State-of-the-Art on Biomarkers for Anaphylaxis in Obstetrics
Abstract
1. Introduction
2. Clinical Diagnosis of Anaphylaxis
3. The Immunology of Anaphylaxis and Pregnancy-Brief Overview
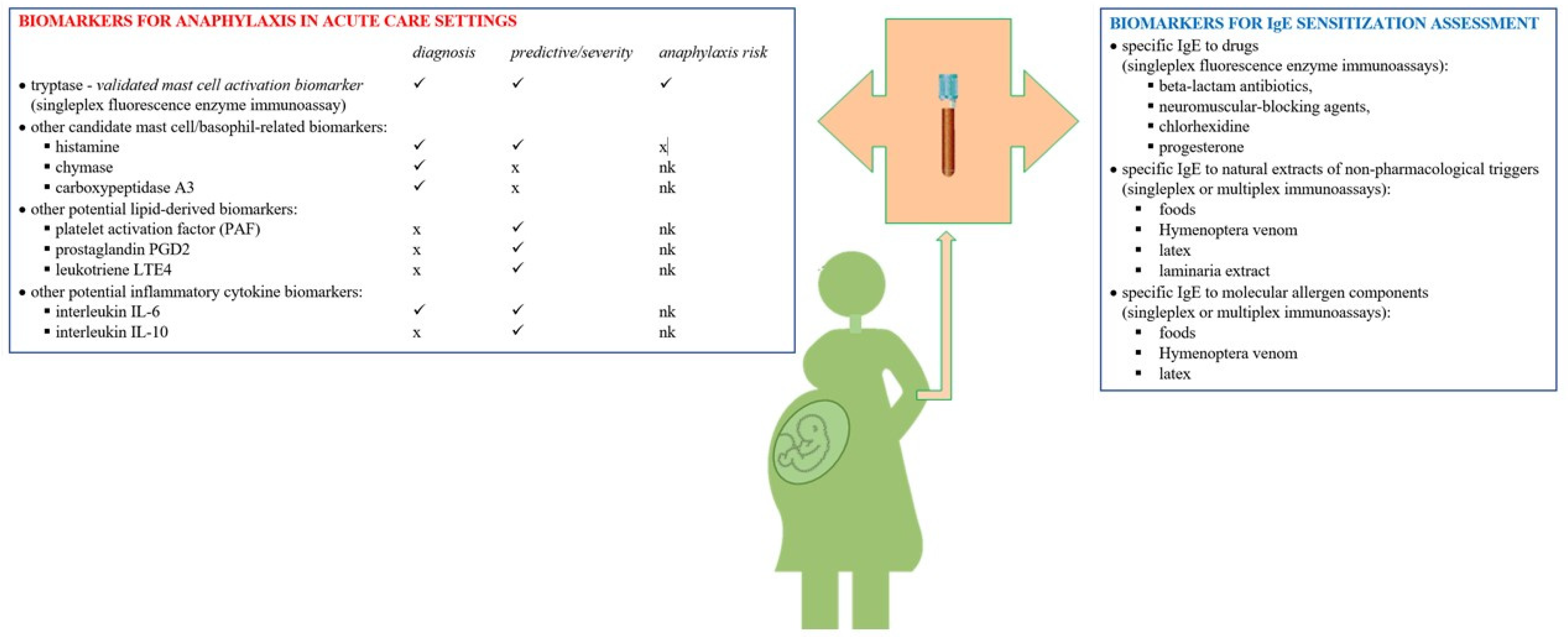
4. Biomarkers
4.1. Mast Cell Tryptase
4.2. Other Potential Biomarkers for Diagnosis of Anaphylaxis in the Acute Care Setting
4.3. Biomarkers for IgE Sensitization Assessment
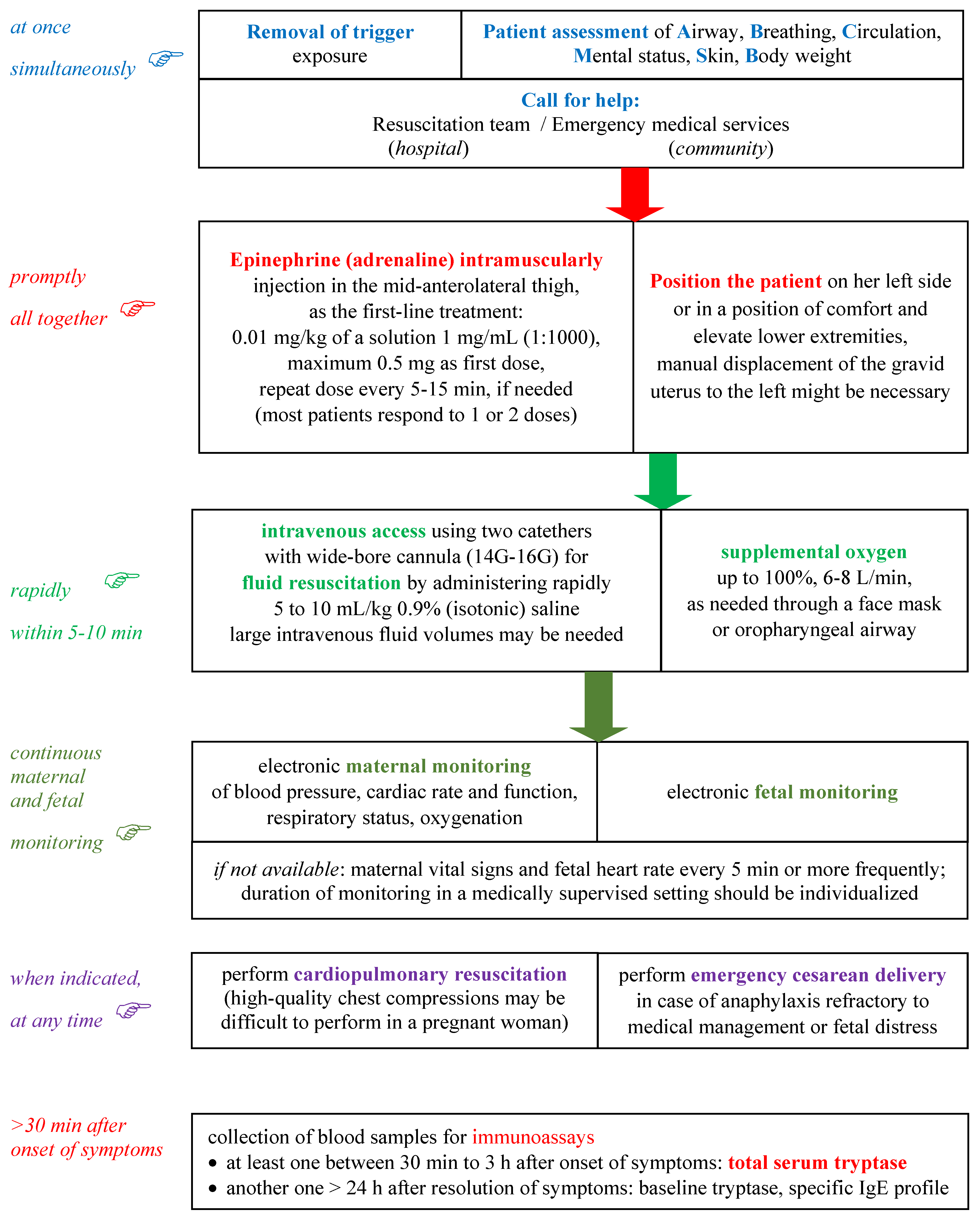
5. Management of Anaphylaxis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liccardi, G.; Milanese, M.; Bilò, M.B.; Liccardi, M.V.; Gargano, D.; Giordano, A.; Habetswallner, F.; Schiavo, M.L.; Madonna, F.; Montera, M.C.; et al. Lessons from peculiar cases of anaphylaxis: Why allergists should be prepared for the unexpected. Eur. Ann. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bilò, M.B.; Martini, M.; Tontini, C.; Corsi, A.; Antonicelli, L. Anaphylaxis. Eur. Ann. Allergy Clin. Immunol. 2020, 53, 4. [Google Scholar] [CrossRef] [PubMed]
- Liew, W.K.; Williamson, E.; Tang, M.L. Anaphylaxis fatalities and admissions in Australia. J. Allergy Clin. Immunol. 2009, 123, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Poirot, E.; He, F.; Gould, L.H.; Hadler, J.L. Deaths, Hospitalizations, and Emergency Department Visits from Food-Related Anaphylaxis, New York City, 2000–2014: Implications for Fatality Prevention. J. Public Health Manag. Pract. 2020, 26, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Tacquard, C.; Chassard, D.; Malinovsky, J.-M.; Saucedo, M.; Deneux-Tharaux, C.; Mertes, P.M. Anaphylaxis-related mortality in the obstetrical setting: Analysis of the French National Confidential Enquiry into Maternal Deaths from 2001 to 2012. Br. J. Anaesth. 2019, 123, e151–e153. [Google Scholar] [CrossRef]
- McCall, S.J.; Bonnet, M.; Äyräs, O.; Vandenberghe, G.; Gissler, M.; Zhang, W.; Van Leeuw, V.; Deneux-Tharaux, C.; Kurinczuk, J.J.; Knight, M.; et al. Anaphylaxis in pregnancy: A population-based multinational European study. Anaesthesia 2020, 75, 1469–1475. [Google Scholar] [CrossRef]
- Mulla, Z.D.; Ebrahim, M.S.; Gonzalez, J.L. Anaphylaxis in the obstetric patient: Analysis of a statewide hospital discharge database. Ann. Allergy Asthma Immunol. 2010, 104, 55–59. [Google Scholar] [CrossRef]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Worm, M.; Ansotegui, I.J.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Tanno, L.; et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ. J. 2019, 12, 100066. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ardusso, L.R.; Bilò, M.B.; El-Gamal, Y.M.; Ledford, D.K.; Ring, J.; Sanchez-Borges, M.; Senna, G.; Sheikh, A.; Thong, B. World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis. World Allergy Organ. J. 2011, 4, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.; Gonzales, J.; Jesurun, C.; Ambat, M.; Mandal-Chaudhuri, S. Anaphylactic shock in pregnancy: A case study and review of the literature. Int. J. Obstet. Anesth. 2008, 17, 350–357. [Google Scholar] [CrossRef]
- Berenguer, A.; Couto, A.; Brites, V.; Fernandes, R. Anaphylaxis in pregnancy: A rare cause of neonatal mortality. BMJ Case Rep. 2013, 2013, bcr2012007055. [Google Scholar] [CrossRef]
- Tsuzuki, Y.; Narita, M.; Nawa, M.; Nakagawa, U.; Wakai, T. Management of maternal anaphylaxis in pregnancy: A case report. Acute Med. Surg. 2016, 4, 202–204. [Google Scholar] [CrossRef]
- McCall, S.J.; Kurinczuk, J.J.; Knight, M. Anaphylaxis in Pregnancy in the United States: Risk Factors and Temporal Trends Using National Routinely Collected Data. J. Allergy Clin. Immunol. Pract. 2019, 7, 2606–2612.e3. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Rossi, K.; Cavalleri, F.; Simoni, A.; Aguzzoli, L.; Masellis, G.; Ferrari, F. Maternal anaphylaxis and fetal brain damage after intrapartum chemoprophylaxis. J. Perinat. Med. 2004, 32, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Reiner, K.; Zah Bogović, T.; Ćaćić, M.; Mihaljević, S.; Krišto, B. Anaphylaxis during pregnancy. Psychiatr. Danub. 2019, 31 (Suppl. 1), 60–62. [Google Scholar] [PubMed]
- Montañez, M.I.; Mayorga, C.; Bogas, G.; Barrionuevo, E.; Fernandez-Santamaria, R.; Martin-Serrano, A.; Laguna, J.J.; Torres, M.J.; Fernandez, T.D.; Doña, I. Epidemiology, Mechanisms, and Diagnosis of Drug-Induced Anaphylaxis. Front. Immunol. 2017, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R.; Schatz, M. Anaphylaxis during pregnancy. J. Allergy Clin. Immunol. 2012, 130, 597–606. [Google Scholar] [CrossRef]
