https://doi.org/10.3390/life15111754






