Phenolic Compounds with Antimicrobial Properties in Mushrooms Frequently Encountered in Temperate Deciduous Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction Protocol
2.4. Total Phenolic Content
2.5. Total Flavonoid Content
2.6. DPPH Radical-Scavenging Activity
2.7. ABTS Radical Cation Decolorization Assay (ABTS+)
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.9. Liquid Chromatography-Diode Array Detection–Electro-Spray Ionization Mass Spectrometry (HPLC-DAD-ESI MS)
2.10. Antimicrobial Activity
2.11. Time-Kill Assay
2.12. Statistical Analysis
3. Results
3.1. Total Polyphenols, Flavonoids, and Antioxidant Activities of Mushroom Extracts
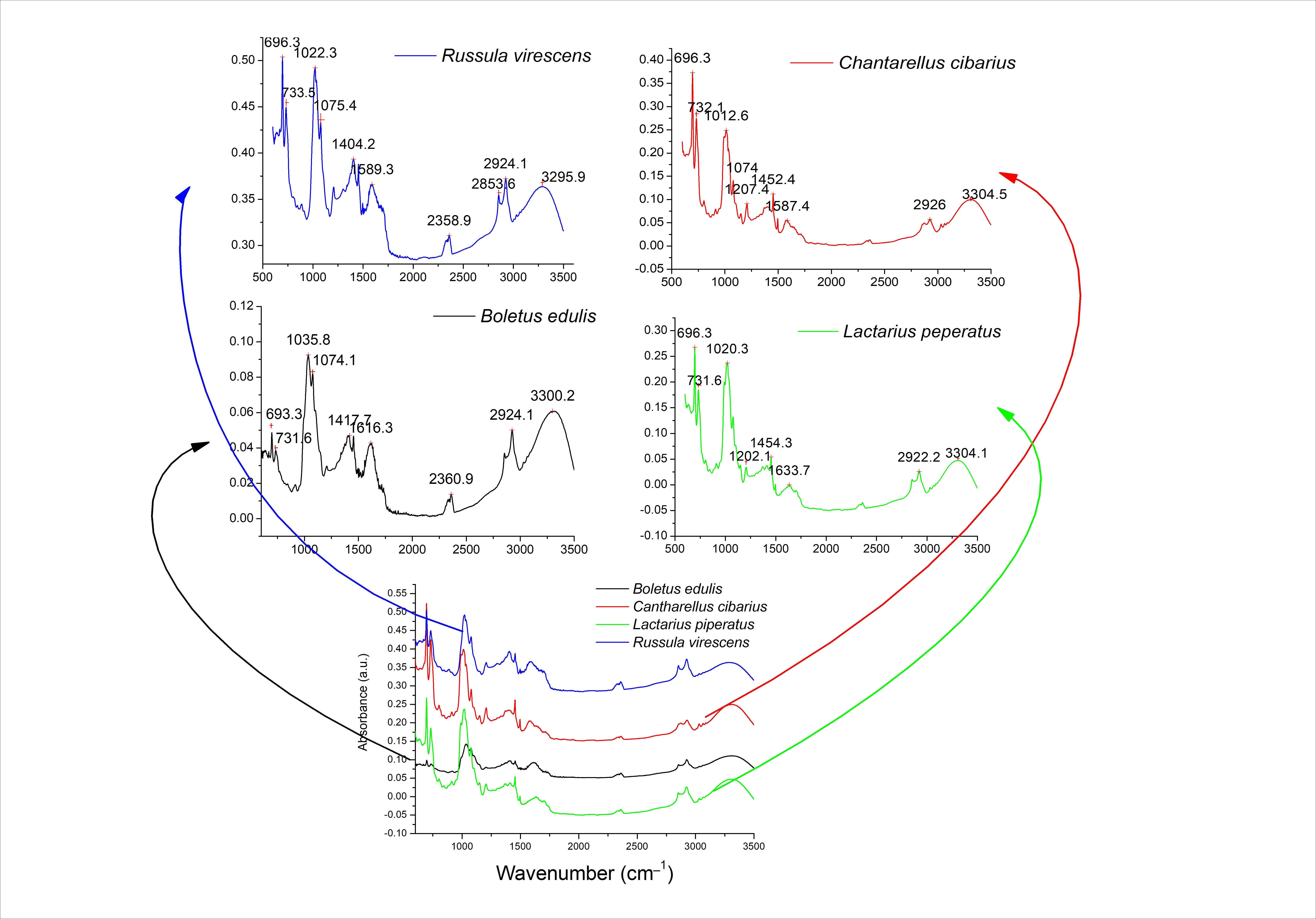
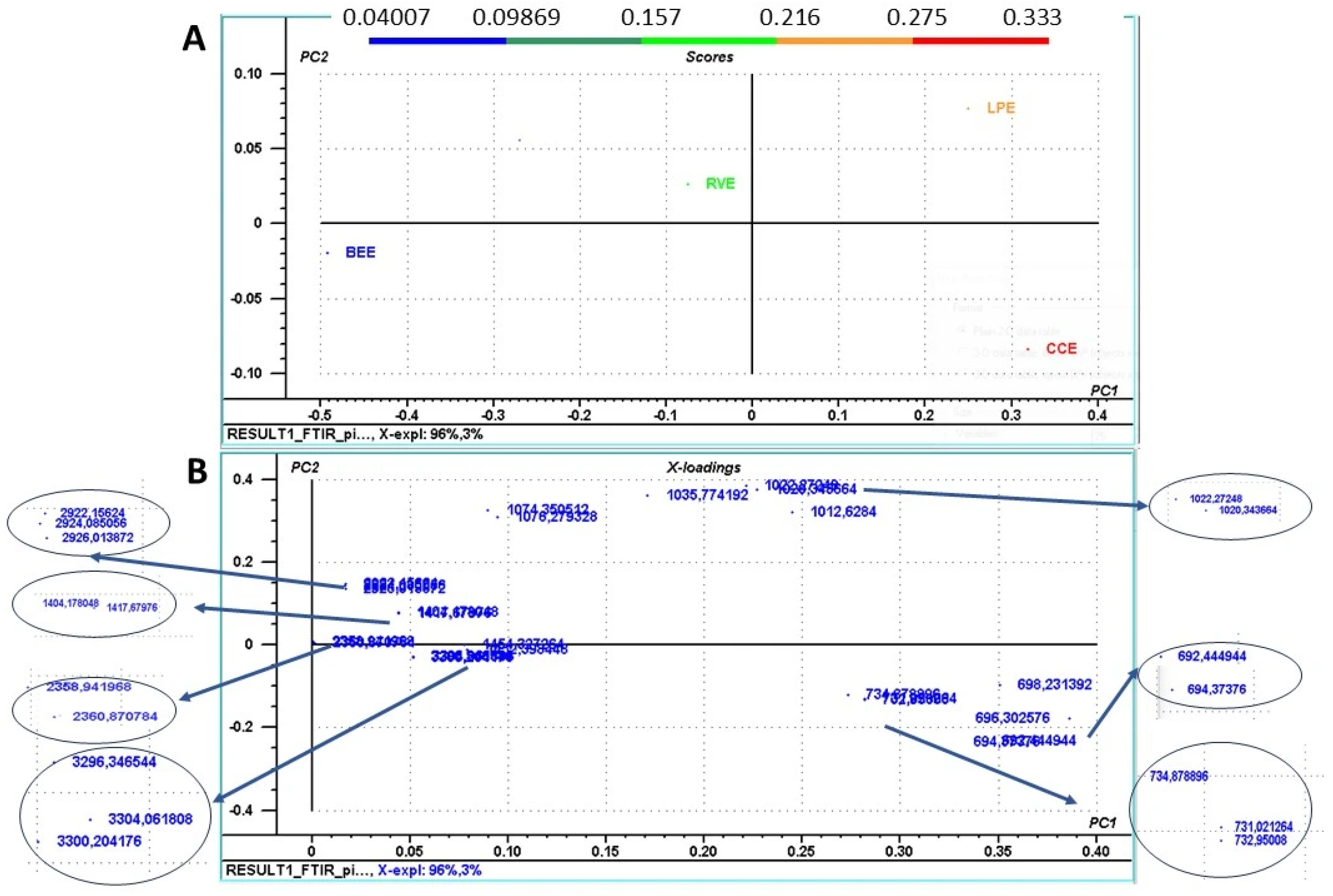
3.2. Chemical Fingerprinting of Mushroom Extracts Using FTIR Analysis
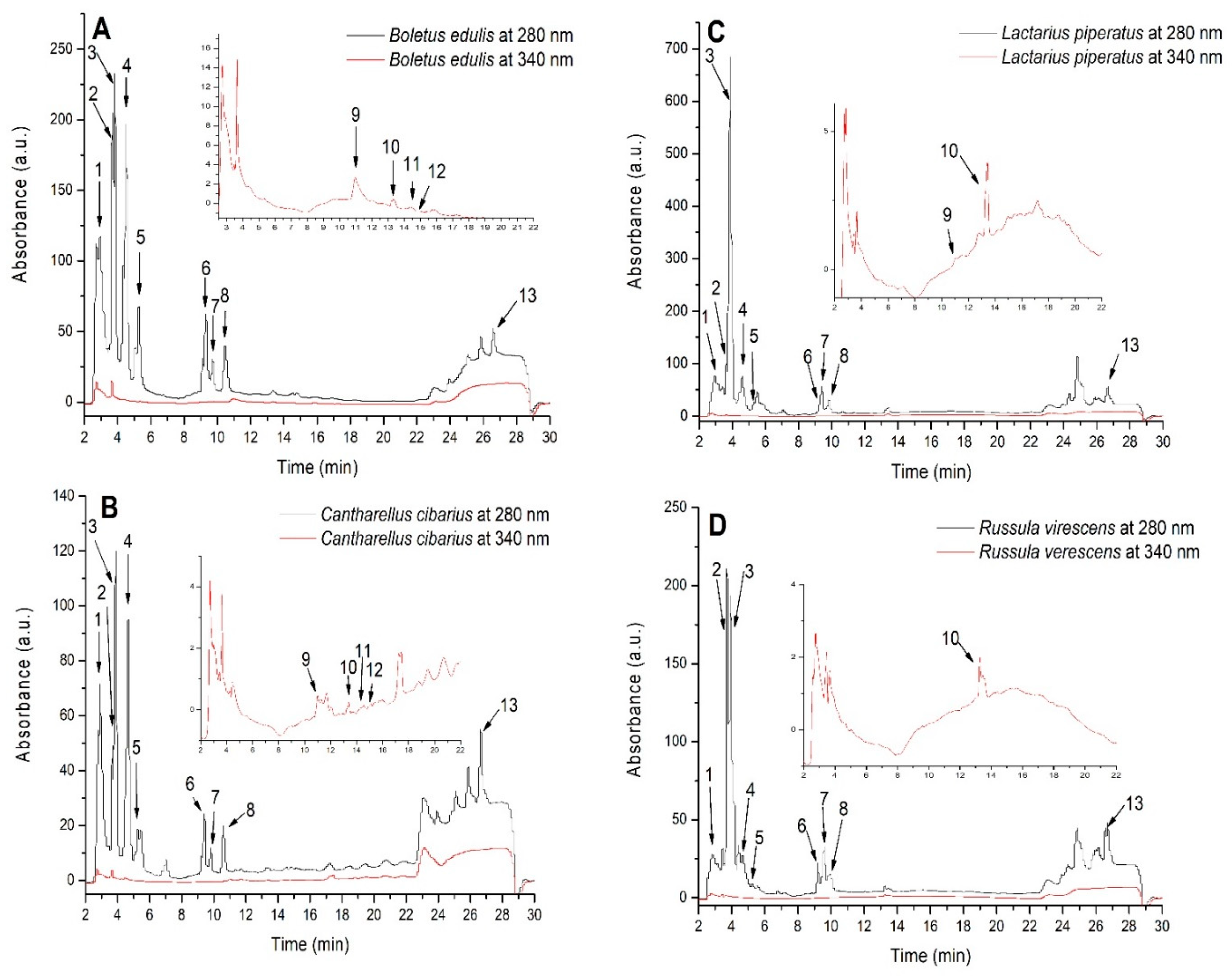
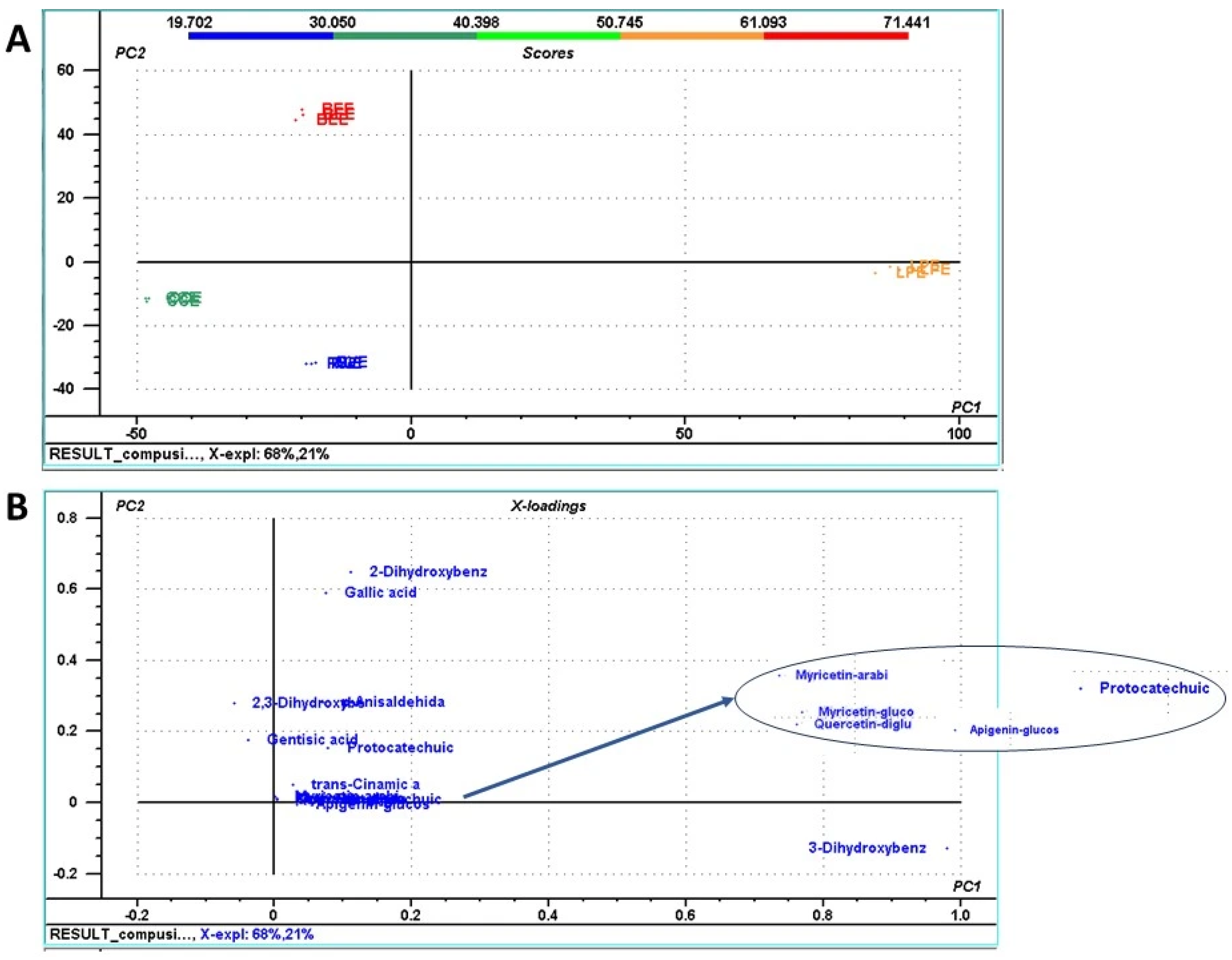
3.3. HPLC-DAD-ESI(+)MS-Based Profiling of Phenolic Constituents in Mushroom Extracts
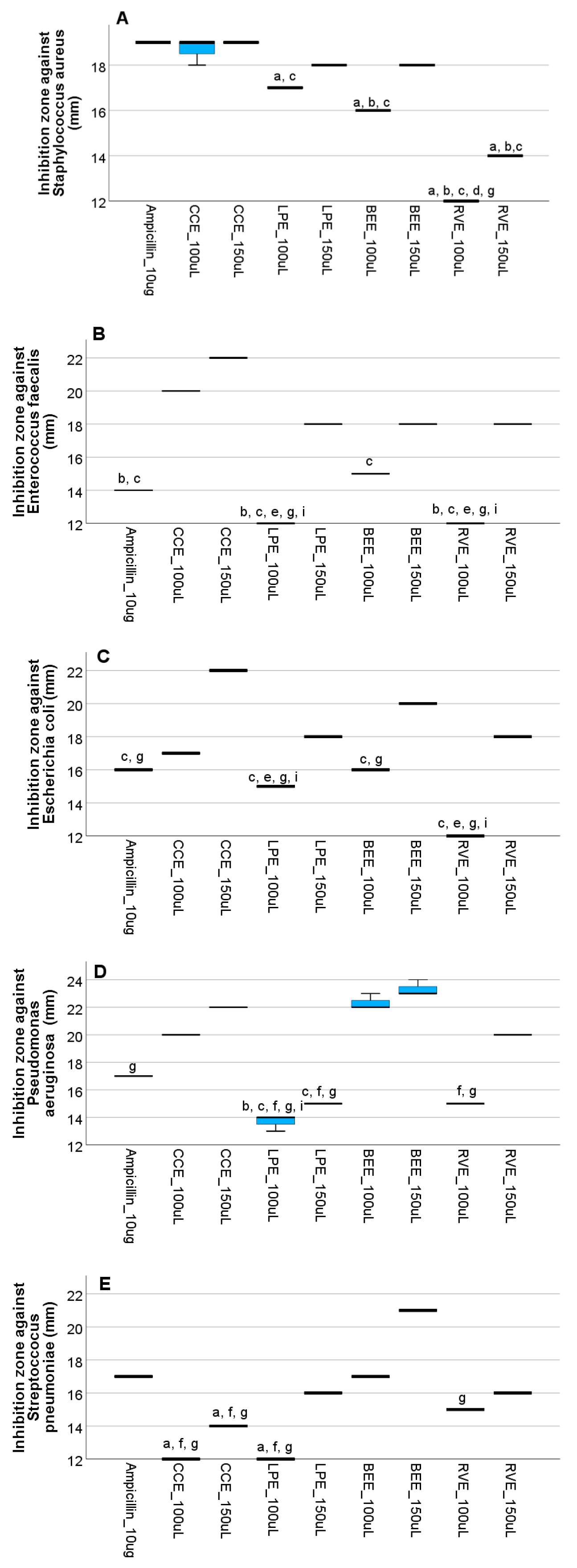
3.4. In Vitro Assessment of the Antimicrobial Efficacy of Mushroom Extracts
