Integration of Postbiotics into Adult Diapers: In Vitro Evaluation of Biocompatibility and Effect on Skin Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Postbiotic Characterization and Quantification
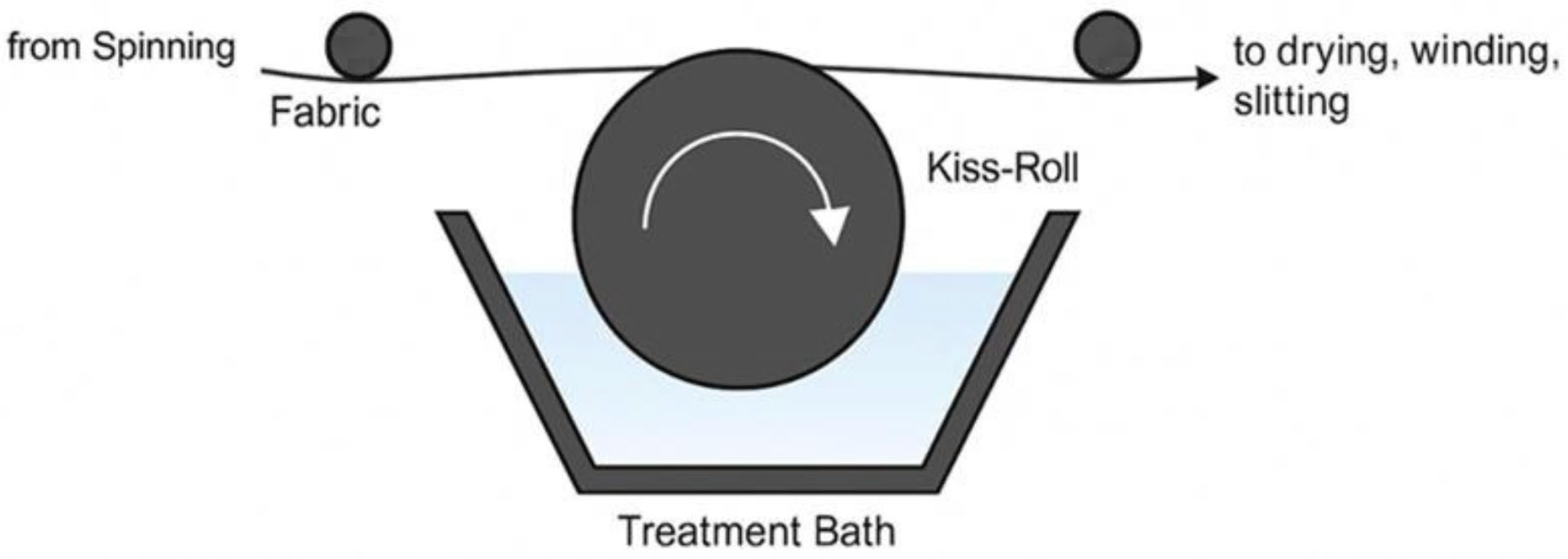
2.2.1. Gravimetric Determination of Oil Pick-Up Capacity in Posbiotic-Treated Nonwoven Fabrics
2.2.2. Quantitative Determination of Lactic Acid in Postbiotic-Containing Nonwoven Textiles by HPLC-UV
2.3. In Vitro Cytotoxicity Assays
2.3.1. Keratinocyte Cytotoxicity Test (ISO 10993-5)
2.3.2. Reconstructed Human Epidermis Irritation Test (OECD 439)
2.4. Microbiota Interaction Studies
2.4.1. Selection of Skin Microorganisms
2.4.2. Assessment of Microbial Load of Postbiotic-Containing Nonwoven
2.4.3. Inoculation and Exposure Conditions
2.4.4. Microbial Balance and Microbial Diversity Assessment
2.5. Statistical Analysis
3. Results
3.1. Gravimetric Determination of Oil Pick-Up Capacity in Posbiotic-Treated Nonwoven Fabrics
3.2. Quantitative Determination of Lactic Acid in Postbiotic-Containing Nonwoven Textiles by HPLC-UV
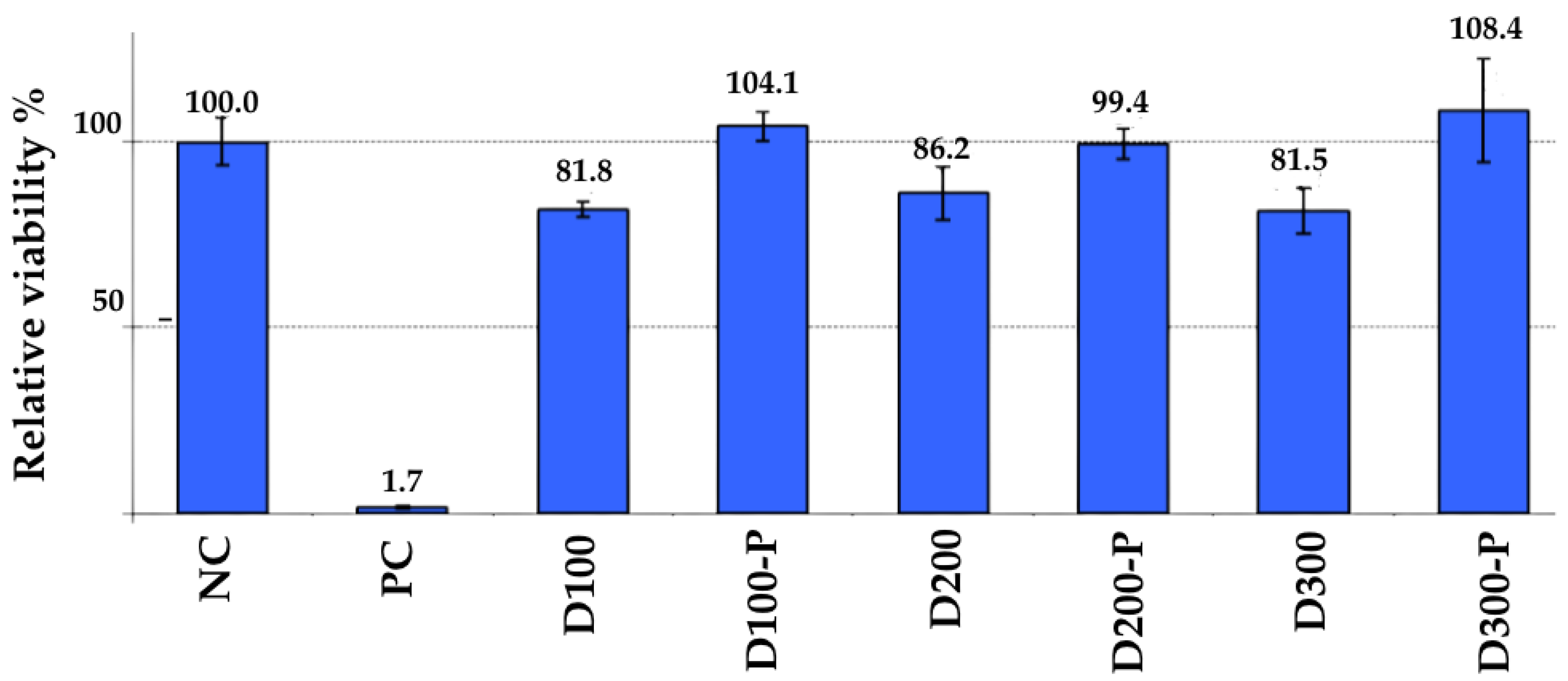
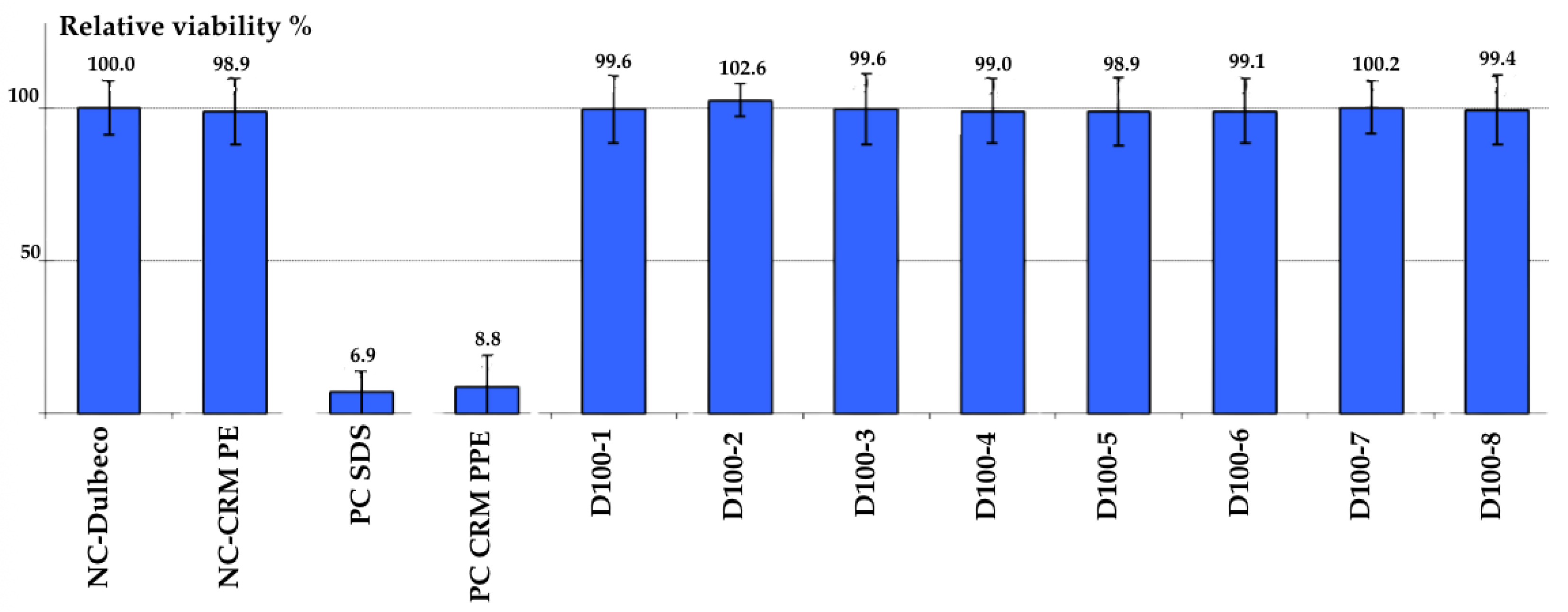
3.3. In Vitro Cytotoxicity and Tissue Viability
3.3.1. Keratinocyte Cytotoxicity (ISO 10993-5)
3.3.2. Epidermal Irritation Assay (OECD 439)
3.4. Effects on Skin Microbiota
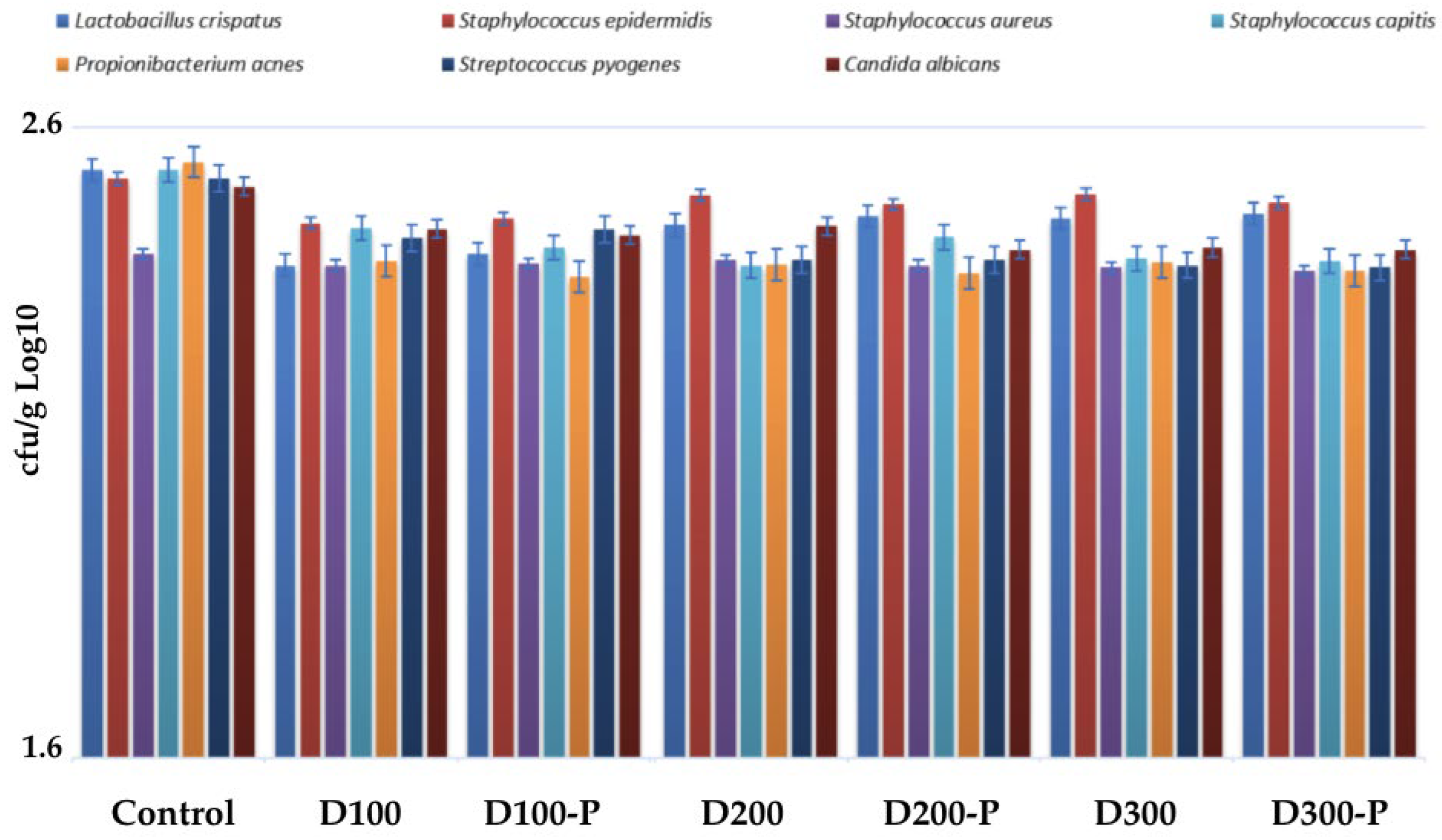
3.4.1. Assessment of Microbial Load of Postbiotic-Containing Nonwoven
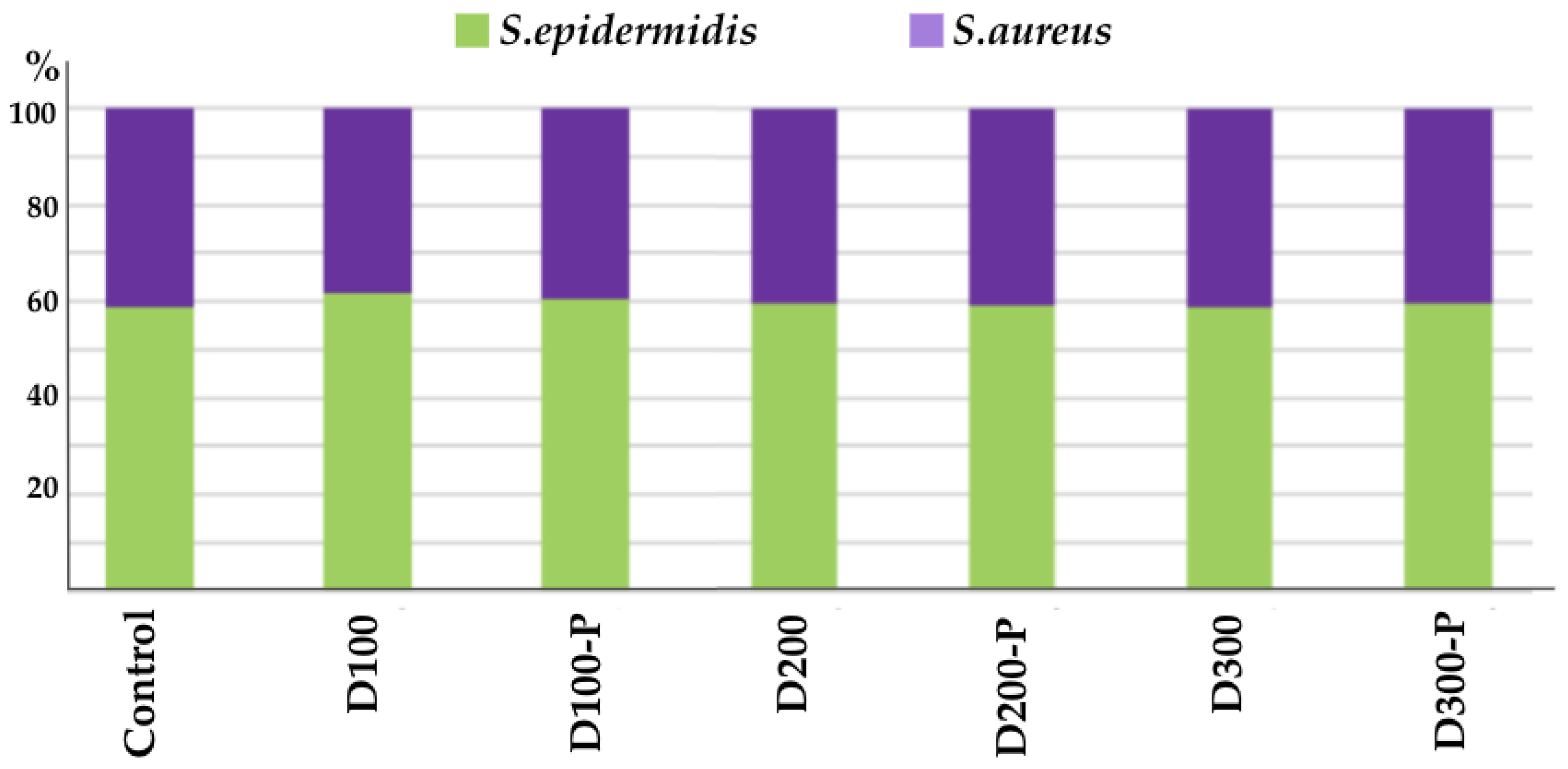
3.4.2. S. epidermidis/S. aureus Balance
3.4.3. Microbial Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| MATRIX | NON WOVEN FABRIC (2.2 mL) | NON WOVEN FABRIC (2 mL) | ||
| OPU% | Analyst-1 | Analyst-2 | Analyst-1 | Analyst-2 |
| 0.673 | 0.666 | 0.645 | 0.655 | |
| 0.680 | 0.669 | 0.642 | 0.646 | |
| 0.667 | 0.680 | 0.648 | 0.641 | |
| 0.667 | 0.667 | 0.639 | 0.644 | |
| 0.676 | 0.676 | 0.641 | 0.646 | |
| 0.673 | 0.667 | 0.651 | 0.642 | |
| MEAN | 0.673 | 0.671 | 0.644 | 0.645 |
| STANDARD DEVIATION | 0.005 | 0.006 | 0.005 | 0.005 |
| MIN | 0.667 | 0.666 | 0.639 | 0.641 |
| MAX | 0.680 | 0.680 | 0.651 | 0.655 |
| GRUBBS LOW (G) | 1.159 | 0.788 | 1.202 | 0.918 |
| GRUBBS HIGH (G) | 1.427 | 1.581 | 1.421 | 1.857 |
