Hydrogen Supplementation in SI Engines: Enhancing Efficiency and Reducing Emissions with a Focus on Knock Phenomena
Abstract
1. Introduction
2. Materials and Methods
3. Results
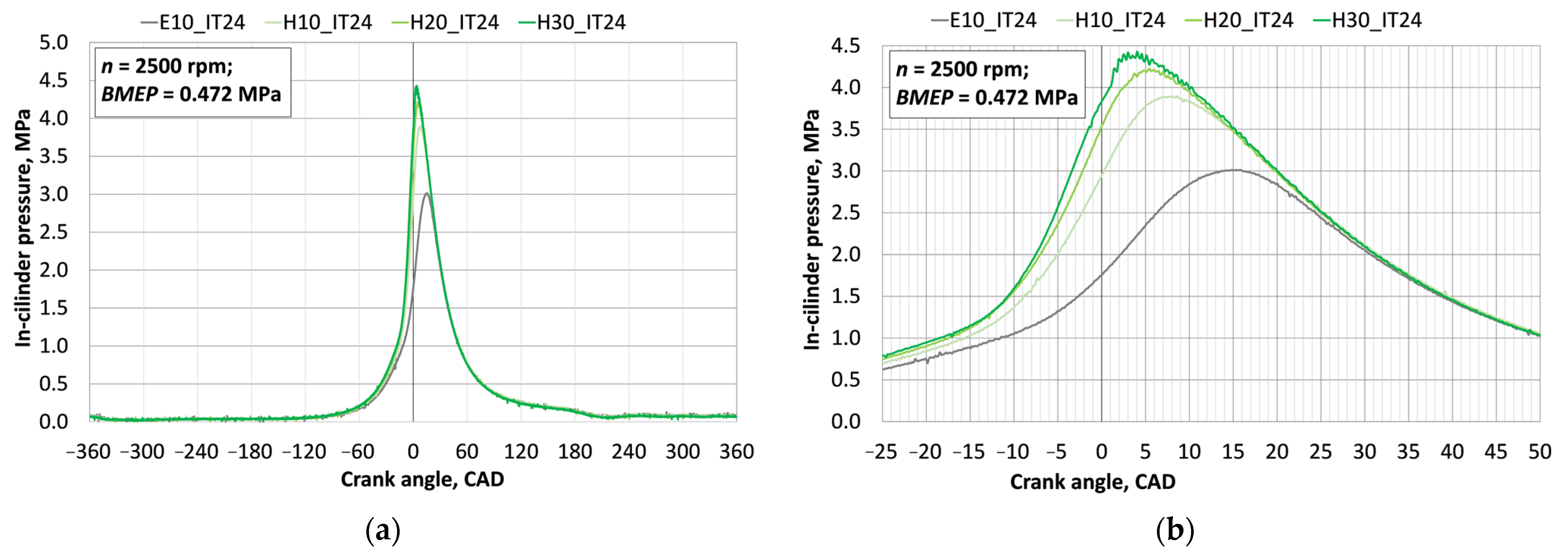
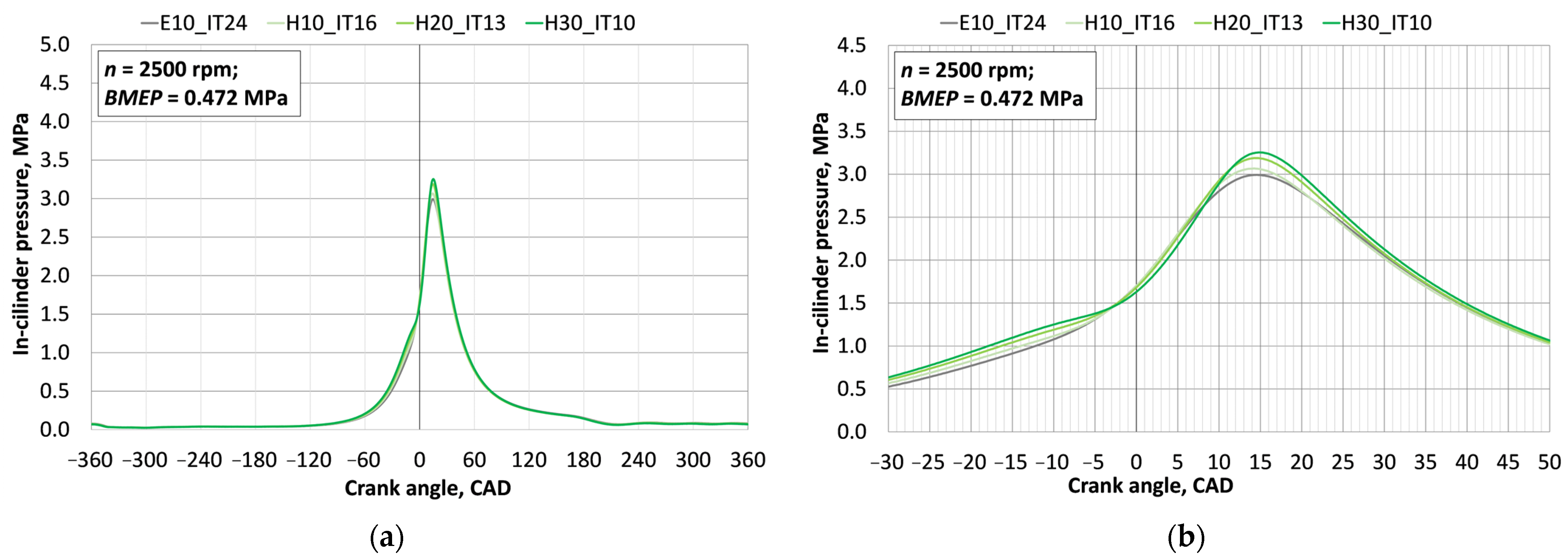
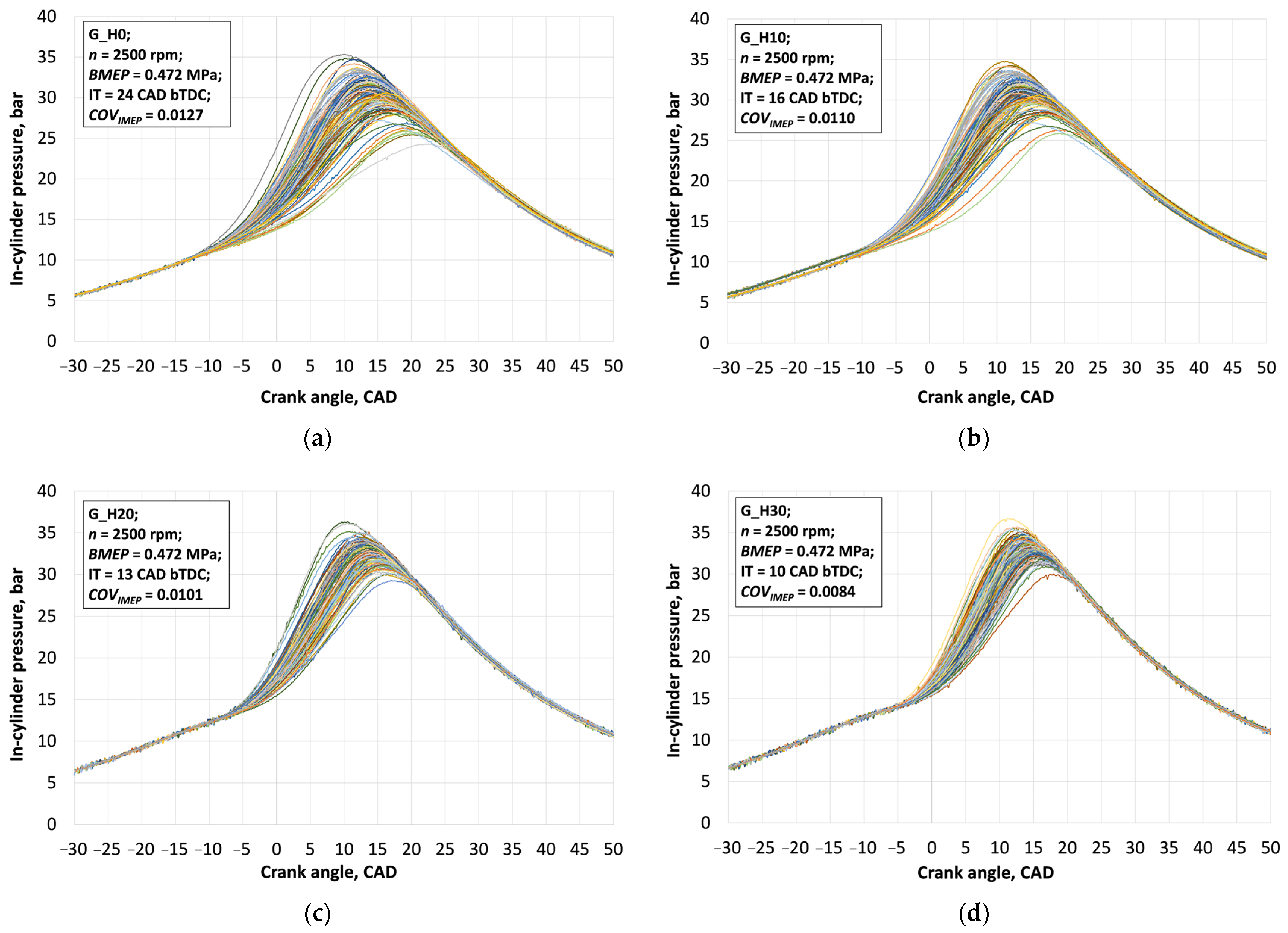
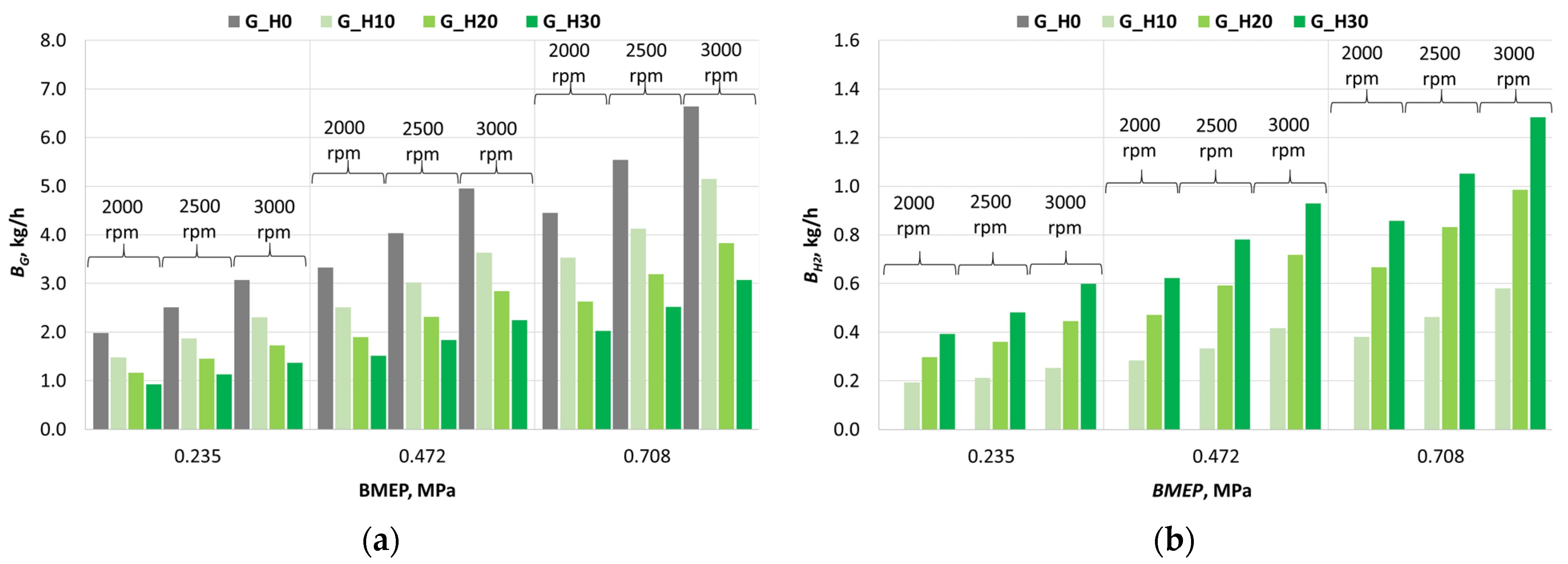
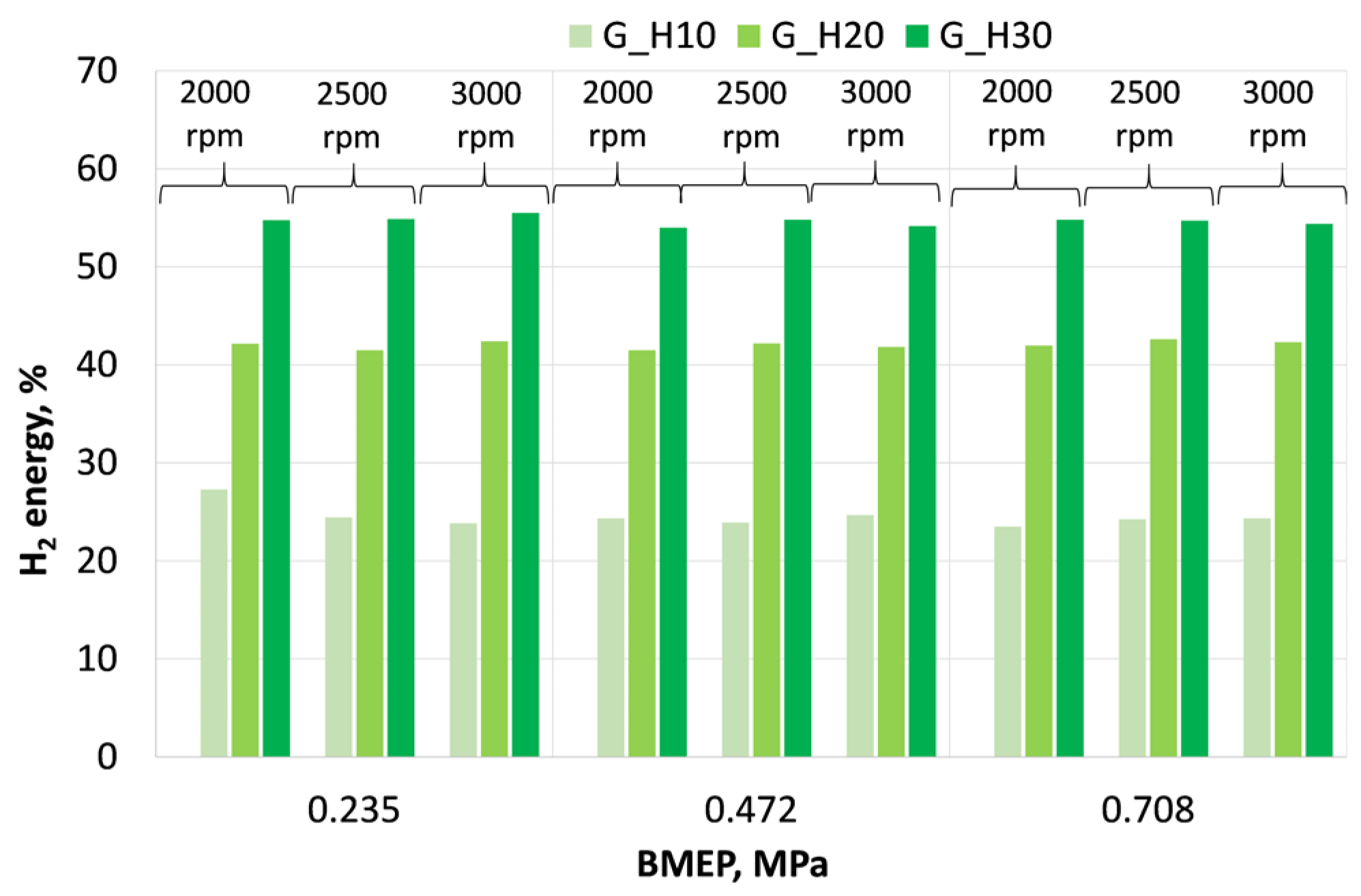
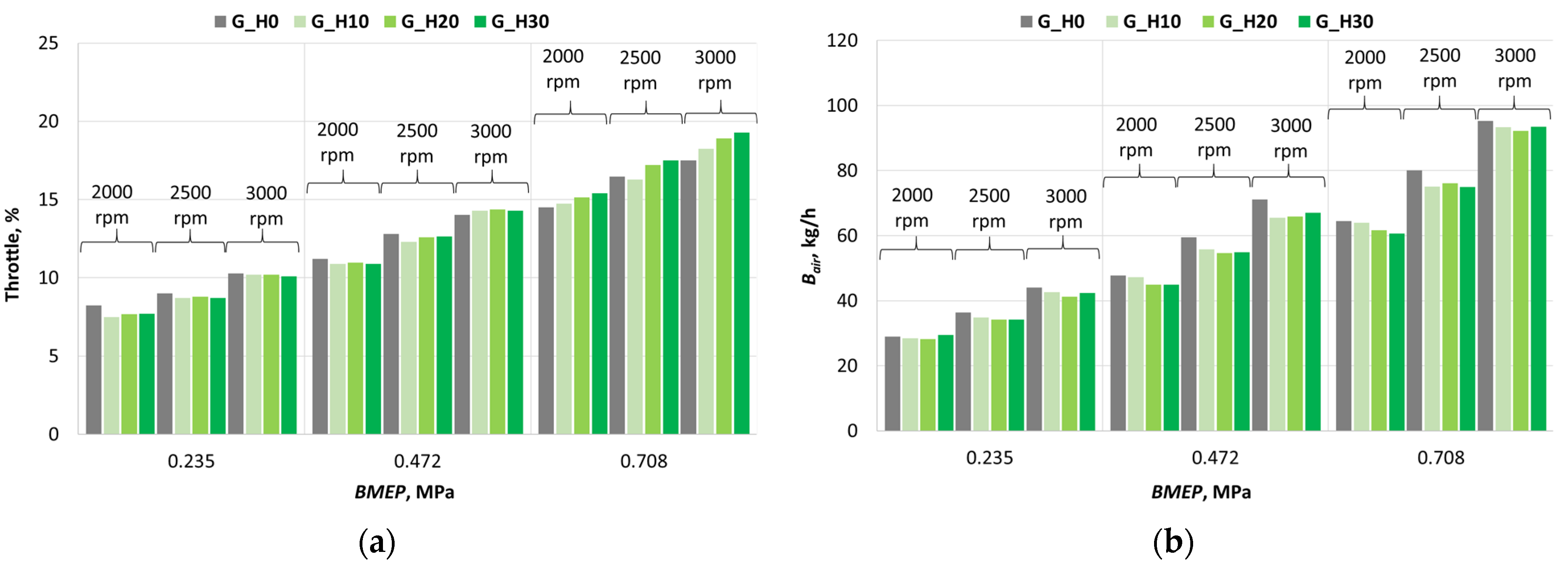
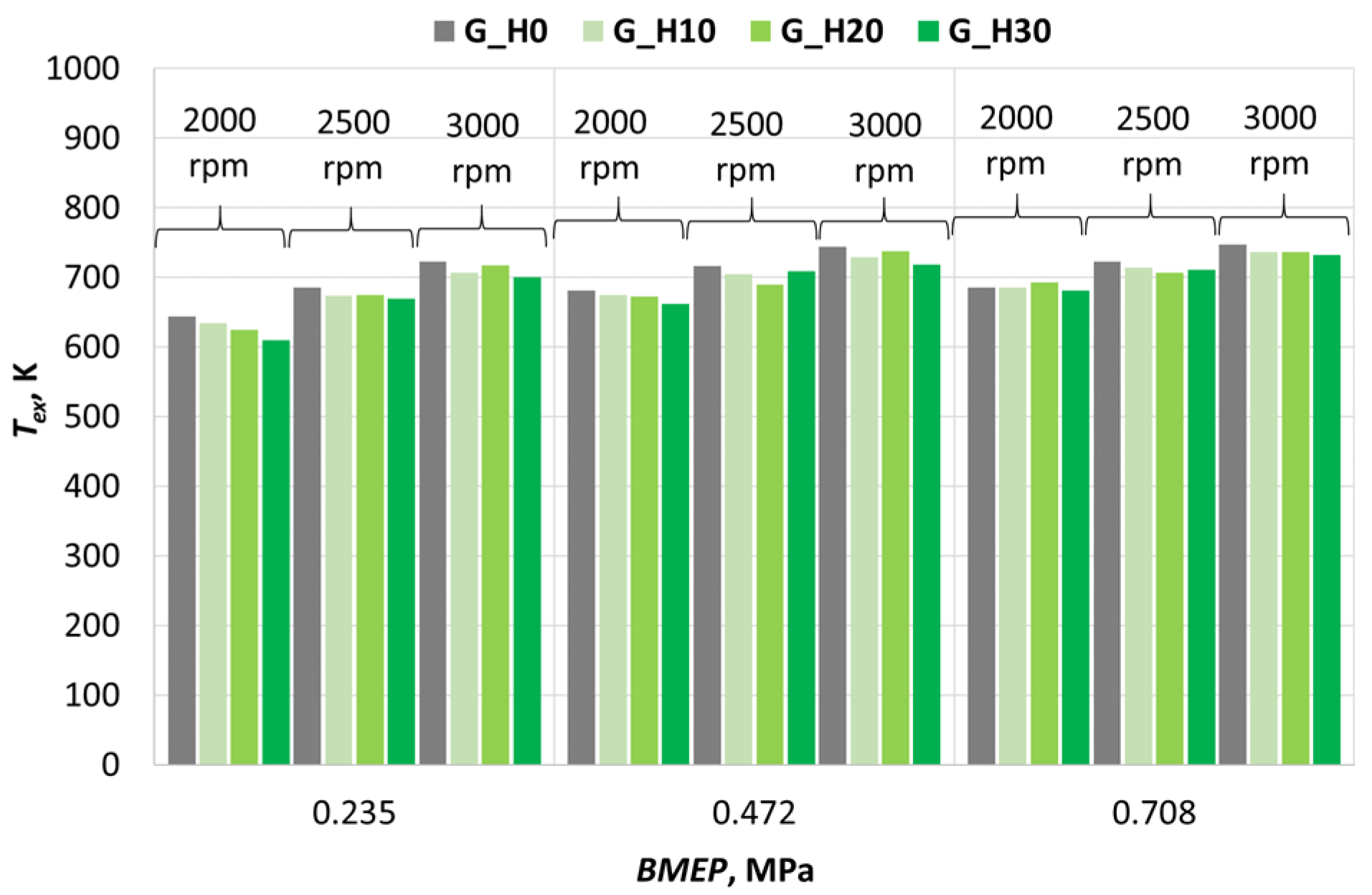
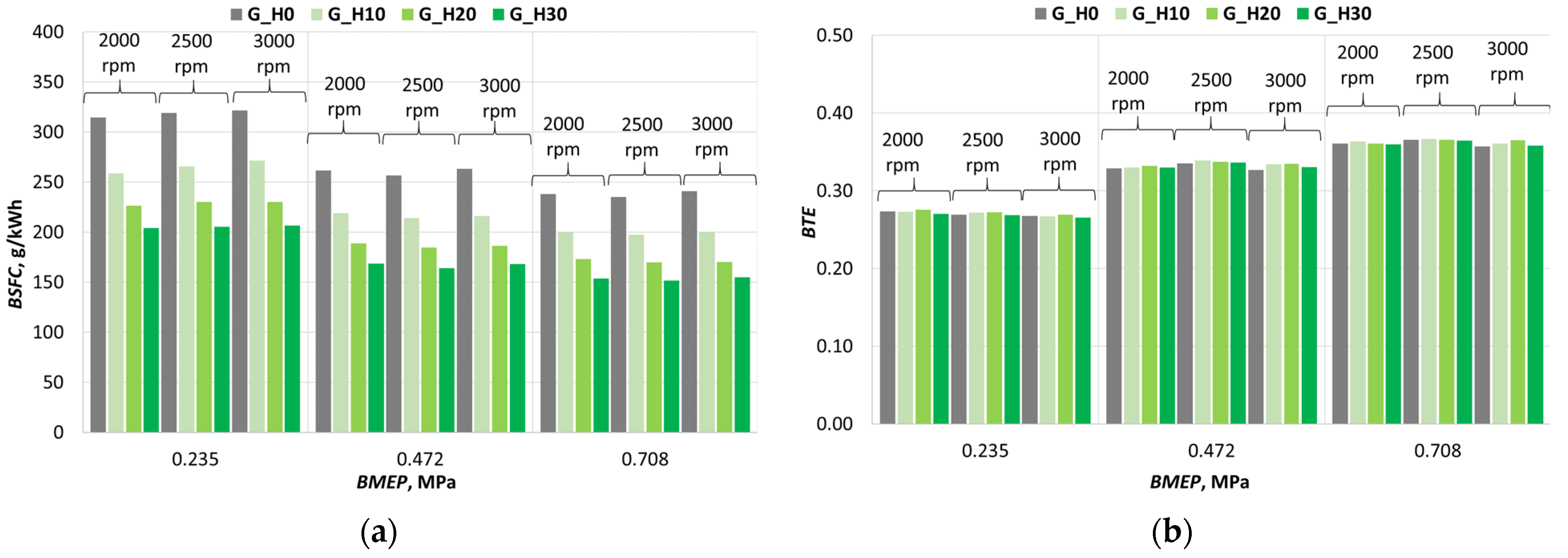
3.1. Investigation of Engine Combustion Behavior and Energy Performance Indicators
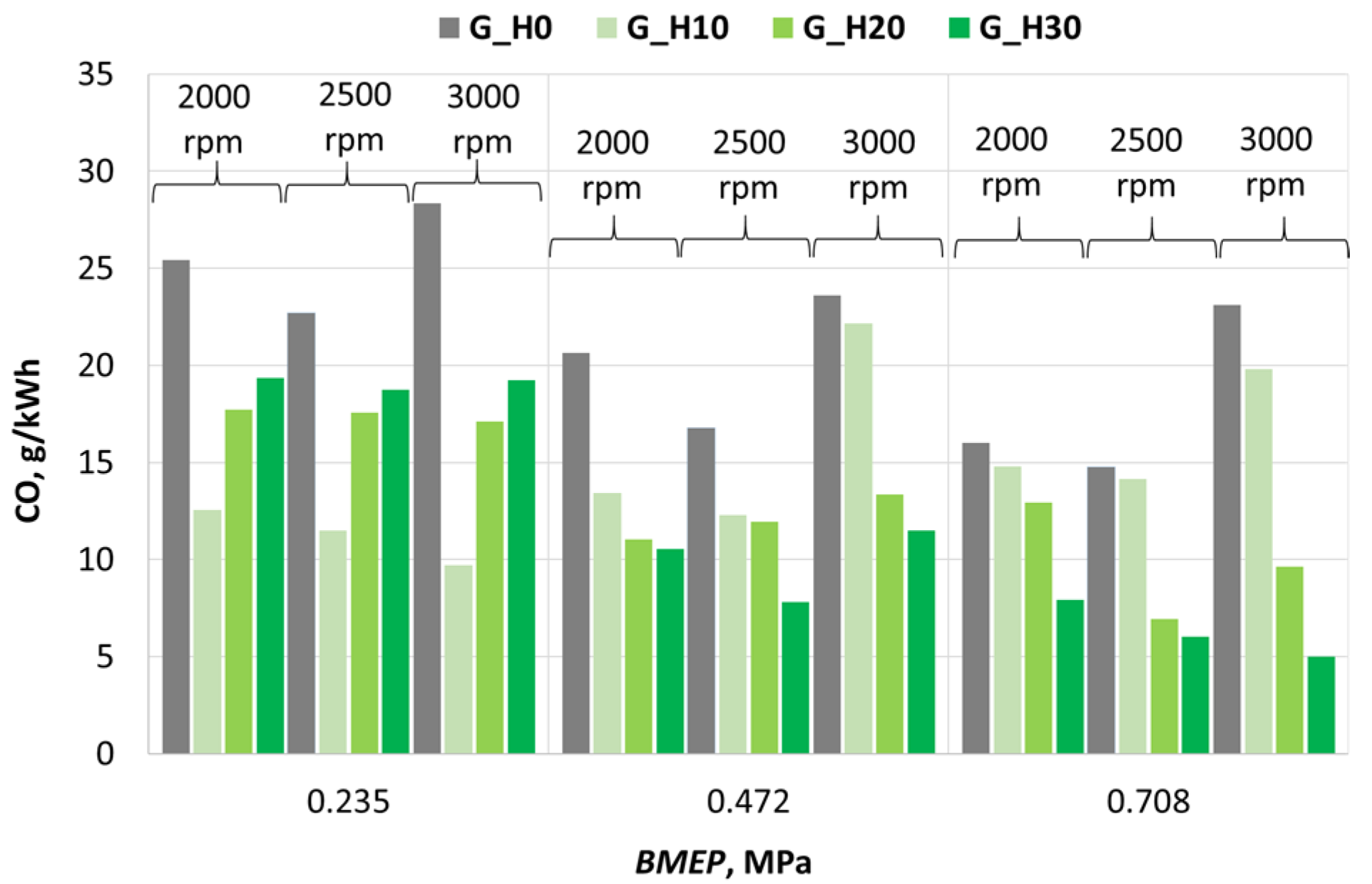
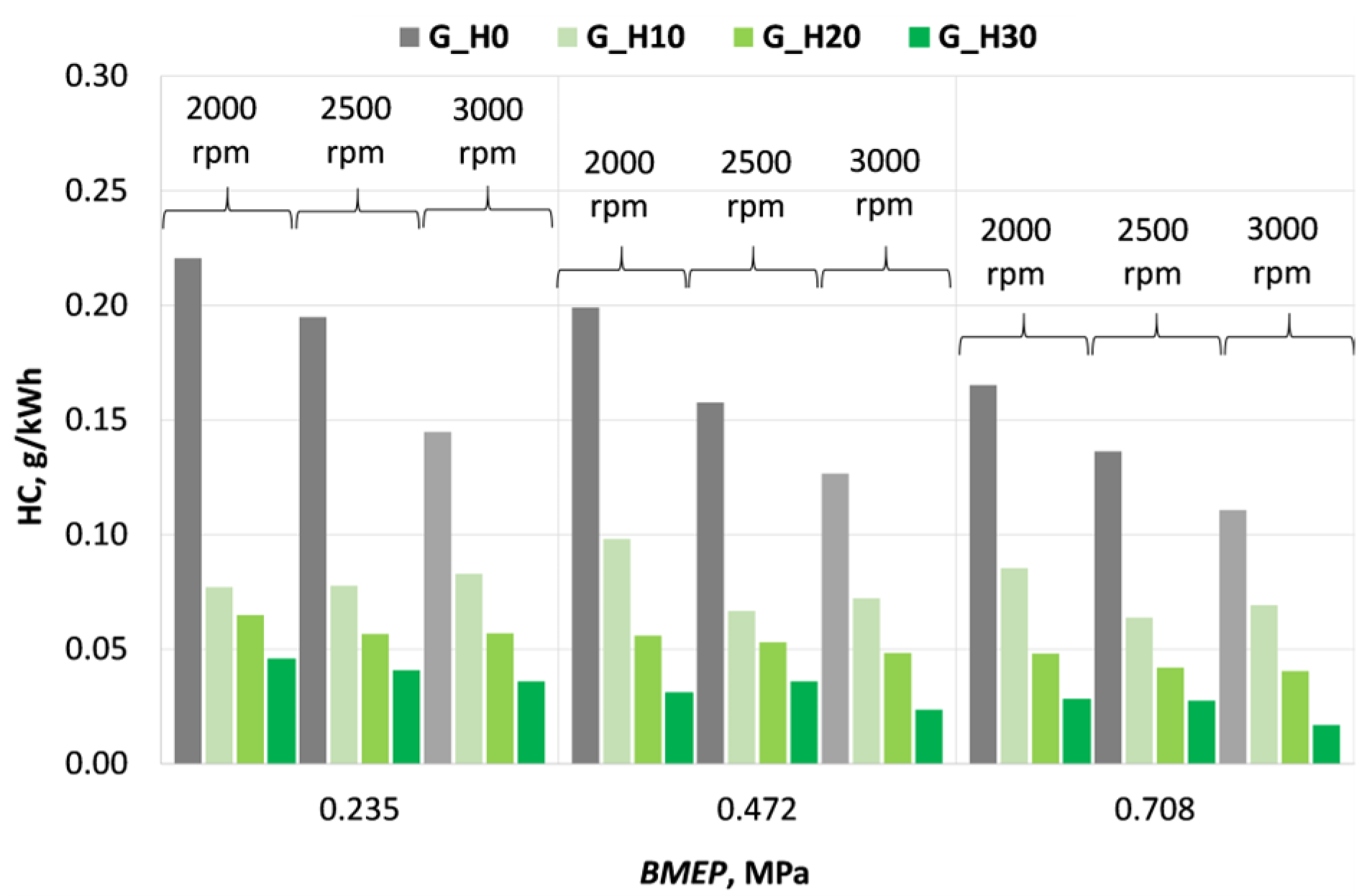
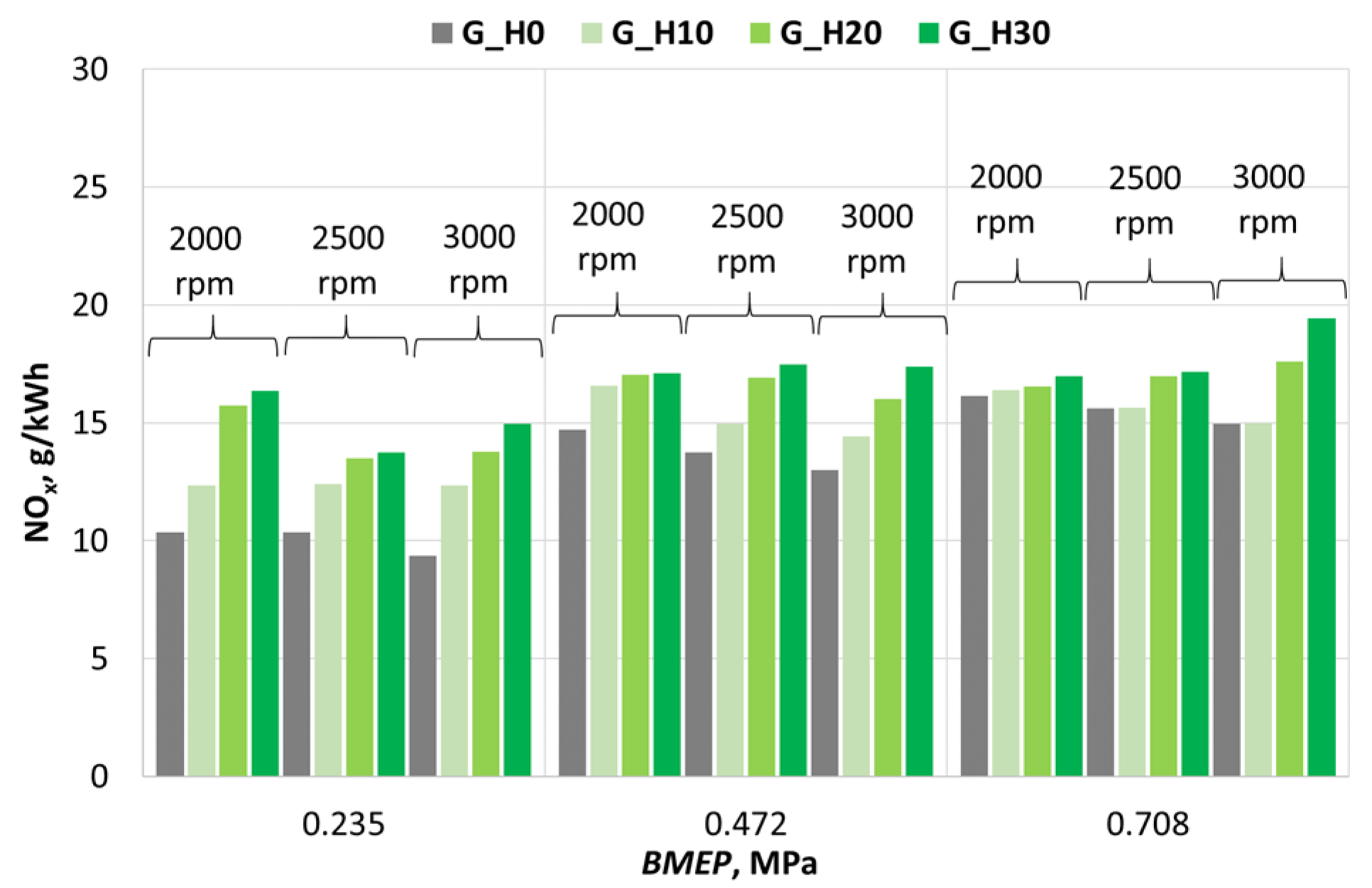
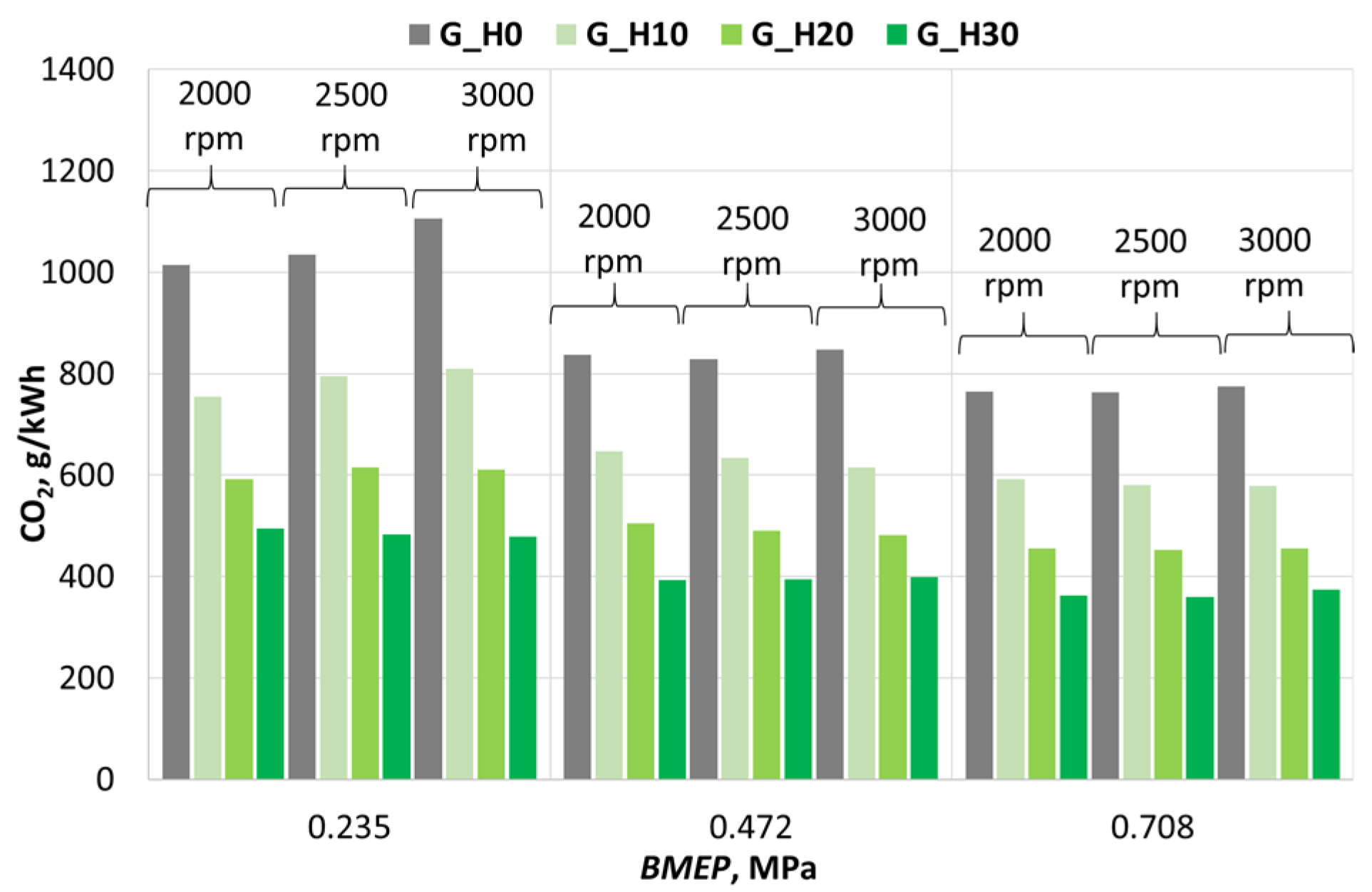
3.2. Investigation of Engine Environmental Performance Indicators
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A/F | Air/fuel mass ratio |
| aTDC | After top dead center |
| Bair | Intake air mass |
| Bf | Hourly fuel consumption |
| BG | Hourly gasoline consumption |
| BH2 | Hourly hydrogen consumption |
| BMEP | Brake mean effective pressure |
| BSFC | Brake specific fuel consumption |
| BTE | Brake thermal efficiency |
| bTDC | Before top dead center |
| CAD | Crank angle degrees |
| CI | Compression-ignition |
| COVIMEP | Coefficient of variation of indicated mean effective pressure |
| ECU | Engine control unit |
| EGR | Exhaust gas recirculation |
| FCEV | Fuel cell electric vehicle |
| H2 | Hydrogen |
| ICE | Internal combustion engine |
| IT | Ignition timing |
| LHV | Lower heating value |
| MPI | Multi-port injection |