3.5. Antibacterial Activity of Mushroom Extracts Assessed by Time-Kill Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khatua, S.; Paul, S.; Acharya, K. Mushroom as the potential source of new generation of antioxidant: A review. Res. J. Pharm. Technol. 2013, 6, 496–505. [Google Scholar]
- Shukla, K.; Giri, B.; Shukla, R.V. Occurrence and distribution of mushrooms in semi-evergreen Sal (Shorea robusta) forest Chhattisgarh, Central India. In Developments in Fungal Biology and Applied Mycology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 501–523. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef] [PubMed]
- Stojek, K.; Gillerot, L.; Jaroszewicz, B. Predictors of mushroom production in the European temperate mixed deciduous forest. For. Ecol. Manage. 2022, 522, 120451. [Google Scholar] [CrossRef]
- Bala, N.; Aitken, E.A.B.; Cusack, A.; Steadman, K.J. Antimicrobial potential of Australian macrofungi extracts against foodborne and other pathogens. Phyther Res. 2012, 26, 465–469. [Google Scholar] [CrossRef]
- Shalaby, S.; Horwitz, B.A. Plant phenolic compounds and oxidative stress: Integrated signals in fungal—Plant interactions. Curr. Genet. 2015, 61, 347–357. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Sharma, V.P.; Annepu, S.K.; Gautam, Y.; Singh, M.; Kamal, S. Status of mushroom production in India. Mushroom Res. 2017, 26, 111–120. [Google Scholar]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT 2014, 59, 689–694. [Google Scholar] [CrossRef]
- Zavastin, D.E.; Bujor, A.; Tuchiluş, C.; Zavastin Mircea, C.G.; Gherman, S.P.; Aprotosoaie, A.C.; Miron, A. Studies on Antioxidant, Antihyperglycemic and Antimicrobial Effects of Edible Mushrooms Boletus Edulis and Cantharellus Cibarius. J. Plant Dev. 2016, 23, 87–95. [Google Scholar]
- Fogarasi, M.; Socaciu, M.I.; Sălăgean, C.D.; Ranga, F.; Fărcaș, A.C.; Socaci, S.A.; Socaciu, C.; Țibulcă, D.; Fogarasi, S.; Semeniuc, C.A. Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus edulis and Cantharellus cibarius Mushrooms from Romania. Molecules 2021, 26, 7508. [Google Scholar] [CrossRef] [PubMed]
- Direkov, L.; Gaberov, V.; Vakova, V. Study of Boletus Edulis Mushrooms in South-Western Bulgaria for the Presence of Natural and Technogenic Radioactive Substances. In Proceedings of the 6 International Scientific Conference, Blagoevgrad, Bulgaria, 10–14 June 2015; pp. 17–21. [Google Scholar]