- McCall, S.J.; Bunch, K.J.; Brocklehurst, P.; D’Arcy, R.; Hinshaw, K.; Kurinczuk, J.J.; Lucas, D.N.; Stenson, B.; Tuffnell, D.; Knight, M. The incidence, characteristics, management and outcomes of anaphylaxis in pregnancy: A population-based descriptive study. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 965–971. [Google Scholar] [CrossRef]
- Piccinni, M.-P. T-cell Cytokines in Pregnancy. Am. J. Reprod. Immunol. 2002, 47, 289–294. [Google Scholar] [CrossRef]
- Krishnan, L.; Guilbert, L.J.; Russell, A.S.; Wegmann, T.G.; Mosmann, T.R.; Belosevic, M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased pro-duction of T helper 2 cytokines. J. Immunol. 1996, 156, 644–652. [Google Scholar]
- Chaouat, G.; Assal Meliani, A.; Martal, J.; Raghupathy, R.; Elliott, J.F.; Mosmann, T.; Wegmann, T.G. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone com-bination is corrected by in vivo injection of IFN-tau. J. Immunol. 1995, 154, 4261–4268. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Sleth, J. Anaphylaxis in late pregnancy: Plasma concentrations of histamine, tryptase and IgE in the neonate. Int. J. Obstet. Anesth. 2018, 36, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Derbala, Y.; Elazzamy, H.; Bilal, M.; Reed, R.; Garcia, M.D.S.; Skariah, A.; Dambaeva, S.; Fernandez, E.; Germain, A.; Gilman-Sachs, A.; et al. Mast cell–induced immunopathology in recurrent pregnancy losses. Am. J. Reprod. Immunol. 2019, 82, e13128. [Google Scholar] [CrossRef] [PubMed]
- Shawkat, E.; Hussain, N.; Myers, J.; Gillham, J.; Helbert, M. Breast milk: Friend or foe? BMJ Case Rep. 2011, 2011, bcr0120113766. [Google Scholar] [CrossRef]
- Kraft, S.; Kinet, J.-P. New developments in FcεRI regulation, function and inhibition. Nat. Rev. Immunol. 2007, 7, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, M.C. The Use of Bruton’s Tyrosine Kinase Inhibitors to Treat Allergic Disorders. Curr. Treat. Options Allergy 2021, 1–13. [Google Scholar] [CrossRef]
- Amoudruz, P.; Minang, J.T.; Sundström, Y.; Nilsson, C.; Lilja, G.; Troye-Blomberg, M.; Sverremark-Ekström, E. Pregnancy, but not the allergic status, influences spontaneous and induced interleukin-1β (IL-1β), IL-6, IL-10 and IL-12 responses. Immunology 2006, 119, 18–26. [Google Scholar] [CrossRef]
- Noe, M.H.; Messingham, K.A.; Brandt, D.S.; Andrews, J.I.; Fairley, J.A. Pregnant women have increased incidence of IgE autoantibodies reactive with the skin and placental antigen BP180 (type XVII collagen). J. Reprod. Immunol. 2010, 85, 198–204. [Google Scholar] [CrossRef]
- Yamani, A.; Wu, D.; Waggoner, L.; Noah, T.; Koleske, A.J.; Finkelman, F.; Hogan, S.P. The vascular endothelial specific IL-4 receptor alpha–ABL1 kinase signaling axis regulates the severity of IgE-mediated anaphylactic reactions. J. Allergy Clin. Immunol. 2017, 142, 1159–1172.e5. [Google Scholar] [CrossRef]
- Tomar, S.; Hogan, S.P. Recent advances in mechanisms of food allergy and anaphylaxis. F1000Research 2020, 9, 863. [Google Scholar] [CrossRef]
- Finkelman, F.D. Anaphylaxis: Lessons from mouse models. J. Allergy Clin. Immunol. 2007, 120, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Akin, C.; Scott, L.M.; Kocabas, C.N.; Kushnir-Sukhov, N.; Brittain, E.; Noel, P.; Metcalfe, D.D. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood 2007, 110, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Escribese, M.M.; Rosace, D.; Chivato, T.; Fernandez, T.D.; Corbí, A.L.; Barber, D. Alternative Anaphylactic Routes: The Potential Role of Macrophages. Front. Immunol. 2017, 8, 515. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Bruhns, P.; Horiuchi, K.; Ravetch, J.V. FcγRIV: A Novel FcR with Distinct IgG Subclass Specificity. Immunity 2005, 23, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P.; Jönsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Kortenhorst, M.S.Q.; Harmsze, A.M.; Hasaart, T.H.M. Anaphylaxis after iron dextran administration in a pregnant woman. Ned. Tijdschr. Geneeskd. 2012, 156, A5264. [Google Scholar]
- Kounis, N.G.; Soufras, G.D.; Kounis, G.N. Fatal anaphylactic reaction to iron sucrose in pregnancy: Iron-induced Kounis syndrome? Indian J. Pharmacol. 2013, 45, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Santosh, S.; Podaralla, P.; Miller, B. Anaphylaxis with elevated serum tryptase after administration of intravenous ferumoxytol. Clin. Kidney J. 2010, 3, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Nemes, R.; Mészáros, T.; Urbanics, R.; Kok, R.J.; Jackman, J.A.; Cho, N.-J.; Storm, G.; Szebeni, J. Complement activation in vitro and reactogenicity of low-molecular weight dextran-coated SPIONs in the pig CARPA model: Correlation with physicochemical features and clinical information. J. Control. Release 2018, 270, 268–274. [Google Scholar] [CrossRef]
