Dynamic Correlations and Disorder in the Masticatory Musculature Network
Abstract
1. Introduction
2. Objectives and Aims
3. Materials and Methods
4. Results
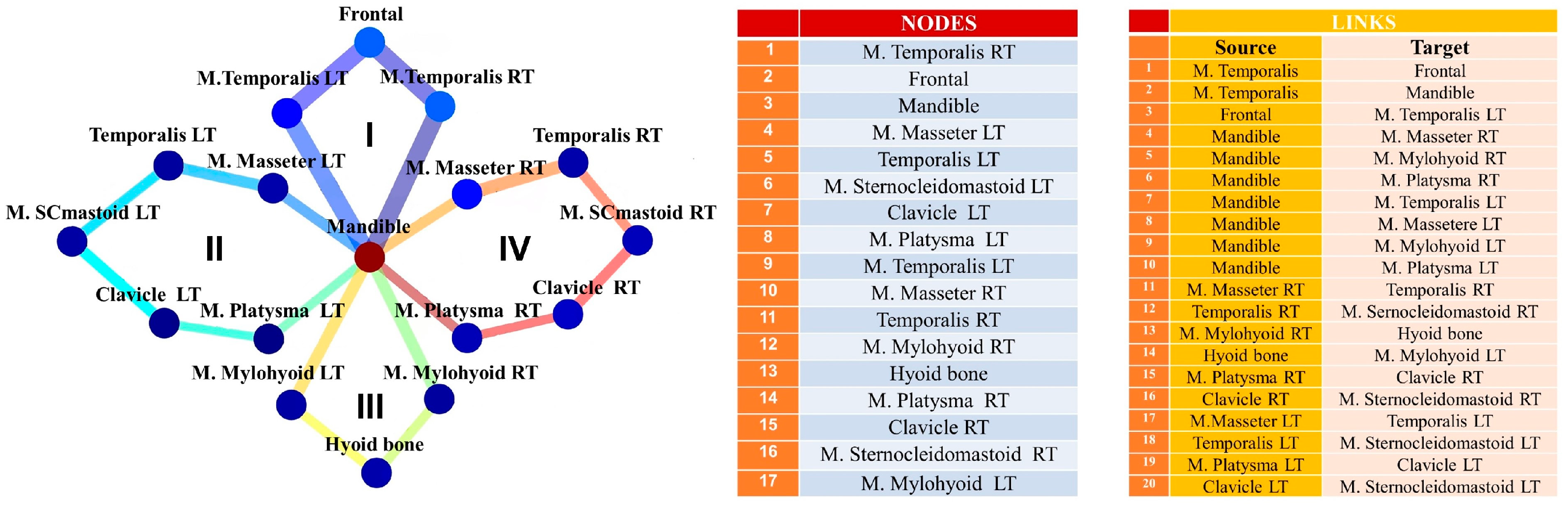
4.1. Measurement Maps Am
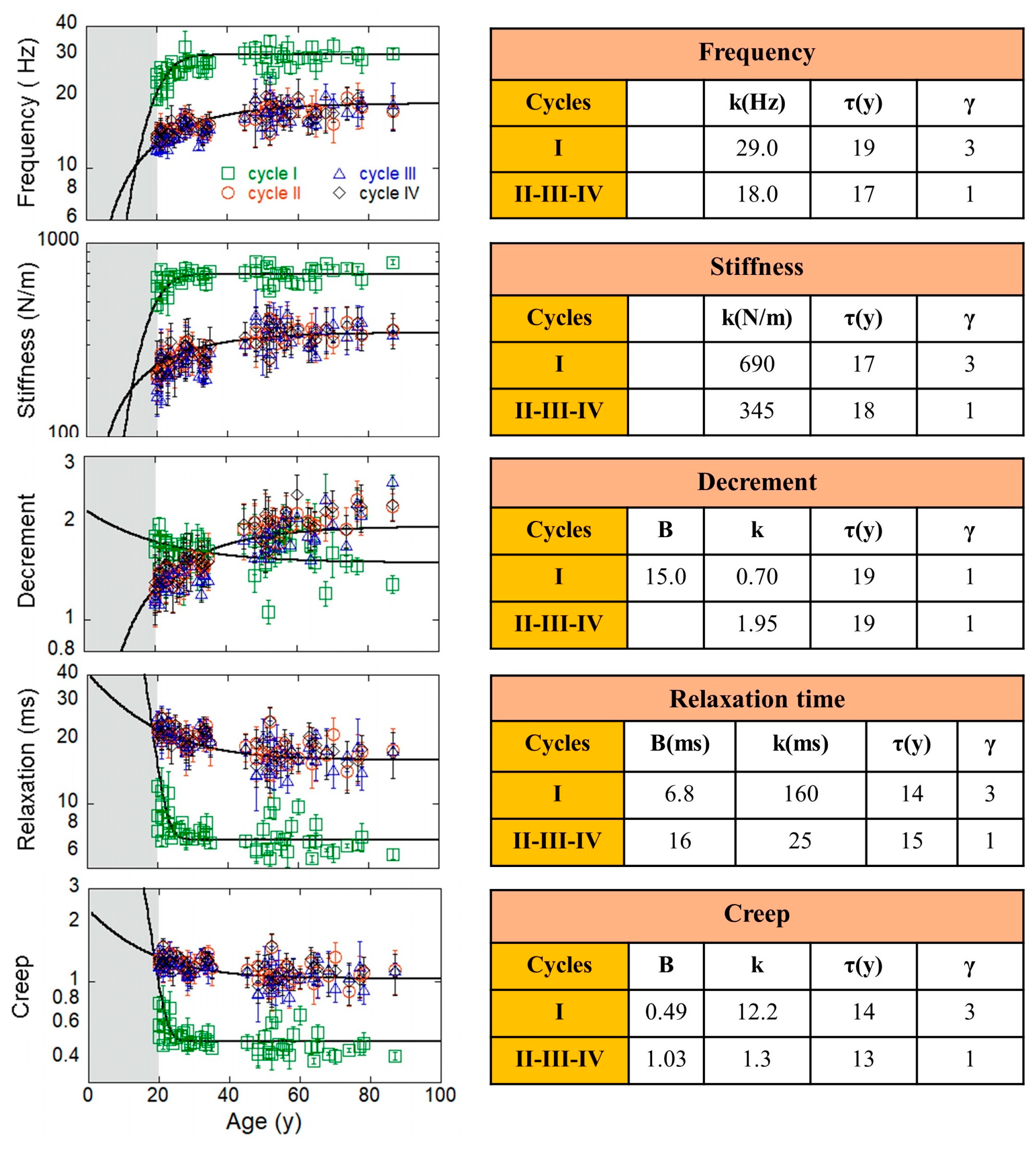
4.2. Cycle Dynamics: Exponential Growth and Decaying of Amcy Measurements
4.3. Correlation Matrix Analysis of MyotonPRO Measurements
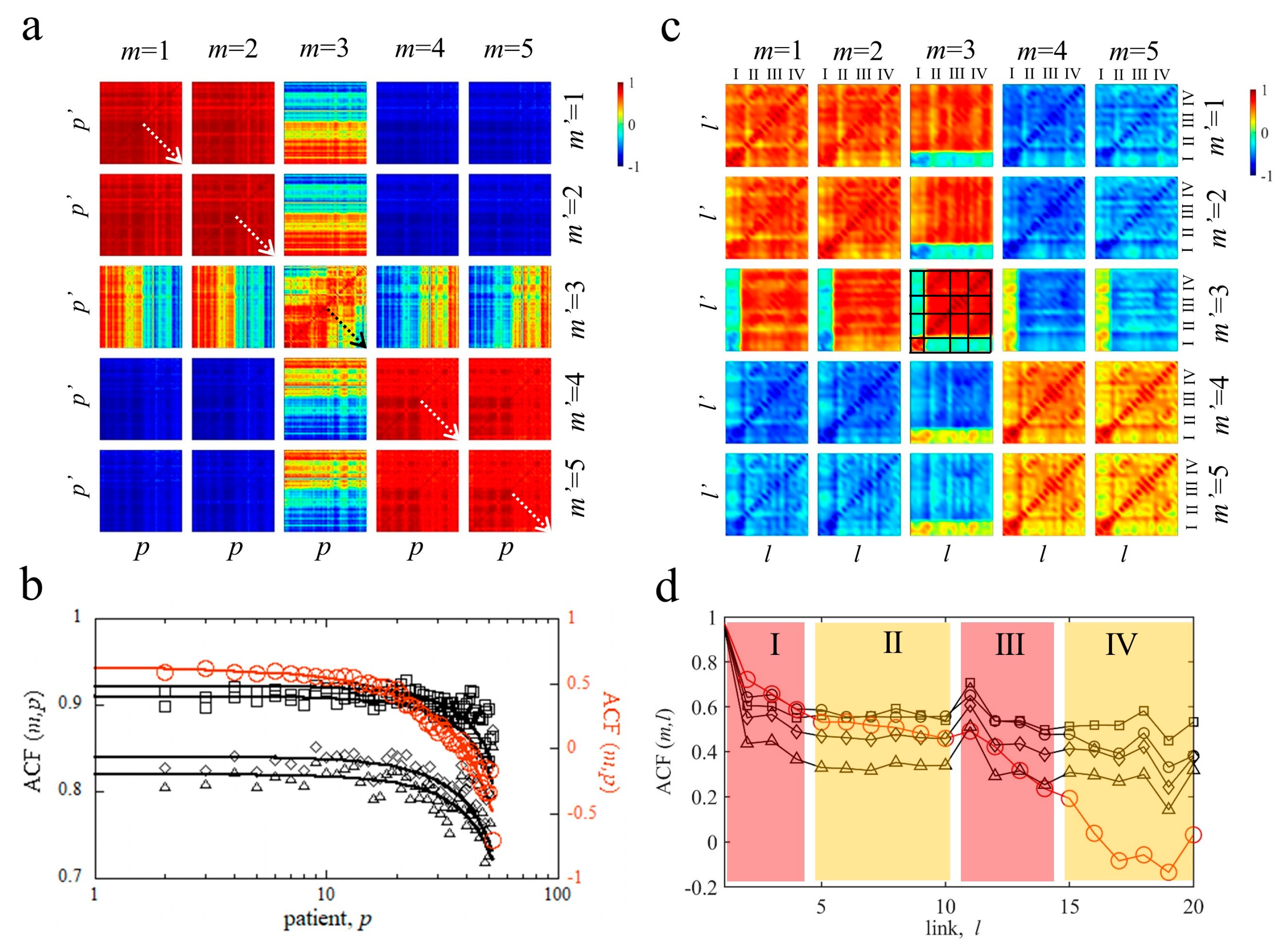
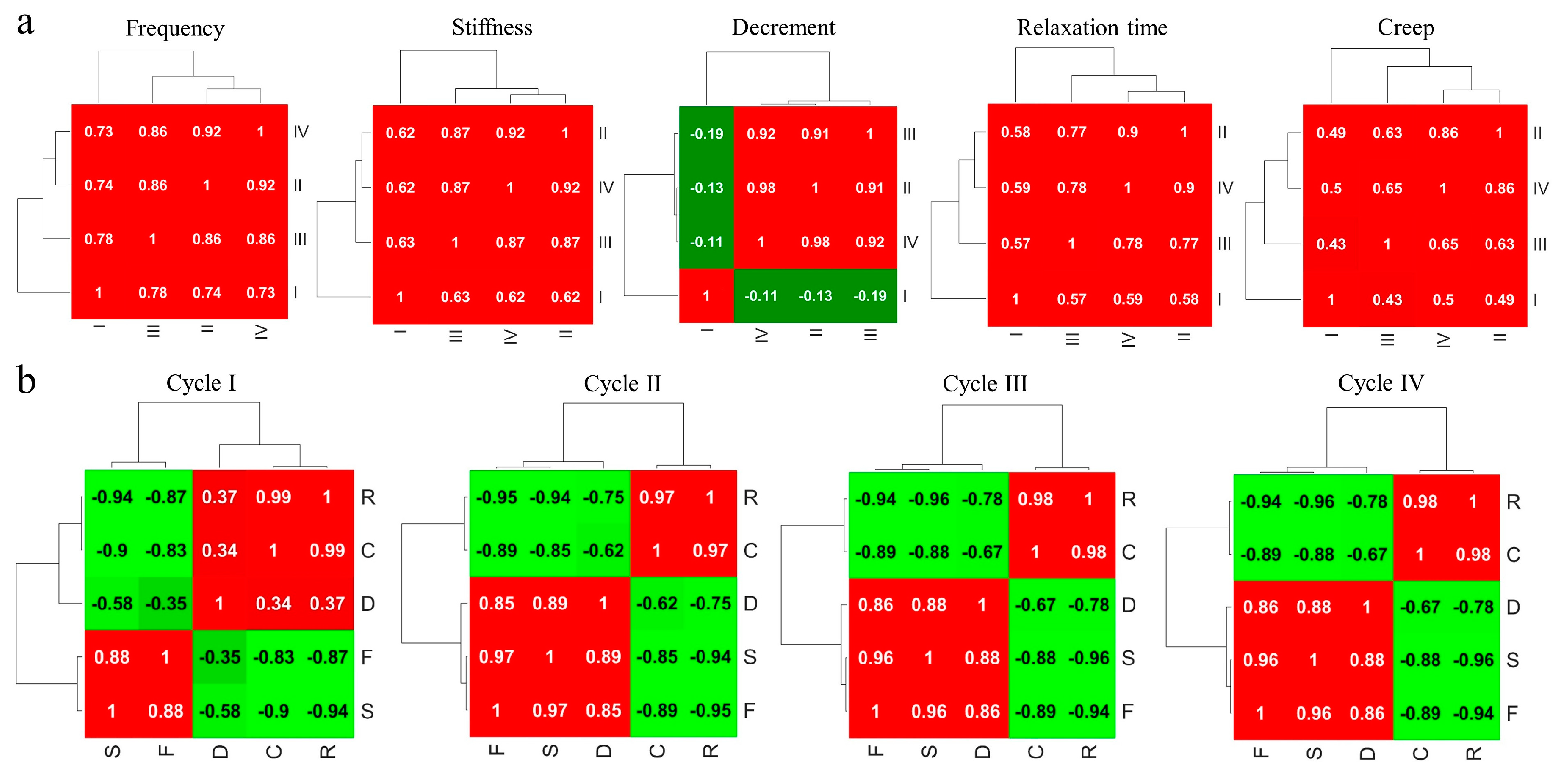
4.3.1. Pairwise Patient–Patient and Link–Link Correlations of MyotonPRO Measurements Am
4.3.2. Correlations between Network Cycles of MyotonPRO Measurements
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Della Posta, D.; Branca, J.J.V.; Guarnieri, G.; Veltro, C.; Pacini, A.; Paternostro, F. Modularity of the Human Musculoskeletal System: The Correlation between Functional Structures by Computer Tools Analysis. Life 2022, 12, 1186. [Google Scholar] [CrossRef]
- Nakata, M. Masticatory Function and Its Effects on General Health. Int. Dent. J. 1998, 48, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Suvinen, T.I.; Reade, P.C.; Kemppainen, P.; Könönen, M.; Dworkin, S.F. Review of Aetiological Concepts of Temporomandibular Pain Disorders: Towards a Biopsychosocial Model for Integration of Physical Disorder Factors with Psychological and Psychosocial Illness Impact Factors. Eur. J. Pain 2005, 9, 613. [Google Scholar] [CrossRef]
- Hickey, J.C.; Kreider, J.A.; Boucher, C.O.; Storz, O. A Method of Studying the Influence of Occlusal Schemes on Muscular Activity. J. Prosthet. Dent. 1959, 9, 498–505. [Google Scholar] [CrossRef]
