Abstract
Seawater has been considered as an alternative to freshwater for flotation. However, many ions in seawater were reported to depress molybdenite (MoS2), with the depressing mechanisms being insufficiently understood. In this study, the influence of divalent ions (e.g., Ca2+ and Mg2+) and dispersant on MoS2 flotation was systematically investigated. It was found that the detrimental effects of Ca2+ and Mg2+ on the natural flotability of MoS2 were mainly due to the attachment of formed CaMoO4 precipitates and Mg(OH)2 colloids onto MoS2 surface. However, the addition of sodium hexametaphosphate (SHMP) reduced the negative effects. Various measurements, including contact angle, zeta potential, fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS) and atomic force microscope (AFM), were conducted to understand the influencing mechanisms of divalent ions and the beneficial effects of SHMP on MoS2 flotation. In addition, the Extended Derjguin–Landau–Verwey–Overbeek (EDLVO) theory was applied to investigate the total interaction energy between MoS2 particles and formed colloids, revealing that the reduced attraction force between MoS2 and Mg(OH)2 colloids in the presence of SHMP primarily resulted in the increased MoS2 recovery. In addition, SHMP combined with Mg2+ and Ca2+ to form dissolvable complexes, thereby reducing insoluble Mg2+ and Ca2+ compounds or precipitation. Thus, this study demonstrated for the first time two influencing mechanisms of SHMP in improving MoS2 recovery in the presence of Ca2+ and Mg2+.
1. Introduction
Molybdenite (MoS2) is the most important molybdenum (Mo) containing mineral source with a sandwich-like S–Mo–S hexagonal layer structure. The adjacent S–S sheets are bonded to each other by van der Waals forces while the individual Mo–S is covalently bonded [1,2,3]. MoS2 presents two types of surfaces, namely hydrophobic faces and hydrophilic edges, which are formed by the rupture of weak S–S molecular bonds and strong covalent Mo–S bonds at different crystal faces, respectively, resulting in an anisotropic surface property [3,4,5]. For instance, López-Valdivieso et al. [6] found a heterogeneous face consisting of many micro-edges on MoS2 particle surface via atomic force microscopy (AFM). In addition, the face/edge aspect influences MoS2 flotation significantly [3,7], e.g., high face/edge aspect ratio indicates a high recovery while low face/edge aspect ratio normally results in a low recovery. In addition, the MoS2 particle is normally deformed during grinding, resulting in a high exposure of edges and micro-edges on the MoS2 surface [5].
MoS2 is usually recovered with copper minerals from Cu-Mo ores [8,9], followed by selective flotation of MoS2 from Cu-Mo concentrate [10]. However, a massive amount of freshwater is consumed in flotation every year while the continuous growth of population and industrial development decrease the overall water quality, resulting in the scarcity of high quality freshwater [11] and a series of economic and environmental problems [12].
Therefore, some flotation plants use saline water including seawater, showing a promising way to relieve freshwater shortages [11], especially for sulfide mineral flotation [13,14]. For instance, Las Luces in Chile utilizes seawater and tailing dam water to grind and float Cu-Mo sulfides [15] while the Mt Keith plant in Australia utilizes bore water to process nickel minerals [16].
However, seawater usually contains many inorganic ions (e.g., K+, Na+, Ca+, Mg+, Cl−, and SO42−) which can change the frothing properties (e.g., froth stability and bubble coalescence) of the pulp as well as the surface properties (e.g., hydrophobicity and electrostatic force) of the minerals particles, further influencing mineral flotability [11,13,17,18]. Laskowski et al. [18] found a reduced mineral recovery in aqueous solutions containing primary ions (e.g., Na+, Cl−, Mg2+, Ca2+, and SO42−) in seawater. Some monovalent salts (e.g., NaCl and KCl) were found to compress electrical double layers, reducing the energy barrier for particle-bubble attachment, further enhancing mineral flotability. For instance, Lucay et al. [10] reported that NaCl decreased the electrostatic repulsion between bubbles and the molybdenite particles, enhancing its flotation kinetics and recovery. In addition, increased hydrophobicity of some minerals treated in a saline environment was found by Troncoso [19]. Moreover, Liu et al. [12] and Ramos et al. [20] reported that salt ions at high concentrations inhibited bubble coalescence and stabilized the froth layer, further increasing mineral recovery. Furthermore, increased electrolyte concentration reduced the bubble size and increased the bubble rise velocity, providing adequate frothing ability, similar to the role of frother [12]. Therefore, the flotation process in some concentrators has been carried out without frothers when using seawater. For instance, Raglan concentrator in Northern Quebec, Canada, utilizes saline water with a salt concentration ranging from 20,000 to 35,000 ppm in the absence of frother [21]. However, the presence of some divalent cations such as Ca2+ and Mg2+ was reported to have a negative effect on mineral flotation due to the adsorption of metal hydroxyl-complexes and colloidal precipitates onto mineral surfaces, reducing hydrophobicity [22,23,24,25].
Many investigators indicated that seawater played a negative role on MoS2 flotation under alkaline conditions [20,26,27,28,29], mainly due to the formation of divalent metallic complexes and colloidal precipitates on mineral surfaces, reducing hydrophobicity and the adsorption of collectors. Therefore, it is necessary to investigate the effect of divalent cations in seawater on MoS2 flotation. However, limited work has been attempted to investigate the effective methods to relieve MoS2 depression using seawater. For instance, Suyantara et al. [28] and Hirajima et al. [22] reported that the addition of emulsified kerosene in the flotation process hindered the adsorption of hydrophilic Mg(OH)2 precipitates on the MoS2 surface. Jeldres et al. [30] showed an improved MoS2 recovery when using CaO-Na2CO3 to remove divalent cations before flotation. Although these studies investigated the ways to increase mineral recovery, the mechanisms were not clear.
SHMP commonly plays a strong dispersing role in the flotation process [31,32,33,34]. For instance, Li et al. [34] reported that the adsorption of SHMP on serpentine prevented the aggregation between serpentine and ascharite. Xu et al. [32] found that SHMP adhered on a particle surface had a dispersing effect which increased the electrostatic repulsion between valuable minerals and gangues. Some published studies showed an ability of SHMP to dissolve metallic ions from mineral surface into solution by complexation [35,36,37,38]. However, the influencing mechanisms of SHMP on MoS2 flotation when using seawater as the flotation media were not sufficiently investigated.
In this work, the roles of SHMP in improving MoS2 recovery in the presence of divalent ions (i.e., Ca2+ and Mg2+) were investigated. The influencing mechanisms were systematically studied by various measurements such as contact angle, zeta potential, XPS and AFM. Furthermore, the extended Derjaguin–Landau–Verwey–Overbeek (EDLVO) theory model was applied to examine the interaction force between MoS2 and colloids formed during the flotation process.
2. Materials and Methods
2.1. Minerals and Reagents
MoS2 was obtained from Guilin, Guangxi province, China. The original sample chunk was crushed, ground in a three head grinding machine (RK/XPM, Wuhan Rock Grinding Equipment Manufacturing Co., Ltd., Wuhan, China) and wet sieved using filter sieve. The obtained powders were thoroughly washed using ethanol to remove fines and the surface filmy oxide layer was removed via sonication. After drying at 30 °C in a DZ-2BC ІІ-type vacuum oven (Tianjin Tester instrument Co., Ltd., Tianjin, China) for 24 h, the obtained particles were sealed in plastic tubes and transferred into a freezer prior to use. The X-ray diffraction (XRD) analysis shown in Figure 1 indicated that the majority of this sample was well-crystallized MoS2.

