Endovascular Repair of Thoracic Aortic Atresia in Adults: A Narrative Review of a Rare Entity and Emerging Technique
Abstract
1. Introduction
1.1. Purpose and Scope of the Review
1.2. Methodology of Literature Selection
- Studies including patients with confirmed diagnosis of aortic coarctation (native or recurrent)
- Studies directly comparing surgical repair versus endovascular intervention (stent placement or balloon angioplasty) OR studies reporting outcomes for either treatment modality that allow for indirect comparison
- Original research articles including randomized controlled trials, cohort studies, case–control studies, and prospective/retrospective observational studies
- Studies published in English between January 2000 and July 2025
- Studies with clear treatment outcome measures (mortality, morbidity, recurrence, re-intervention rates)
- Studies with minimum sample size of 10 patients
- Human studies only
- Studies reporting follow-up data of at least 30 days post-intervention
- Studies with clearly defined patient populations and treatment protocols
- Animal studies, in vitro studies, or experimental models
- Case reports or case series with fewer than 10 patients
- Editorials, opinion pieces, narrative reviews, and commentaries
- Conference abstracts without full-text availability
- Duplicate publications or studies with overlapping patient datasets
- Studies focusing primarily on other congenital cardiac defects without specific focus on aortic coarctation or atresia
- Studies without clinical outcomes or treatment-related endpoints
- Studies published before January 2000
- Studies not published in English
- Studies with insufficient data for outcome assessment
- Reviews and meta-analyses
1.3. Anatomy and Embryology
- Embryological and Developmental Theories
- 2.
- Acquired and Secondary Factors: Inflammation and maternal factors
- 3.
- Associated Cardiac Anomalies
2. How to Approach a Patient with Thoracic Aortic Atresia
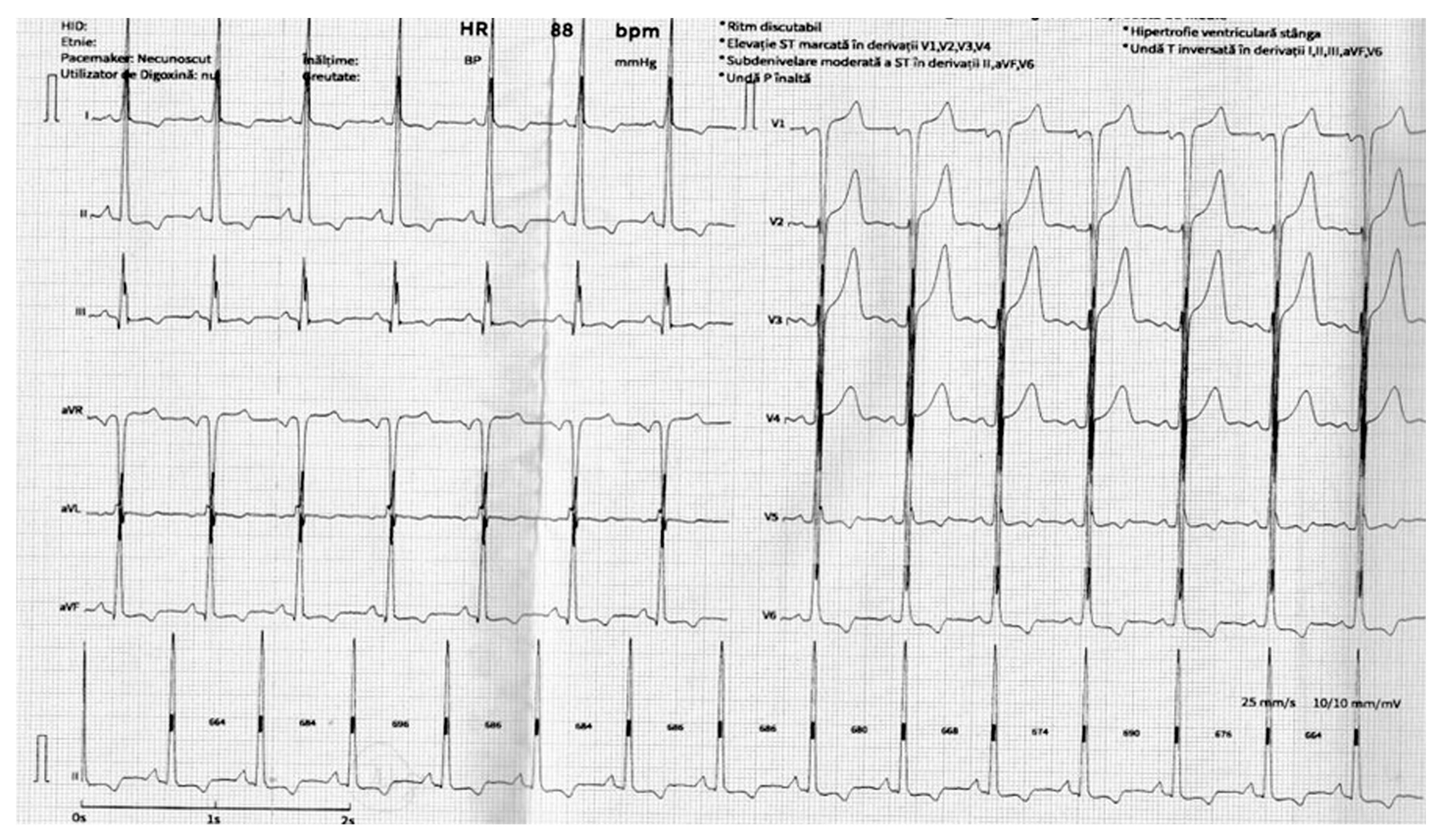
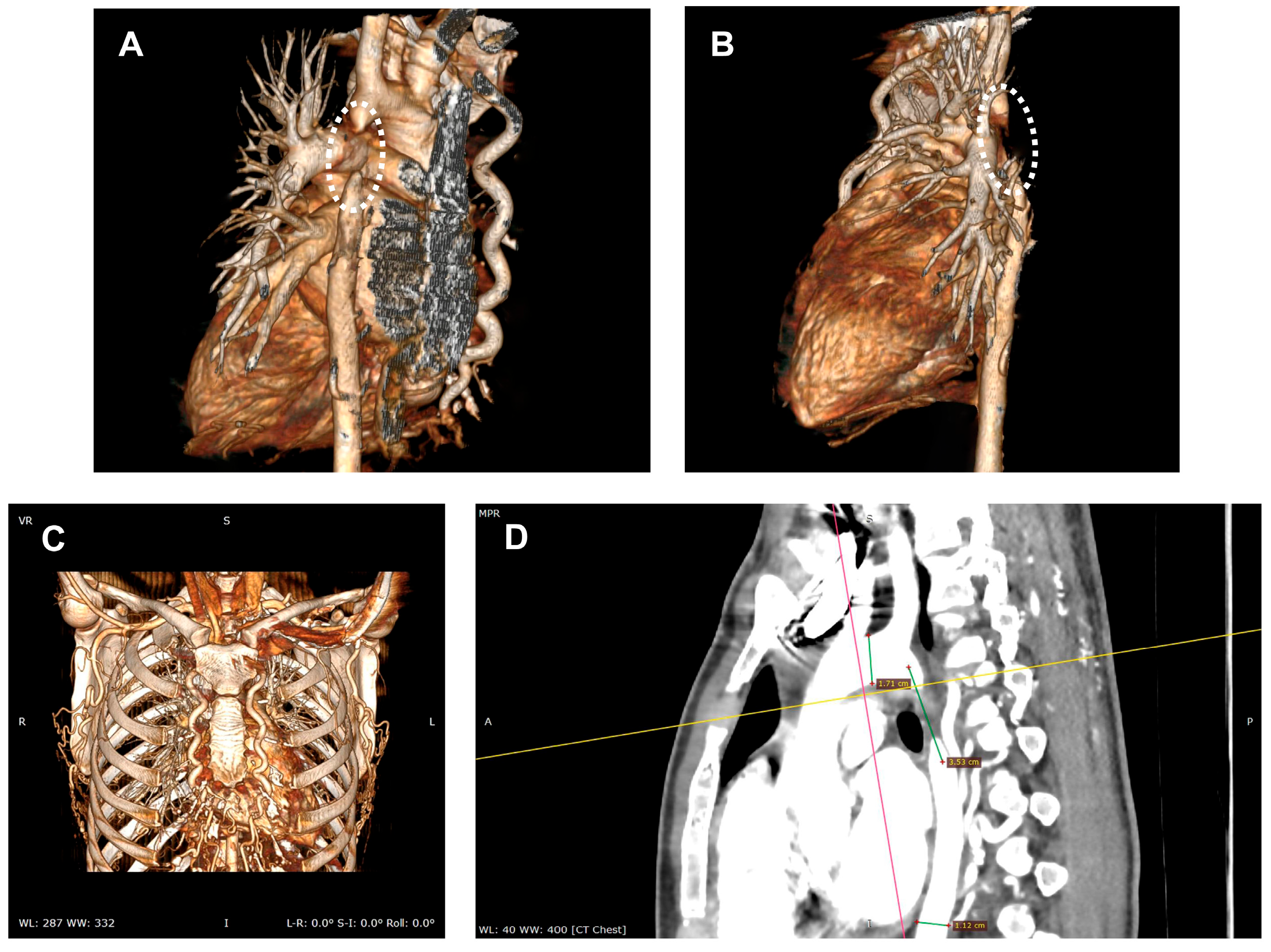
2.1. Diagnosis
Clinical Manifestations
2.2. Treatment
- Individual clinical expertise and institutional experience
- Extrapolation from related conditions (standard coarctation)
- Single-center case series with inherent selection bias
- Theoretical considerations based on anatomic principles
2.2.1. Core Steps of Endovascular Technique for AAA
2.2.2. Crossing the Atretic Segment
2.2.3. Balloon Angioplasty
2.2.4. Stent Placement
2.2.5. Branch Vessel Management
2.2.6. Imaging and Hemodynamic Assessment
2.3. Post-Procedure Care
2.3.1. Surgery vs. Endovascular Treatment
| Parameter | Surgical Repair | Endovascular Treatment |
|---|---|---|
| Procedural Success Rate | 98.7% (1-year freedom from reintervention) [34] | 97% (pooled technical success) [44] |
| Early Mortality (30-day) | 3.9–8.6% (varies by complexity) [45] | 2.7–3.9% (generally lower) [45] |
| Late Mortality (5-year) | Variable by indication | 98.1% survival at 5 years [46] |
| Major Neurological Complications | Higher stroke risk in complex cases [47] | Reduced paraplegia (RR 0.70) |
| Hospital Stay | 7–14 days (longer ICU stay) [48] | 2–5 days (shorter recovery) [48] |
| Restenosis/Recoarctation | 5–15% at 10 years [49] | 10–25% at 5–10 years |
| Reintervention Rates | 2–8% at 5 years [50] | 15–30% at 10 years [50,51] |