- Choi, H.W.; Suwanpradid, J.; Kim, I.H.; Staats, H.F.; Haniffa, M.; MacLeod, A.S.; Abraham, S.N. Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science 2018, 362, eaao0666. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Drucker, E.; Krapfenbauer, K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.-D. Molecular biomarkers for grass pollen immunotherapy. World J. Methodol. 2014, 4, 26–45. [Google Scholar] [CrossRef]
- Sala-Cunill, A.; Cardona, V. Anaphylaxis viewed by experts. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 435–441. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Frew, A.J.; Ansotegui, I.J.; Bochner, B.S.; Golden, D.B.K.; Finkelman, F.D.; Leung, D.Y.M.; Lotvall, J.; Marone, G.; Metcalfe, D.D. Risk assessment in anaphylaxis: Current and future approaches. J. Allergy Clin. Immunol. 2007, 120, S2–S24. [Google Scholar] [CrossRef]
- Cunill, A.S.; Guilarte, M.; Cardona, V. Phenotypes, endotypes and biomarkers in anaphylaxis: Current insights. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 370–376. [Google Scholar] [CrossRef]
- Cunill, A.S.; Cardona, V. Biomarkers of anaphylaxis, beyond tryptase. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B. Diagnostic Value of Tryptase in Anaphylaxis and Mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.C.; Wilding, T.; Buka, R.; Baretto, R.L.; Huissoon, A.P.; Krishna, M.T. Biomarkers in Human Anaphylaxis: A Critical Appraisal of Current Evidence and Perspectives. Front. Immunol. 2019, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Rodrigues-Cernadas, J.; Vultaggio, A.; Brockow, K.; Caubet, J.-C.R.J.-P.; Makowska, J.; et al. In vitrotests for drug hypersensitivity reactions: An ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2016, 71, 1103–1134. [Google Scholar] [CrossRef] [PubMed]
- Popescu, F.-D.; Vieru, M. Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules. World J. Methodol. 2018, 8, 17–36. [Google Scholar] [CrossRef]
- Sheldon, J.; Philips, B. Laboratory investigation of anaphylaxis: Not as easy as it seems. Anaesthesia 2014, 70, 1–5. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Arock, M.; Brockow, K.; Butterfield, J.H.; Carter, M.C.; Castells, M.; Escribano, L.; Hartmann, K.; Lieberman, P.; et al. Definitions, Criteria and Global Classification of Mast Cell Disorders with Special Reference to Mast Cell Activation Syndromes: A Consensus Proposal. Int. Arch. Allergy Immunol. 2012, 157, 215–225. [Google Scholar] [CrossRef]
- Weiler, C.R.; Austen, K.F.; Akin, C.; Barkoff, M.S.; Bernstein, J.A.; Bonadonna, P.; Butterfield, J.H.; Carter, M.; Fox, C.C.; Maitland, A.; et al. AAAAI Mast Cell Disorders Committee Work Group Report: Mast cell activation syndrome (MCAS) diagnosis and management. J. Allergy Clin. Immunol. 2019, 144, 883–896. [Google Scholar] [CrossRef]
- Sun, K.-J.; He, J.-T.; Huang, H.-Y.; Xue, Y.; Xie, X.-L.; Wang, Q. Diagnostic role of serum tryptase in anaphylactic deaths in forensic medicine: A systematic review and meta-analysis. Forensic Sci. Med. Pathol. 2018, 14, 209–215. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Sakai, K.; Bradford, T.R.; Ren, S.; Zweiman, B.; Worobec, A.S.; Metcalfe, D.D. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J. Clin. Investig. 1995, 96, 2702–2710. [Google Scholar] [CrossRef]
- Luskin, K.T.; White, A.A.; Lyons, J.J. The Genetic Basis and Clinical Impact of Hereditary Alpha-Tryptasemia. J. Allergy Clin. Immunol. Pract. 2021, 9, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Greiner, G.; Sprinzl, B.; Górska, A.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; et al. Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 2021, 137, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, S.; Seyfarth, F.; Hipler, U.; Elsner, P. Impact of Age and Heterophilic Interference on the Basal Serum Tryptase, a Risk Indication for Anaphylaxis, in 1,092 Dermatology Patients. Acta Derm. Venereol. 2012, 92, 484–489. [Google Scholar] [CrossRef]