Figure 1.
X-ray Diffraction pattern (XRD) of MoS2.
The elemental composition of the MoS2 sample is given in Table 1, indicating a high Mo concentration with a small portion of impurities, consistent with the XRD results.

Table 1.
Chemical composition of MoS2 sample.
In addition, Figure 2 shows the cumulative size distribution of the MoS2 sample used for flotation experiment, indicating that more than 90% of the particles were within 38–75 μm as the d10, d50 and d90 (which refer to the particle size of MoS2 sample when its cumulative size distribution reaches 10%, 50%, 90%, respectively) were 48, 66 and 74 μm, respectively.
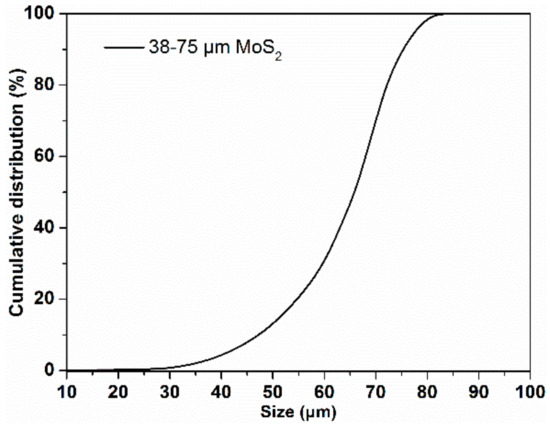
Figure 2.
Cumulative size distribution of MoS2.
Reagents including SHMP, anhydrous calcium chloride (CaCl2) and magnesium chloride hexahydrate (MgCl2·6H2O) from China Sinaopharm Chemical Reagent Co., Ltd. were analytical grade and used as supplied. Sodium hydroxide (NaOH) and hydrochloric acid (HCl) supplied by China Sinaopharm Chemical Reagent Co., Ltd. (Shanghai, China) were used as pH regulators. All experimental solutions were prepared using Millipore® ultrapure water (Billerica, MA, USA) with a resistivity of 18.2 MΩ·cm.
2.2. Flotation
MoS2 flotation tests were performed in a mechanical agitation XFG ІІ-type flotation machine made by Wuhan Exploration Machinery Factory (Wuhan, China). First, 0.25 g MoS2 (38–75 μm) and 25 mL conditioned solution were added into the 40 mL flotation cell, followed by maintaining pulp pH at a desired value during the first 6 min with an agitation speed of 1200 rpm. The geometry of the flotation cell is shown in Figure 3. Thereafter, froth collection was consecutively carried out every 10 s with an air flow rate of 0.1 L/min. Both froth concentrates and tailing were filtered and air dried at 70 °C prior to weighing. The concentrations of Ca2+ (0.01 M) and Mg2+ (0.05 M) selected in this study were the same as those contained in seawater [23], thereby providing evidence to understand the primary ions playing the most significant inhibition role. The recovery shown in the Figure 4, Figure 5 and Figure 6 was the average value of three repeated experiments, with the error bar being as one standard deviation.
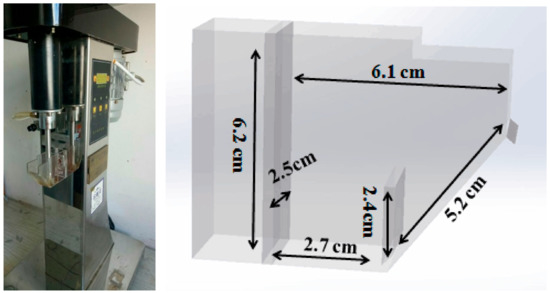
Figure 3.
Geometry of flotation cell.
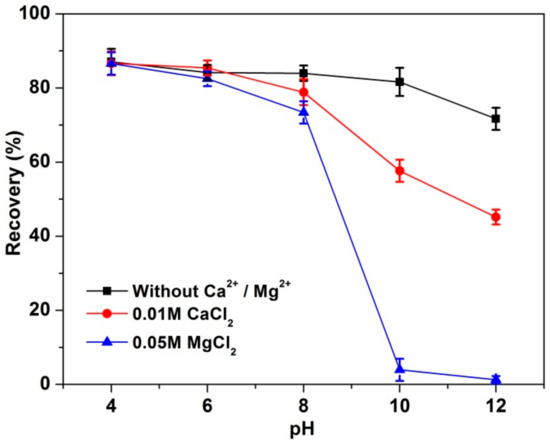
Figure 4.
MoS2 recovery at various pH values.
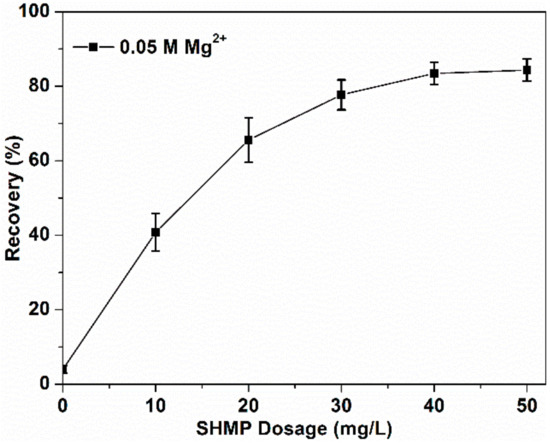
Figure 5.
MoS2 recovery as a function of SHMP dosage at pH 10.
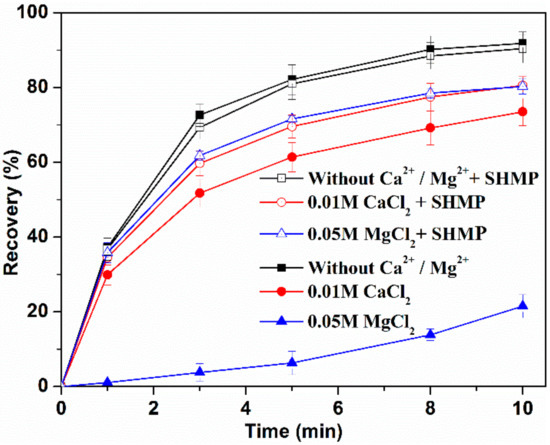
Figure 6.
MoS2 recovery at pH 10.
2.3. Contact Angle Measurements
A JC2000C type measuring device (Shanghai Zhongchen Digital Technology Company, Shanghai, China) was used to measure the contact angle of MoS2 treated under different conditions. The freshly cleaved surface was obtained by peeling off the top layers of the MoS2 sample using adhesive tape and then conditioning in the testing solution for 10 min. After rinsing three times using ultrapure water and air drying, a 0.25 µL drop of ultrapure water was placed onto the sample surface through a microliter syringe. Thereafter, the contact angle was obtained by analyzing the drop profile. The average value of three different measurements was presented herein as the final contact angle.
