A Modified NAR Scoring Model Incorporating Immune Infiltration Characteristics to Better Predict Long-Term Survival Following Neoadjuvant Radiotherapy in Rectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Treatment and Follow-Up
2.3. NAR Scores
2.4. TRG
2.5. Clinicopathological Variable Stratification
2.6. Statistical Analyses
3. Results
3.1. Patient and Tumor Characteristics
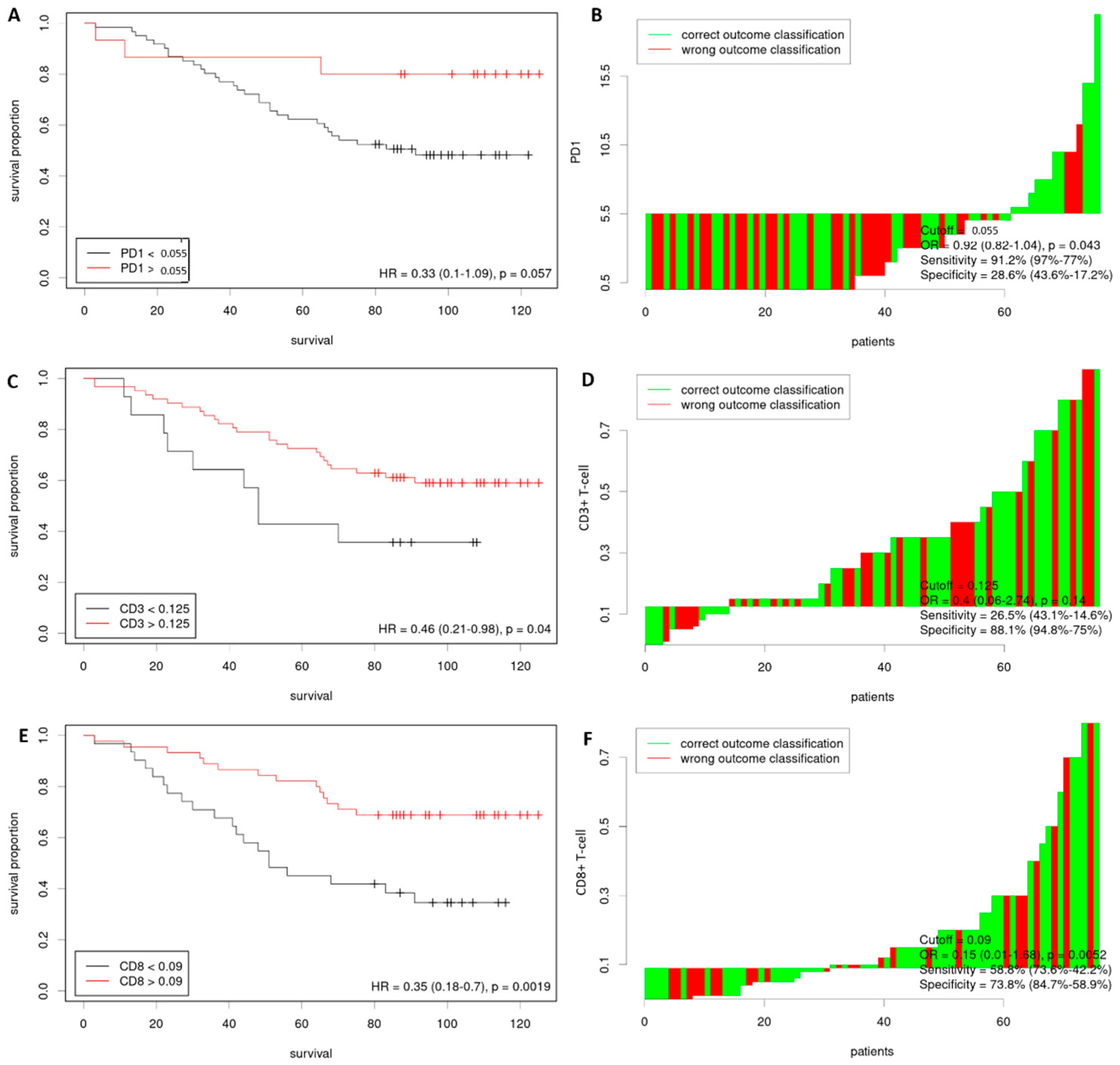
3.2. Cutoff Values for Immune Cell Infiltration Characteristics
3.3. Independent Prognostic Factors for OS
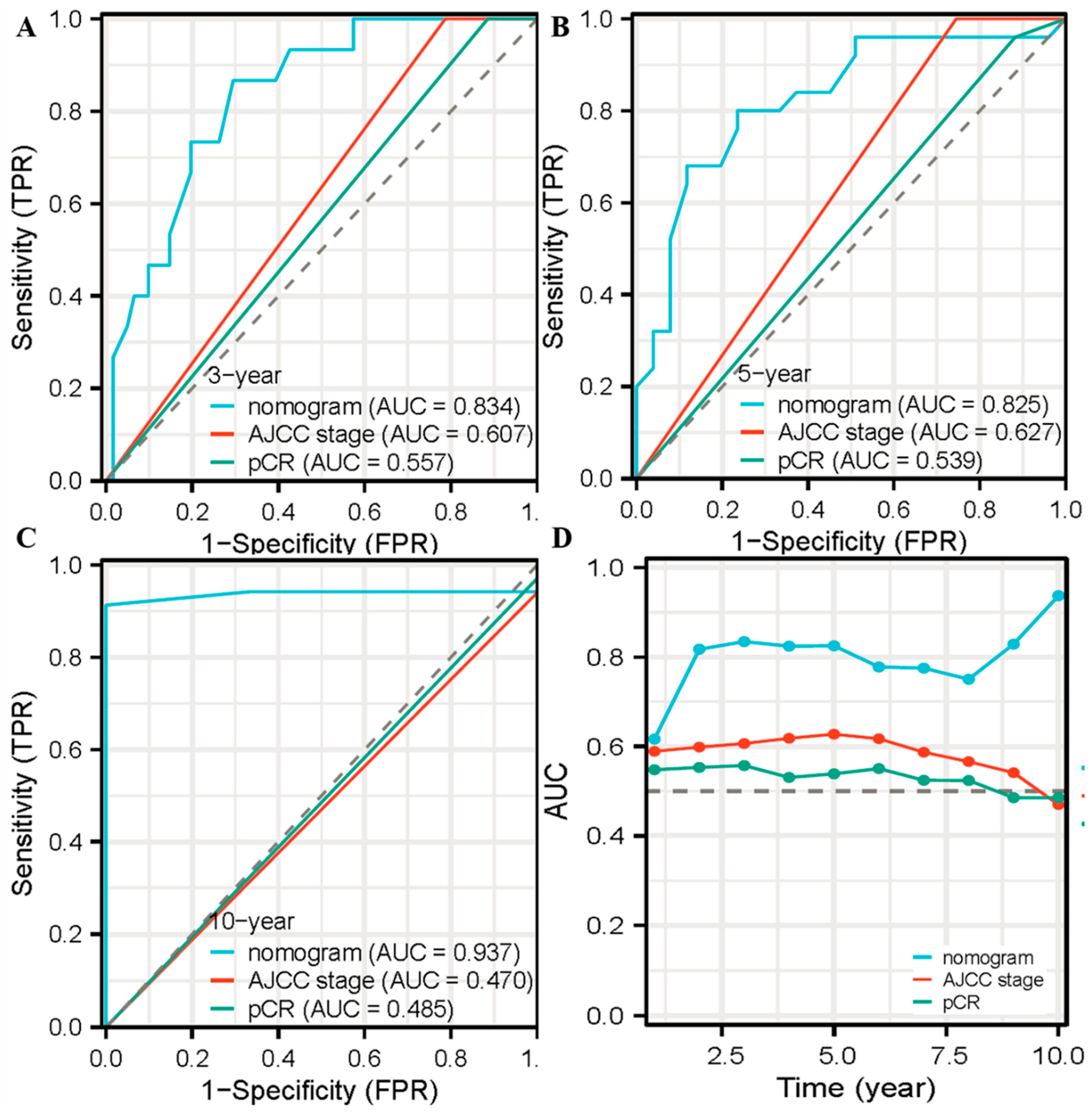
3.4. The Nomogram for OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Luo, D.; Yang, Y.; Zhang, R.; Li, Q.; Li, X. Effect of interval between neoadjuvant chemoradiotherapy and surgery on oncological outcomes in poor responders with locally advanced rectal cancer: A retrospective cohort study. Int. J. Surg. 2023, 109, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Buyse, M.; Molenberghs, G.; Paoletti, X.; Oba, K.; Alonso, A.; Van der Elst, W.; Burzykowski, T. Statistical evaluation of surrogate endpoints with examples from cancer clinical trials. Biom. J. 2016, 58, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Quah, H.M.; Chou, J.F.; Gonen, M.; Shia, J.; Schrag, D.; Saltz, L.B.; Goodman, K.A.; Minsky, B.D.; Wong, W.D.; Weiser, M.R. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008, 113, 57–64. [Google Scholar] [CrossRef]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Sauer, R.; Wittekind, C.; Rodel, C. Downstage migration after neoadjuvant chemoradiotherapy for rectal cancer: The reverse of the Will Rogers phenomenon? Cancer 2015, 121, 1724–1727. [Google Scholar] [CrossRef]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the CAO/ARO/AIO-94 trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.J.; Zhao, X.X.; Yang, Y.; Liang, C.Y.; Feng, L.L.; Wan, X.B.; Ding, Y.; Zhang, Y.W. Development of a Joint Prediction Model Based on Both the Radiomics and Clinical Factors for Predicting the Tumor Response to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer. Cancer Manag. Res. 2021, 13, 3235–3246. [Google Scholar] [CrossRef]
- Germani, P.; Di Candido, F.; Léonard, D.; Cuicchi, D.; Elmore, U.; Allaix, M.E.; Barbieri, V.P.; D’Allens, L.; Faes, S.; Milani, M.; et al. Contemporary snapshot of tumor regression grade (TRG) distribution in locally advanced rectal cancer: A cross sectional multicentric experience. Updates Surg. 2021, 73, 1795–1803. [Google Scholar] [CrossRef]