| SI | Spark-ignition |
| Tex | Exhaust gas temperature |
| TDC | Top dead center |
References
- Singh, M.; Singla, M.K.; Beryozkina, S.; Gupta, J.; Safaraliev, M. Hydrogen Vehicles and Hydrogen as a Fuel for Vehicles: A-State-of-the-Art Review. Int. J. Hydrogen Energy 2024, 64, 1001–1010. [Google Scholar] [CrossRef]
- Fakhreddine, O.; Gharbia, Y.; Derakhshandeh, J.F.; Amer, A.M. Challenges and Solutions of Hydrogen Fuel Cells in Transportation Systems: A Review and Prospects. World Electr. Veh. J. 2023, 14, 156. [Google Scholar] [CrossRef]
- Cybulsky, A.; Allroggen, F.; Shao-Horn, Y.; Mallapragada, D.S. Decarbonization of Aviation via Hydrogen Propulsion: Technology Performance Targets and Energy System Impacts. arXiv 2023, arXiv:2309.14629. [Google Scholar]
- Pardhi, S.; Chakraborty, S.; Tran, D.-D.; El Baghdadi, M.; Wilkins, S.; Hegazy, O. A Review of Fuel Cell Powertrains for Long-Haul Heavy-Duty Vehicles: Technology, Hydrogen, Energy and Thermal Management Solutions. Energies 2022, 15, 9557. [Google Scholar] [CrossRef]
- Cheekatamarla, P. Hydrogen and the Global Energy Transition—Path to Sustainability and Adoption across All Economic Sectors. Energies 2024, 17, 807. [Google Scholar] [CrossRef]
- Ihsan Shahid, M.; Rao, A.; Farhan, M.; Liu, Y.; Ahmad Salam, H.; Chen, T.; Ma, F. Hydrogen Production Techniques and Use of Hydrogen in Internal Combustion Engine: A Comprehensive Review. Fuel 2024, 378, 132769. [Google Scholar] [CrossRef]