- Salerni, E.; Perini, C. Experimental study for increasing productivity of Boletus edulis s.l. in Italy. For. Ecol. Manage. 2004, 201, 161–170. [Google Scholar] [CrossRef]
- Režić Mužinić, N.; Veršić Bratinčević, M.; Grubić, M.; Matas, R.F.; Čagalj, M.; Visković, T.; Popović, M. Golden Chanterelle or a Gold Mine? Metabolites from Aqueous Extracts of Golden Chanterelle (Cantharellus cibarius) and Their Antioxidant and Cytotoxic Activities. Molecules 2023, 28, 2110. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Karunarathna, S.C.; Patabendige, N.; Tarafder, E.; Lou, D.; Zhou, Y.; Hapuarachchi, K. Unveiling the Bioactive Compounds and Therapeutic Potential of Russula: A Comprehensive Review. J. Fungi. 2025, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Szilagyi, B.; Majdik, C.; Katona, G.; Indolean, C.; Măicăneanu, A. Cd (II) and Zn (II) biosorption on Lactarius piperatus macrofungus: Equilibrium isotherm and kinetic studies. Environ. Prog. Sustain. Energy. 2014, 33, 1158–1170. [Google Scholar] [CrossRef]
- Kovač, M.; Žel, J. The effect of aluminium on the cytokinins in the mycelia of Lactarius piperatus. Plant Sci. 1994, 97, 137–142. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- Chu, M.; Khan, R.D.; Zhou, Y.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Phenolic Compounds in Common Commercial Mushrooms and Their Potential Antioxidant Activities. Processes 2023, 11, 1711. [Google Scholar] [CrossRef]
- Ulusu, F.; Emsen, B.; Karapinar, H.S.; Uzun, Y.; Kaya, A. HPLC based identification of phenolics and biological activities in Elaphomyces mushroom extracts. Sci. Rep. 2025, 15, 27182. [Google Scholar] [CrossRef]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes versicolor: Medicinal mushroom with important health benefits. Not. Bot. Horti Agrobot. Cluj-Napoca. 2018, 46, 343–349. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnao, M.B.; Casas, J.L.; del Río, J.A.; Acosta, M.; García-Cánovas, F. An enzymatic colorimetric method for measuring naringin using 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) in the presence of peroxidase. Anal. Biochem. 1990, 185, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100-S26; Performance Standards for Antimicrobial Susceptibility Testing Supplement M100S. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016.