- Erlandsson, J.; Lörinc, E.; Ahlberg, M.; Pettersson, D.; Holm, T.; Glimelius, B.; Martling, A. Tumour regression after radiotherapy for rectal cancer—Results from the randomised Stockholm III trial. Radiother. Oncol. 2019, 135, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; You, Y.N.; Agarwal, A.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Eng, C.; Feig, B.W.; Das, P.; Krishnan, S.; Crane, C.H.; et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J. Clin. Oncol. 2012, 30, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Hughes, R. Critical appraisal of the ‘wait and see’ approach in rectal cancer for clinical complete responders after chemoradiation. Br. J. Surg. 2012, 99, 897–909. [Google Scholar] [CrossRef]
- Valentini, V.; van Stiphout, R.G.; Lammering, G.; Gambacorta, M.A.; Barba, M.C.; Bebenek, M.; Bonnetain, F.; Bosset, J.F.; Bujko, K.; Cionini, L.; et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J. Clin. Oncol. 2011, 29, 3163–3172. [Google Scholar] [CrossRef] [PubMed]
- George, T.J., Jr.; Allegra, C.J.; Yothers, G. Neoadjuvant Rectal (NAR) Score: A New Surrogate Endpoint in Rectal Cancer Clinical Trials. Curr. Color. Cancer Rep. 2015, 11, 275–280. [Google Scholar] [CrossRef]
- Fokas, E.; Fietkau, R.; Hartmann, A.; Hohenberger, W.; Grutzmann, R.; Ghadimi, M.; Liersch, T.; Strobel, P.; Grabenbauer, G.G.; Graeven, U.; et al. Neoadjuvant rectal score as individual-level surrogate for disease-free survival in rectal cancer in the CAO/ARO/AIO-04 randomized phase III trial. Ann. Oncol. 2018, 29, 1521–1527. [Google Scholar] [CrossRef]
- Rahma, O.E.; Yothers, G.; Hong, T.S.; Russell, M.M.; You, Y.N.; Parker, W.; Jacobs, S.A.; Colangelo, L.H.; Lucas, P.C.; Gollub, M.J.; et al. Use of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Initial Results From the Pembrolizumab Arm of a Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1225–1230. [Google Scholar] [CrossRef]
- Drake, C.G.; Lipson, E.J.; Brahmer, J.R. Breathing new life into immunotherapy: Review of melanoma, lung and kidney cancer. Nat. Rev. Clin. Oncol. 2014, 11, 24–37. [Google Scholar] [CrossRef]