| Blood Pressure Control | Excellent long-term (85–90%) [52] | Good short-term (80–85%) |
| Procedural Mortality | 1–3% (isolated CoA) [53] | 0.5–2% (balloon/stent) |
| Cost-Effectiveness | Higher initial cost, lower long-term [54] | Lower initial cost, higher follow-up [54] |
2.3.2. Covered Stents vs. Bare Metal Stents in Endovascular Treatment
2.3.3. Surgical vs. Endovascular Treatment Effect on Post-Procedural Residual Hypertension
3. Discussion
3.1. Gaps in Knowledge and Future Directions
3.2. Limitations of This Article
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Aortic Atresia |
| AV | Aortic Valve |
| BIB | Balloon-in-Balloon (catheter) |
| BP | Blood Pressure |
| BMW | Balance Middle Weight (guidewire, Abbott Vascular) |
| CT | Computed Tomography |
| CTO | Chronic Total Occlusion |
| ECG | Electrocardiogram |
| EF | Ejection Fraction |
| F | French (catheter size) |
| JR | Judkins Right (catheter) |
| LV | Left Ventricle |
| LVH | Left Ventricular Hypertrophy |
| MPA2 | Multipurpose A2 (catheter) |
| MV | Mitral Valve |
| TTE | Transthoracic Echocardiogram |
| Confianza Pro 12 | Asahi Intecc coronary guidewire |
| FineCross | Terumo microcatheter |
| Cheatham Platinum D’Vill | Covered stent (NuMed Inc.) Introducer sheath system (NuMed Inc.) |
| CT angiography | Computed tomography angiography |
References
- Kenny, D.; Hijazi, Z.M. Coarctation of the aorta: From fetal life to adulthood. Cardiol. J. 2011, 19, 487–495. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Andrade, L.; Cook, S.C. Aortic Coarctation. Cardiol. Clin. 2020, 38, 337–351. [Google Scholar] [CrossRef]
- Egbe, A.; Uppu, S.; Stroustrup, A.; Lee, S.; Ho, D.; Srivastava, S. Incidences and Sociodemographics of Specific Congenital Heart Diseases in the United States of America: An Evaluation of Hospital Discharge Diagnoses. Pediatr. Cardiol. 2014, 35, 975–982. [Google Scholar] [CrossRef]
- Cribbs, M.G. Coarctation: A Review. US Cardiol. Rev. 2020, 13, 99–104. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Morrow, A.G.; Braunwald, E. Complete Interruption of the Aortic Arch. Circulation 1962, 26, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Niu, S.; Adachi, S.; Ohga, K. Adult aortic arch atresia. Jpn. J. Thorac. Cardiovasc. Surg. 2000, 48, 599–602. [Google Scholar] [CrossRef]
- Nigro Stimato, V.; Didier, D.; Beghetti, M.; Tissot, C. Atresia of the Aortic Arch in 4-Year-Old Child: A Clinical Case Study. Front. Pediatr. 2015, 3, 19. [Google Scholar] [CrossRef]
- Pujitha, V.; Pandey, N.N.; Verma, M.; Kumar, S.; Ramakrishnan, S.; Jagia, P. Interrupted Aortic Arch: Assessment of Morphology and Associated Cardiovascular Anomalies on Computed Tomography Angiography. J. Card. Surg. 2024, 2024, 1-8. [Google Scholar] [CrossRef]
- Liberman, L.; Gersony, W.M.; Flynn, P.A.; Lamberti, J.J.; Cooper, R.S.; Starc, T.J. Effectiveness of Prostaglandin E 1 in Relieving Obstruction in Coarctation of the Aorta Without Opening the Ductus Arteriosus. Pediatr. Cardiol. 2004, 25, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U. Prostaglandin E-mediated molecular mechanisms driving remodeling of the ductus arteriosus. Pediatr. Int. 2015, 57, 820–827. [Google Scholar] [CrossRef]