2.4. Zeta Potential Measurements
Zeta potentials of MoS2 were determined in different aqueous solutions using a Nano-ZS90 zeta potential analyzer (Malvern Co., Ltd., Malvern, UK). The MoS2 with a particle size of −38 µm was further ball ground to less than 5 µm for zeta potential measurements. Then, 0.05 g of finely ground sample was poured into 50 mL test solution and conditioned by magnetic stirring for 10 min so that the suspension was homogenized. The pulp pH was adjusted to a desired value using NaOH or HCl. Subsequently, the agitated suspension was transferred into a sample cell and then tested. Each experiment was repeated at least thrice with a typical variation of ±5 mV and the average was reported as the result presented herein. The zeta potential measurement of Mg(OH)2 precipitate formed in the 0.05 M MgCl2 solution was determined by a similar same way as that for MoS2 particles, i.e., the 0.05 M MgCl2 solution was firstly adjusted for 10 min to pH 10 to allow for precipitation. The solution with precipitation was then stirred to homogeneity and transferred into a sample cell for zeta potential tests.
2.5. XPS Measurements
First, 0.25 g of 38–75 μm MoS2 samples was placed into 25 mL solution containing 0.01 M CaCl2 or 0.05 M MgCl2 with or without 50 mg/L SHMP, followed by pH adjustment and magnetic stirring for 30 min. After that, the sample was filtered and freeze-dried for XPS analysis using Thermo Fisher ESCALAB 250Xi spectrometer (Waltham, MA, USA) with an Al Kα monochromatic X-ray source. All wide survey spectra were collected from 1350 to 0 eV with a pass energy of 100 eV and a step size of 1.0 eV while the high resolution XPS spectra for each element were collected with a pass energy of 30 eV and a step size of 0.1 eV. Both survey and high resolution spectra had a dwell time of 0.1 s and 5 sweeps. The XPS Peak 4.1 software was used for data analysis. The charge compensation for all spectra was calibrated based on the C 1s binding energy at 284.8 eV.
2.6. AFM Measurements
A MultiMode 8 atomic force microscope (AFM, Bruker, Santa Barbara, CA, USA) with tapping mode in air was applied to investigate the morphology (256 × 256 pixel resolution) of MoS2 surfaces, thereby providing the layer thickness and roughness. ScanAsyst-Air Si3N4 probe with a radius of 2 nm was used. For each test, freshly cleaved MoS2 surface was obtained by peeling off the top layers of the molybdenite sample using a sticky tape, followed by dropping 10 mL conditioned solution (e.g., 0.05 M MgCl2 solution at pH 10 with or without 50 mg/L SHMP addition) on the freshly cleaved surface for 10 min. After that, the MoS2 surface was washed 3 times with ultrapure water, and then air-dried prior to imaging.
2.7. Theory Calculation
Usually, the total interaction energies (VT) between particles in aqueous solution are quantitatively predicted by the extended Derjaguin–Landau–Verwey–Overbeek (EDLVO) theory, in which the energies of the Van der Waals interaction VW, the electrostatic interaction VE, and steric hindrance effects VSR are taken into consideration, as described in Equation (1) [31,38,39,40].
VW and A can be calculated according to Equations (2) and (3).
The Hamaker constant of MoS2 (A11) in vacuum is 9.38 × 10−20 J [41]. As Hamaker constant of Mg(OH)2 in vacuum cannot be found in the literature, it is replaced by that of MgO, A22 = 10.6 × 10−20 J [42]. The Hamaker constant of water A33 is 3.7 × 10−20 J [42,43]. H (nm) refers to the distance between particles.
The electrostatic interaction energy VE between MoS2 particles and Mg(OH)2 colloids can be expressed by Equation (4) [42].
where ε0 and εr are the vacuum dielectric constant and the relative dielectric constant of the continuous phase and the value of ε0εr is 6.95 × 10−10 C2/(J·m) [31]. ψ1 and ψ2 refer to the surface potentials of MoS2 particles and Mg(OH)2 colloids, respectively, usually represented by zeta potentials [39]. κ−1 is the thickness of electric double-layer, κ = 0.180 nm−1 [44].
The adsorption of SHMP on the mineral surface can increase the steric repulsion among particles [27,38]. The steric hindrance interaction energy VSR is calculated according to Equation (5) [30,37].
where R represents the radius of particles. δ stands for the thickness of adsorbed layer after SHMP adsorption, with a given value of 5.45 nm [31]. Z is the covering area of the macromolecules (i.e., SHMP molecule) on the particle surface, 1.9 × 10−16 m2 [31]. k refers to the Boltzmann constant, k = 1.381 × 10−23 J/K [38].
3. Results
3.1. Flotation Results
Figure 4 shows MoS2 recovery at 5 min as a function of pH from 4 to 12 in the absence of flotation reagents. All experiments named Without Ca2+/Mg2+ represent the experiments treated in ultrapure water but with pH being adjusted using NaOH or HCl. MoS2 recovery was over 80% at pH 4–10 without Ca2+ and Mg2+, which was dramatically decreased to 72% at pH 12. However, in the presence of 0.01 M CaCl2 and 0.05 M MgCl2, MoS2 recovery decreased with increased pH value, achieving a minimum value of 45% and 1% at pH 12, respectively. This indicates that both divalent ions have a negative effect on MoS2 flotation. It should be noted that the depressing effect due to MgCl2 was more apparent than that of CaCl2. Although Nagaraj and Farinato [45] reported that Ca2+ had a negligible effect on Mo floatability, many other studies indicated that both Mg2+ and Ca2+ played negative roles on sulfide mineral flotation, [22,23,26,29,46], consistent with this study. The differences were probably due to the different mineral samples and flotation conditions used.
In the flotation of Cu-Mo sulfide minerals, pH adjustment to 9.5–12 is usually used to depress pyrite flotation [30]. The selected pH of 10 for MoS2 flotation was consistent with many other studies [22,47,48,49].
Figure 5 shows the MoS2 flotation in 0.05 M MgCl2 solution as a function of SHMP dosage. With the increase of SHMP dosage from 0 to 40 mg/L, MoS2 recovery was increased significantly. A further but slight increase was found when SHMP dosage was increased from 40 to 50 mg/L, indicating that the optimal SHMP dosage was 50 mg/L.
Figure 6 shows the effects of SHMP (50 mg/L) on MoS2 flotation at pH 10. Insignificant difference was observed for MoS2 recovery without Ca2+ and Mg2+, regardless of SHMP addition, suggesting that SHMP had a negligible impact on MoS2 recovery in the absence of flotation reagents and salts. In addition, MoS2 recovery was increased dramatically within the first 3 min, achieving a recovery of more than 70%. Afterwards, MoS2 recovery was increased slowly and eventually achieved approximately 91% at 10 min. This indicates fast flotation kinetics and a high recovery of MoS2.
When 50 mg/L SHMP was added, MoS2 recovery in CaCl2 solution was increased from 73% to 79% while a more significant increase from 25% to 79% was observed when MoS2 was exposed to MgCl2 solutions. This means that the increase of MoS2 recovery in MgCl2 solution due to SHMP was 54% while that in CaCl2 solution was only 6% within 10 min, indicating that SHMP played a more beneficial role on MoS2 flotation in the presence of MgCl2 as compared to CaCl2.
3.2. Contact Angle Analyses
Figure 7 shows that the contact angle of the MoS2 surface (fresh surface treated in solution for 10 min) was gradually decreased to various extents with increased pH under the conditions examined. Specifically, the contact angle of MoS2 surface was decreased from 88° to 82° from pH 4 to 12 without divalent ions, exhibiting the inherent hydrophobicity of MoS2 and the insignificant role of pH. Tabares et al. [1] also reported that higher pH resulted in a slightly lower hydrophobicity of the MoS2 surface. More significant declines from 88° to 75° and 48° were found in the presence of 0.01 M CaCl2 and 0.05 M MgCl2, respectively when pH was increased from 4 to 12, indicating that both Ca2+ and Mg2+ ions reduced MoS2 hydrophobicity, consistent with flotation results, e.g., MgCl2 increased MoS2 wettability more significantly than that of CaCl2.