- Goyal, H.; Jones, P.; Bajwa, A.; Parsons, D.; Akehurst, S.; Davy, M.H.; Leach, F.C.; Esposito, S. Design Trends and Challenges in Hydrogen Direct Injection (H2DI) Internal Combustion Engines—A Review. Int. J. Hydrogen Energy 2024, 86, 1179–1194. [Google Scholar] [CrossRef]
- Turner, J.W.G. Future Technological Directions for Hydrogen Internal Combustion Engines in Transport Applications. Appl. Energy Combust. Sci. 2025, 21, 100302. [Google Scholar] [CrossRef]
- Kourougianni, F.; Arsalis, A.; Olympios, A.V.; Yiasoumas, G.; Konstantinou, C.; Papanastasiou, P.; Georghiou, G.E. A Comprehensive Review of Green Hydrogen Energy Systems. Renew. Energy 2024, 231, 120911. [Google Scholar] [CrossRef]
- Kharel, S.; Shabani, B. Hydrogen as a Long-Term Large-Scale Energy Storage Solution to Support Renewables. Energies 2018, 11, 2825. [Google Scholar] [CrossRef]
- Pizzuti, I.; Conti, M.; Delibra, G.; Corsini, A.; Castorrini, A. Energy Storage and Management of Offshore Wind-Based Green Hydrogen Production. Processes 2025, 13, 643. [Google Scholar] [CrossRef]
- Serrano-Arévalo, T.I.; Tovar-Facio, J.; Ponce-Ortega, J.M. Optimal Incorporation of Intermittent Renewable Energy Storage Units and Green Hydrogen Production in the Electrical Sector. Energies 2023, 16, 2609. [Google Scholar] [CrossRef]
- Anastasiadis, A.G.; Papadimitriou, P.; Vlachou, P.; Vokas, G.A. Management of Hybrid Wind and Photovoltaic System Electrolyzer for Green Hydrogen Production and Storage in the Presence of a Small Fleet of Hydrogen Vehicles—An Economic Assessment. Energies 2023, 16, 7990. [Google Scholar] [CrossRef]
- Zun, M.T.; McLellan, B.C. Cost Projection of Global Green Hydrogen Production Scenarios. Hydrogen 2023, 4, 932–960. [Google Scholar] [CrossRef]