- Van Toorenenbergen, A.W.; Van Daele, P.L.; Boonstra, J.G. False-elevated serum tryptase assay result caused by heterophilic antibodies. J. Allergy Clin. Immunol. 2005, 116, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Van Toorenenbergen, A.W.; Hooijkaas, H.; Heerenbrink, G.K.; Goorbergh, D.M.D.-V.D. Heterophilic antibody interference in a tryptase immunoassay. Clin. Biochem. 2008, 41, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Sperr, W.R.; Jordan, J.-H.; Baghestanian, M.; Kiener, H.-P.; Samorapoompichit, P.; Semper, H.; Hauswirth, A.; Schernthaner, G.; Chott, A.; Natter, S.; et al. Expression of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia. Blood 2001, 98, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Sperr, W.R.; Stehberger, B.; Wimazal, F.; Baghestanian, M.; Schwartz, L.B.; Kundi, M.; Semper, H.; Jordan, J.-H.; Chott, A.; Drach, J.; et al. Serum tryptase measurements in patients with myelodysplastic syndromes. Leuk. Lymphoma 2002, 43, 1097–1105. [Google Scholar] [CrossRef]
- Klion, A.D.; Noel, P.; Akin, C.; Law, M.A.; Gilliland, D.G.; Cools, J.; Metcalfe, D.D.; Nutman, T.B. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood 2003, 101, 4660–4666. [Google Scholar] [CrossRef]
- Desai, A.; Sowerwine, K.; Liu, Y.; Lawrence, M.G.; Chovanec, J.; Hsu, A.P.; O’Connell, M.; Kim, J.; Boris, L.; Jones, N.; et al. GATA-2–deficient mast cells limit IgE-mediated immediate hypersensitivity reactions in human subjects. J. Allergy Clin. Immunol. 2019, 144, 613–617.e14. [Google Scholar] [CrossRef]
- Dugas-Breit, S.; Schopf, P.; Dugas, M.; Schiffl, H.; Rueff, F.; Przybilla, B. Baseline serum levels of mast cell tryptase are raised in hemodialysis patients and associated with severity of pruritus. J. Dtsch. Dermatol. Ges. 2005, 3, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, A.E.; González, C.; Enríquez, R.; Fernández, J.; Millán, I.; Barber, X.; Amorós, F. Serum tryptase levels and markers of renal dysfunction in a population with chronic kidney disease. J. Nephrol. 2010, 23, 282–290. [Google Scholar] [PubMed]
- Yamaoka, M.; Deguchi, M.; Ninomiya, K.; Kurasako, T.; Matsumoto, M. A suspected case of rocuronium–sugammadex complex-induced anaphylactic shock after cesarean section. J. Anesth. 2016, 31, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Béné, J.; Alarcon, P.; Faucon, M.; Auffret, M.; Delfosse, F.; Becker, T.; De Zorzi, S.; Gautier, S. Anaphylactic shock after misoprostol in voluntary termination of pregnancy—A case report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Farrar, S.C.; Gherman, R.B. Serum tryptase analysis in a woman with amniotic fluid embolism. A case report. J. Reprod. Med. 2001, 46. [Google Scholar]
- A Gilmore, D.; Wakim, J.; Secrest, J.; Rawson, R. Anaphylactoid syndrome of pregnancy: A review of the literature with latest management and outcome data. AANA J. 2003, 71, 120. [Google Scholar] [PubMed]
- Gist, R.S.; Stafford, I.P.; Leibowitz, A.B.; Beilin, Y. Amniotic Fluid Embolism. Anesth. Analg. 2009, 108, 1599–1602. [Google Scholar] [CrossRef]
- Clark, S.L.; Romero, R.; Dildy, G.A.; Callaghan, W.M.; Smiley, R.M.; Bracey, A.W.; Hankins, G.D.; D’Alton, M.E.; Foley, M.; Pacheco, L.D.; et al. Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am. J. Obstet. Gynecol. 2016, 215, 408–412. [Google Scholar] [CrossRef]
- Harboe, T.; Benson, M.D.; Oi, H.; Softeland, E.; Bjorge, L.; Guttormsen, A.B. Cardiopulmonary distress during obstetrical anaesthesia: Attempts to diagnose amniotic fluid embolism in a case series of suspected allergic anaphylaxis. Acta Anaesthesiol. Scand. 2006, 50, 324–330. [Google Scholar] [CrossRef]
- Benson, M.D. Immunologic studies in presumed amniotic fluid embolism. Obstet. Gynecol. 2001, 97, 510–514. [Google Scholar] [CrossRef]
- Clark, S.L.; Hankins, G.D.; Dudley, D.A.; Dildy, G.; Porter, T. Amniotic fluid embolism: Analysis of the national registry. Am. J. Obstet. Gynecol. 1995, 172, 1158–1169. [Google Scholar] [CrossRef]
- Busardò, F.P.; Frati, P.; Zaami, S.; Fineschi, V. Amniotic Fluid Embolism Pathophysiology Suggests the New Diagnostic Armamentarium: β-Tryptase and Complement Fractions C3-C4 Are the Indispensable Working Tools. Int. J. Mol. Sci. 2015, 16, 6557–6570. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Ohi, H.; Terao, T. A simple, noninvasive, sensitive method for diagnosis of amniotic fluid embolism by monoclonal antibody TKH-2 that recognizes NeuAcα2-6GalNAc. Am. J. Obstet. Gynecol. 1993, 168, 848–853. [Google Scholar] [CrossRef]