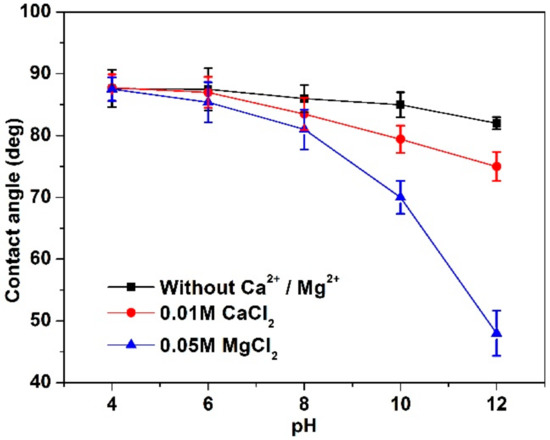
Figure 7.
Contact angle of MoS2 surface at different pH.
Figure 8 shows that the contact angle of the MoS2 surface treated without Ca2+ and Mg2+ was not obviously changed after the addition of SHMP. A slight increase in contact angle was observed in 0.01 M CaCl2 solutions when 50 mg/L SHMP was added into the solution, indicating that the addition of SHMP reduced the negative effects of Ca2+ on MoS2 surface. A more apparent increase in contact angle was found for MoS2 treated in 0.05 M MgCl2 solution when SHMP was present.
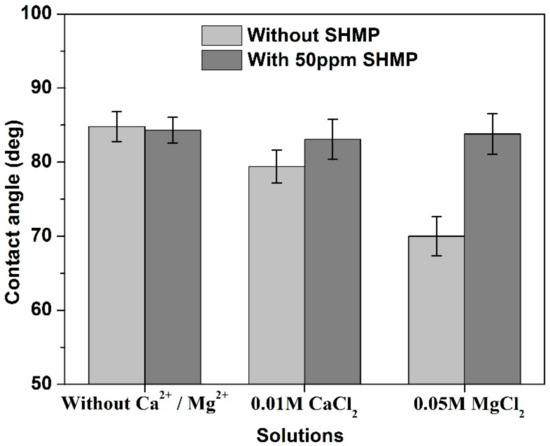
Figure 8.
Contact angle of MoS2 treated in various solutions at pH 10.
3.3. Zeta Potential Analyses
Figure 9 shows the zeta potentials of MoS2 in the absence of SHMP. Without Ca2+ and Mg2+, zeta potential was always negative over the pH range tested, consistent with that found in other study [26]. The zeta potential was more negative with increased pH value. An increased zeta potential was observed in MgCl2 solution within pH 2–7, which was reversed when pH was greater than 9, e.g., a zeta potential of 18.2 mV was observed when solution pH was 10.
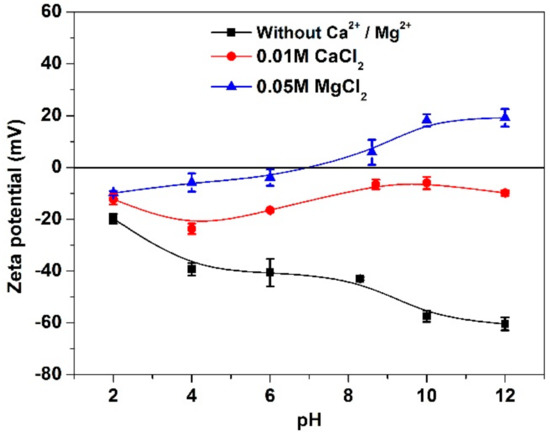
Figure 9.
Zeta potential of MoS2 at different pH valules.
Table 2 indicates that the zeta potentials of MoS2 treated without Ca2+/Mg2+ or in CaCl2 solution were more negative in the presence of 50 mg/L SHMP. Li et al. [23] found a similar change of zeta potential on chalcopyrite surface once adding SHMP into the solution. However, the zeta potential was reversed again from a positive value to a negative value in 0.05 M MgCl2 solution, which might be due to the reduced formation or adsorption of Mg precipitation on MoS2 surface.

Table 2.
Zeta potential of MoS2 treated in various solutions at pH 10.
4. Discussion
4.1. XPS Analyses
4.1.1. Survey Spectra
A previous study [23] showed that the calcium hydroxide precipitate (Ca(OH)2(s)) and magnesium hydroxide precipitate (Mg(OH)2(s)) was formed at pH greater than 12.4 and 9.3 in 0.01 M CaCl2 and 0.05 M MgCl2 solution, respectively. When pulp was controlled at pH 10, three Mg complexes, namely MgOH+, Mg(OH)2(aq) and (Mg(OH)2(s)), were present in 0.05 M MgCl2 solution while two Ca complexes, namely CaOH+ and Ca(OH)2(aq), were present in 0.01 M CaCl2 solution. These complexes may be adsorbed on the negatively charged mineral surface. Therefore, XPS was conducted to obtain the chemical information of surface species formed on MoS2 surface [23,50,51]. The broad survey spectra of untreated and treated MoS2 and the main elemental quantifications are presented in Figure 10 and Table 3, respectively. As shown in Figure 10, no obvious Ca 2p peak was formed on all MoS2 surfaces. Table 3 shows that only approximately 1% Ca was found on the surface when MoS2 was treated in 0.01 M CaCl2 solution, indicating same adsorption of Ca species on MoS2 surface. However, Ca was removed after 50 mg/L SHMP was added, suggesting that SHMP prevented the formation of Ca species on the MoS2 surface.
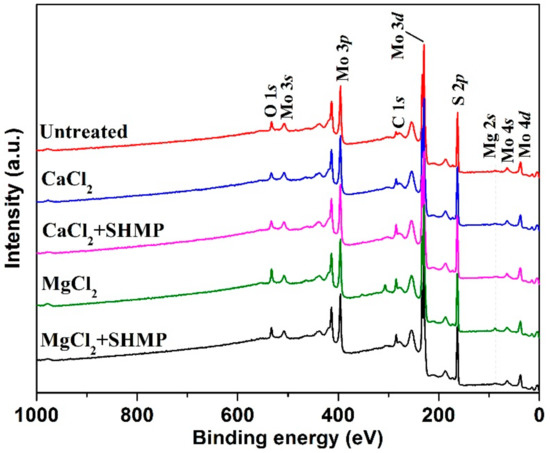
Figure 10.
XPS survey spectra of MoS2 treated in different solution at pH 10.

Table 3.
Elemental quantification (at %) of MoS2 surface.
In addition, Figure 10 shows that untreated MoS2 presented a relatively weak peak for Mg 2s at 89.5 eV, while a relatively stronger Mg 2s peak was observed on MoS2 surface treated in MgCl2 solution, indicating the adsorption of Mg precipitate on MoS2. However, SHMP prevented Mg precipitation on the MoS2 surface seen by significantly decreased Mg 2s peak intensity, consistent with contact angle and zeta potential measurements. Furthermore, Mg 1s and O 1s were decreased from 4 at. % to 2 at. % and from 11 at. % to 7 at. %, respectively, when 50 mg/L SHMP was added in 0.05 M MgCl2 solution. The simultaneous and stoichiometric declines in Mg and O highly supported that SHMP reduced the formation and adsorption of Mg(OH)2 on the MoS2 surface, thereby improving recovery.
