Effects of Surface Treatments on Innovative Additively Manufactured Scaffolds: Implications for Biocompatibility in Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Zn-1Mg Alloy Coins and Zn-1Mg Alloy Scaffolds
2.2. Surface Treatments
2.2.1. Electrochemical Polishing
2.2.2. Coin Particle Blasting
2.2.3. Eigengut Blasting
2.2.4. Sterilization
2.2.5. Grouping
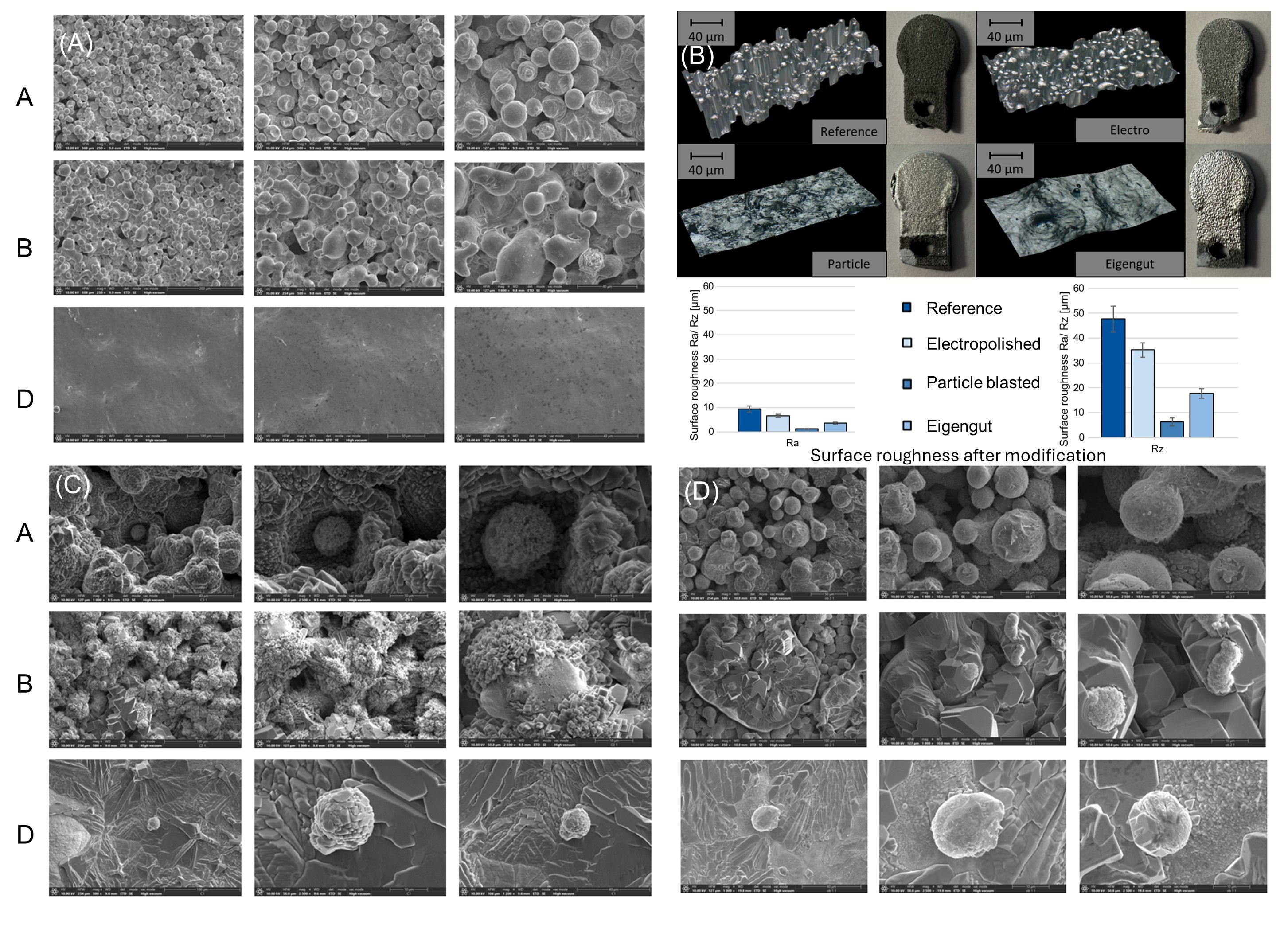
2.2.6. Surface Analysis
2.3. Scanning Electron Microscopy (SEM) Analysis
2.4. Evaluation of Cell Growth DAPI Staining
2.5. Evaluation of Cell Morphology Phalloidin/DAPI Staining
2.6. Flow Cytometry
2.7. Statistical Analysis
3. Results
3.1. Additive Manufacturing of Specimen
3.2. Zn-1Mg Alloy Coins
3.2.1. Zn-1Mg Coin Surface Observation
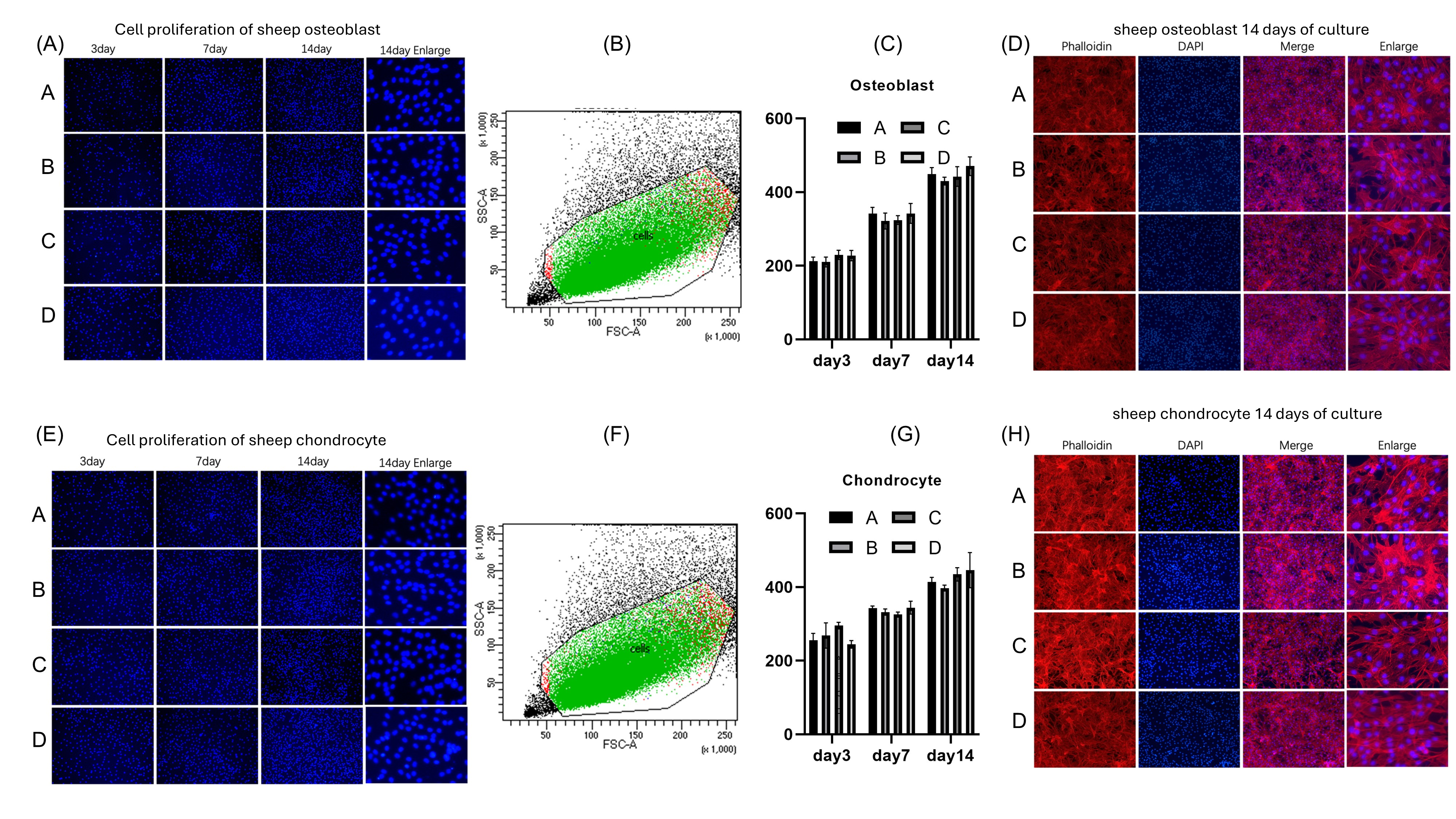
3.2.2. Co-Culture of Zn-1Mg Coins with Osteoblasts and Chondrocytes
3.2.3. SEM Analysis of Co-Culture of Coins with Osteoblasts and Chondrocytes
3.3. Zn-1Mg Scaffolds
3.3.1. Zn-1Mg Scaffold Surface Observation
3.3.2. Co-Culture of Zn-1Mg Scaffolds with Osteoblasts and Chondrocytes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prządka, M.; Pająk, W.; Kleinrok, J.; Pec, J.; Michno, K.; Karpiński, R.; Baj, J. Advances in 3D Printing Applications for Personalized Orthopedic Surgery: From Anatomical Modeling to Patient-Specific Implants. J. Clin. Med. 2025, 14, 3989. [Google Scholar] [CrossRef]
- Brachet, A.; Bełżek, A.; Furtak, D.; Geworgjan, Z.; Tulej, D.; Kulczycka, K.; Karpiński, R.; Maciejewski, M.; Baj, J. Application of 3D Printing in Bone Grafts. Cells 2023, 12, 859. [Google Scholar] [CrossRef]