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The lignicolous fungus Trametes versicolor (L.) Lloyd (1920): A promising natural source of antiradical and AChE inhibitory agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef]
- Tabtimmai, L.; Srisook, P.; Kuaprasert, B.; Thumanu, K.; Choowongkomon, K. FTIR spectra signatures reveal different cellular effects of EGFR inhibitors on nonsmall cell lung cancer cells. J. Biophotonics. 2020, 13, e201960012. [Google Scholar] [CrossRef] [PubMed]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef]
- Al Qutaibi, M.; Kagne, S.R. Unearthing Nature’s Pharmacy: Exploring the Antimicrobial Potency of Mushrooms. J. Food Process. Preserv. 2024, 2024, 1–14. [Google Scholar] [CrossRef]
- Church, N.; Mckillip, J. Antibiotic resistance crisis: Challenges and imperatives. Biologia 2021, 76, 1535–1550. [Google Scholar] [CrossRef]
- Shen, H.S.; Shao, S.; Chen, J.C.; Zhou, T. Antimicrobials from Mushrooms for Assuring Food Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 316–329. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Fogarasi, M.; Diaconeasa, Z.M.; Pop, C.R.; Fogarasi, S.; Semeniuc, C.A.; Fărcaş, A.C.; Țibulcă, D.; Sălăgean, C.-D.; Tofană, M.; Socaci, S.A. Elemental composition, antioxidant and antibacterial properties of some wild edible mushrooms from Romania. Agronomy 2020, 10, 1972. [Google Scholar] [CrossRef]
- Srikram, A.; Supapvanich, S. Proximate compositions and bioactive compounds of edible wild and cultivated mushrooms from Northeast Thailand. Agric. Nat. Resour. 2016, 50, 432–436. [Google Scholar] [CrossRef]
- Vamanu, E.; Nita, S. Antioxidant Capacity and the Correlation with Major Phenolic Compounds, Anthocyanin, and Tocopherol Content in Various Extracts from the Wild Edible Boletus edulis Mushroom. Biomed. Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.; Paesa, M.; Morales, C.; Mendoza, G.; Osada, J.; Teixeira, J.A.; Ferreira-Santos, P.; Rodríguez-Yoldi, M.J. Biological Properties of Boletus edulis Extract on Caco-2 Cells: Antioxidant, Anticancer, and Anti-Inflammatory Effects. Antioxidants 2024, 13, 908. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.; Farcas, A.C.; Tofana, M.; Semeniuc, C.A. Bioactive Compounds and Volatile Profiles of Five Transylvanian Wild Edible Mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chu, M.; Ahmadi, F.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. A Comprehensive Review on Phytochemical Profiling in Mushrooms: Occurrence, Biological Activities, Applications and Future Prospective. Food Rev. Int. 2024, 40, 924–951. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Fourtaka, K.; Melanouri, E.M.; Dedousi, M.; Diamantis, I.; Gardeli, C.; Papanikolaou, S. Examining the Impact of Substrate Composition on the Biochemical Properties and Antioxidant Activity of Pleurotus and Agaricus Mushrooms. Fermentation 2023, 9, 689. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-derived and dietary hydroxybenzoic acids—A comprehensive study of structural, anti-/pro-oxidant, lipophilic, antimicrobial, and cytotoxic activity in mda-mb-231 and mcf-7 cell lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Bai, L.; Xu, D.; Zhou, Y.M.; Zhang, Y.-B.; Zhang, H.; Chen, Y.-B.; Cui, Y.-L. Antioxidant Activities of Natural Polysaccharides and Their Derivatives for Biomedical and Medicinal Applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Mardani-Ghahfarokhi, A.; Farhoosh, R. Antioxidant activity and mechanism of inhibitory action of gentisic and α-resorcylic acids. Sci. Rep. 2020, 10, 19487. [Google Scholar] [CrossRef]