| GRUBBS CRITERION (GÖ) | 1.890 | 1.890 | 1.890 | 1.890 |
| EVALUATION (G<GÖ) | TRUE | TRUE | TRUE | TRUE |
| RSD | 0.008 | 0.008 | 0.007 | 0.008 |
| % RSD | 0.750 | 0.848 | 0.712 | 0.775 |
| Decimal Mean | 0.007 | 0.007 | 0.006 | 0.006 |
| PRSD | 4.246 | 4.247 | 4.273 | 4.272 |
| 1/2PRSD | 2.123 | 2.124 | 2.137 | 2.136 |
| EVALUATION (%RSD<1/2PRSD) | TRUE | TRUE | TRUE | TRUE |
| MATRIX | NON WOVEN FABRIC (2.2 mL) | NON WOVEN FABRIC (2 mL) | ||
| LACTIC ACID % | Analyst-1 | Analyst-2 | Analyst-1 | Analyst-2 |
| 0.0044 | 0.0045 | 0.0042 | 0.0040 | |
| 0.0045 | 0.0042 | 0.0041 | 0.0041 | |
| 0.0044 | 0.0044 | 0.0042 | 0.0040 | |
| 0.0043 | 0.0045 | 0.0041 | 0.0041 | |
| 0.0043 | 0.0044 | 0.0040 | 0.0042 | |
| 0.0045 | 0.0045 | 0.0041 | 0.0041 | |
| MEAN | 0.0044 | 0.0044 | 0.0041 | 0.0041 |
| STANDARD DEVIATION | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| MIN | 0.0043 | 0.0042 | 0.0040 | 0.0040 |
| MAX | 0.0045 | 0.0045 | 0.0042 | 0.0042 |
| GRUBBS LOW (G) | 1.2898 | 1.7699 | 1.0349 | 1.0747 |
| GRUBBS HIGH G) | 1.2263 | 0.8337 | 1.2571 | 1.5503 |
| GRUBBS CRITERION (GÖ) | 1.8900 | 1.8900 | 1.8900 | 1.8900 |
| EVALUATION (G<GÖ) | TRUE | TRUE | TRUE | TRUE |
| RSD | 0.0236 | 0.0238 | 0.0197 | 0.0166 |
| % RSD | 2.3555 | 2.3841 | 1.9671 | 1.6608 |
| Decimal Mean | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| PRSD | 9.0497 | 9.0483 | 9.1373 | 9.1545 |
| 1/2PRSD | 4.5248 | 4.5241 | 4.5686 | 4.5773 |
| EVALUATION (%RSD<1/2PRSD) | TRUE | TRUE | TRUE | TRUE |
References
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the Human Skin Microbiota and Its Roles in Cutaneous Diseases. Microb. Cell Factories 2022, 21, 176. [Google Scholar] [CrossRef]
- Smythe, P.; Wilkinson, H.N. The Skin Microbiome: Current Landscape and Future Opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Surface Proteins of Staphylococcus Epidermidis. Front. Microbiol. 2020, 11, 1829. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus Aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus Aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Farahmand, S. Microbiome of Compromised Skin. In Skin Microbiome Handbook; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 143–169. ISBN 978-1-119-59305-8. [Google Scholar]