- Otegbeye, E.E.; Mitchem, J.B.; Park, H.; Chaudhuri, A.A.; Kim, H.; Mutch, M.G.; Ciorba, M.A. Immunity, immunotherapy, and rectal cancer: A clinical and translational science review. Transl. Res. 2021, 231, 124–138. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, L.; Wan, J.; Zhang, H.; Wu, R.; Wang, J.; Wang, Y.; Xu, Y.; Cai, S.; Zhang, Z.; et al. Neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer: A new era for anal preservation. Front. Immunol. 2022, 13, 1067036. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cai, M.; Zhang, P.; Li, G.; Liu, T.; Li, X.; Cai, K.; Nie, X.; Wang, J.; Liu, J.; et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J. Immunother. Cancer 2021, 9, e003554. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Oh, Y.T.; Noh, O.K.; Chun, M.; Park, J.E.; Cho, S.R. Nodal tumor response according to the count of peripheral blood lymphocyte subpopulations during preoperative chemoradiotherapy in locally advanced rectal cancer. Radiat. Oncol. J. 2016, 34, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Jiang, W.; Davuluri, R.; Xu, C.; Krishnan, S.; Mohan, R.; Koong, A.C.; Hsu, C.C.; Lin, S.H. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother. Oncol. 2018, 128, 584–590. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Brase, J.C.; Sinn, B.V.; Gade, S.; Kronenwett, R.; Pfitzner, B.M.; Salat, C.; Loi, S.; Schmitt, W.D.; et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 2015, 33, 983–991. [Google Scholar] [CrossRef]
- Chen, C.C.; Wu, M.L.; Huang, K.C.; Huang, I.P.; Chung, Y.L. The Effects of Neoadjuvant Treatment on the Tumor Microenvironment in Rectal Cancer: Implications for Immune Activation and Therapy Response. Clin. Color. Cancer 2020, 19, e164–e180. [Google Scholar] [CrossRef]
- Mandard, A.M.; Dalibard, F.; Mandard, J.C.; Marnay, J.; Henry-Amar, M.; Petiot, J.F.; Roussel, A.; Jacob, J.H.; Segol, P.; Samama, G.; et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef]
- Shah, S.; Asawa, P.; Abel, S.; Wegner, R.E. Validation of the Neoadjuvant Rectal Cancer (NAR) Score for Prognostication Following Total Neoadjuvant Therapy (TNT) for Locally Advanced Rectal Cancer. J. Gastrointest. Cancer 2022, 108, E33. [Google Scholar] [CrossRef]
- Naffouje, S.A.; Manguso, N.; Imanirad, I.; Sahin, I.H.; Xie, H.; Hoffe, S.; Frakes, J.; Sanchez, J.; Dessureault, S.; Felder, S. Neoadjuvant rectal score is prognostic for survival: A population-based propensity-matched analysis. J. Surg. Oncol. 2022, 126, 1219–1231. [Google Scholar] [CrossRef]