- Ćirković, D.; Matijašević, S.; Deletić, N.; Ćirković, B.; Gašić, U.; Sredojević, M.; Jovanović, Z.; Djurić, V.; Tešić, Ž. The Effect of Early and Late Defoliation on Phenolic Composition and Antioxidant Properties of Prokupac Variety Grape Berries (Vitis vinifera L.). Agronomy 2019, 9, 822. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control. 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Yilmaz, S.; Sova, M.; Ergün, S. Antimicrobial activity of trans-cinnamic acid and commonly used antibiotics against important fish pathogens and nonpathogenic isolates. J. Appl. Microbiol. 2018, 125, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Flores-Maldonado, O.; Dávila-Aviña, J.; González, G.M.; Becerril-García, M.A.; Ríos-López, A.L. Antibacterial activity of gallic acid and methyl gallate against emerging non-fermenting bacilli. Folia Microbiol. 2025, 70, 127–135. [Google Scholar] [CrossRef]
- Ahn, Y.-J.; Lee, C.-O.; Kweon, J.-H.; Ahn, J.-W.; Park, J.-H. Growth-inhibitory effects of Galla Rhois-derived tannins on intestinal bacteria. J. Appl. Microbiol. 1998, 84, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, Q.; Liu, L.; Jin, W.; Wang, J.; Sun, Y. The specific effect of gallic acid on Escherichia coli biofilm formation by regulating pgaABCD genes expression. Appl. Microbiol. Biotechnol. 2018, 102, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Efimova, S.S.; Zakharova, A.A.; Medvedev, R.Y.; Ostroumova, O.S. Ion Channels Induced by Antimicrobial Agents in Model Lipid Membranes are Modulated by Plant Polyphenols Through Surrounding Lipid Media. J. Membr. Biol. 2018, 251, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Asante-Donyinah, D.; Letsyo, E.; Dzikunoo, J.; Adams, Z.S. Dietary Polyphenols and Obesity: A Review of Polyphenol Effects on Lipid and Glucose Metabolism, Mitochondrial Homeostasis, and Starch Digestibility and Absorption. Plant Foods Hum. Nutr. 2023, 78, 1–12. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A. Polyphenols and DNA damage: A mixed blessing. Nutrients 2016, 8, 785. [Google Scholar] [CrossRef]
- Calvo, L.G.; Castillo, A.; Villarino, R.A.; Rama, J.L.R.; Abril, A.G.; de Miguel, T. Study of the Antibacterial Activity of Rich Polyphenolic Extracts Obtained from Cytisus scoparius against Foodborne Pathogens. Antibiotics 2023, 12, 1645. [Google Scholar] [CrossRef]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [CrossRef] [PubMed]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. BBA-Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Caturla, N.; Vera-Samper, E.; Villalaín, J.; Mateo, C.R.; Micol, V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Letsididi, K.S.; Lou, Z.; Letsididi, R.; Mohammed, K.; Maguy, B.L. Antimicrobial and antibiofilm effects of trans-cinnamic acid nanoemulsion and its potential application on lettuce. LWT 2018, 94, 25–32. [Google Scholar] [CrossRef]
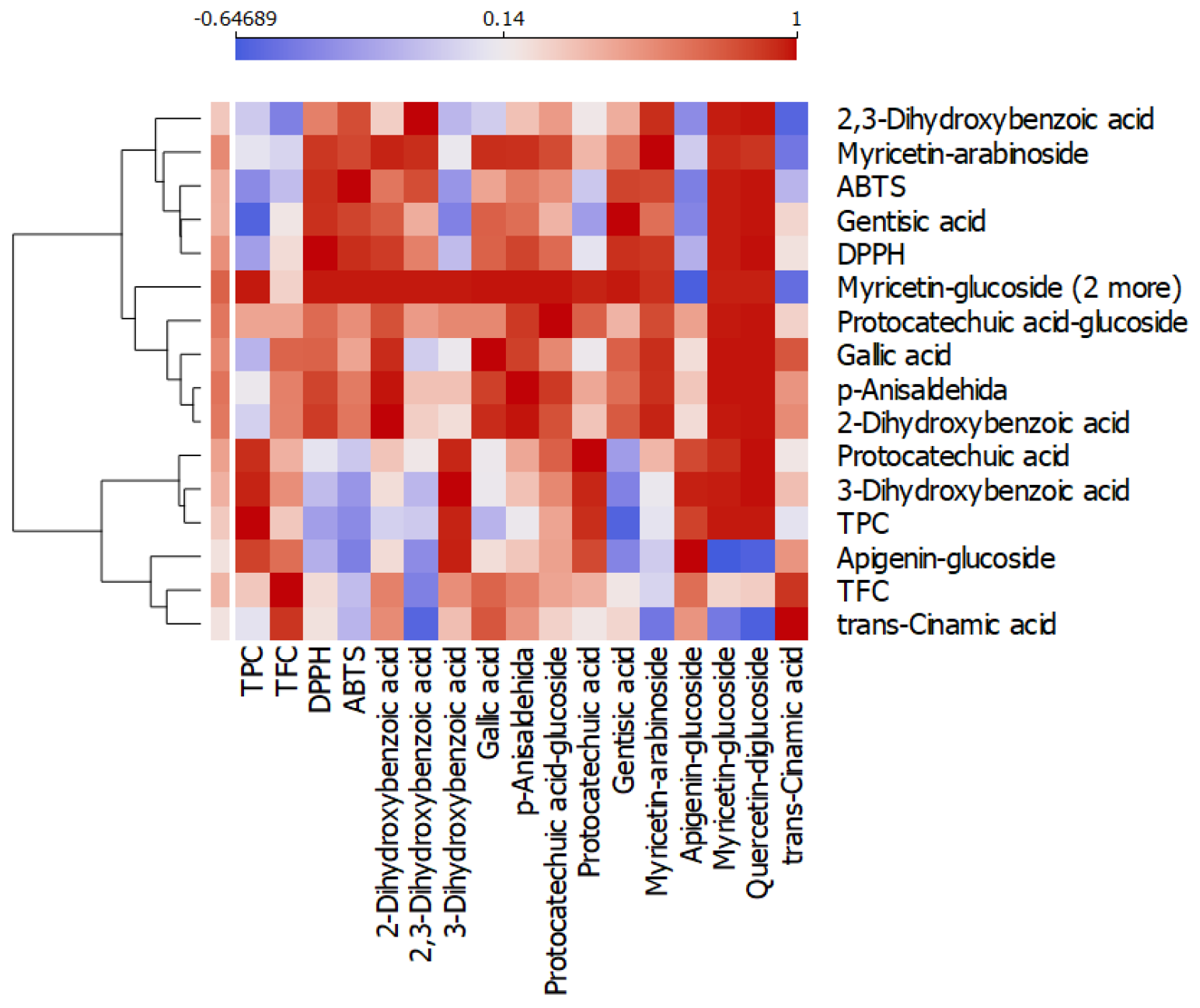


| Mushroom Variety | TPC (mg GAE/100 g FW) | TFC (mg QE/100 g FW) | DPPH (%) | ABTS (%) |
|---|---|---|---|---|
| Boletus edulis (BEE) | 59.64 ± 0.54 b | 5.90 ± 0.07 b | 44.73 ± 0.67 c | 63.99 ± 0.52 c |
| Lactarius piperatus (LPE) | 200.84 ± 0.90 d | 6.16 ± 0.12 c | 25.37 ± 0.20 b | 30.77 ± 0.55 a |