- Hamdi, A.; Lloyd, C.; Eri, R.; Van, T.T.H. Postbiotics: A Promising Approach to Combat Age-Related Diseases. Life 2025, 15, 1190. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Wang, D.; Li, Y.; Yip, R.C.S.; Chen, H. Postbiotics-Peptidoglycan, Lipoteichoic Acid, Exopolysaccharides, Surface Layer Protein and Pili Proteins—Structure, Activity in Wounds and Their Delivery Systems. Int. J. Biol. Macromol. 2024, 274, 133195. [Google Scholar] [CrossRef]
- Zhong, B.; Zhao, Y.; Gao, L.; Yang, G.; Gao, Y.; Li, F.; Li, S. Anticancer Effects of Weizmannia Coagulans MZY531 Postbiotics in CT26 Colorectal Tumor-Bearing Mice by Regulating Apoptosis and Autophagy. Life 2024, 14, 1334. [Google Scholar] [CrossRef]
- Roberts, K.D.; Ahmed, S.; San Valentin, E.; Di Martino, L.; McCormick, T.S.; Ghannoum, M.A. Immunomodulatory Properties of Multi-Strain Postbiotics on Human CD14+ Monocytes. Life 2024, 14, 1673. [Google Scholar] [CrossRef]
- Rusic, D.; Ivic, M.; Slugan, A.; Leskur, D.; Modun, D.; Durdov, T.; Vukovic, D.; Bukic, J.; Bozic, J.; Seselja Perisin, A. Pilot Study on the Effects of a Cosmetic Serum Containing Niacinamide, Postbiotics and Peptides on Facial Skin in Healthy Participants: A Randomized Controlled Trial. Life 2024, 14, 1677. [Google Scholar] [CrossRef]
- Golkar, N.; Ashoori, Y.; Heidari, R.; Omidifar, N.; Abootalebi, S.N.; Mohkam, M.; Gholami, A. A Novel Effective Formulation of Bioactive Compounds for Wound Healing: Preparation, In Vivo Characterization, and Comparison of Various Postbiotics Cold Creams in a Rat Model. Evid. Based Complement. Alternat. Med. 2021, 2021, 8577116. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, J.-H.; Baek, J.; Kim, W. Improvement of Cutaneous Wound Healing via Topical Application of Heat-Killed Lactococcus Chungangensis CAU 1447 on Diabetic Mice. Nutrients 2021, 13, 2666. [Google Scholar] [CrossRef]
- Demirhan, H.K.; Aksoy, Z.B.; Karaca, B.; Kankilic, T.; Akcali, K.C.; Kiran, F. In Vitro Wound Healing Effects of Postbiotics Derived from the Gut Microbiota of Long-Lived Blind Mole Rats, a Model of Healthy Ageing. Wound Repair Regen. 2025, 33, e70023. [Google Scholar] [CrossRef]