- Mekonnin, A.S.; Wacławiak, K.; Humayun, M.; Zhang, S.; Ullah, H. Hydrogen Storage Technology, and Its Challenges: A Review. Catalysts 2025, 15, 260. [Google Scholar] [CrossRef]
- Hossain Bhuiyan, M.M.; Siddique, Z. Hydrogen as an Alternative Fuel: A Comprehensive Review of Challenges and Opportunities in Production, Storage, and Transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Maka, A.O.M.; Mehmood, M.; Chaudhary, T.N. Green Hydrogen Revolution and Its Pathway towards Sustainable Development. Clean Energy 2025, 9, 124–131. [Google Scholar] [CrossRef]
- Satyapal, S. U.S. DOE Hydrogen Program and National Clean Hydrogen Strategy. Available online: https://www.energy.gov/sites/default/files/2024-02/doe-h2-program-national-strategy-remarks-jan2024.pdf?utm_source=chatgpt.com (accessed on 1 May 2025).
- Department of Energy Announces $10.5 Million to Advance Hydrogen Combustion Engine Innovation. Available online: https://www.energy.gov/eere/fuelcells/articles/department-energy-announces-105-million-advance-hydrogen-combustion-engine (accessed on 1 May 2025).
- U.S. National Hydrogen Strategy and Roadmap|Hydrogen Program. Available online: https://www.hydrogen.energy.gov/library/roadmaps-vision/clean-hydrogen-strategy-roadmap?utm_source=chatgpt.com (accessed on 1 May 2025).
- Talus, K.; Martin, M. A Guide to Hydrogen Legislation in the USA: A Renewed Effort. J. World Energy Law Bus. 2022, 15, 449–461. [Google Scholar] [CrossRef]