- Yokoyama, U.; Ichikawa, Y.; Minamisawa, S.; Ishikawa, Y. Pathology and molecular mechanisms of coarctation of the aorta and its association with the ductus arteriosus. J. Physiol. Sci. 2017, 67, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, E.-K.; Kim, W.-H.; Shim, G.H.; Kim, H.-S.; Park, J.D.; Bae, E.J.; Kim, B.I.; Noh, C.I.; Choi, J.-H. Abnormally extended ductal tissue into the aorta is indicated by similar histopathology and shared apoptosis in patients with coarctation. Int. J. Cardiol. 2010, 145, 177–182. [Google Scholar] [CrossRef]
- Priya, S.; Thomas, R.; Nagpal, P.; Sharma, A.; Steigner, M. Congenital anomalies of the aortic arch. Cardiovasc. Diagn. Ther. 2018, 8, S26–S44. [Google Scholar] [CrossRef]
- Hanneman, K.; Newman, B.; Chan, F. Congenital Variants and Anomalies of the Aortic Arch. RadioGraphics 2017, 37, 32–51. [Google Scholar] [CrossRef]
- Kellenberger, C.J. Aortic arch malformations. Pediatr. Radiol. 2010, 40, 876–884. [Google Scholar] [CrossRef]
- Ho, S.Y.; Anderson, R.H. Coarctation, tubular hypoplasia, and the ductus arteriosus. Histological study of 35 specimens. Heart 1979, 41, 268–274. [Google Scholar] [CrossRef]
- Yildirim, A.; Karabulut, N.; Dogan, S.; Herek, D. Congenital thoracic arterial anomalies in adult: CT overview. Diagn. Interv. Radiol. 2011, 17, 352–362. [Google Scholar] [CrossRef]
- Ellegård, R.; Malm, T.; Weismann, C.G.; Fernlund, E.; Björnlert, A.N.; Årstrand, H.K.; Ellnebo-Svedlund, K.; Gunnarsson, C. Transcriptome analysis of the aortic coarctation area. J. Mol. Cell. Cardiol. Plus 2024, 10, 100094. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Favoccia, C.; Jha, N.K.; Carminati, M.; Gonzalez, L.F.; Eicken, A.; Butera, G.; Martins, J.D.F.; Pinto, F.; Tofeig, M.; et al. Long-term follow-up after recanalisation of aortic arch atresia. EuroIntervention 2021, 16, e1274–e1280. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, Q.; Huang, W.; Wu, H.; Li, W.; Lai, Q. Diagnosis of Congenital Coarctation of the Aorta and Accompany Malformations in Infants by Multi-Detector Computed Tomography Angiography and Transthoracic Echocardiography: A Chinese Clinical Study. Med. Sci. Monit. 2017, 23, 2308–2314. [Google Scholar] [CrossRef][Green Version]
- Hazuková, R. Frequency of emergencies in adults due to unrecognized coarctation of the aorta. Anatol. J. Cardiol. 2016, 16, 74. [Google Scholar] [CrossRef]
- Norwood, W.I.; Stellin, G.J. Aortic atresia with interrupted aortic arch: Reparative operation. J. Thorac. Cardiovasc. Surg. 1981, 81, 239–244. [Google Scholar] [CrossRef]
- Sirak, H.D.; Ressallat, M.; Hosier, D.M.; Delorimier, A.A. A New Operation for Repairing Aortic Arch Atresia in Infancy. Circulation 1968, 37 (Suppl. S4), 43–50. [Google Scholar] [CrossRef]
- Suradi, H.; Hijazi, Z.M. Current management of coarctation of the aorta. Glob. Cardiol. Sci. Pract. 2015, 2015, 44. [Google Scholar] [CrossRef] [PubMed]
- D’oNofrio, A.; Piazza, M.; Andreatta, G.; Cao, I.; Lombardi, V.; Pittarello, D.; Grego, F.; Antonello, M.; Gerosa, G. Endovascular aortic arch repair under monitored anaesthesia care: Maximizing microinvasiveness. Eur. J. Cardio-Thorac. Surg. 2024, 65, ezae032. [Google Scholar] [CrossRef] [PubMed]
- Butera, G.; Heles, M.; Carminati, M. Percutaneous treatment of aortic isthmus atresia. Catheter. Cardiovasc. Interv. 2011, 78, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Tefera, E.; Leye, M.; Chanie, Y.; Raboisson, M.J.; Miro, J. Percutaneous recanalization of totally occluded coarctation of the aorta in children using Brockenbrough needle and covered stents. Ann. Pediatr. Cardiol. 2016, 9, 153. [Google Scholar] [CrossRef]