4.1.2. Mo 3d, S 2p and O 1s XPS Spectra
To further estimate the change of surface species, high resolution Mo 3d, S 2p and O 1s XPS spectra were collected, as presented in Figure 11. Each Mo spectra consisted of two Gaussian–Lorentzian bands separated by 3.2 eV with the intensity of Mo 3d5/2 being doubled that of Mo 3d3/2 [51,52]. As shown in Figure 11a, three peaks located at 229.8 eV, 232.7 eV and 227.0 eV corresponded to MoO3, MoS2 and S 2s components [2,51,52], respectively. The Mo 3d spectra indicate that both untreated MoS2 and MoS2 treated in various solutions experienced slight oxidation.

Figure 11.
XPS spectra of: (a) Mo 3d; (b) S 2p; and (c) O 1s.
Each S component of the S 2p spectrum was composed of two peaks separated by 1.2 eV with the intensity of the lower binding energy peak (S 2p3/2) being double that of the peak (S 2p1/2) at a higher binding energy, based on two Gaussian–Lorentzian bands [53]. Figure 11b shows that the S 2p spectra was divided into two components at 162.6 eV and 172.7 eV, representing MoS2 and SO42−, respectively, with the latter being likely derived from the oxidation of MoS2. Figure 11c indicates the O 1s binding energies of 533.3 eV, 531.1 eV and 530.9 eV for attached oxygen on the MoS2 surface (O2/MoS2), Mo trioxide (MoO3) and hydroxide/sulfate (OH−/SO42−), respectively. It should be noted that the binding energies of hydroxide and sulfate overlapped at 530.9 ± 0.15 eV [54].
Table 4 presents the binding energy and atomic percentage of the elements investigated. The percentage of Mo due to MoO3 was decreased from 3.6% Mo (untreated) to 3.0% Mo and 2.2% Mo in 0.01 M CaCl2 solution and 0.05 M MgCl2 solution, respectively, probably due to the dissolution of MoO3 into solution. In addition, the MoO3 content in CaCl2 solution was greater than that in MgCl2 solution, highly due to the formation of CaMoO4 precipitate on the surface, which increased the MoS2 oxide content. The MoO3 was further reduced to 2.2% Mo when SHMP was added into the CaCl2 solution, indicating that the addition of SHMP prevented the formation of CaMoO4 on the surface.

Table 4.
Species content (% element) on MoS2 surfaces.
The atomic proportion of S due to MoS2 and SO42− of untreated MoS2 was 95.4% S and 4.6% S, respectively, indicating a weak oxidation of MoS2, consistent with Mo 3d XPS measurements. The SO42− was further reduced to 3.3% S and 3.9% S in CaCl2 and MgCl2 solution, respectively, possibly due to the dissolution of SO42− from surface into solution. In addition, a further decrease was observed when 50 mg/L SHMP was added, indicating that the addition of SHMP was beneficial to SO42− dissolution, in accordance with a previous study [23].
In addition, the MoO3 concentration was decreased from 45.2% O to 23.4% O and 17.9% O when MoS2 was treated in CaCl2 and MgCl2 solution, respectively. The atomic proportion of OH−/SO42− species was increased from 7.9% O to 8.4% O and 16.0% O when MoS2 was treated in CaCl2 and MgCl2 solution, respectively. As S 2p XPS analysis indicated that SO42− was decreased for the MoS2 surface treated in both salt solutions, the increase of OH−/SO42− further confirmed the adsorption of more hydroxide species on the MoS2 surface, especially treated in MgCl2 solution. However, a decrease was observed when 50 mg/L SHMP was added, e.g., from 8.4% O to 6.1% O and from 16% O to 6.6% O, respectively, further suggesting that the addition of SHMP decreased the adsorption of Ca and Mg complexes onto the MoS2 surface due primarily to its dispersion and complexing effects.
4.2. TMAFM Imaging
Figure 12 shows the tapping mode AFM images of the MoS2 surface exposed to different conditions. A clean surface with some scratches was observed on the untreated MoS2 surface. The scratches were probably due to the peeling treatments, consistent with other published studies [6,22,55]. The height and root mean square (RMS) roughness for the untreated surface were 0.42 nm and 0.07 nm, respectively, illustrating that the untreated MoS2 surface was relatively flat and smooth [56].
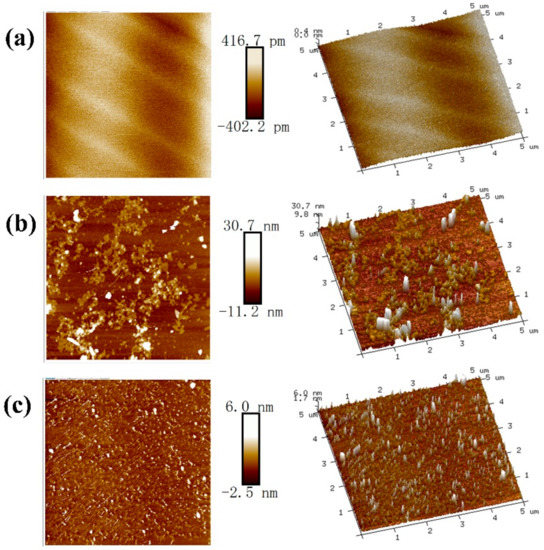
Figure 12.
Typical 2D and 3D AFM images (5 μm × 5 μm) of: (a) untreated MoS2; and MoS2 treated in: (b) 0.05 M MgCl2; and (c) 0.05 M MgCl2 with 50 mg/L SHMP at pH of 10.
However, Figure 12b shows many colloidal particles with various sizes and irregular shapes on the MoS2 surface treated in 0.05 M MgCl2 solution, which can be explained by the adsorption of Mg(OH)2 colloid aggregation. Suyantara et al. [24] also reported that MoS2 surface treated in MgCl2 solution at high alkaline condition presented a number of white spots and mountainous features. Compared to the untreated surface, the treated surface gave a significant greater height (30.7 nm) and RMS roughness (5.47 nm). These changes revealed that the adsorption of Mg(OH)2(s) on the MoS2 surface increased the surface roughness and decreased the surface homogeneity. In contrast, when MoS2 was treated in 0.05 M MgCl2 solution with 50 mg/L SHMP, the size of bright spots became smaller while its height (6.0 nm) and RMS roughness (1.36 nm) apparently decreased, suggesting that the presence of SHMP reduced the adsorption Mg(OH)2 precipitates on the MoS2 surface, thereby increasing the contact angle and improving MoS2 flotation.