- Palade, C.-M.; Vulpoi, G.-A.; Vulpoi, R.-A.; Drug, V.L.; Barboi, O.-B.; Ciocoiu, M. The Biotics Family: Current Knowledge and Future Perspectives in Metabolic Diseases. Life 2022, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Rescigno, M. Postbiotics: What Else? Benef. Microbes 2013, 4, 101–107. [Google Scholar] [CrossRef]
- Hong, K.-B.; Hong, Y.; Jung, E.; Jo, K.; Suh, H. Changes in the Diversity of Human Skin Microbiota to Cosmetic Serum Containing Prebiotics: Results from a Randomized Controlled Trial. J. Pers. Med. 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Sivamaruthi, B.; Tansrisook, C.; Peerajan, S.; Chaiyasut, K.; Bharathi, M. Influence of Paraprobiotics-Containing Moisturizer on Skin Hydration and Microbiome: A Preliminary Study. Appl. Sci. 2022, 12, 12483. [Google Scholar] [CrossRef]
- Alves, E.; Gregório, J.; Ríjo, P.; Rosado, C.; Rodrigues, L.M. The Impact of Kefir on Epidermal Water Homeostasis in Healthy Human Skin. Life 2022, 12, 1075. [Google Scholar] [CrossRef]
- Aslan, İ.; Çelebi, L.T. Postbiotics Cosmetic Formulation: In Vitro Efficacy Studies on a Microbiome Friendly Antiperspirant. J. Res. Pharm. 2025, 27, 2095–2105. [Google Scholar] [CrossRef]
- Gökçe, H.B.; Aslan, İ. Novel Liposome–Gel Formulations Containing a Next Generation Postbiotic: Characterization, Rheological, Stability, Release Kinetic, and In Vitro Antimicrobial Activity Studies. Gels 2024, 10, 746. [Google Scholar] [CrossRef]
- Tarhan Çelebi, L.; Bursalioglu, E.O.; Çakici, B.; Genel, N.; Kalbisen, H.T.; Aslan, I. Can Buttermilk (Ayran) with Its Postbiotic Content Be Used in the Protection of Colon Health? J. Immunol. Clin. Microbiol. 2024, 9, 127–137. [Google Scholar] [CrossRef]
- Myoung, K.; Choi, E.-J.; Kim, S.; Hwang, J.A.; Lee, J.Y.; Kim, H.-J.; Hwang, J.S. Nano-Sized Lysate of Lactiplantibacillus Plantarum Isolated from Green Tea Leaves as a Potential Skin Care Ingredient. Biotechnol. Bioprocess Eng. 2024, 29, 1071–1080. [Google Scholar] [CrossRef]
- Mukarram, M.; Rizzo, D.; Omari, S.; Grand, Z.; Jackson, S.; Jaing, J.; Vinagolu-Baur, J.; Jimoh, L. The Impact of Topical Probiotic Lactobacillus Plantarum on Skin Barrier Repair in Atopic Dermatitis. ARC J. Dermatol. 2025, 8, 19–36. [Google Scholar] [CrossRef]