- Tanidir, I.C. Radiofrequency resistant pulmonary atresia with intact septum: The use of Conquest Pro 12 coronary guidewire. Turk. Kardiyol. Dern. Ars. Arch. Turk. Soc. Cardiol. 2014, 42, 568–570. [Google Scholar] [CrossRef]
- Hamid, T.; Motwani, M.; Schneider, H.; Dua, J.S.; Hoschtitzky, A.; Clarke, B.; Mahadevan, V.S. Benefit of endovascular stenting for aortic coarctation on systemic hypertension in adults. Arch. Cardiovasc. Dis. 2015, 108, 626–633. [Google Scholar] [CrossRef]
- Contrafouris, C.; Antonopoulos, C.N.; Rammos, S.; Kanakis, M.; Petsios, K.; Kakisis, J.D.; Geroulakos, G. Evaluating the Effectiveness of Stenting for Aortic Coarctation. AORTA 2022, 10, 235–241. [Google Scholar] [CrossRef]
- Kische, S.; D’Ancona, G.; Stoeckicht, Y.; Ortak, J.; Elsässer, A.; Ince, H. Percutaneous Treatment of Adult Isthmic Aortic Coarctation. Circ. Cardiovasc. Interv. 2015, 8, e001799. [Google Scholar] [CrossRef]
- Sachdeva, R.; Valente, A.M.; Armstrong, A.K.; Cook, S.C.; Han, B.K.; Lopez, L.; Lui, G.K.; Pickard, S.S.; Powell, A.J.; Bhave, N.M.; et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 Appropriate Use Criteria for Multimodality Imaging During the Follow-Up Care of Patients With Congenital Heart Disease. J. Am. Coll. Cardiol. 2020, 75, 657–703. [Google Scholar]
- Dikmen, N.; Ozcinar, E.; Eyileten, Z.; Hasde, A.I.; Yazicioglu, L.; Kaya, B.; Uysalel, A. Comparative analysis of surgical and endovascular approaches for isolated aortic coarctation repair across age groups: Outcomes and long-term efficacy. J. Clin. Med. 2024, 13, 5814. [Google Scholar] [CrossRef]
- Klocker, J.; Koell, A.; Erlmeier, M.; Goebel, G.; Jaschke, W.; Fraedrich, G. Ischemia and functional status of the left arm and quality of life after left subclavian artery coverage during stent grafting of thoracic aortic diseases. J. Vasc. Surg. 2014, 60, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Bharati, S.; Lev, M. The surgical anatomy of hypoplasia of aortic tract complex. J. Thorac. Cardiovasc. Surg. 1984, 88, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Forbes, T.J.; Kim, D.W.; Du, W.; Turner, D.R.; Holzer, R.; Amin, Z.; Hijazi, Z.; Ghasemi, A.; Rome, J.J.; Nykanen, D.; et al. Comparison of Surgical, Stent, and Balloon Angioplasty Treatment of Native Coarctation of the Aorta. J. Am. Coll. Cardiol. 2011, 58, 2664–2674. [Google Scholar]
- Arulrajah, K.; Spanos, K.; Panuccio, G.; Gandet, T.; Rickers, C.; Kölbel, T. Endovascular Recanalization of Aortic Isthmus Atresia with an “Electrified Wire Technique”. J. Endovasc. Ther. 2025, 32, 994–998. [Google Scholar]
- Mondal, S.; Gopalakrishnan, A.; Pant, B.P.; Iliyas, M.; Sasidharan, B. Stenting of aortic isthmus atresia—A case series. IHJ Cardiovasc. Rep. 2024, 8, 60–65. [Google Scholar]
- Goel, P.K.; Syal, S.K. Percutaneous reconstruction of aortic isthmus atresia using coronary total occlusion technique. J. Cardiol. Cases 2014, 10, 121–124. [Google Scholar] [CrossRef]
- Gheorghe, L.; Arzamendi, D.; Li, C.; Barros-Membrilla, A.; Muñoz, J.D.; Peñaranda, A.S.; Sultan, S.; Spence, M.; Collins, A. How should I treat an asymptomatic aortic coarctation with a concomitant dissection of the descending aorta? EuroIntervention 2017, 12, 2037–2040. [Google Scholar] [CrossRef]