4.3. Mechanisms
The variation in MoS2 recovery might be attributed to different mechanisms. The crystal structure of MoS2 consists of hydrophobic faces and hydrophilic edges with the face consisting of micro-faces and micro-edges [3]. Generally, MoS2 edges and micro-edges are easily oxidized to form molybdate ions (MoO42−) or hydromolybdate ions (HMoO4−) (Equations (6) and (7)):
2MoS2 + 9O2 + 10OH− → 2HMoO4− + 4SO42− + 4H2O
HMoO4− + OH− → MoO42− + H2O
HMoO4− is the main oxidation product when pH is lower than 6 while the main oxidation product is MoO42− under neutral and alkaline conditions [3,55]. With increased solution pH, more negative MoO42− can be formed on the edge and micro-edge of the face, causing a more negatively charged MoS2 surface [55], as shown in Figure 9. Moreover, the increased electric charge due to the formation of MoO42− ions on the micro-edges of the face, decreases MoS2 hydrophobicity due to oxidation [1]. Tabares et al. [1] also reported that the oxidation occurring on the micro-edges adjacent to the face of MoS2 enhanced the surface hydration layer, thereby decreasing surface hydrophobicity and MoS2 recovery. Therefore, the slightly reduced recovery and contact angle in solution without Ca2+ and Mg2+ was highly likely due to the oxidation of the MoS2 surface.
When MoS2 was treated in either CaCl2 or MgCl2 solution, the metal ions were easily adsorbed on the MoS2 edge, reducing the hydrophobicity of MoS2 particles [5,57]. Specifically, the adsorbed Ca2+ onto the edges spontaneously reacts with MoO42− to form CaMoO4 precipitation based on Equation (8) [58,59]. In addition, as described in a previous study [23], two Ca species including CaOH+ and Ca(OH)2(aq) were increased in 0.01 M CaCl2 solution when solution pH was increased from 4 to 12. Therefore, in addition to the adsorption of CaMoO4, the adsorption of positive CaOH+ on the edges of negative MoS2 particles by electrostatic interaction contributes to the increased zeta potential [1]. Therefore, the CaMoO4 precipitate depositing on both the edges and micro-edges of the faces reduced MoS2 hydrophobicity and further deteriorated MoS2 flotation [1].
MoO42− + Ca2+ → CaMoO4(s)
In 0.05 M MgCl2 solution, the MgOH+ and Mg(OH) 2(aq) concentrations were increased when pH was increased from 4 to 9.3. With the formed MgOH+ being mainly adsorbed on micro-edges, MoS2 recovery (Figure 6) was decreased due to the decreased hydrophobicity (Figure 7) [26]. When pH was greater than 9.3, the positively charged Mg(OH)2 precipitate was formed [23] and deposited on both the micro-edges on the faces and edges of MoS2 particles [26] due to electrostatic interaction [46], thereby resulting in a lower recovery.
Some published studies showed that SHMP had a good complexing ability for hydrolyzed metallic ions [35,36,37,38]. For instance, Ding et al. [35] and Feng et al. [37] reported that Ca2+ from the minerals surface was selectively dissolved into solution due to the complexation of SHMP. In other words, SHMP can react with Ca2+ or Mg2+ to form soluble complexes (Equations (9)–(11)), thereby reducing the formation of Ca and Mg precipitates (e.g., CaMoO4(s) and Mg(OH)2(s)) [36,38] and relieving their negative effects on MoS2 flotation.
(NaPO3)6 → Na4P6O182− +2Na+
Na4P6O182− + 2Ca2+ → CaNa4P6O18
Na4P6O182− + 2Mg2+ → MgNa4P6O18
In addition, SHMP can disperse slime that might attach on valuable mineral surfaces, through changing the surface potential of the particle and increasing the electrostatic repulsive energy between particles [31,32,33]. Therefore, the presence of SHMP may prevent Mg(OH)2 colloids attaching onto the MoS2 surface via its dispersing role. Therefore, the interaction energy between MoS2 particles and Mg(OH)2 colloids were investigated based on EDLVO theory model.
In the absence of SHMP, the zeta potentials of MoS2 and Mg(OH)2 colloids were −57.5 mV and 11.6 mV, respectively, which were further decreased to −71.9 mV and −23.2 mV after 50 mg/L SHMP addition. Particle size analysis showed that the average diameter (d50) of MoS2 particles was approximately 66 µm and that of Mg(OH)2 colloids was 7.6 µm. Consequently, the radius of MoS2 particle (R1) and Mg(OH)2 particle R2 were 33 μm and 3.8 μm, respectively.
Figure 13 shows the interaction energy between MoS2 particles and Mg(OH)2 colloids with and without SHMP. In the absence of SHMP, both Van der Waals interaction energy VW1 and electrostatic interaction energy VE1 were negative within all particle distances examined. In addition, the total interaction energy VT between MoS2 and Mg(OH)2 colloids was negative, revealing that the attraction force dominated the interparticle aggregation between MoS2 and Mg(OH)2 colloids. Specifically, the absolute value of VE1 was significantly greater than that of VW, indicating that the negative VE played a dominant role on VT between MoS2 and Mg(OH)2 colloids when no SHMP was added.
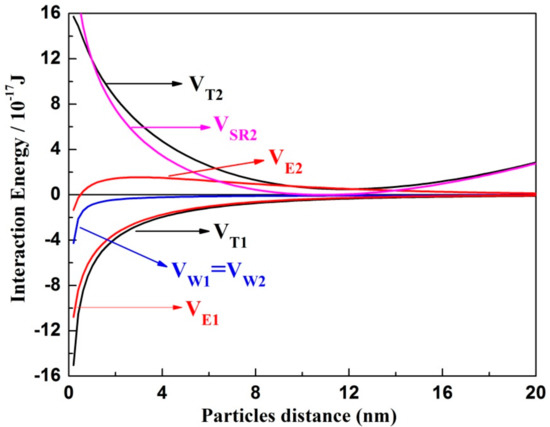
Figure 13.
Interaction energy between MoS2 and Mg(OH)2 at pH 10.
When 50 mg/L SHMP was added, VE2 was increased from negative to positive at longer particle distance. Meanwhile, the steric hindrance interaction energy VSR appeared due to the increased steric repulsion force among particles when SHMP molecules with a long chain were adsorbed on the minerals surface. More importantly, the absolute value of VSR was significantly greater than others within a short particle distance, indicating that the positive VSR dominated the force between MoS2 and Mg(OH)2 colloids when approaching each other. Accordingly, the presence of SHMP reversed VT from negative to positive values, indicating the appearance of repulsion force among particles. Therefore, the increased MoS2 recovery in the presence of SHMP was mainly due to the prevention of Mg(OH)2 attaching onto MoS2 surface.
5. Conclusions
The flotability of MoS2 was depressed in either 0.01 M CaCl2 or 0.05 M MgCl2 solution at alkaline condition, mainly due to the adsorption of complex Ca and Mg species including CaMoO4, CaOH+, Mg(OH)2, and MgOH+ on the edges and micro-edges of face. The addition of SHMP had a negligible effect on MoS2 recovery in solution without Ca2+ and Mg2+, but eliminated the negative effects of Ca2+ and Mg2+ on MoS2 flotation when present. TMAFM analyses indicated that the adsorption of precipitates increased surface roughness but decreased surface homogeneity while SHMP reduced the adsorption of precipitates on the MoS2 surface.
Two mechanisms were proposed based on various measurements and theoretical calculation, with the latter being more significant for improved MoS2 recovery in the presence of divalent ions. Firstly, SHMP can complex with Ca2+ and Mg2+ to form dissolvable complexes in the solution, decreasing the formation of hydrophilic Ca and Mg complexes and precipitates. Secondly, EDLVO calculation indicated that the presence of SHMP in 0.05 M MgCl2 solution changed the total interaction force between MoS2 and Mg(OH)2 colloids from attraction to repulsion, thereby preventing the adsorption of hydrophilic Mg(OH)2 on MoS2 surface.