- EU Hydrogen Strategy under the EU Green Deal|European Hydrogen Observatory. Available online: https://observatory.clean-hydrogen.europa.eu/eu-policy/eu-hydrogen-strategy-under-eu-green-deal (accessed on 1 May 2025).
- Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions. A Hydrogen Strategy for a Climate-Neutral Europe. 2020. Available online: https://energy.ec.europa.eu/system/files/2020-07/hydrogen_strategy_0.pdf (accessed on 5 May 2025).
- Optimisation of Hydrogen Powered Internal Combustion Engines (HYICE)|FP6. Available online: https://cordis.europa.eu/project/id/506604/reporting (accessed on 1 May 2025).
- Hydrogen Europe Position Paper. CO2 Emission Standards for Heavy-Duty Vehicles; Hydrogen Europe: Brussels, Belgium, 2023; p. 5. [Google Scholar]
- European Union. Regulation (EU) 2019/1242 of the European Parliament and of the Council of 20 June 2019 Setting CO2 Emission Performance Standards for New Heavy-Duty Vehicles and Amending Regulations (EC) No 595/2009 and (EU) 2018/956 of the European Parliament and of the Council and Council Directive 96/53/EC (Text with EEA Relevance); European Union: Brussels, Belgium, 2019; Volume 198. [Google Scholar]
- Kazmin, A. Combustion Engine Ban Threatens ‘Grave Crisis’ for Europe, Italy Says. Financial Times, 25 September 2024. [Google Scholar]
- Collins, P. Bosch to Launch H2 Engine for Trucks and Construction Vehicles; Hydrogen Europe: Brussels, Belgium, 2024. [Google Scholar]
- Collins, P. Ferrari Patents Hydrogen Internal-Combustion Engine; Hydrogen Europe: Brussels, Belgium, 2024. [Google Scholar]