- Panagiotou, D.; Filidou, E.; Gaitanidou, M.; Tarapatzi, G.; Spathakis, M.; Kandilogiannakis, L.; Stavrou, G.; Arvanitidis, K.; Tsetis, J.K.; Gionga, P.; et al. Role of Lactiplantibacillus Plantarum UBLP-40, Lactobacillus Rhamnosus UBLR-58 and Bifidobacterium Longum UBBL-64 in the Wound Healing Process of the Excisional Skin. Nutrients 2023, 15, 1822. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of Human Epithelial Tight Junction Proteins by Lactobacillus Plantarum in Vivo and Protective Effects on the Epithelial Barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef]
- Anderson, R.; Cookson, A.; McNabb, W.; Park, Z.; McCann, M.J.; Kelly, W.; Roy, N. Lactobacillus Plantarum MB452 Enhances the Function of the Intestinal Barrier by Increasing the Expression Levels of Genes Involved in Tight Junction Formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef]
- Ogawa-Fuse, C.; Morisaki, N.; Shima, K.; Hotta, M.; Sugata, K.; Ichihashi, T.; Oguri, M.; Yoshida, O.; Fujimura, T. Impact of Water Exposure on Skin Barrier Permeability and Ultrastructure. Contact Dermat. 2019, 80, 228–233. [Google Scholar] [CrossRef]
- Biological Evaluation of Medical Devices. Part 5: Tests for in Vitro Cytotoxicity. In ANSI/AAMI/ISO 10993-5:2009/(R)2022; Biological Evaluation of Medical Devices? Part 5: Tests for In Vitro Cytotoxicity; Default Book Series; ACS Publications: Washington, DC, USA, 2022; ISBN 978-1-57020-355-8. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar] [CrossRef]
- ISO 21150:2015; Cosmetics-Microbiology-Detection of Escherichia coli. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 18416:2015; Cosmetics-Microbiology-Detection of Candida albicans. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 16212:2017; Cosmetics-Microbiology-Enumeration of Yeast and Mould. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 22717:2015; Cosmetics-Microbiology-Detection of Pseudomonas aeruginosa. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 22718:2015; Cosmetics-Microbiology-Detection of Staphylococcus aureus. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 21149:2017; Cosmetics-Microbiology-Enumeration and Detection of Aerobic Mesophilic Bacteria. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 17516:2014; Cosmetics—Microbiology—Microbiological Limits. International Organization for Standardization: Geneva, Switzerland, 2014.