- Pizolato, R.A.; Fernandes, F.S.D.F.; Gavião, M.B.D. Speech Evaluation in Children with Temporomandibular Disorders. J. Appl. Oral Sci. 2011, 19, 493–499. [Google Scholar] [CrossRef]
- Giannakopoulos, N.N.; Hellmann, D.; Schmitter, M.; Krüger, B.; Hauser, T.; Schindler, H.J. Neuromuscular Interaction of Jaw and Neck Muscles During Jaw Clenching. J. Orofac. Pain 2013, 27, 61–71. [Google Scholar] [CrossRef]
- Davies, P.L. Electromyographic Study of Superficial Neck Muscles in Mandibular Function. J. Dent. Res. 1979, 58, 537–538. [Google Scholar] [CrossRef]
- Lopez-Chaichio, L.; Padial-Molina, M.; O’Valle, F.; Gil-Montoya, J.A.; Catena, A.; Galindo-Moreno, P. Oral Health and Healthy Chewing for Healthy Cognitive Ageing: A Comprehensive Narrative Review. Gerodontology 2021, 38, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Parker, P. (Eds.) Electromyography Physiology, Engineering, and Noninvasive Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-471-67580-8. [Google Scholar]
- Vena, P.; Royston, T.J. Dilatational and Shear Waves in Poro-Viscoelastic Media. J. Mech. Behav. Biomed. Mater. 2019, 97, 99–107. [Google Scholar] [CrossRef]
- Nordez, A.; Hug, F. Muscle Shear Elastic Modulus Measured Using Supersonic Shear Imaging Is Highly Related to Muscle Activity Level. J. Appl. Physiol. 2010, 108, 1389–1394. [Google Scholar] [CrossRef]
- Carlsen, J.F.; Pedersen, M.R.; Ewertsen, C.; Săftoiu, A.; Lönn, L.; Rafaelsen, S.R.; Nielsen, M.B. A Comparative Study of Strain and Shear-Wave Elastography in an Elasticity Phantom. Am. J. Roentgenol. 2015, 204, W236–W242. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, X.; Liu, J.; Ding, J.; Ren, Y.; Hua, K. Evaluation of Levator Ani with No Defect on Elastography in Women with POP. Int. J. Clin. Exp. Med. 2015, 8, 10204–10212. [Google Scholar]