- Gunung Capital. Hydrogen Fuel Cell Vehicles: Current Status and Future Growth; Gunung Capital: Singapore, 2023; Available online: https://gunungcapital.com/hydrogen-fuel-cell-vehicles-current-status-and-future-growth/ (accessed on 1 May 2025).
- Shin, J.-E. Hydrogen Technology Development and Policy Status by Value Chain in South Korea. Energies 2022, 15, 8983. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S. Economic Analysis on Hydrogen Pipeline Infrastructure Establishment Scenarios: Case Study of South Korea. Energies 2022, 15, 6824. [Google Scholar] [CrossRef]
- Cho, M.; Lee, Y.; Kim, Y.; Lee, M.C. Strategic Public Relations Policy for Accelerating Hydrogen Acceptance: Insights from an Expert Survey in South Korea. Energies 2024, 17, 4325. [Google Scholar] [CrossRef]
- Hydrogen Fuel Cell Vehicle Market Size|Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/hydrogen-fuel-cell-vehicle-market (accessed on 1 May 2025).
- Zhang, P.; Lu, B.; Qu, Y.; Ibrahim, H.; Ding, H. Efficiency Measurement and Trend Analysis of the Hydrogen Energy Industry Chain in China. Sustainability 2025, 17, 3140. [Google Scholar] [CrossRef]
- Liu, W.; Xue, T.; Adam, N.A.; Jero, A.; Yang, H. Hydrogen Economy in China: Integrating Biomass for Renewable Ernergy Transition and Economic Growth. Int. J. Hydrogen Energy 2025, 121, 171–188. [Google Scholar] [CrossRef]
- The Mainichi. Tokyo Bets Big on Hydrogen with Moves to Boost Commercial Fuel Cell Vehicles. Mainichi Daily News, 7 April 2024.
- Japan: Hydrogen Station Number by Region 2025. Available online: https://www.statista.com/statistics/1236606/japan-hydrogen-fuel-station-number-by-region/ (accessed on 1 May 2025).