- Kohda, K.; Li, X.; Soga, N.; Nagura, R.; Duerna, T.; Nakajima, S.; Nakagawa, I.; Ito, M.; Ikeuchi, A. An in vitro mixed infection model with commensal and pathogenic staphylococci for the exploration of interspecific interactions and their impacts on skin physiology. Frontiers in Cellular and Infection Microbiology. 2021, 11, 712360. [Google Scholar] [CrossRef]
- D’Arcangelo, S.; Fermo, P.D.; Diban, F.; Ferrone, V.; D’Ercole, S.; Giulio, M.D.; Lodovico, S.D. Staphylococcus Aureus/Staphylococcus Epidermidis from Skin Microbiota Are Balanced by Pomegranate Peel Extract: An Eco-Sustainable Approach. PLoS ONE 2024, 19, e0308211. [Google Scholar] [CrossRef]
- Aslan, İ.; Çelebi, L.T.; Bursalıoğlu, E.; Çakıcı, B.; Aydin, M.A.; Ibrahim, B.; Kurt, A.A. Novel Microbiome Friendly Purifying Oil Cleanser Formulation with Oil-Soluble Postbiotics. J. Immunol. Clin. Microbiol. 2025, 10, 1–2. [Google Scholar]
- Suellen Ferro de Oliveira, C.; Kekhasharú Tavaria, F. The Impact of Bioactive Textiles on Human Skin Microbiota. Eur. J. Pharm. Biopharm. 2023, 188, 66–77. [Google Scholar] [CrossRef]
- Broadhead, R.; Craeye, L.; Callewaert, C. The Future of Functional Clothing for an Improved Skin and Textile Microbiome Relationship. Microorganisms 2021, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Lee, H.; Kim, M.; Lee, J.W.; Saeed, M.; Lee, H.; Jung, S.-H.; Shim, J.-J.; Lee, J.-L.; Heo, K.; et al. Development and Metabolic Profiling of a Postbiotic Complex Exhibiting Antibacterial Activity against Skin Microorganisms and Anti-Inflammatory Effect on Human Keratinocytes. Food Sci. Biotechnol. 2022, 31, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Cho, M.; Kang, D.-J. Anti-Inflammatory Response of New Postbiotics in TNF-α/IFN-γ-Induced Atopic Dermatitis-like HaCaT Keratinocytes. Curr. Issues Mol. Biol. 2024, 46, 6100–6111. [Google Scholar] [CrossRef] [PubMed]
- Torres Salazar, B.O.; Dema, T.; Schilling, N.A.; Janek, D.; Bornikoel, J.; Berscheid, A.; Elsherbini, A.M.A.; Krauss, S.; Jaag, S.J.; Lämmerhofer, M.; et al. Commensal Production of a Broad-Spectrum and Short-Lived Antimicrobial Peptide Polyene Eliminates Nasal Staphylococcus Aureus. Nat. Microbiol. 2024, 9, 200–213. [Google Scholar] [CrossRef]