- Zuo, Z.L.; Tsauo, J.Y.; Chen, M.; Feng, Y. Successful percutaneous stent implantation for isolated dismal transverse aortic arch kinking. Medicine 2017, 96, e6089. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.A. Use of Covered Stents to Treat Coarctation of the Aorta. Korean Circ. J. 2009, 39, 261. [Google Scholar] [CrossRef][Green Version]
- Nana, P.; Spanos, K.; Brodis, A.; Kouvelos, G.; Rickers, C.; Kozlik-Feldmann, R.; Giannoukas, A.; Kölbel, T. A Systematic Review and Meta-analysis on Stenting for Aortic Coarctation Management in Adults. J. Endovasc. Ther. 2025, 32, 548–557. [Google Scholar] [CrossRef]
- McClure, R.S.; Rommens, K.L.; Herget, E.J.; Keir, M.; Gregory, A.J.; Smith, H.N.; Moore, R.D. The Aortic Team Model for the Management of the Distal Arch, Descending Thoracic and Thoracoabdominal Aorta: Appraisal at 3 Years. AORTA 2023, 11, 165–173. [Google Scholar] [CrossRef]
- Schleiger, A.; Al Darwish, N.; Meyer, M.; Kramer, P.; Berger, F.; Nordmeyer, J. Long-term follow-up after endovascular treatment of aortic coarctation with bare and covered Cheatham platinum stents. Catheter. Cardiovasc. Interv. 2023, 102, 672–682. [Google Scholar] [PubMed]
- Salsano, A.; Salsano, G.; Spinella, G.; Zaottini, F.; Mavilio, N.; Perocchio, G.; Pane, B.; Ricci, D.; Pratesi, G.; Castellan, L.; et al. Endovascular Versus Open Surgical Repair for Ruptured Descending Aortic Pathologies: A Systematic Review and Meta-Analysis of Observational Studies. Cardiovasc. Interv. Radiol. 2021, 44, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gou, D.; Xu, K.; Lu, Z.; Li, P.; Lei, Y.; Wang, Y.; Yang, Y.; Liu, S.; Zhu, G. Comparison of short-and long-term outcomes between endovascular and open repair for descending thoracic aortic aneurysm: A systematic review and meta-analysis. Int. J. Surg. 2025, 111, 2662–2674. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Cao, Z.; Wang, C.; Premaratne, S.; Starnes, B.W.; Shu, C.; Zhang, W.W. Endovascular treatment of aortic coarctation using covered balloon-expandable stents—A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1439458. [Google Scholar] [CrossRef]
- Naazie, I.N.; Chang, M.; Dosluoglu, H.H.; Dryjski, M.; Khan, S.; Montross, B.; Harris, L. Outcomes of Endovascular vs Surgical Left Subclavian Artery Revascularization in Thoracic Endovascular Aortic Repair Requiring Left Subclavian Artery Coverage. J. Vasc. Surg. 2024, 79, e146–e147. [Google Scholar] [CrossRef]
- Verzini, F.; Gibello, L.; Varetto, G.; Frola, E.; Boero, M.; Porro, L.; Gattuso, A.; Peretti, T.; Rispoli, P. Proportional meta-analysis of open surgery or fenestrated endograft repair for postdissection thoracoabdominal aneurysms. J. Vasc. Surg. 2021, 74, 1377–1385.e9. [Google Scholar] [CrossRef]
- Meijs, T.A.; Minderhoud, S.C.S.; Muller, S.A.; de Winter, R.J.; Mulder, B.J.M.; van Melle, J.P.; Hoendermis, E.S.; van Dijk, A.P.J.; Zuithoff, N.P.A.; Krings, G.J.; et al. Cardiovascular Morbidity and Mortality in Adult Patients With Repaired Aortic Coarctation. J. Am. Heart Assoc. 2021, 10, e023199. [Google Scholar] [CrossRef]
- Xie, X.; Shu, X.; Zhang, W.; Guo, D.; Zhang, W.W.; Wang, L.; Fu, W. A Comparison of Clinical Outcomes of Endovascular Repair Versus Open Surgery for Ruptured Descending Thoracic Aorta. J. Endovasc. Ther. 2022, 29, 307–318. [Google Scholar] [CrossRef]