- Yap, J.; McLellan, B. Evaluating the Attitudes of Japanese Society towards the Hydrogen Economy: A Comparative Study of Recent and Past Community Surveys. Int. J. Hydrogen Energy 2024, 54, 66–83. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Xu, N.; Li, W.; Yao, Y.; Li, P.; Yu, Q.; Wen, C.; Song, X.; Shibasaki, R.; et al. Roadmap to Hydrogen Society of Tokyo: Locating Priority of Hydrogen Facilities Based on Multiple Big Data Fusion. Appl. Energy 2022, 313, 118688. [Google Scholar] [CrossRef]
- Khan, U.; Yamamoto, T.; Sato, H. Understanding Attitudes of Hydrogen Fuel-Cell Vehicle Adopters in Japan. Int. J. Hydrogen Energy 2021, 46, 30698–30717. [Google Scholar] [CrossRef]
- Wrightbus to Build 1,000 Zero-Emission Buses. Available online: https://www.thetimes.com/business-money/companies/article/wrightbus-to-build-1000-zero-emission-buses-bffn35b60?utm_source=chatgpt.com®ion=global (accessed on 1 May 2025).
- Dimitrov, E.; Gigov, B.; Pantchev, S.; Michaylov, P.; Peychev, M. A Study of Hydrogen Fuel Impact on Compression Ignition Engine Performance. MATEC Web Conf. 2018, 234, 03001. [Google Scholar] [CrossRef]
- Ji, C.; Wang, S. Effect of Hydrogen Addition on Combustion and Emissions Performance of a Spark Ignition Gasoline Engine at Lean Conditions. Int. J. Hydrogen Energy 2009, 34, 7823–7834. [Google Scholar] [CrossRef]
- Aghahasani, M.; Gharehghani, A.; Mahmoudzadeh Andwari, A.; Mikulski, M.; Pesyridis, A.; Megaritis, T.; Könnö, J. Numerical Study on Hydrogen–Gasoline Dual-Fuel Spark Ignition Engine. Processes 2022, 10, 2249. [Google Scholar] [CrossRef]
- Georgescu, R.; Pană, C.; Negurescu, N.; Cernat, A.; Nuțu, C.; Panait, A. Analysis of the Combustion at the Use of Low Doses of Hydrogen in the Automotive Spark Ignition Engine. In CONAT 2024 International Congress of Automotive and Transport Engineering; Chiru, A., Covaciu, D., Eds.; Proceedings in Automotive Engineering; Springer Nature: Cham, Switzerland, 2025; pp. 15–27. ISBN 978-3-031-77626-7. [Google Scholar]
- Stępień, Z. A Comprehensive Overview of Hydrogen-Fueled Internal Combustion Engines: Achievements and Future Challenges. Energies 2021, 14, 6504. [Google Scholar] [CrossRef]
- Heywood, J. Internal Combustion Engine Fundamentals 2E, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 978-1-260-11610-6. [Google Scholar]
- Zhang, F.; Chen, G.; Wu, D.; Li, T.; Zhang, Z.; Wang, N. Characterising Premixed Ammonia and Hydrogen Combustion for a Novel Linear Joule Engine Generator. Int. J. Hydrogen Energy 2021, 46, 23075–23090. [Google Scholar] [CrossRef]
- Jarvis, D.J.; Adamkiewicz, G.; Heroux, M.-E.; Rapp, R.; Kelly, F.J. Nitrogen Dioxide. In WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-890-0213-4. [Google Scholar]
- White, C.; Steeper, R.; Lutz, A. The Hydrogen-Fueled Internal Combustion Engine: A Technical Review. Int. J. Hydrogen Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- Lewis, B.; von Elbe, G. Combustion, Flames, and Explosions of Gases, 3rd ed.; Academic Press: Orlando, FL, USA, 1987; ISBN 978-0-12-446751-4. [Google Scholar]
- Pukalskas, S.; Vipartas, T.; Rimkus, A.; Kriaučiūnas, D.; Žaglinskis, J.; Stravinskas, S.; Ušinskas, A.; Juknelevičius, R.; Mejeras, G.; Žuraulis, V.; et al. Numerical Modelling Assessment of the Impact of Hydrogen on the Energy and Environmental Performance of a Car Using Dual Fuel (Gasoline–Hydrogen). Appl. Sci. 2025, 15, 1939. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Jia, B.; Zhang, Z.; Roskilly, A. Numerical Investigation on NOx Emission of a Hydrogen-Fuelled Dual-Cylinder Free-Piston Engine. Appl. Sci. 2023, 13, 1410. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, S.; Zou, J.; Shreka, M. A Numerical Investigation on De-NOx Technology and Abnormal Combustion Control for a Hydrogen Engine with EGR System. Processes 2020, 8, 1178. [Google Scholar] [CrossRef]
- Falfari, S.; Cazzoli, G.; Mariani, V.; Bianchi, G. Hydrogen Application as a Fuel in Internal Combustion Engines. Energies 2023, 16, 2545. [Google Scholar] [CrossRef]
- Negurescu, N.; Pana, C.; Popa, M.G.; Soare, D. Aspects Regarding the Combustion of Hydrogen in Spark Ignition Engine; SAE Paper 2006-01-0651; SAE: Warrendale, PA, USA, 2006; pp. 51–64. [Google Scholar]
- Verhelst, S.; Sierens, R.; Verstraeten, S. A Critical Review of Experimental Research on Hydrogen Fueled SI Engines; SAE Technical Paper 2006-01-0430; SAE: Warrendale, PA, USA, 2006. [Google Scholar]
- Duan, X.; Xu, L.; Xu, L.; Jiang, P.; Gan, T.; Liu, H.; Ye, S.; Sun, Z. Performance Analysis and Comparison of the Spark Ignition Engine Fuelled with Industrial By-Product Hydrogen and Gasoline. J. Clean. Prod. 2023, 424, 138899. [Google Scholar] [CrossRef]
- Marwaha, A.; Subramanian, K.A. Performance Enhancement and Emissions Reduction of Ethanol-fueled Spark Ignition Engine with Hydrogen. Biofuels Bioprod. Biorefining 2024, 18, 701–719. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, C.; Xu, C.; Yu, Z. Experimental and Numerical Study on the Effect of Hydrogen Addition on Laminar Burning Velocity of Ethanol–Air Mixtures. Energies 2022, 15, 3114. [Google Scholar] [CrossRef]
- 2007 Nissan Qashqai I (J10) 1.6 (114 Hp)|Technical Specs, Data, Fuel Consumption, Dimensions. Available online: https://www.auto-data.net/en/nissan-qashqai-i-j10-1.6-114hp-730 (accessed on 22 May 2025).
- Purayil, S.T.P.; Martini, E.A.; Elsaid, A.; Khalil, M.; Zoghbour, T.; Seyam, M.; Elnajjar, E. Influence of Steam Induction on the Performance and Hydrogen Knock Limit of a Hydrogen-Gasoline Spark Ignition Engine. Int. J. Thermofluids 2024, 24, 100933. [Google Scholar] [CrossRef]
- Shivaprasad, K.; Rajesh, R.; Anteneh Wogasso, W.; Nigatu, B.; Addisu, F. Usage of Hydrogen as a Fuel in Spark Ignition Engine. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012037. [Google Scholar] [CrossRef]
- Pressure Sensor for Combustion Analysis. Data Sheet. Available online: https://www.avl.com/documents/10138/885983/AT3377E_ZI31.pdf (accessed on 22 May 2025).