- Dong, J.; Lin, P.; Putra, N.E.; Tumer, N.; Leeflang, M.A.; Huan, Z.; Fratila-Apachitei, L.E.; Chang, J.; Zadpoor, A.A.; Zhou, J. Extrusion-Based Additive Manufacturing of Mg-Zn/Bioceramic Composite Scaffolds. Acta Biomater. 2022, 151, 628–646. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, B.; Wang, P.; Wang, X.; Zhang, B.; Shi, Q.; Xi, T.; Chen, M.; Guan, S. Enhanced in Vitro and in Vivo Performance of Mg-Zn-Y-Nd Alloy Achieved with APTES Pretreatment for Drug-Eluting Vascular Stent Application. ACS Appl. Mater. Interfaces 2016, 8, 17842–17858. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xue, Y.; Wang, F.; Guo, D.; He, Y.; Zhao, X.; Yan, F.; Xu, Y.; Xia, D.; Liu, Y. Sustained Release of Magnesium and Zinc Ions Synergistically Accelerates Wound Healing. Bioact. Mater. 2023, 26, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef]
- da Silva, V.A.; Bobotis, B.C.; Correia, F.F.; Lima-Vasconcellos, T.H.; Chiarantin, G.M.D.; De La Vega, L.; Lombello, C.B.; Willerth, S.M.; Malmonge, S.M.; Paschon, V.; et al. The Impact of Biomaterial Surface Properties on Engineering Neural Tissue for Spinal Cord Regeneration. Int. J. Mol. Sci. 2023, 24, 13642. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 948–963. [Google Scholar] [CrossRef]