- Chen, T.L.-W.; Agresta, C.E.; Lipps, D.B.; Provenzano, S.G.; Hafer, J.F.; Wong, D.W.-C.; Zhang, M.; Zernicke, R.F. Ultrasound Elastographic Assessment of Plantar Fascia in Runners Using Rearfoot Strike and Forefoot Strike. J. Biomech. 2019, 89, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, P.; Zellers, J.A.; Balascio, P.; Silbernagel, K.G.; Cortes, D.H. Quantification of Mechanical Properties in Healthy Achilles Tendon Using Continuous Shear Wave Elastography: A Reliability and Validation Study. Ultrasound Med. Biol. 2019, 45, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the Elastic Properties of Skeletal Muscle and Tendon Using Shearwave Ultrasound Elastography and MyotonPRO. Sci. Rep. 2018, 8, 17064. [Google Scholar] [CrossRef]
- Nowicki, A.; Dobruch-Sobczak, K. Introduction to ultrasound elastography. J. Ultrason. 2016, 16, 113–124. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.M.H.; Chen, X.; Hagemann, H.; Tang, M.-X.; Bull, A.M.J. Developing and Using Fast Shear Wave Elastography to Quantify Physiologically-Relevant Tendon Forces. Med. Eng. Phys. 2019, 69, 116–122. [Google Scholar] [CrossRef]
- Kelly, J.P.; Koppenhaver, S.L.; Michener, L.A.; Proulx, L.; Bisagni, F.; Cleland, J.A. Characterization of Tissue Stiffness of the Infraspinatus, Erector Spinae, and Gastrocnemius Muscle Using Ultrasound Shear Wave Elastography and Superficial Mechanical Deformation. J. Electromyogr. Kinesiol. 2018, 38, 73–80. [Google Scholar] [CrossRef]