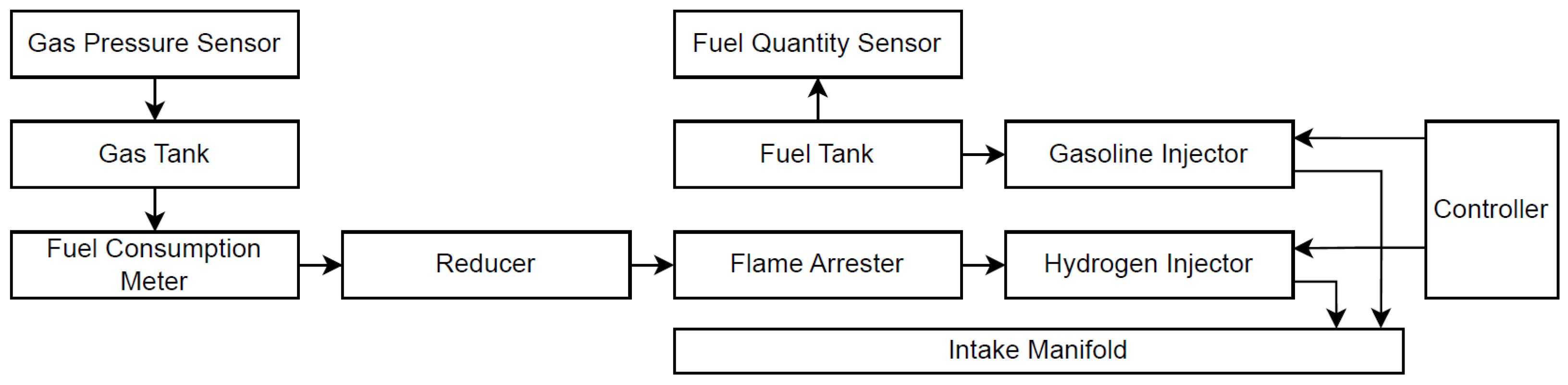
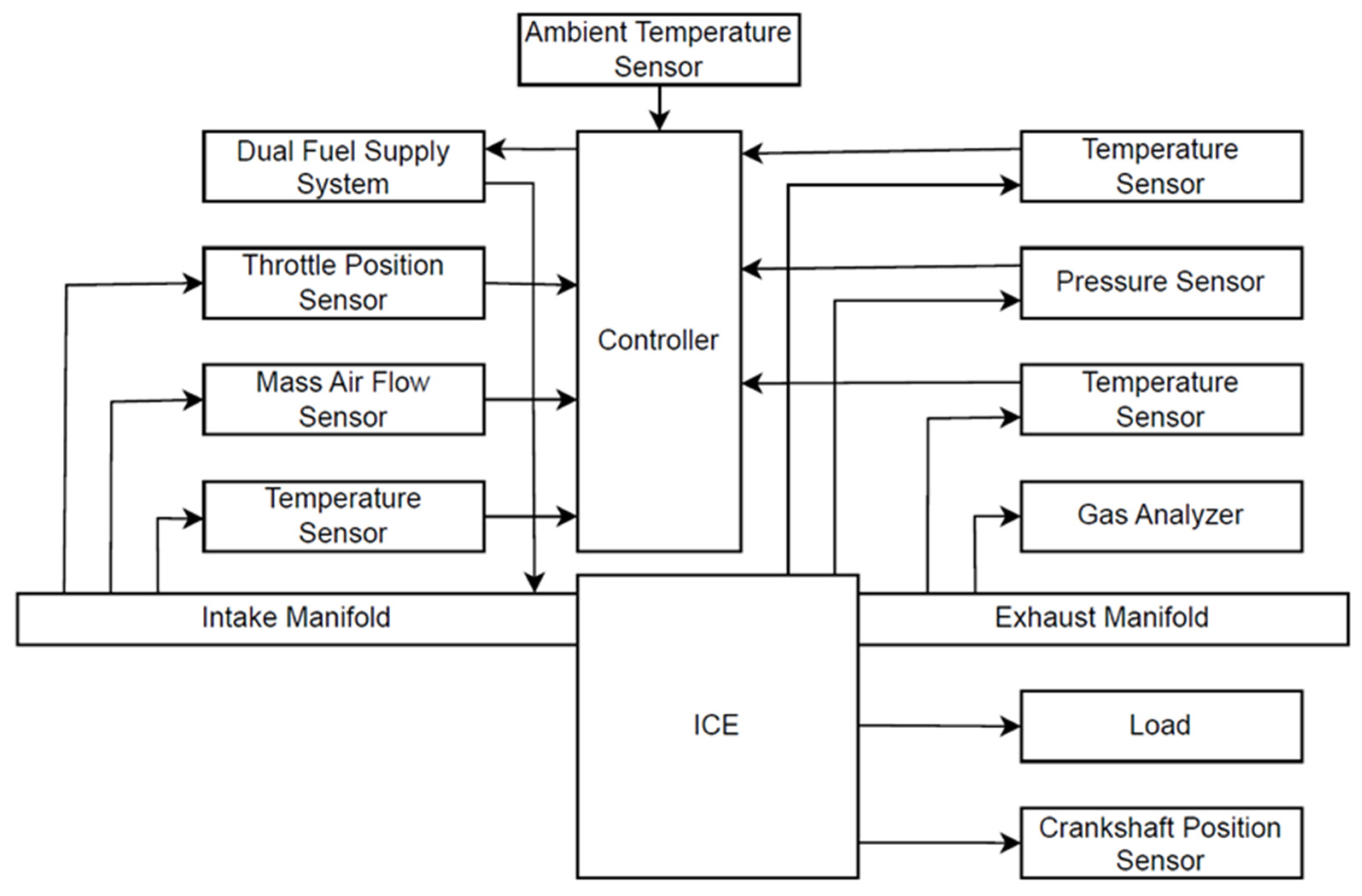
| Parameter | Value |
|---|---|
| Displacement, cm3/in3 | 1598/97.4 |
| Bore, mm/in | 78/3.07 |
| Stroke, mm/in | 83.6/3.29 |
| Compression ratio | 10.7:1 |
| Cylinder alignment | In-line 4 |
| Power, hp (at rpm) | 114 (6000) |
| Torque, Nm (at rpm) | 156 (4400) |
| Charge system | naturally aspirated |
| Valves per cylinder | 4 |
| Property | Gasoline | Hydrogen |
|---|---|---|
| Chemical formula | CnH2n+2 (C4–C12) | H2 |
| Carbon concentration in fuels, C (Wt%) | 82.81 | - |
| Hydrogen concentration in fuels, H (Wt%) | 13.66 | 100 |
| Oxygen concentration in fuels, O (Wt%) | 3.53 | - |
| C/H ratio | 6.06 | 0 |
| Flammability limits (excess air) | 0.7–4 | 0.1–7.1 |
| Density (20 °C), kg/m3 | 752.4 | 0.09 |
| Minimum ignition energy (mJ) | 0.25 | 0.02 |
| Octane number | 95 | 130 |
| Stoichiometric air to fuel ratio (A/F), kg air/1 kg fuel | 14.2 | 34.78 |
| Lower heating value (mass) (LHV_m), MJ/kg | 41.88 | 120 |
| Flame velocity (m/s) | 0.3–0.5 | 2.65–3.25 |
| Marking | Gasoline, Mass% | H2, Mass% | LHV, MJ/kg | A/F Ratio, kg air/1 kg Fuel | C/H Ratio | Ignition Timing, CAD BTDC 1 |
|---|---|---|---|---|---|---|
| G_H0_IT24 | 100 | 0 | 41.88 | 14.20 | 6.06 | 24 |
| G_H10_IT24 | 90 | 10 | 49. 69 | 16.26 | 3.34 | 24 |
| G_H20_IT24 | 80 | 20 | 57.50 | 18.32 | 2.14 | 24 |
| G_H30_IT24 | 70 | 30 | 65.31 | 20.37 | 1.47 | 24 |
| G_H0_IT24 | 100 | 0 | 41.88 | 14.20 | 6.06 | 24 |
| G_H10_IT16 | 90 | 10 | 49.69 | 16.26 | 3.34 | 16 |
| G_H20_IT13 | 80 | 20 | 57.50 | 18.32 | 2.14 | 13 |
| G_H30_IT10 | 70 | 30 | 65.31 | 20.37 | 1.47 | 10 |
| Parameter | Value |
|---|---|
| Sensor type | Piezoelectric pressure sensor |
| Lifetime, load cycles | |
| Measurement range, bar | 0–200 |
| Overload, bar | 250 |
| Sensitivity, pC/bar nominal | 12 |
| Linearity, % | ≤±0.5 |
| Natural frequency, kHz | ∼130 |
| Acceleration sensitivity, bar/g | ≤0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pukalskas, S.; Rimkus, A.; Vipartas, T.; Stravinskas, S.; Kriaučiūnas, D.; Mejeras, G.; Ušinskas, A. Hydrogen Supplementation in SI Engines: Enhancing Efficiency and Reducing Emissions with a Focus on Knock Phenomena. Machines 2025, 13, 571. https://doi.org/10.3390/machines13070571
Pukalskas S, Rimkus A, Vipartas T, Stravinskas S, Kriaučiūnas D, Mejeras G, Ušinskas A. Hydrogen Supplementation in SI Engines: Enhancing Efficiency and Reducing Emissions with a Focus on Knock Phenomena. Machines. 2025; 13(7):571. https://doi.org/10.3390/machines13070571
Chicago/Turabian StylePukalskas, Saugirdas, Alfredas Rimkus, Tadas Vipartas, Saulius Stravinskas, Donatas Kriaučiūnas, Gabrielius Mejeras, and Andrius Ušinskas. 2025. "Hydrogen Supplementation in SI Engines: Enhancing Efficiency and Reducing Emissions with a Focus on Knock Phenomena" Machines 13, no. 7: 571. https://doi.org/10.3390/machines13070571
APA StylePukalskas, S., Rimkus, A., Vipartas, T., Stravinskas, S., Kriaučiūnas, D., Mejeras, G., & Ušinskas, A. (2025). Hydrogen Supplementation in SI Engines: Enhancing Efficiency and Reducing Emissions with a Focus on Knock Phenomena. Machines, 13(7), 571. https://doi.org/10.3390/machines13070571