- Wang, G.; Tang, Z.; Ye, J.; Tang, H.; Yao, K.; Zeng, Q.; Yang, Y.; Fu, M.; Luo, L.; Shen, Q.; et al. Development and validation of neoadjuvant rectal score-based signature nomograms to predict overall survival and disease-free survival in locally advanced rectal cancer: A retrospective, double center, cohort study. Int. J. Clin. Oncol. 2023, 28, 268–279. [Google Scholar] [CrossRef]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- El Sissy, C.; Kirilovsky, A.; Van den Eynde, M.; Muşină, A.M.; Anitei, M.G.; Romero, A.; Marliot, F.; Junca, A.; Doyen, J.; Mlecnik, B.; et al. A Diagnostic Biopsy-Adapted Immunoscore Predicts Response to Neoadjuvant Treatment and Selects Patients with Rectal Cancer Eligible for a Watch-and-Wait Strategy. Clin. Cancer Res. 2020, 26, 5198–5207. [Google Scholar] [CrossRef] [PubMed]
- Shinto, E.; Hase, K.; Hashiguchi, Y.; Sekizawa, A.; Ueno, H.; Shikina, A.; Kajiwara, Y.; Kobayashi, H.; Ishiguro, M.; Yamamoto, J. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann. Surg. Oncol. 2014, 21 (Suppl. 3), S414–S421. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Akiyoshi, T.; Yamamoto, N.; Hiyoshi, Y.; Mukai, T.; Yamaguchi, T.; Nagasaki, T.; Taketomi, A.; Fukunaga, Y.; Kawachi, H. Intratumoral Budding and CD8-Positive T-cell Density in Pretreatment Biopsies as a Predictor of Response to Neoadjuvant Chemoradiotherapy in Advanced Rectal Cancer. Clin. Color. Cancer 2023, in press. [Google Scholar] [CrossRef]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- Ogura, A.; Akiyoshi, T.; Yamamoto, N.; Kawachi, H.; Ishikawa, Y.; Mori, S.; Oba, K.; Nagino, M.; Fukunaga, Y.; Ueno, M. Pattern of programmed cell death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer. Eur. J. Cancer 2018, 91, 11–20. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koh, J.; Kim, S.; Jeon, S.R.; Chie, E.K.; Kim, K.; Kang, G.H.; Han, S.W.; Kim, T.Y.; Jeong, S.Y.; et al. Chemoradiation-Induced Alteration of Programmed Death-Ligand 1 and CD8(+) Tumor-Infiltrating Lymphocytes Identified Patients With Poor Prognosis in Rectal Cancer: A Matched Comparison Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1216–1224. [Google Scholar] [CrossRef]