- Leonard, C.T.; Brown, J.S.; Price, T.R.; Queen, S.A.; Mikhailenok, E.L. Comparison of Surface Electromyography and Myotonometric Measurements during Voluntary Isometric Contractions. J. Electromyogr. Kinesiol. 2004, 14, 709–714. [Google Scholar] [CrossRef]
- Chuang, L.; Wu, C.; Lin, K. Reliability, Validity, and Responsiveness of Myotonometric Measurement of Muscle Tone, Elasticity, and Stiffness in Patients With Stroke. Arch. Phys. Med. Rehabil. 2012, 93, 532–540. [Google Scholar] [CrossRef]
- Korhonen, R.K.; Vain, A.; Vanninen, E.; Viir, R.; Jurvelin, J.S. Can Mechanical Myotonometry or Electromyography Be Used for the Prediction of Intramuscular Pressure? Physiol. Meas. 2005, 26, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.; Masi, A.T.; Andonian, B.J.; Barry, A.J.; Coates, B.A.; Dougherty, J.; Schaefer, E.; Henderson, J.; Kelly, J. Stiffness of Resting Lumbar Myofascia in Healthy Young Subjects Quantified Using a Handheld Myotonometer and Concurrently with Surface Electromyography Monitoring. J. Bodyw. Mov. Ther. 2015, 20, P388–P396. [Google Scholar] [CrossRef]
- Alev, K.; Vain, A.; Aru, M.; Pehme, A.; Purge, P.; Kaasik, P.; Seene, T. Glucocorticoid-Induced Changes in Rat Skeletal Muscle Biomechanical and Viscoelastic Properties: Aspects of Aging. J. Manip. Physiol. Ther. 2018, 41, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-E. Influential Factors of Masticatory Performance in Older Adults: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 4286. [Google Scholar] [CrossRef]