- Chen, B.; You, Y.; Ma, A.; Song, Y.; Jiao, J.; Song, L.; Shi, E.; Zhong, X.; Li, Y.; Li, C. Zn-Incorporated TiO2 Nanotube Surface Improves Osteogenesis Ability Through Influencing Immunomodulatory Function of Macrophages. Int. J. Nanomed. 2020, 15, 2095–2118. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A Review on Current Research Status of the Surface Modification of Zn-Based Biodegradable Metals. Bioact. Mater. 2022, 7, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, X.; Xia, F.; Jiang, Y.; Zhang, H.; Huang, M.; Niu, C.; Wu, J.; Zhao, Y.; Wang, X.; et al. Large-Scale Integration of a Zinc Metasilicate Interface Layer Guiding Well-Regulated Zn Deposition. Adv. Mater. 2022, 34, e2202188. [Google Scholar] [CrossRef]
- Kast, M.; Schroeder, P.; Hyun, Y.J.; Pongratz, P.; Bruuckl, H. Synthesis of Single-Crystalline Zn Metal Nanowires Utilizing Cold-Wall Physical Vapor Deposition. Nano Lett. 2007, 7, 2540–2544. [Google Scholar] [CrossRef]
- Rabadjieva, D.; Tepavitcharova, S.; Gergulova, R.; Sezanova, K.; Titorenkova, R.; Petrov, O.; Dyulgerova, E. Mg- and Zn-Modified Calcium Phosphates Prepared by Biomimetic Precipitation and Subsequent Treatment at High Temperature. J. Mater. Sci. Mater. Med. 2011, 22, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Roi, A.; Ardelean, L.C.; Roi, C.I.; Boia, E.R.; Boia, S.; Rusu, L.C. Oral Bone Tissue Engineering: Advanced Biomaterials for Cell Adhesion, Proliferation and Differentiation. Materials 2019, 12, 2296. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.; Miculescu, M.; Manescu Paltanea, V.; Stere, A.; Quan, P.H.; Paltanea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef]