- Chen, T.W.; Huang, K.C.; Chiang, S.F.; Chen, W.T.; Ke, T.W.; Chao, K.S.C. Prognostic relevance of programmed cell death-ligand 1 expression and CD8+ TILs in rectal cancer patients before and after neoadjuvant chemoradiotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1043–1053. [Google Scholar] [CrossRef]

| Characteristics | Descriptive Analysis | |
|---|---|---|
| n | % | |
| NAR score | MEAN 20.4 ± 16.3 | |
| Age | ||
| <60 | 56 | 73.7 |
| ≥60 | 20 | 26.3 |
| Gender | ||
| Male | 49 | 64.5 |
| Female | 27 | 35.5 |
| BMI (kg/m2) | ||
| <18.5 | 7 | 9.2 |
| 18.5–24.9 | 53 | 69.7 |
| ≥25 | 16 | 21.1 |
| Distance to anal margin (cm) | ||
| <5 | 49 | 64.5 |
| ≥5 | 27 | 35.5 |
| Lymphovascular invasion | ||
| Yes | 15 | 19.7 |
| No | 61 | 80.3 |
| Neural invasion | ||
| Yes | 14 | 18.4 |
| No | 62 | 81.6 |
| Tumor nodules | ||
| Yes | 16 | 21.1 |
| No | 60 | 78.9 |
| Mucinous adenocarcinoma | ||
| Yes | 8 | 10.5 |
| No | 68 | 89.5 |
| Pathologic differentiation | ||
| Poorly | 16 | 21.1 |
| Moderately | 60 | 78.9 |
| cT stage | ||
| 2 | 4 | 5.3 |
| 3 | 41 | 53.9 |
| 4 | 31 | 40.8 |
| cN stage | ||
| 0 | 28 | 36.8 |
| 1 | 38 | 50.0 |
| 2 | 10 | 13.2 |
| ypT stage | ||
| 0 | 4 | 5.3 |
| 2 | 17 | 22.4 |
| 3 | 47 | 61.8 |
| 4 | 8 | 10.5 |
| ypN stage | ||
| 0 | 33 | 43.4 |
| 1 | 27 | 35.5 |
| 2 | 16 | 21.1 |
| PD-1 | ||
| <5.5% | 61 | 80.2 |
| ≥5.5% | 15 | 19.8 |
| CD3+ T-cell | ||
| <12.5% | 14 | 18.4 |
| ≥12.5% | 62 | 81.6 |
| CD8+ T-cell | ||
| <9.0% | 41 | 53.9 |
| ≥9.0% | 35 | 46.1 |
| pCR | ||
| Yes | 7 | 9.2 |
| No | 69 | 90.8 |
| Neoadjuvant radiotherapy | ||
| Short course | 36 | 47.4 |
| Long course | 40 | 52.6 |
| Neoadjuvant chemotherapy | ||
| Yes | 63 | 82.9 |
| No | 13 | 17.1 |
| Adjuvant radiotherapy | ||
| Yes | 6 | 7.9 |
| No | 70 | 92.1 |
| Adjuvant chemotherapy | ||
| Yes | 52 | 68.4 |
| No | 24 | 31.6 |
| Death | ||
| Yes | 34 | 44.7 |
| No | 42 | 55.3 |
| Characteristics | Univariable Analysis | ||
|---|---|---|---|
| HR | 95% CI | p | |
| Age | 1.774 | 0.878–3.587 | 0.110 |
| Gender | 0.629 | 0.301–1.317 | 0.219 |
| BMI | 1.306 | 0.685–2.491 | 0.417 |
| Distance to anal margin (cm) | 1.248 | 0.625–2.494 | 0.530 |
| Lymphovascular invasion | 5.148 | 2.516–10.533 | 0.000 |
| Neural invasion | 2.232 | 1.064–4.681 | 0.034 |
| Tumor nodules | 1.844 | 0.879–3.868 | 0.106 |
| Mucinous adenocarcinoma | 1.990 | 0.769–5.154 | 0.156 |