- Ramazanoglu, E.; Turhan, B.; Usgu, S. Evaluation of the Tone and Viscoelastic Properties of the Masseter Muscle in the Supine Position, and Its Relation to Age and Gender. Dent. Med. Probl. 2021, 58, 155–161. [Google Scholar] [CrossRef]
- Görürgöz, C.; İçen, M.; Kurt, M.; Aksoy, S.; Bakırarar, B.; Rozylo-Kalinowska, I.; Orhan, K. Degenerative Changes of the Mandibular Condyle in Relation to the Temporomandibular Joint Space, Gender and Age: A Multicenter CBCT Study. Dent. Med. Probl. 2023, 60, 127–135. [Google Scholar] [CrossRef]
- Florjanski, W.; Malysa, A.; Orzeszek, S.; Smardz, J.; Olchowy, A.; Paradowska-Stolarz, A.; Wieckiewicz, M. Evaluation of Biofeedback Usefulness in Masticatory Muscle Activity Management—A Systematic Review. J. Clin. Med. 2019, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Eriksson, P.-O.; Häggman-Henrikson, B.; Nordh, E.; Zafar, H. Co-Ordinated Mandibular and Head-Neck Movements during Rhythmic Jaw Activities in Man. J. Dent. Res. 2000, 79, 1378–1384. [Google Scholar] [CrossRef]
- Häggman-Henrikson, B.; Eriksson, P.-O. Head Movements during Chewing: Relation to Size and Texture of Bolus. J. Dent. Res. 2004, 83, 864–868. [Google Scholar] [CrossRef]
- Nilsson, E.; Häggman-Henrikson, B.; Domellöf, E.; Hellström, F.; Häger, C.K.; Österlund, C. Development of Integrated Jaw–Neck Motor Function in Children at 6, 10 and 13 Years of Age Compared to Adults: A Kinematic Longitudinal Study. J. Oral Rehabil. 2023, 50, 1002–1011. [Google Scholar] [CrossRef]