- Cercamondi, C.I.; Bendik, I.; Eckhardt, E.; Mak, T.; Seifert, N.; Kuratli, K.; Richard, N.; Tamasi, B.; Mussler, B.; Wintergerst, E. A Postbiotic Derived from Lactobacillaceae Protects Intestinal Barrier Function in a Challenge Model Using Colon Organoid Tubules. Foods 2025, 14, 1173. [Google Scholar] [CrossRef]
- Martorell, P.; Alvarez, B.; Llopis, S.; Navarro, V.; Ortiz, P.; Gonzalez, N.; Balaguer, F.; Rojas, A.; Chenoll, E.; Ramón, D.; et al. Heat-Treated Bifidobacterium Longum CECT-7347: A Whole-Cell Postbiotic with Antioxidant, Anti-Inflammatory, and Gut-Barrier Protection Properties. Antioxidants 2021, 10, 536. [Google Scholar] [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Oliveira, A.C.; de Carvalho Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, C.R. Production, Formulation, and Application of Postbiotics in the Treatment of Skin Conditions. Fermentation 2023, 9, 264. [Google Scholar] [CrossRef]
- Duarte, M.; Oliveira, A.L.; Oliveira, C.; Pintado, M.; Amaro, A.; Madureira, A.R. Current Postbiotics in the Cosmetic Market-an Update and Development Opportunities. Appl. Microbiol. Biotechnol. 2022, 106, 5879–5891. [Google Scholar] [CrossRef] [PubMed]
- Aslan, I.; Tarhan Celebi, L.; Kayhan, H.; Kizilay, E.; Gulbahar, M.Y.; Kurt, H.; Cakici, B. Probiotic Formulations Containing Fixed and Essential Oils Ameliorates SIBO-Induced Gut Dysbiosis in Rats. Pharmaceuticals 2023, 16, 1041. [Google Scholar] [CrossRef]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and Topical Probiotics and Postbiotics in Skincare and Dermatological Therapy: A Concise Review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Lekkala, L.; Yadav, D.; Jain, S.; Yadav, H. Microbiome and Postbiotics in Skin Health. Biomedicines 2025, 13, 791. [Google Scholar] [CrossRef]
- Jin, X.; Nguyen, T.T.M.; Yi, E.-J.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Kim, M.-J.; Yi, T.-H. Emerging Trends in Skin Anti-Photoaging by Lactic Acid Bacteria: A Focus on Postbiotics. Chemistry 2024, 6, 1495–1508. [Google Scholar] [CrossRef]