- Giavaresi, G.; Bellavia, D.; De Luca, A.; Costa, V.; Raimondi, L.; Cordaro, A.; Sartori, M.; Terrando, S.; Toscano, A.; Pignatti, G.; et al. Magnesium Alloys in Orthopedics: A Systematic Review on Approaches, Coatings and Strategies to Improve Biocompatibility, Osteogenic Properties and Osteointegration Capabilities. Int. J. Mol. Sci. 2023, 25, 282. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of Bone Development and Repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Wang, S.H.; Lee, S.P.; Yang, C.W.; Lo, C.M. Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. Materials 2021, 14, 441. [Google Scholar] [CrossRef]
- Che, X.; Jin, X.; Park, N.R.; Kim, H.J.; Kyung, H.S.; Kim, H.J.; Lian, J.B.; Stein, J.L.; Stein, G.S.; Choi, J.Y. Cbfbeta Is a Novel Modulator against Osteoarthritis by Maintaining Articular Cartilage Homeostasis through TGF-Beta Signaling. Cells 2023, 12, 1064. [Google Scholar] [CrossRef]
- Majumdar, T.; Cooke, M.E.; Lawless, B.M.; Bellier, F.; Hughes, E.A.B.; Grover, L.M.; Jones, S.W.; Cox, S.C. Formulation and Viscoelasticity of Mineralised Hydrogels for Use in Bone-Cartilage Interfacial Reconstruction. J. Mech. Behav. Biomed. Mater. 2018, 80, 33–41. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Gebhardt, A.; Kessler, J.; Thurn, L. 3D-Drucken; Hanser: München, Germany, 2016. [Google Scholar]
- DIN EN ISO/ASTM 52900; Additive manufacturing—General principles—Fundamentals and vocabulary. Beuth Verlag: Berlin, Germany, 2022.