- Verscheure, D.; Haulon, S.; Tsilimparis, N.; Resch, T.; Wanhainen, A.; Mani, K.; Dias, N.; Sobocinski, J.; Eagleton, M.; Ferreira, M.; et al. Endovascular Treatment of Post Type A Chronic Aortic Arch Dissection With a Branched Endograft. Ann. Surg. 2021, 273, 997–1003. [Google Scholar] [CrossRef]
- Sasikumar, D.; Sasidharan, B.; Rashid, A.; Ayyappan, A.; Goplakrishnan, A.; Krishnamoorthy, K.M.; Sivasubramonian, S. Early and late outcome of covered and non-covered stents in the treatment of coarctation of aorta—A single centre experience. Indian Heart J. 2020, 72, 278–282. [Google Scholar] [CrossRef]
- Castaldi, B.; Ciarmoli, E.; Di Candia, A.; Sirico, D.; Tarantini, G.; Scattolin, F.; Padalino, M.; Vida, V.; Di Salvo, G. Safety and efficacy of aortic coarctation stenting in children and adolescents. Int. J. Cardiol. Congenit. Heart Dis. 2022, 8, 100389. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, N.; Ozcinar, E.; Akça, F.; Sen, E.; Karacuha, A.F.; Kayan, A.; Yazicioglu, L. The Long-Term Results of Covered Endovascular Aortic Bifurcation Repair in Complex Aortoiliac Disease: A Two-Year Follow-Up. J. Clin. Med. 2024, 13, 5684. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Castaldi, B.; Cattapan, I.; Pozza, A.; Fumanelli, J.; Di Salvo, G. Hypertension in aortic coarctation. Front. Cardiovasc. Med. 2025, 12, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Canniffe, C.; Ou, P.; Walsh, K.; Bonnet, D.; Celermajer, D. Hypertension after repair of aortic coarctation—A systematic review. Int. J. Cardiol. 2013, 167, 2456–2461. [Google Scholar] [CrossRef]
- Früh, S.; Knirsch, W.; Dodge-Khatami, A.; Dave, H.; Prêtre, R.; Kretschmar, O. Comparison of surgical and interventional therapy of native and recurrent aortic coarctation regarding different age groups during childhood. Eur. J. Cardio-Thorac. Surg. 2011, 39, 898–904. [Google Scholar]
- Pan, M.; Pericet, C.; González-Manzanares, R.; Díaz, M.A.; de Lezo, J.S.; Hidalgo, F.; Alvarado, M.; Dueñas, G.; Gómez, E.; Espejo, S.; et al. Very long-term follow-up after aortic stenting for coarctation of the aorta. Rev. Española De Cardiol. (Engl. Ed.) 2024, 77, 332–341. [Google Scholar]
- Godart, F. Intravascular stenting for the treatment of coarctation of the aorta in adolescent and adult patients. Arch. Cardiovasc. Dis. 2011, 104, 627–635. [Google Scholar] [CrossRef]
- Holzer, R.J.; Gauvreau, K.; McEnaney, K.; Watanabe, H.; Ringel, R. Long-Term Outcomes of the Coarctation of the Aorta Stent Trials. Circ. Cardiovasc. Interv. 2021, 14, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.C. Transcatheter Intervention for Coarctation of Aorta: Current Status. Indian J. Clin. Cardiol. 2021, 2, 44–50. [Google Scholar] [CrossRef]
- Momenah, T.S.; Khan, M.A.; Qureshi, S.; Hijazi, Z.M. Acquired aortic atresia: Catheter therapy using covered stents. Catheter. Cardiovasc. Interv. 2015, 86, 1063–1067. [Google Scholar] [CrossRef]
- Crafoord, C.; Nylin, G. Congenital coarctation of the aorta and its surgical treatment. J. Thorac. Surg. 1945, 14, 347–361. [Google Scholar] [CrossRef]
- Omeje, I.; Poruban, R.; Sagát, M.; Nosál, M.; Hraška, V. Surgical treatment of aortic coarctation. Images Paediatr. Cardiol. 2004, 6, 18–28. [Google Scholar]