- Uluca, H.; Çelebi, L.T.; Çakıcı, B.; Ekşi, Ö.; Bursalıoğlu, E.O.; Bayır, İ.; Kurt, A.A.; Aslan, İ. Differences between the Feeding of Hydroponically Grown Curly Vegetables with Postbiotic Free Nutrient Solution and ATAGREEN Postbiotic Supplemented Nutrient Solution with Probiotics. Curr. Perspect. Med. Aromat. Plants 2025, 8, 13–23. [Google Scholar]
| Analysis | Result (CFU/g) | Limit for Products Used Around the Eyes and for Children Under 3 Years | Limit for Other Products | Compliance Status |
|---|---|---|---|---|
| Total Aerobic Mesophilic Bacterial Count | <1.0 × 101 | 1.0 × 102 | 1.0 × 103 | Complies |
| Pseudomonas aeruginosa Detection | Not detected | Absent | Absent | Complies |
| Escherichia coli Detection | Not detected | Absent | Absent | Complies |
| Candida albicans Detection | Not detected | Absent | Absent | Complies |
| Staphylococcus aureus Detection | Not detected | Absent | Absent | Complies |
| Total Yeast and Mold Count | <1.0 × 101 | 1.0 × 102 | 1.0 × 103 | Complies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahimoglu, O.O.; Tarhan Celebi, L.; Dikbiyik, D.E.; Gokce, H.B.; Cakici, B.; Türkoğlu, Z.; Atsu, A.N.; Aslan, I. Integration of Postbiotics into Adult Diapers: In Vitro Evaluation of Biocompatibility and Effect on Skin Microbiota. Life 2025, 15, 1652. https://doi.org/10.3390/life15111652
Ibrahimoglu OO, Tarhan Celebi L, Dikbiyik DE, Gokce HB, Cakici B, Türkoğlu Z, Atsu AN, Aslan I. Integration of Postbiotics into Adult Diapers: In Vitro Evaluation of Biocompatibility and Effect on Skin Microbiota. Life. 2025; 15(11):1652. https://doi.org/10.3390/life15111652
Chicago/Turabian StyleIbrahimoglu, Oznur Ozlem, Leyla Tarhan Celebi, Dilan Ece Dikbiyik, Halise Betul Gokce, Bekir Cakici, Zafer Türkoğlu, Ayse Nilhan Atsu, and Ismail Aslan. 2025. "Integration of Postbiotics into Adult Diapers: In Vitro Evaluation of Biocompatibility and Effect on Skin Microbiota" Life 15, no. 11: 1652. https://doi.org/10.3390/life15111652
APA StyleIbrahimoglu, O. O., Tarhan Celebi, L., Dikbiyik, D. E., Gokce, H. B., Cakici, B., Türkoğlu, Z., Atsu, A. N., & Aslan, I. (2025). Integration of Postbiotics into Adult Diapers: In Vitro Evaluation of Biocompatibility and Effect on Skin Microbiota. Life, 15(11), 1652. https://doi.org/10.3390/life15111652