- Lippert, R.B. Restriktionsgerechte Gestaltung Innerer Strukturen Für Das Selektive Laserstrahlschmelzen. In Additive Manufacturing Quantifiziert: Visionäre Anwendungen und Stand der Technik; Lachmayer, R., Lippert, R.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 23–48. [Google Scholar]
- King, W.E.; Anderson, A.T.; Ferencz, R.M.; Hodge, N.E.; Kamath, C.; Khairallah, S.A.; Rubenchik, A.M. Laser Powder Bed Fusion Additive Manufacturing of Metals; Physics, Computational, and Materials Challenges. Appl. Phys. Rev. 2015, 2, 041304. [Google Scholar] [CrossRef]
- Gebhardt, A. Rapid Prototyping: Werkzeug Für Die Schnelle Produktentwicklung; Mit 37 Tabellen; Hanser: München, Germany, 1996. [Google Scholar]
- Meiners, W. Direktes Selektives Laser-Sintern Einkomponentiger Metallischer Werkstoffe. Ph.D. Thesis, Shaker, Aachen, Germany, 1999. [Google Scholar]
- Voshage, M.A. Additive Verarbeitung von Zink-Magnesium-Legierungen; RWTH Aachen University: Aachen, Germany, 2023; p. 1. [Google Scholar]
- Wen, P.; Qin, Y.; Chen, Y.; Voshage, M.; Jauer, L.; Poprawe, R.; Schleifenbaum, J.H. Laser Additive Manufacturing of Zn Porous Scaffolds: Shielding Gas Flow, Surface Quality and Densification. J. Mater. Sci. Technol. 2019, 35, 368–376. [Google Scholar] [CrossRef]
- Kniha, K.; Buhl, E.M.; Mohlhenrich, S.C.; Bock, A.; Holzle, F.; Hellwig, E.; Al-Ahmad, A.; Modabber, A. In Vivo and in Vitro Analysis in a Rat Model Using Zoledronate and Alendronate Medication: Microbiological and Scanning Electron Microscopy Findings on Peri-Implant Rat Tissue. BMC Oral. Health 2021, 21, 672. [Google Scholar] [CrossRef] [PubMed]
- Givan, A.L. Flow Cytometry: An Introduction; Springer: Berlin/Heidelberg, Germany, 2011; Volume 699. [Google Scholar]
- Laczko-Rigo, R.; Bakos, E.; Jojart, R.; Tomboly, C.; Mernyak, E.; Ozvegy-Laczka, C. Selective Antiproliferative Effect of C-2 Halogenated 13alpha-Estrones on Cells Expressing Organic Anion-Transporting Polypeptide 2B1 (OATP2B1). Toxicol. Appl. Pharmacol. 2021, 429, 115704. [Google Scholar] [CrossRef]
- Nourse, J.L.; Leung, V.M.; Abuwarda, H.; Evans, E.L.; Izquierdo-Ortiz, E.; Ly, A.T.; Truong, N.; Smith, S.; Bhavsar, H.; Bertaccini, G.; et al. Piezo1 Regulates Cholesterol Biosynthesis to Influence Neural Stem Cell Fate during Brain Development. J. Gen. Physiol. 2022, 154, e202213084. [Google Scholar] [CrossRef]
- Siddiqui, S.; Livak, F. Principles of Advanced Flow Cytometry: A Practical Guide; Springer: New York, NY, USA, 2023; Volume 2580. [Google Scholar]
- Ujas, T.A.; Obregon-Perko, V.; Stowe, A.M. A Guide on Analyzing Flow Cytometry Data Using Clustering Methods and Nonlinear Dimensionality Reduction (tSNE or UMAP); Springer: New York, NY, USA, 2023; Volume 2616. [Google Scholar]
- Chou, J.; Hao, J.; Hatoyama, H.; Ben-Nissan, B.; Milthorpe, B.; Otsuka, M. Effect of Biomimetic Zinc-Containing Tricalcium Phosphate (Zn-TCP) on the Growth and Osteogenic Differentiation of Mesenchymal Stem Cells. J. Tissue Eng. Regen. Med. 2015, 9, 852–858. [Google Scholar] [CrossRef]
- Roh, H.J.; Park, J.; Lee, S.H.; Kim, D.H.; Lee, G.C.; Jeon, H.; Chae, M.; Lee, K.S.; Sun, J.Y.; Lee, D.H.; et al. Optimization of the Clinically Approved Mg-Zn Alloy System through the Addition of Ca. Biomater. Res. 2022, 26, 41. [Google Scholar] [CrossRef]