- Sohrabi, B.; Jamshidi, P.; Yaghoubi, A.; Habibzadeh, A.; Hashemi-Aghdam, Y.; Moin, A.; Kazemi, B.; Ghaffari, S.; Baghayi, M.R.A.; Mahmoody, K. Comparison Between Covered and Bare Cheatham-Platinum Stents for Endovascular Treatment of Patients With Native Post-Ductal Aortic Coarctation. JACC Cardiovasc. Interv. 2014, 7, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Kische, S.; Schneider, H.; Akin, I.; Ortak, J.; Rehders, T.C.; Chatterjee, T.; Nienaber, C.A.; Ince, H. Technique of interventional repair in adult aortic coarctation. J. Vasc. Surg. 2010, 51, 1550–1559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alkashkari, W.; Albugami, S.; Hijazi, Z.M. Management of coarctation of the aorta in adult patients: State of the art. Korean Circ. J. 2019, 49, 298–313. [Google Scholar] [CrossRef]
- Lock, J.E.; Bass, J.L.; Amplatz, K.; Fuhrman, B.P.; Castaneda-Zuniga, W. Balloon dilation angioplasty of aortic coarctations in infants and children. Circulation 1983, 68, 109–116. [Google Scholar] [CrossRef]
- Mendelsohn, A.M.; Lloyd, T.R.; Crowley, D.C.; Sandhu, S.K.; Kocis, K.C.; Beekman, R.H. Late follow-up of balloon angioplasty in children with a native coarctation of the aorta. Am. J. Cardiol. 1994, 74, 696–700. [Google Scholar] [CrossRef][Green Version]
- Meadows, J.; Minahan, M.; McElhinney, D.B.; McEnaney, K.; Ringel, R. Intermediate Outcomes in the Prospective, Multicenter Coarctation of the Aorta Stent Trial (COAST). Circulation 2015, 131, 1656–1664. [Google Scholar] [CrossRef]
- Zhu, W.; Xia, Z.; Zhou, C.; Wan, J.; Wang, J.; Li, Y.; Zhang, J.; Henein, M.; Fang, F.; Zhang, G. Prognostic implications of residual mild coarctation gradient after interventional repair. J. Clin. Hypertens. 2024, 26, 1098–1109. [Google Scholar] [CrossRef]
- Mohammadzadeh Shabestari, M.; Eshraghi, A.; Hakim Attar, F.; Ghaderi, F.; Poorzand, H.; Mohammadzadeh Shabestari, A.H.; Alizadeh, B.; Morovatdar, N.; Shahri, B.; Alimi, H.; et al. Evaluation of short and mid-term clinical outcomes in patients with aortic coarctation treated with self-expandable stents. Sci. Rep. 2024, 14, 11748. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, X.; Liu, S.; Yu, C. Efficacy and safety of debranching technique with zone 1 thoracic endovascular aortic repair in high-risk patients with distal aortic arch lesions. J. Cardiothorac. Surg. 2025, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, E.G.; Krings, G.J.; Meijs, T.A.; Franken, A.C.; Driesen, B.W.; Sieswerda, G.T.; Meijboom, F.J.; Doevendans, P.A.F.; Molenschot, M.M.C.; Voskuil, M. Safety and efficacy of stenting for aortic arch hypoplasia in patients with coarctation of the aorta. Neth. Heart J. 2020, 28, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.S.; Gadelha Júnior Hde, P.; dos Santos, M.A. Hybrid Repair versus Conventional Open Repair Approaches for Aortic Arch Disease: A Comprehensive Review. Braz. J. Cardiovasc. Surg. 2021, 36, 244–252. [Google Scholar] [CrossRef]
- Carrel, T.P.; Do, D.D.; Triller, J.; Schmidli, J. A Less Invasive Approach to Completely Repair the Aortic Arch. Ann. Thorac. Surg. 2005, 80, 1475–1478. [Google Scholar] [CrossRef]
- Beckmann, E.; Jassar, A.S. Coarctation repair—Redo challenges in the adults: What to do? J. Vis. Surg. 2018, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.R.; Patel, A.; Kundan, S.; Radhakrishnan, H.B.; Rao, S.G. Hypoplastic left heart syndrome: Current modalities of treatment and outcomes. Indian J. Thorac. Cardiovasc. Surg. 2021, 37 (Suppl. S1), 26–35. [Google Scholar] [CrossRef]