- Lee, H.-M.; Chen, J.-J.J.; Ju, M.-S.; Lin, C.-C.K.; Poon, P.P.W. Validation of Portable Muscle Tone Measurement Device for Quantifying Velocity-Dependent Properties in Elbow Spasticity. J. Electromyogr. Kinesiol. 2004, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Gavronski, G.; Veraksitš, A.; Vasar, E.; Maaroos, J. Evaluation of Viscoelastic Parameters of the Skeletal Muscles in Junior Triathletes. Physiol. Meas. 2007, 28, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Peipsi, A.; Stokes, M.; Knicker, A.; Abeln, V. Feasibility of Monitoring Muscle Health in Microgravity Environments Using Myoton Technology. Med. Biol. Eng. Comput. 2015, 53, 57–66. [Google Scholar] [CrossRef]
- Peipsi, A.; Kosemets, M. MyotonPRO User Manual; MYOTON AS: Tallinn, Estonia, 2023. [Google Scholar]
- Campi, G. Structural Fluctuations at Nanoscale in Complex Functional Materials (Chapter 14). In Synchrotron Radiation Science and Applications, Proceedings of the 2019 Meeting of the Italian Synchrotron Radiation Society—Dedicated to Carlo Lamberti, Camerino, Italy, 9–11 September 2019; Di Cicco, A., Giuli, G., Trapananti, A., Eds.; Springer Proceedings in Physics; Springer International Publishing: Cham, Switzerland, 2021; Volume 220, ISBN 978-3-030-72004-9. [Google Scholar]
- Campi, G.; Di Gioacchino, M.; Poccia, N.; Ricci, A.; Burghammer, M.; Ciasca, G.; Bianconi, A. Nanoscale Correlated Disorder in Out-of-Equilibrium Myelin Ultrastructure. ACS Nano 2018, 12, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Karri, R.R. Evaluating and Estimating the Complex Dynamic Phenomena in Nonlinear Chemical Systems. Int. J. Chem. React. Eng. 2011, 9, A94. [Google Scholar] [CrossRef]
- Bär, M.; Billing, G.D. The Role of Degenerate States in Chemistry; J. Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-471-46151-7. [Google Scholar]