| Pathologic differentiation | 2.273 | 1.086–4.761 | 0.029 |
| cT stage | 0.592 | 0.332–1.056 | 0.076 |
| cN stage | 1.220 | 0.742–2.007 | 0.432 |
| ypT stage | 1.136 | 0.762–1.694 | 0.531 |
| ypN stage | 2.460 | 1.587–3.814 | 0.000 |
| PD-1 | 0.334 | 0.102–1.095 | 0.070 |
| CD3+ T-cell | 0.459 | 0.213–0.987 | 0.046 |
| CD8+ T-cell | 0.354 | 0.178–0.703 | 0.003 |
| NAR score | 1.036 | 1.017–1.056 | 0.000 |
| pCR | 0.250 | 0.034–1.832 | 0.173 |
| Neoadjuvant Radiotherapy | 0.582 | 0.295–1.147 | 0.118 |
| Neoadjuvant chemotherapy | 0.667 | 0.290–1.532 | 0.340 |
| Adjuvant radiotherapy | 1.080 | 0.330–3.535 | 0.898 |
| Adjuvant chemotherapy | 0.893 | 0.435–1.833 | 0.759 |
| Variable | β Coefficient | HR | 95% CI | p |
|---|---|---|---|---|
| Lymphovascular invasion | 1.217 | 3.375 | 1.254–9.087 | 0.016 |
| Neural invasion | −0.393 | 0.675 | 0.218–2.089 | 0.496 |
| Pathologic differentiation | 0.251 | 1.286 | 0.578–2.861 | 0.538 |
| CD3+ T-cell | −0.298 | 0.742 | 0.279–1.976 | 0.551 |
| CD8+ T-cell | −0.836 | 0.433 | 0.198–0.948 | 0.036 |
| NAR score | 0.027 | 1.028 | 1.005–1.051 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zeng, Y.; Li, H.; Zhuang, Q.; Tang, L.; Wu, J.; Li, J. A Modified NAR Scoring Model Incorporating Immune Infiltration Characteristics to Better Predict Long-Term Survival Following Neoadjuvant Radiotherapy in Rectal Cancer. Life 2023, 13, 2106. https://doi.org/10.3390/life13112106
Zhang X, Zeng Y, Li H, Zhuang Q, Tang L, Wu J, Li J. A Modified NAR Scoring Model Incorporating Immune Infiltration Characteristics to Better Predict Long-Term Survival Following Neoadjuvant Radiotherapy in Rectal Cancer. Life. 2023; 13(11):2106. https://doi.org/10.3390/life13112106
Chicago/Turabian StyleZhang, Xueqing, Yibin Zeng, Hui Li, Qingyang Zhuang, Lirui Tang, Junxin Wu, and Jinluan Li. 2023. "A Modified NAR Scoring Model Incorporating Immune Infiltration Characteristics to Better Predict Long-Term Survival Following Neoadjuvant Radiotherapy in Rectal Cancer" Life 13, no. 11: 2106. https://doi.org/10.3390/life13112106
APA StyleZhang, X., Zeng, Y., Li, H., Zhuang, Q., Tang, L., Wu, J., & Li, J. (2023). A Modified NAR Scoring Model Incorporating Immune Infiltration Characteristics to Better Predict Long-Term Survival Following Neoadjuvant Radiotherapy in Rectal Cancer. Life, 13(11), 2106. https://doi.org/10.3390/life13112106