- Pinho, A.R.; Martins, F.; Costa, M.E.V.; Senos, A.M.R.; Da Cruz E Silva, O.A.B.; Pereira, M.D.L.; Rebelo, S. In Vitro Cytotoxicity Effects of Zinc Oxide Nanoparticles on Spermatogonia Cells. Cells 2020, 9, 1081. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Endothelial Cellular Responses to Biodegradable Metal Zinc. ACS Biomater. Sci. Eng. 2015, 1, 1174–1182. [Google Scholar] [CrossRef]
- Fernández-Bertólez, N.; Alba-González, A.; Touzani, A.; Ramos-Pan, L.; Méndez, J.; Reis, A.T.; Quelle-Regaldie, A.; Sánchez, L.; Folgueira, M.; Laffon, B.; et al. Toxicity of Zinc Oxide Nanoparticles: Cellular and Behavioural Effects. Chemosphere 2024, 363, 142993. [Google Scholar] [CrossRef]
- Müller, E.; Schoberwalter, T.; Mader, K.; Seitz, J.-M.; Kopp, A.; Baranowsky, A.; Keller, J. The Biological Effects of Magnesium-Based Implants on the Skeleton and Their Clinical Implications in Orthopedic Trauma Surgery. Biomater. Res. 2024, 28, 0122. [Google Scholar] [CrossRef] [PubMed]
- Fathi, E.; Farahzadi, R. Enhancement of Osteogenic Differentiation of Rat Adipose Tissue-Derived Mesenchymal Stem Cells by Zinc Sulphate under Electromagnetic Field via the PKA, ERK1/2 and Wnt/β-Catenin Signaling Pathways. PLoS ONE 2017, 12, e0173877. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wang, X.; Xu, F.; Dai, T.; Zhou, J.G.; Liu, J.; Song, K.; Tian, L.; Liu, B.; Liu, Y. In Vivo Biocompatibility and Degradability of a Zn–Mg–Fe Alloy Osteosynthesis System. Bioact. Mater. 2022, 7, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Klíma, K.; Ulmann, D.; Bartoš, M.; Španko, M.; Dušková, J.; Vrbová, R.; Pinc, J.; Kubásek, J.; Ulmannová, T.; Foltán, R.; et al. Zn-0.8Mg-0.2Sr (Wt.%) Absorbable Screws-An In-Vivo Biocompatibility and Degradation Pilot Study on a Rabbit Model. Materials 2021, 14, 3271. [Google Scholar] [CrossRef]
- Gao, J.; Wu, S.; Qiao, L.; Wang, Y. Corrosion Behavior of Mg and Mg-Zn Alloys in Simulated Body Fluid. Trans. Nonferrous Met. Soc. China 2008, 18, 588–592. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Fischer, F.; Voshage, M.; Jauer, L.; Kopp, A.; Praster, M.; Groven, R.V.M.; Schleifenbaum, J.H.; Eschweiler, J.; Kobbe, P.; et al. Effects of Surface Treatments on Innovative Additively Manufactured Scaffolds: Implications for Biocompatibility in Bone Tissue Engineering. Life 2025, 15, 1755. https://doi.org/10.3390/life15111755
Zhao Q, Fischer F, Voshage M, Jauer L, Kopp A, Praster M, Groven RVM, Schleifenbaum JH, Eschweiler J, Kobbe P, et al. Effects of Surface Treatments on Innovative Additively Manufactured Scaffolds: Implications for Biocompatibility in Bone Tissue Engineering. Life. 2025; 15(11):1755. https://doi.org/10.3390/life15111755
Chicago/Turabian StyleZhao, Qun, Florian Fischer, Maximilian Voshage, Lucas Jauer, Alexander Kopp, Maximilian Praster, Rald Victor Maria Groven, Johannes Henrich Schleifenbaum, Jörg Eschweiler, Philipp Kobbe, and et al. 2025. "Effects of Surface Treatments on Innovative Additively Manufactured Scaffolds: Implications for Biocompatibility in Bone Tissue Engineering" Life 15, no. 11: 1755. https://doi.org/10.3390/life15111755
APA StyleZhao, Q., Fischer, F., Voshage, M., Jauer, L., Kopp, A., Praster, M., Groven, R. V. M., Schleifenbaum, J. H., Eschweiler, J., Kobbe, P., Buhl, E. M., Hildebrand, F., Balmayor, E. R., & Greven, J. (2025). Effects of Surface Treatments on Innovative Additively Manufactured Scaffolds: Implications for Biocompatibility in Bone Tissue Engineering. Life, 15(11), 1755. https://doi.org/10.3390/life15111755