- Augieri, A.; Rizzo, F.; Galluzzi, V.; Mancini, A.; Fabbri, F.; Armenio, A.A.; Vannozzi, A.; Pinto, V.; Rufoloni, A.; Piperno, L.; et al. Correlated Disorder in YBCO and Composite YBCO Films Revealed by Means of Synchrotron X-Ray Diffraction. IEEE Trans. Appl. Supercond. 2018, 28, 7500604. [Google Scholar] [CrossRef]
- Montroll, E.W.; Bendler, J.T. On Lévy (or Stable) Distributions and the Williams- Watts Model of Dielectric Relaxation. J. Stat. Phys. 1984, 34, 129–162. [Google Scholar] [CrossRef]
- Dagotto, E. Complexity in Strongly Correlated Electronic Systems. Science 2005, 309, 257–262. [Google Scholar] [CrossRef]
- Campi, G.; Bianconi, A.; Poccia, N.; Bianconi, G.; Barba, L.; Arrighetti, G.; Innocenti, D.; Karpinski, J.; Zhigadlo, N.D.; Kazakov, S.M.; et al. Inhomogeneity of Charge-Density-Wave Order and Quenched Disorder in a High-Tc Superconductor. Nature 2015, 525, 359–362. [Google Scholar] [CrossRef]
- Liu, X.; Hersam, M.C. 2D Materials for Quantum Information Science. Nat. Rev. Mater. 2019, 4, 669–684. [Google Scholar] [CrossRef]
- Peck, C.C.; Goulet, J.; Lobbezoo, F.; Schiffman, E.L.; Alstergren, P.; Anderson, G.C.; De Leeuw, R.; Jensen, R.; Michelotti, A.; Ohrbach, R.; et al. Expanding the Taxonomy of the Diagnostic Criteria for Temporomandibular Disorders. J. Oral Rehabil. 2014, 41, 2–23. [Google Scholar] [CrossRef] [PubMed]

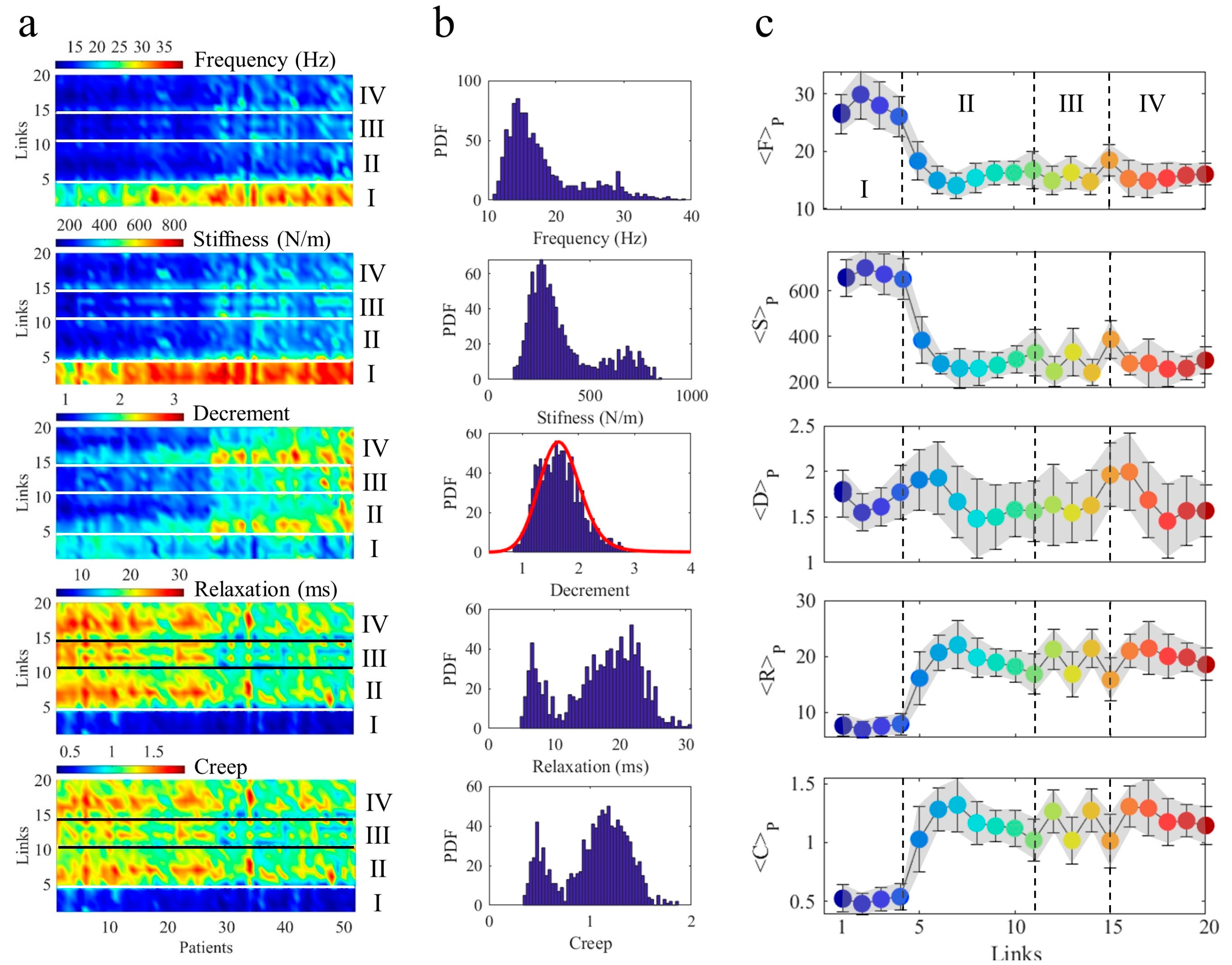
| Cycles | F (%) | S (%) | R (%) | C (%) | <F> | <S> | <R> | <C> |
|---|---|---|---|---|---|---|---|---|
| I | 0.32 | 0.27 | 0.20 | 0.20 | 25.50 | 636.0 | 7.17 | 0.49 |
| II–IV | 0.68 | 0.73 | 0.80 | 0.80 | 15.35 | 284.4 | 18.90 | 1.16 |
| Frequency | Stiffness | Decrement | Relaxation | Creep | |