- Lindman, B.R.; Arnold, S.V.; Bagur, R.; Clarke, L.; Coylewright, M.; Evans, F.; Hung, J.; Lauck, S.B.; Peschin, S.; Sachdev, V.; et al. Priorities for patient-Centered Research in Valvular Heart Disease: A report from the National Heart, Lung, and Blood Institute Working Group. J. Am. Heart Assoc. 2020, 9, e015975. [Google Scholar] [CrossRef]
- Turner, D.R.; Gaines, P.A. Endovascular management of coarctation of the aorta. Semin. Interv. Radiol. 2007, 24, 153–166. [Google Scholar] [CrossRef][Green Version]
- Berman, N.; Pozailov, S.; Krymko, H.; Slanovic, L.; Murninkas, M.; Grunseid, M.; Levitas, A. Hypertension at diagnosis of coarctation of the aorta as a risk factor for recoarctation. Pediatr. Res. 2025, 98, 237–240. [Google Scholar] [CrossRef]
- Dhanekula, A.S.; Sweet, M.P.; Desai, N.; Burke, C.R. Aortic arch stenting: Current strategies, new technologies and future directions. Heart 2021, 107, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- García-Pavía, P.; Ruigómez, J.G.; López-Mínguez, J.R.; Roldán, P.F.; Asensio, J.M.N.; Domínguez, J.R.; Segovia, J.; Alonso-Pulpón, L. Endovascular treatment of long-term complications following surgical repair of aortic coarctation. Rev. Esp. Cardiol. (Engl. Ed) 2010, 63, 473–477. [Google Scholar] [CrossRef]
- Williams, K.; Khan, A.; Lee, Y.S.; Hare, J.M. Cell-based therapy to boost right ventricular function and cardiovascular performance in hypoplastic left heart syndrome: Current approaches and future directions. Semin. Perinatol. 2023, 47, 151725. [Google Scholar] [CrossRef]
- Bokhari, S.F.H.; Sattar, S.M.F.; Mehboob, U.; Umais, M.; Ahmad, M.; Malik, A.; Bakht, D.; Iqbal, A.; Dost, W. Advancements in prenatal diagnosis and management of hypoplastic left heart syndrome: A multidisciplinary approach and future directions. World J. Cardiol. 2025, 17, 103668. [Google Scholar] [CrossRef]
- Gahlan, P.; Nair, R.; Ali, F.M.; Pasupati, S. Transcatheter endovascular management of complex coarctation of the aorta in adults. JACC Case Rep. 2025, 30, 103537. [Google Scholar] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rășinar, C.F.; Liuba, P.; Brie, A.D.; Tîrziu, A.; Mornoș, C.; Brie, D.M.; Gaiță, D.I.; Luca, C.T. Endovascular Repair of Thoracic Aortic Atresia in Adults: A Narrative Review of a Rare Entity and Emerging Technique. Life 2025, 15, 1651. https://doi.org/10.3390/life15111651
Rășinar CF, Liuba P, Brie AD, Tîrziu A, Mornoș C, Brie DM, Gaiță DI, Luca CT. Endovascular Repair of Thoracic Aortic Atresia in Adults: A Narrative Review of a Rare Entity and Emerging Technique. Life. 2025; 15(11):1651. https://doi.org/10.3390/life15111651
Chicago/Turabian StyleRășinar, Claudiu Florin, Petru Liuba, Alina Diduța Brie, Alexandru Tîrziu, Cristian Mornoș, Daniel Miron Brie, Dan Ion Gaiță, and Constantin Tudor Luca. 2025. "Endovascular Repair of Thoracic Aortic Atresia in Adults: A Narrative Review of a Rare Entity and Emerging Technique" Life 15, no. 11: 1651. https://doi.org/10.3390/life15111651
APA StyleRășinar, C. F., Liuba, P., Brie, A. D., Tîrziu, A., Mornoș, C., Brie, D. M., Gaiță, D. I., & Luca, C. T. (2025). Endovascular Repair of Thoracic Aortic Atresia in Adults: A Narrative Review of a Rare Entity and Emerging Technique. Life, 15(11), 1651. https://doi.org/10.3390/life15111651