- Passia, E.; Jandus, P. Using Baseline and Peak Serum Tryptase Levels to Diagnose Anaphylaxis: A Review. Clin. Rev. Allergy Immunol. 2020, 58, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Gülen, T.; Teufelberger, A.; Ekoff, M.; Westerberg, C.M.; Lyberg, K.; Dahlén, S.-E.; Dahlén, B.; Nilsson, G. Distinct plasma biomarkers confirm the diagnosis of mastocytosis and identify increased risk of anaphylaxis. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Mochizuki, A.; McEuen, A.R.; Buckley, M.G.; Walls, A. The release of basogranulin in response to IgE-dependent and IgE-independent stimuli: Validity of basogranulin measurement as an indicator of basophil activation. J. Allergy Clin. Immunol. 2003, 112, 102–108. [Google Scholar] [CrossRef]
- Nishio, H.; Takai, S.; Miyazaki, M.; Horiuchi, H.; Osawa, M.; Uemura, K.; Yoshida, K.-I.; Mukaida, M.; Ueno, Y.; Suzuki, K. Usefulness of serum mast cell–specific chymase levels for postmortem diagnosis of anaphylaxis. Int. J. Leg. Med. 2005, 119, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Whitworth, H.; E-Khedr, M.; Brown, T.; Goswami, R.; Eren, E.; Lucas, J.; Erlewyn-Lajeunesse, M.; Summers, C.; Pumphrey, R. Mast Cell Chymase: A Useful Serum Marker in Anaphylaxis. J. Allergy Clin. Immunol. 2011, 127, AB143. [Google Scholar] [CrossRef]
- Zhou, X.; Buckley, M.; Lau, L.; Summers, C.W.; Pumphrey, R.; Walls, A.F. Mast Cell Carboxypeptidase as a New Clinical Marker for Anaphylaxis. J. Allergy Clin. Immunol. 2006, 117, S85. [Google Scholar] [CrossRef]
- Korosec, P.; Turner, P.; Silar, M.; Kopac, P.; Kosnik, M.; Gibbs, B.F.; Shamji, M.H.; Custovic, A.; Rijavec, M. Basophils, high-affinity IgE receptors, and CCL2 in human anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 750–758.e15. [Google Scholar] [CrossRef] [PubMed]
- Vadas, P.; Gold, M.; Perelman, B.; Liss, G.M.; Lack, G.; Blyth, T.; Simons, F.E.R.; Simons, K.J.; Cass, D.; Yeung, J. Platelet-Activating Factor, PAF Acetylhydrolase, and Severe Anaphylaxis. N. Engl. J. Med. 2008, 358, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Vadas, P.; Perelman, B.; Liss, G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 144–149. [Google Scholar] [CrossRef]
- Reber, L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Taniguchi, M.; Mita, H.; Fukutomi, Y.; Higashi, N.; Miyazaki, E.; Kumamoto, T.; Akiyama, K. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin. Exp. Allergy 2009, 39, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Higashi, N.; Mita, H.; Ono, E.; Fukutomi, Y.; Yamaguchi, H.; Kajiwara, K.; Tanimoto, H.; Sekiya, K.; Akiyama, K.; Taniguchi, M. Profile of eicosanoid generation in aspirin-intolerant asthma and anaphylaxis assessed by new biomarkers. J. Allergy Clin. Immunol. 2010, 125, 1084–1091.e6. [Google Scholar] [CrossRef] [PubMed]
- Callesen, K.T.; Poulsen, L.K.; Garvey, L.H.; Jensen, B.M. Comparing baseline and reaction samples of perioperative anaphylaxis patients reveals IL-6 and CCL2 as potential biomarkers. Clin. Exp. Allergy 2021. [Google Scholar] [CrossRef] [PubMed]
- Dass, C.; Eyck, P.T.; Ballas, Z.; Lee, S. Characterization of serum biomarkers during anaphylaxis in emergency department patients. J. Allergy Clin. Immunol. Pract. 2020, 8, 3213–3215.e1. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cano, R.; Pascal, M.; Araujo, G.; Goikoetxea, M.J.; Valero, A.L.; Picado, C.; Bartra, J. Mechanisms, Cofactors, and Augmenting Factors Involved in Anaphylaxis. Front. Immunol. 2017, 8, 1193. [Google Scholar] [CrossRef]
- Yuste-Montalvo, A.; Fernandez-Bravo, S.; Oliva, T.; Pastor-Vargas, C.; Betancor, D.; Goikoetxea, M.J.; Laguna, J.J.; López, J.A.; Alvarez-Llamas, G.; Cuesta-Herranz, J.; et al. Proteomic and Biological Analysis of an In Vitro Human Endothelial System in Response to Drug Anaphylaxis. Front. Immunol. 2021, 12, 692569. [Google Scholar] [CrossRef]
- Stone, S.F.; Isbister, G.; Shahmy, S.; Mohamed, F.; Abeysinghe, C.; Karunathilake, H.; Ariaratnam, A.; Jacoby-Alner, T.E.; Cotterell, C.L.; Brown, S.G.A. Immune Response to Snake Envenoming and Treatment with Antivenom; Complement Activation, Cytokine Production and Mast Cell Degranulation. PLoS Negl. Trop. Dis. 2013, 7, e2326. [Google Scholar] [CrossRef]
- Cunill, A.S.; Björkqvist, J.; Senter, R.; Guilarte, M.; Cardona, V.; Labrador-Horrillo, M.; Nickel, K.F.; Butler, L.; Luengo, O.; Kumar, P.; et al. Plasma contact system activation drives anaphylaxis in severe mast cell–mediated allergic reactions. J. Allergy Clin. Immunol. 2015, 135, 1031–1043.e6. [Google Scholar] [CrossRef]