| Russula virescens (RVE) | 123.22 ± 0.82 c | 5.37 ± 0.03 a | 20.99 ± 0.08 a | 38.84 ± 0.61 b |
| Cantharellus cibarius (CCE) | 37.60 ± 0.84 a | 5.85 ± 0.04 b | 21.48 ± 0.20 a | 30.50 ± 0.33 a |
| Peak No. | Rt (min) | λmax (nm) | [M+H]+ (m/z) | Compound | Boletus edulis Extract (BEE) | Cantharellus cibaris Extract (CCE) | Lactarius piperatus Extract (LPE) | Russula virescens Extract (RVE) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.96 | 270 | 139 | 2-Dihydroxyben zoic acid 1 | 71.21 ± 0.31 d | 32.46 ± 0.83 b | 52.47 ± 0.83 c | 19.85 ± 0.14 a |
| 2 | 3.69 | 270 | 155 | 2,3-Dihydroxyben zoic acid 1 | 56.16 ± 0.65 d | 7.33 ± 0.03 a | 20.14 ± 0.08 b | 48.16 ± 0.49 c |
| 3 | 3.84 | 270 | 139 | 3-Dihydroxyben zoic acid 1 | 59.97 ± 0.55 b | 37.01 ± 0.30 a | 170.07 ± 2.10 d | 73.2 ± 0.91 c |
| 4 | 4.51 | 275 | 171 | Gallic acid 1 | 63.39 ± 1.85 d | 41.59 ± 0.23 b | 49.44 ± 0.25 c | 11.55 ± 0.13 a |
| 5 | 5.27 | 260 | 137 | p-Anisaldehyde 1 | 24.07 ± 1.08 d | 5.01 ± 0.01 b | 17.50 ± 1.32 c | 2.48 ± 0.01 a |
| 6 | 9.31 | 280 | 317 | Protocatechuic acid-glucoside 1 | 20.71 ± 0.55 d | 5.16 ± 0.04 a | 19.58 ± 0.62 c | 10.75 ± 0.14 b |
| 7 | 9.72 | 280 | 155 | Protocatechuic 1 acid | 6.17 ± 0.52 b | 2.03 ± 0.03 a | 11.46 ± 0.12 c | 6.43 ± 0.36 b |
| 8 | 10.46 | 280 | 155 | Gentisic acid 1 | 14.02 ± 0.28 d | 4.83 ± 0.12 c | 1.20 ± 0.02 b | 0.07 ± 0.00 a |
| 9 | 10.96 | 360.260 | 451.319 | Myricetin-arabinoside 2 | 2.93 ± 0.29 | 1.74 ± 0.06 | 2.29 ± 0.08 | n.d. |
| 10 | 13.34 | 340.270 | 433 | Apigenin-glucoside 3 | 2.48 ± 0.03 a | 2.55 ± 0.08 a | 6.56 ± 0.37 b | 2.59 ± 0.06 a |
| 11 | 14.53 | 360.260 | 481.319 | Myricetin-glucoside 2 | 2.59 ± 0.16 | 1.81 ± 0.02 | n.d. | n.d. |
| 12 | 14.79 | 360.255 | 627.303 | Quercetin-diglucoside 2 | 2.44 ± 0.11 | 1.77 ± 0.02 | n.d. | n.d. |
| 13 | 26.61 | 290 | 149 | trans-Cinnamic acid 4 | 24.39 ± 1.35 b | 25.67 ± 0.20 b,c | 27.09 ± 0.14 c | 18.54 ± 0.14 a |
| Total phenolics | 350.51 ± 7.54 | 168.95 ± 1.86 | 377.80 ± 5.91 | 193.65 ± 3.39 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puia, A.; Pandrea, S.-L.; Cruceru, J.; Puia, I.C.; Chedea, V.S.; Ciont, C.; Pop, O.L.; Leopold, L.F.; Ranga, F.; Urcan, A.C.; et al. Phenolic Compounds with Antimicrobial Properties in Mushrooms Frequently Encountered in Temperate Deciduous Forests. Life 2025, 15, 1653. https://doi.org/10.3390/life15111653
Puia A, Pandrea S-L, Cruceru J, Puia IC, Chedea VS, Ciont C, Pop OL, Leopold LF, Ranga F, Urcan AC, et al. Phenolic Compounds with Antimicrobial Properties in Mushrooms Frequently Encountered in Temperate Deciduous Forests. Life. 2025; 15(11):1653. https://doi.org/10.3390/life15111653
Chicago/Turabian StylePuia, Aida, Stanca-Lucia Pandrea, Jeanine Cruceru, Ion Cosmin Puia, Veronica Sanda Chedea, Călina Ciont, Oana Lelia Pop, Loredana Florina Leopold, Floricuța Ranga, Adriana Cristina Urcan, and et al. 2025. "Phenolic Compounds with Antimicrobial Properties in Mushrooms Frequently Encountered in Temperate Deciduous Forests" Life 15, no. 11: 1653. https://doi.org/10.3390/life15111653
APA StylePuia, A., Pandrea, S.-L., Cruceru, J., Puia, I. C., Chedea, V. S., Ciont, C., Pop, O. L., Leopold, L. F., Ranga, F., Urcan, A. C., Nicolescu, A., Bobis, O., Bocsan, I. C., Armean, S., Buzoianu, A. D., & Pop, R. M. (2025). Phenolic Compounds with Antimicrobial Properties in Mushrooms Frequently Encountered in Temperate Deciduous Forests. Life, 15(11), 1653. https://doi.org/10.3390/life15111653