Author Contributions
Funding acquisition, Y.L.; Investigation, W.L. and Q.X.; Project administration, Y.L.; Supervision, Y.L.; Validation, Z.W.; Writing—original draft, W.L. and Q.X.; and Writing—review and editing, Y.L. and S.S.
Funding
This research was financially supported by the National Natural Science Foundation of China (51604205 and 51774223) and Natural Science Foundation of Hubei Province (2016CFB268). The authors gratefully acknowledge the support from Fundamental Research Funds for the Central Universities (WUT: 2016IVA046 and 2017IVB018).
Acknowledgments
A special thank is given to the financial support for the Excellent Dissertation Cultivation Fund of Wuhan University of Technology (2017-YS-052).
Conflicts of Interest
The authors declare no conflict of interests.
References
- Tabares, J.O.; Ortega, I.M.; Bahena, J.L.R.; López, A.A.S.; Pérez, D.V.; Valdivieso, A.L. Surface properties and flotability of molybdenite. In Proceedings of the 2006 China-Mexico Workshop on Minerals Particle Technology, San Luis Potosí, Mexico, 4–6 December 2006. [Google Scholar]
- Hirajima, T.; Mori, M.; Ichikawa, O.; Sasaki, K.; Miki, H.; Farahat, M.; Sawada, M. Selective flotation of chalcopyrite and molybdenite with plasma pre-treatment. Miner. Eng. 2014, 66–68, 102–111. [Google Scholar] [CrossRef]
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the flotation of molybdenite. Part І: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Lince, J.R.; Frantz, P. Anisotropic oxidation of MoS2 crystallites studied by angle-resolved X-ray photoelectron spectroscopy. Tribol. Lett. 2000, 9, 3–4. [Google Scholar]
- Zanin, M.; Ametov, I.; Grano, S.; Zhou, L.; Skinner, W. A study of mechanisms affecting molybdenite recovery in a bulk copper/molybdenum flotation circuit. Int. J. Miner. Process. 2009, 93, 256–266. [Google Scholar] [CrossRef]
- López-Valdivieso, A.; Madrid Ortega, I.; Reyes Bahena, J.L.; Sanchez Lopez, A.A.; Song, S. Properties of the molybdenite/aqueous solution interface and their relationship with the mineral natural floatability. In Proceedings of the XVI International Congress in Extractive Metallurgy, Saltillo, México, 24–28 April 2006; pp. 299–310. (In Spanish). [Google Scholar]
- Yang, B.; Song, S.; Lopez-Valdivieso, A. Effect of particle size on the contact angle of molybdenite powders. Miner. Process. Extr. Metall. Rev. 2014, 35, 208–215. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice Flotation of Sulfide Ores; Elsevier Socience: Burlington, VT, USA, 2007; p. 685. [Google Scholar]
- Liu, G.Y.; Lu, Y.P.; Zhong, H.; Cao, Z.F.; Xu, Z.H. A novel approach for preferential flotation recovery of molybdenite from a porphyry copper-molybdenum ore. Miner. Eng. 2012, 36–38, 37–44. [Google Scholar] [CrossRef]
- Lucay, F.; Cisternas, L.A.; Gálvez, E.D.; López-Valdivieso, A. Study of the natural floatability of molybdenite fines in saline solutions and effect of gypsum precipitation. Miner. Metall. Process. 2015, 32, 203–208. [Google Scholar]
- Wang, B.; Peng, Y. The effect of saline water on mineral flotation—A critical review. Miner. Eng. 2014, 66–68, 13–24. [Google Scholar] [CrossRef]
- Liu, W.; Moran, C.; Vink, S. A review of the effect of water quality on flotation. Miner. Eng. 2013, 53, 91–100. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Forbes, L.; Cisternas, L.A. Effect of seawater on sulfide ore flotation: A review. Miner. Process. Extr. Metall. Rev. 2016, 37, 369–384. [Google Scholar] [CrossRef]
- Castro, S. Challenges in flotation of Cu-Mo sulfide ores in sea water. In Water in Mineral Processing of the First International Symposium; Drelich, J., Ed.; Society for Mining, Metallurgy and Exploration: Seattle, WA, USA, 2012. [Google Scholar]
- Moreno, P.A.; Aral, H.; Cuevas, J.; Monardes, A.; Adaro, M.; Norgate, T.; Bruckard, W. The use of seawater as process water at las luces copper-molybdenum beneficiation plant in taltal (Chile). Miner. Eng. 2011, 24, 852–858. [Google Scholar] [CrossRef]
- Peng, Y.; Seaman, D. The flotation of slime–fine fractions of Mt. Keith pentlandite ore in de-ionised and saline water. Miner. Eng. 2011, 5, 479–481. [Google Scholar] [CrossRef]
- Romero, C.P.; Jeldres, R.I.; Quezada, G.R.; Concha, F.; Toledo, P.G. Zeta potential and viscosity of colloidal silica suspensions: Effect of seawater salts, pH, flocculant, and shear rate. Collids Surf. A Physicochem. Eng. Asp. 2018, 538, 210–218. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Castro, S.; Ramos, O. Effect of seawater main components on frothability in the flotation of Cu-Mo sulfide ore. Physicochem. Probl. Mineral Pro. 2014, 50, 17–29. [Google Scholar]
- Troncoso, P.; Saavedra, J.; Acuna, S.; Jeldres, R.; Concha, F.; Toledo, P. Nanoscale adhesive forces between silica surfaces in aqueous solutions. J. Colloid Interface Sci. 2014, 424, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.; Castro, S.; Laskowski, J.S. Copper–molybdenum ores flotation in sea water: Floatability and frothability. Miner. Eng. 2013, 53, 108–112. [Google Scholar] [CrossRef]
- Quinn, J.J.; Kracht, W.; Gomez, C.O.; Gagnon, C.; Finch, J.A. Comparing the effect of salts and frother (MIBC) on gas dispersion and froth properties. Miner. Eng. 2007, 20, 1296–1302. [Google Scholar] [CrossRef]
- Hirajima, T.; Suyantara, G.P.W.; Ichikawa, O.; Elmahdy, A.M.; Miki, H.; Sasaki, K. Effect of Mg2+ and Ca2+ as divalent seawater cations on the floatability of molybdenite and chalcopyrite. Miner. Eng. 2016, 96–97, 83–93. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Xiao, Q.; Wei, Z.; Song, S. The influencing mechanisms of sodium hexametaphosphate on chalcopyrite flotation in the presence of MgCl2 and CaCl2. Minerals 2018, 8, 150. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Elmahdy, A.M.; Miki, H.; Sasaki, K. Effect of kerosene emulsion in MgCl2 solution on the kinetics of bubble interactions with molybdenite and chalcopyrite. Collids Surf. A Physicochem. Eng. Asp. 2016, 501, 98–113. [Google Scholar] [CrossRef]
- Uribe, L.; Gutierrez, L.; Laskowski, J.; Castro, S. Role of calcium and magnesium cations in the interactions between kaolinite and chalcopyrite in seawater. Physicochem. Probl. Miner. Process. 2017, 53, 737–749. [Google Scholar]
- Qiu, Z.; Liu, G.; Liu, Q.; Zhong, H. Understanding the roles of high salinity in inhibiting the molybdenite flotation. Collids Surf. A Physicochem. Eng. Asp. 2016, 509, 123–129. [Google Scholar] [CrossRef]