- Ballesteros-Martinez, C.; Mendez-Barbero, N.; Montalvo-Yuste, A.; Jensen, B.M.; Gomez-Cardenosa, A.; Klitfod, L.; Garrido-Arandia, M.; Alvarez-Llamas, G.; Pastor-Vargas, C.; Vivanco, F.; et al. Endothelial Regulator of Calcineurin 1 Promotes Barrier Integrity and Modulates Histamine-Induced Barrier Dysfunction in Anaphylaxis. Front. Immunol. 2017, 8, 1323. [Google Scholar] [CrossRef]
- Mendez-Barbero, N.; Yuste-Montalvo, A.; Nuñez-Borque, E.; Jensen, B.M.; Gutiérrez-Muñoz, C.; Tome-Amat, J.; Arandia, M.G.; Díaz-Perales, A.; Ballesteros-Martinez, C.; Laguna, J.J.; et al. The TNF-like weak inducer of the apoptosis/fibroblast growth factor–inducible molecule 14 axis mediates histamine and platelet-activating factor–induced subcutaneous vascular leakage and anaphylactic shock. J. Allergy Clin. Immunol. 2019, 145, 583–596.e6. [Google Scholar] [CrossRef]
- Nuñez-Borque, E.; Fernandez-Bravo, S.; Del Rio, P.R.; Alwashali, E.M.; Lopez-Dominguez, D.; Gutierrez-Blazquez, M.D.; Laguna, J.J.; Tome-Amat, J.; Gallego-Delgado, J.; Gomez-Lopez, A.; et al. Increased miR-21-3p and miR-487b-3p serum levels during anaphylactic reaction in food allergic children. Pediatr. Allergy Immunol. 2021, 32, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.J.; Acharya, U.; Ormerod, A.D.; Herriot, R. Latex/chlorhexidine-induced anaphylaxis in pregnancy. Allergy 1998, 53, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, K.; Nasser, S.M. Management of hypersensitivity reactions to anti-D immunoglobulin preparations. Allergy 2014, 69, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, I.; Evans, S.; Switzer, I.; Underdown, B.; Greenbaum, J.; Dolovich, J. Urticaria from allergy to a purified human anti-Rh antibody preparation. Allergy 1995, 50, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A. Progestogen Sensitization: A Unique Female Presentation of Anaphylaxis. Curr. Allergy Asthma Rep. 2020, 20, 1–6. [Google Scholar] [CrossRef]
- Shank, J.J.; Olney, S.C.; Lin, F.L.; McNamara, M.F. Recurrent Postpartum Anaphylaxis with Breast-Feeding. Obstet. Gynecol. 2009, 114, 415–416. [Google Scholar] [CrossRef]
- Mullins, R.; Russell, A.; Mcgrath, G.; Smith, R.; Sutherland, D. Breastfeeding anaphylaxis. Lancet 1991, 338, 1279–1280. [Google Scholar] [CrossRef]
- MacDonell, J.W.; Ito, S. Breastfeeding Anaphylaxis Case Study. J. Hum. Lact. 1998, 14, 243–244. [Google Scholar] [CrossRef]
- Villalta, D.; Martelli, P. A case of breastfeeding anaphylaxis. Eur. Ann. Allergy Clin. Immunol. 2007, 39, 26–27. [Google Scholar]
- McKinney, K.K.; Scranton, S.E. A case report of breastfeeding anaphylaxis: Successful prophylaxis with oral antihistamines. Allergy 2010, 66, 435–436. [Google Scholar] [CrossRef]
- Yamamoto, K.; Go, R.; Nakai, K.; Tobetto, Y.; Kawanishi, R.; Kato, M. Three cases of latex allergy complicated with anaphy-laxis during cesarean section. Masui 2012, 61, 1080–1084. [Google Scholar] [PubMed]
- Popescu, F.-D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Dubiela, P.; Dölle-Bierke, S.; Aurich, S.; Worm, M.; Hoffmann-Sommergruber, K. Component-resolved diagnosis in adult patients with food-dependent anaphylaxis. World Allergy Organ. J. 2021, 14, 100530. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Ariano, R.; Aruanno, A.; Barzaghi, C.; Borrelli, P.; Busa, M.; Celi, G.; Cinquini, M.; Cortellini, G.; D’Auria, F.; et al. Systemic allergic reactions induced by labile plant-food allergens: Seeking potential cofactors. A multicenter study. Allergy 2020, 76, 1473–1479. [Google Scholar] [CrossRef]
- Popescu, F.-D. Drug allergies due to IgE sensitization to α-GAL. FARMACIA 2019, 67, 43–49. [Google Scholar] [CrossRef]
- Adriaensens, I.; Vercauteren, M.; Soetens, F.; Janssen, L.; Leysen, J.; Ebo, D. Allergic reactions during labour analgesia and caesarean section anaesthesia. Int. J. Obstet. Anesth. 2013, 22, 231–242. [Google Scholar] [CrossRef]
- Vidal, C.; Méndez-Brea, P.; López-Freire, S.; González-Vidal, T. Vaginal Capsules: An Unsuspected Probable Source of Exposure to α-Gal. J. Investig. Allergol. Clin. Immunol. 2016, 26, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Pagán, J.A.; Postigo, I.; Rodríguez-Pacheco, J.R.; Peña, M.; Guisantes, J.A.; Martinez, J. Bovine serum albumin contained in culture medium used in artificial insemination is an important anaphylaxis risk factor. Fertil. Steril. 2008, 90, 2013.e17–2013.e19. [Google Scholar] [CrossRef]