|---|---|---|---|---|---|
| Frequency | 1 | 0.96 | 0.23 | −0.93 | −0.91 |
| Stiffness | 0.96 | 1 | 0.25 | −0.95 | −0.93 |
| Decrement | 0.23 | 0.25 | 1 | −0.28 | −0.19 |
| Relaxation | −0.93 | −0.95 | −0.28 | 1 | 0.99 |
| Creep | −0.91 | −0.93 | −0.19 | 0.99 | 1 |
| M | Measurement | Contrast | β | p0 | Age (y) |
|---|---|---|---|---|---|
| 1 | Frequency | 0.028 | 1.85 | 42 | 60 |
| 2 | Stiffness | 0.028 | 1.85 | 43 | 60 |
| 3 | Decrement | 0.265 | 1.05 | 33 | 50 |
| 4 | Relaxation | 0.050 | 1.85 | 48 | 70 |
| 5 | Creep | 0.040 | 1.85 | 48 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campi, G.; Ricci, A.; Costa, N.; Genovesi, F.; Branca, J.J.V.; Paternostro, F.; Della Posta, D. Dynamic Correlations and Disorder in the Masticatory Musculature Network. Life 2023, 13, 2107. https://doi.org/10.3390/life13112107
Campi G, Ricci A, Costa N, Genovesi F, Branca JJV, Paternostro F, Della Posta D. Dynamic Correlations and Disorder in the Masticatory Musculature Network. Life. 2023; 13(11):2107. https://doi.org/10.3390/life13112107
Chicago/Turabian StyleCampi, Gaetano, Alessandro Ricci, Nicola Costa, Federico Genovesi, Jacopo Junio Valerio Branca, Ferdinando Paternostro, and Daniele Della Posta. 2023. "Dynamic Correlations and Disorder in the Masticatory Musculature Network" Life 13, no. 11: 2107. https://doi.org/10.3390/life13112107
APA StyleCampi, G., Ricci, A., Costa, N., Genovesi, F., Branca, J. J. V., Paternostro, F., & Della Posta, D. (2023). Dynamic Correlations and Disorder in the Masticatory Musculature Network. Life, 13(11), 2107. https://doi.org/10.3390/life13112107