- Rebolledo, E.; Laskowski, J.S.; Gutierrez, L.; Castro, S. Use of dispersants in flotation of molybdenite in seawater. Miner. Eng. 2017, 100, 71–74. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Hirajima, T.; Miki, H.; Sasaki, K. Floatability of molybdenite and chalcopyrite in artificial seawater. Miner. Eng. 2018, 115, 117–130. [Google Scholar] [CrossRef]
- Laskowski, J.; Castro, S. Flotation in concentrated electrolyte solutions. Int. J. Miner. Process. 2015, 144, 50–55. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Arancibia-Bravo, M.P.; Reyes, A.; Aguirre, C.E.; Cortes, L.; Cisternas, L.A. The impact of seawater with calcium and magnesium removal for the flotation of copper-molybdenum sulphide ores. Miner. Eng. 2017, 109, 10–13. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Hu, Y.; Lan, Y. Influences of phosphates on dispersion of fine alumin-silicate minerals. Cent. South Univ. (Sci. Technol.) 2007, 38, 238–244. [Google Scholar]
- Xu, D.; Zhu, S.; Cao, G.; Cu, H. Influences of sodium hexametaphosphate on dispersion of fine montmorillonite in coal flotation. J. China Coal Soc. 2016, 41, 192–198. [Google Scholar]
- Xia, Q.; Li, Z.; Qiu, X.; Dai, Z. Invesitgation of action mechanism between sodium hexametaphosphate and serpenine. Min. Metall. Eng. 2002, 22, 53–56. (In Chinese) [Google Scholar]
- Li, Z.; Han, Y.; Li, Y.; Gao, P. Effect of serpentine and sodium hexametaphosphate on ascharite flotation. Trans. Nonferrous Met. Soc. China 2017, 27, 1841–1848. [Google Scholar] [CrossRef]
- Ding, H.; Lin, H.; Deng, Y. Depressing effect of sodium hexametaphosphate on apatite in flotation of rutile. J. Univ. Sci. Technol. Beijing 2007, 14, 200–203. [Google Scholar] [CrossRef]
- Luo, N.; Wei, D.; Shen, Y.; Han, C.; Zhang, C. Elimination of the adverse effect of calcium ion on the flotation separation of magnesite from dolomite. Minerals 2017, 7, 150. [Google Scholar]
- Feng, Q.; Zhou, Q.; Zhang, G.; Lu, Y.; Yang, S. Inhibition mechanism of sodium hexametaphosphate on calcite. Chin. J. Nonferrous Met. 2011, 21, 436–441. (In Chinese) [Google Scholar]
- Lu, Y.; Zhang, M.; Feng, Q.; Long, T.; Ou, L.; Zhang, G. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Trans. Nonferrous Met. Soc. China 2011, 21, 208–213. [Google Scholar] [CrossRef]
- Missana, T.; Adell, A. On the applicability of DLVO theory to the prediction of clay colloids stability. J. Colloid Interface Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Liu, H.; Wang, Y. Effects of water hardness on the dispersion of fine coal and montmorillonite. J. China Univ. Min. Technol. 2009, 38, 114–118. [Google Scholar]
- Lin, Q.; Gu, G.; Wang, H.; Liu, Y.; Fu, J.; Wang, C. Flotation mechanisms of molybdenite fines by neutral oils. Int. J. Miner. Metall. Mater. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Ren, J.; Shen, J.; Luo, S. Particle Dispersion Science and Technology; Chemical Industry Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Van Oss C, J.; Giese, R.F.; Costanzo, P.M. Dlvo and non-dlvo interactions in hectorite. Clay Clay Miner. 1990, 38, 151–159. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.; Feng, Q.; Li, H. Solution chemistry of sodium silicate and implications for pyrite flotation. Ind. Eng. Chem. Res. 2012, 51, 12089–12094. [Google Scholar] [CrossRef]
- Nagaraj, D.R.; Farinato, R. Chemical factor effects in saline and hypersaline waters in the flotation of Cu and Cu-Mo ores. In Proceedings of the XXVII International Mineral Processing Congress, Santiago, Chile, 20–24 October 2014. [Google Scholar]
- Yuan, Z.; Zhang, Q.; Liu, J. Influence and mechanism of metal ions on flotation of molybdenite. J. Northeast. Univ. (Nat. Sci.) 2016, 37, 1013–1016. [Google Scholar]
- Jacques, S.; Greet, C.J.; Bastin, D. Oxidative weathering of a copper sulphide ore and its influence on pulp chemistry and flotation. Miner. Eng. 2016, 99, 52–59. [Google Scholar] [CrossRef]
- Muganda, S.; Zanin, M.; Grano, S.R. Influence of particle size and contact angle on the flotation of chalcopyrite in a laboratory batch flotation cell. Int. J. Miner. Process. 2011, 98, 150–162. [Google Scholar] [CrossRef]
- Li, Y.; Lartey, C.; Song, S.; Li, Y.; Gerson, A. The fundamental roles of monovalent and divalent cations with sulfates on molybdenite flotation in the absence of flotation reagents. RSC Adv. 2018, 8, 23364–23371. [Google Scholar] [CrossRef]
- Buckley, A.N. A survey of the application of X-ray photoelectron spectroscopy to flotation research. Collids Surf. A Physicochem. Eng. Asp. 1994, 93, 159–172. [Google Scholar] [CrossRef]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; Sasaki, K.; Imaizumi, Y.; Kuroiwa, S. Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Yuehua, H.; Zhang, C.; Guan, Q.; Zhang, C. Separation of molybdenite from chalcopyrite in the presence of novel depressant 4-amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2h)-one. Minerals 2017, 7, 146. [Google Scholar]
- Liu, G.; Huang, Y.; Qu, X.; Xiao, J.; Yang, X.; Xu, Z. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1, 2,4-triazole-5-thione to malachite by tof-sims, xps, ftir, contact angle, zeta potential and micro-flotation. Collids Surf. A Physicochem. Eng. Asp. 2016, 503, 34–42. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Muir, I.J. Oxidation states and speciation of secondary products on pyrite and arsenopyrite reacted with mine waste waters and air. Mineral. Petrol. 1998, 62, 123–144. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Q.; Xu, Z.; Zeng, H. Probing anisotropic surface properties of molybdenite by direct force measurements. Langmuir 2015, 31, 11409–11418. [Google Scholar] [CrossRef] [PubMed]
- Beaussart, A.; Parkinson, L.; Mierczynska-Vasilev, A.; Beattie, D.A. Adsorption of modified dextrins on molybdenite: Afm imaging, contact angle, and flotation studies. J. Colloid Interface Sci. 2012, 368, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Hsu, L.L. Factors affecting the flotation recovery of molybdenite from porphyry copper ores. Int. J. Miner. Process. 1984, 12, 145–162. [Google Scholar] [CrossRef]
- Wan, H.; Yang, W.; He, T.; Yang, J.; Guo, L.; Peng, Y. The influence of Ca2+ and pH on the interaction between PAHs and molybdenite edges. Minerals 2017, 7, 104. [Google Scholar] [CrossRef]
- Wan, H.; Yang, W.; Cao, W.; He, T.; Liu, Y.; Yang, J.; Guo, L.; Peng, Y. The interaction between Ca2+ and molybdenite edges and its effect on molybdenum flotation. Minerals 2017, 7, 141. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).