- Popescu, F.-D.; Ganea, C.S.; Panaitescu, C.; Vieru, M. Molecular diagnosis in cat allergy. World J. Methodol. 2021, 11, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakagawa, Y.; Kotobuki, Y.; Katayama, I. A case of human seminal plasma allergy sensitized with dog prostatic kallikrein, Can f 5. Allergol. Int. 2018, 68, 259–260. [Google Scholar] [CrossRef]
- Mattsson, L.; Lundgren, T.; Everberg, H.; Larsson, H.; Lidholm, J. Prostatic kallikrein: A new major dog allergen. J. Allergy Clin. Immunol. 2009, 123, 362–368.e3. [Google Scholar] [CrossRef] [PubMed]
- Schoos, A.-M.M.; Nwaru, B.I.; Borres, M.P. Component-resolved diagnostics in pet allergy: Current perspectives and future directions. J. Allergy Clin. Immunol. 2021, 147, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chang, Y.H.; Kim, W.K.; Kim, Y.K.; Cho, S.H.; Kim, Y.Y.; Min, K.U. Two Cases of Anaphylaxis After Laminaria Insertion. J. Korean Med. Sci. 2003, 18, 886–888. [Google Scholar] [CrossRef]
- Erasmus, C.; Blackwood, W.; Wilson, J. Infantile multicystic encephalomalacia after maternal bee sting anaphylaxis during pregnancy. Arch. Dis. Child. 1982, 57, 785–787. [Google Scholar] [CrossRef]
- Rizk, D.; Mensah-Brown, E.; Lukic, M. Placental abruption and intrauterine death following an ant sting. Int. J. Gynecol. Obstet. 1998, 63, 71–72. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27, 1–250. [Google Scholar] [CrossRef]
- Schuler, S.; Ferrari, G.; Schmid-Grendelmeier, P.; Harr, T. Microarray-based component-resolved diagnosis of latex allergy: Isolated IgE-mediated sensitization to latexprofilin Hev b8 may act as confounder. Clin. Transl. Allergy 2013, 3, 11. [Google Scholar] [CrossRef]
- Draisci, G.; Zanfini, B.A.; Nucera, E.; Catarci, S.; Sangregorio, R.; Schiavino, D.; Mannocci, A.; Patriarca, G. Latex Sensitization. Anesthesiology 2011, 114, 565–569. [Google Scholar] [CrossRef]
- Ronel, I.; Weiniger, C.F.; Levy, N. Re: The incidence, characteristics, managements and outcomes of anaphylaxis in pregnancy: A population-based descriptive study. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1340. [Google Scholar] [CrossRef]
- Ikeda, N.; Oda, Y.; Tanaka, K.; Nakamura, T.; Asada, A. A case of anaphylactic shock induced by latex during cesarean section. Masui 2010, 59, 1294–1297. [Google Scholar] [PubMed]
- Liccardi, G.; Bilò, M.B.; Mauro, C.; Salzillo, A.; Piccolo, A.; D’Amato, M.; Liccardi, A.; D’Amato, G. Oxytocin: An unexpected risk for cardiologic and broncho-obstructive effects, and allergic reactions in susceptible delivering women. Multidiscip. Respir. Med. 2013, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Ogata, J.; Minami, K. Synthetic oxytocin and latex allergy. Br. J. Anaesth. 2007, 98, 845–846. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liccardi, G.; Biló, M.B.; Mauro, C.; Salzillo, A.; Piccolo, A.; D’Amato, M.; D’Amato, G. Oxytocin: A likely underestimated risk for anaphylactic reactions in delivering women sensitized to latex. Ann. Allergy, Asthma Immunol. 2013, 110, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Amrani, Y.; Syed, F.; Huang, C.; Li, K.; Liu, V.; Jain, D.; Keslacy, S.; Sims, M.W.; Baidouri, H.; Cooper, P.R.; et al. Expression and activation of the oxytocin receptor in airway smooth muscle cells: Regulation by TNFα and IL-13. Respir. Res. 2010, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Gutkowska, J.; Jankowski, M. Oxytocin Revisited: Its Role in Cardiovascular Regulation. J. Neuroendocr. 2011, 24, 599–608. [Google Scholar] [CrossRef]
- Kjaer, B.N.; Krøigaard, M.; Garvey, L.H.; Kjær, B.N. Oxytocin use during Caesarean sections in Denmark—Are we getting the dose right? Acta Anaesthesiol. Scand. 2015, 60, 18–25. [Google Scholar] [CrossRef]
- Liccardi, G.; Calzetta, L.; Salzillo, A.; Puxeddu, E.; Rogliani, P. Relationship between oxytocin/vasopressin and latex in obstetric surgery: How to recognize (and prevent) allergic reactions and differentiate them from side effects? J. Allergy Clin. Immunol. Pract. 2017, 5, 873. [Google Scholar] [CrossRef]
- Shum, M.; Jerschow, E. Reply. J. Allergy Clin. Immunol. Pract. 2017, 5, 873–874. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.Y.; Chiang, W.C.; Huang, C.H.; Woo, C.K.; Ibrahim, I.; Heng, K.; Pramanick, A.; Lee, B.W. An unusual cause of food-induced anaphylaxis in mothers. World Allergy Organ. J. 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, U.; Moreschi, C.; Marega, G.; Tse, R.; Garland, J.; Ondruschka, B.; Palmiere, C. Medicolegal Implications of Biphasic Anaphylaxis. Am. J. Forensic Med. Pathol. 2020, 42, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Beyer, K.; Biedermann, T.; Bircher, A.; Fischer, M.; Fuchs, T.; Heller, A.; Hoffmann, F.; Huttegger, I.; Jakob, T.; et al. Guideline (S2k) on acute therapy and management of anaphylaxis: 2021 update. Allergo J. Int. 2021, 30, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Abella, B.S.; Berger, W.E.; Blaiss, M.S.; Stiell, I.G.; Herres, J.P.; Moellman, J.J.; Suner, S.; Kessler, A.; Klausner, H.A.; Caterino, J.M.; et al. Intravenous Cetirizine Versus Intravenous Diphenhydramine for the Treatment of Acute Urticaria: A Phase III Randomized Controlled Noninferiority Trial. Ann. Emerg. Med. 2020, 76, 489–500. [Google Scholar] [CrossRef]
- Fischer, D.; Leek, T.K.V.; Ellis, A.K.; Kim, H. Anaphylaxis. Allergy, Asthma Clin. Immunol. 2018, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, W.; Ellis, A.K. Do Corticosteroids Prevent Biphasic Anaphylaxis? J. Allergy Clin. Immunol. Pract. 2017, 5, 1194–1205. [Google Scholar] [CrossRef]
- Campbell, D.E. Anaphylaxis Management: Time to Re-Evaluate the Role of Corticosteroids. J. Allergy Clin. Immunol. Pract. 2019, 7, 2239–2240. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, C.; Galappatthy, P.; Seneviratne, S. Corticosteroids in management of anaphylaxis; a systematic review of evidence. Eur. Ann. Allergy Clin. Immunol. 2017, 49, 196–207. [Google Scholar] [CrossRef]
- Lieberman, P. Biphasic anaphylactic reactions. Ann. Allergy Asthma Immunol. 2005, 95, 217–226. [Google Scholar] [CrossRef]
- Dribin, T.E.; Sampson, H.A.; Camargo, C.A.; Brousseau, D.C.; Spergel, J.M.; Neuman, M.I.; Shaker, M.; Campbell, R.L.; Michelson, K.A.; Rudders, S.A.; et al. Persistent, refractory, and biphasic anaphylaxis: A multidisciplinary Delphi study. J. Allergy Clin. Immunol. 2020, 146, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
| Anaphylaxis Is Highly Likely When Any One of the Following 2 Criteria Are Fulfilled: |
|---|
| 1. Acute onset of an illness (minutes to several hours) with simultaneous involvement of the skin, mucosal tissue, or both (e.g., generalized hives, pruritus or flushing, swollen lips-tongue-uvula) |
| AND AT LEAST ONE OF THE FOLLOWING: |
| a. Respiratory compromise (e.g., dyspnea, wheeze-bronchospasm, stridor, reduced PEF, hypoxemia) |
| b. Reduced BP or associated symptoms of end-organ dysfunction (e.g., hypotonia [collapse], syncope, incontinence) |
| c. Severe gastrointestinal symptoms (e.g., severe crampy abdominal pain, repetitive vomiting), especially after exposure to non-food allergens |
| 2. Acute onset of hypotension a or bronchospasm b or laryngeal involvement c after exposure to a known or highly probable allergen d for that patient (minutes to several hours), even in the absence of typical skin involvement. |
| Triggers | References | Mechanism Involved | Effector Cells | Important Mediators |
|---|---|---|---|---|
| penicillins, foods, venom, latex | [2,6,8,11] | IgE-dependent | mast cell/basophil | histamine, tryptase, chymase, carboxypeptidase |
| iron (intravenous) | [2,41,42] | Complement system | mast cells | histamine, PAF, C3a, C5a |
| neuromuscular blockers | [2,6,8,11] | Mast cell/basophil activation MRGPRX2; IgE-dependent | mast cells | histamine, tryptase, chymase, heparin, PAF |
| Candidate Biomarker | Method of Detection | Comments | References |
|---|---|---|---|
| serum/plasma histamine | enzyme immunoassay | short half-life | [53,55] |
| serum/saliva carboxypeptidase A3 | enzyme-linked immunoassay | half-life longer than tryptase | [55,90] |
| serum chemokine ligand CCL2 | sandwich enzyme immunoassay | glycosylation influences half-life | [55,91] |
| serum interleukin IL-6 | sandwich enzyme immunoassay | inflammatory cytokine | [97,98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simionescu, A.A.; Stanescu, A.M.A.; Popescu, F.-D. State-of-the-Art on Biomarkers for Anaphylaxis in Obstetrics. Life 2021, 11, 870. https://doi.org/10.3390/life11090870
Simionescu AA, Stanescu AMA, Popescu F-D. State-of-the-Art on Biomarkers for Anaphylaxis in Obstetrics. Life. 2021; 11(9):870. https://doi.org/10.3390/life11090870
Chicago/Turabian StyleSimionescu, Anca Angela, Ana Maria Alexandra Stanescu, and Florin-Dan Popescu. 2021. "State-of-the-Art on Biomarkers for Anaphylaxis in Obstetrics" Life 11, no. 9: 870. https://doi.org/10.3390/life11090870
APA StyleSimionescu, A. A., Stanescu, A. M. A., & Popescu, F.-D. (2021). State-of-the-Art on Biomarkers for Anaphylaxis in Obstetrics. Life, 11(9), 870. https://doi.org/10.3390/life11090870







