Abstract
Incessant generation and mismanagement of industrial waste, resource scarcity, and environmental degradation have created non-sustainability in human life. Though industrial wastes are hazardous or non-hazardous in nature based on their source, open dumping disposal is commonly done for both types of waste. The adversity associated with waste enhances the environmental and health impacts. However, this waste has the potential to recycle and minimize resource scarcity. The circular economy works on the concept of reuse, recycling, and recovery to convert waste into a resource. Thus, industrial waste can benefit the environment and economic growth to build industrial ecology. However, the opportunities and challenges associated with industrial ecology for the reuse and recycling of waste have to be identified and preserved. Therefore, this study has identified challenges associated with waste, analyzed their impact, and industrial regulations, prioritized their criticality, and developed solution strategies to alleviate them. Two case studies on industrial byproducts, i.e., fly ash and red mud, based on different income groups are discussed in this study. It highlights the circular economy has minimized waste generation and enhanced the recovery of secondary resource materials. In addition, this study supports achieving the sustainable development goals (SDGs) 11 and 12 to build a sustainable industrial ecosystem.
1. Introduction
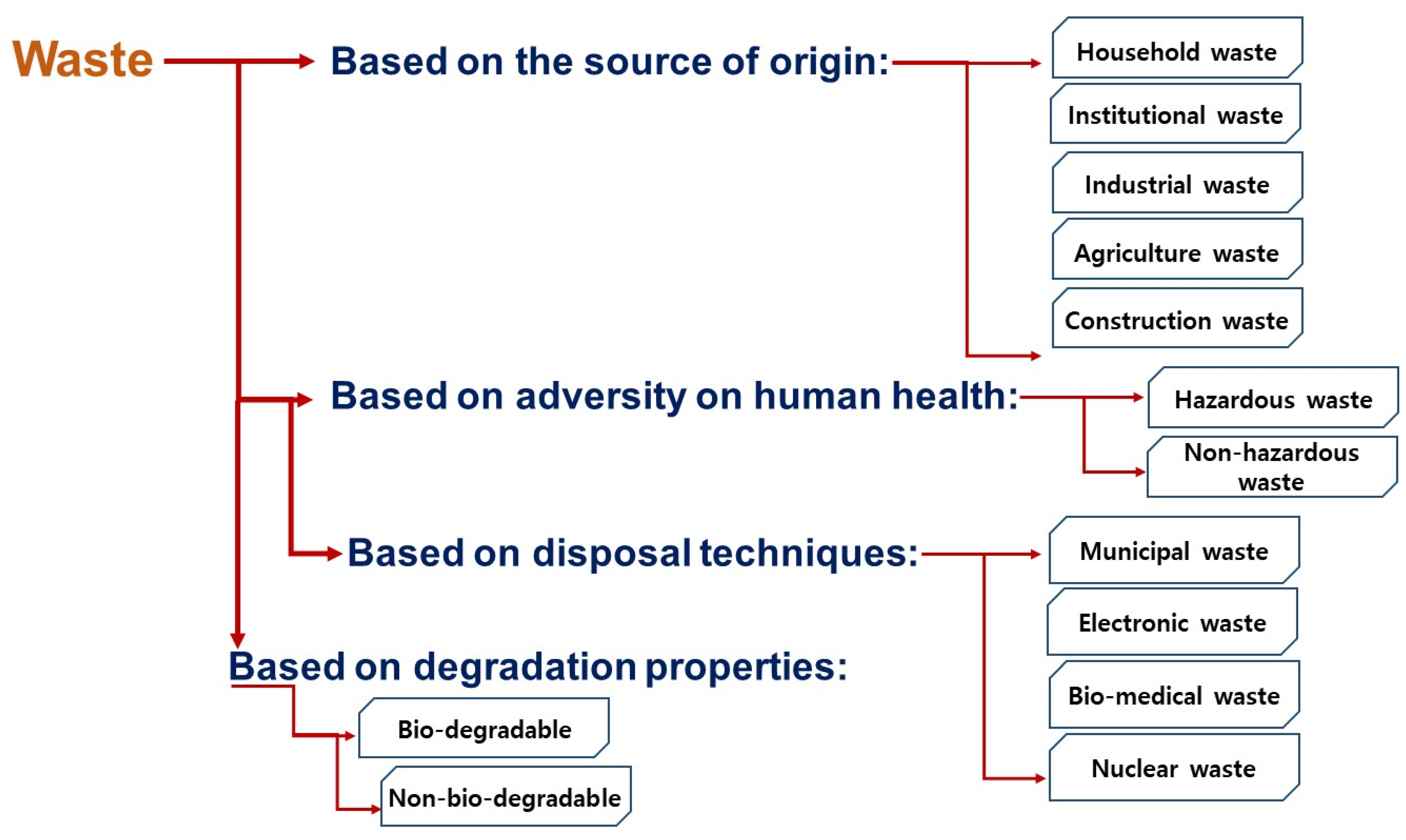
The rapid increase in urban population, rise in living standards, pace in economic growth and, consequently, the change in behavioral lifestyle have resulted in different adversities. Due to the “throw-away” practice in the society, a tremendous increase in waste generation has been recorded, which comes from different sources and different practices. Commonly, “waste” is a substance belonging to the refused, rejected, abandoned mass, and unwanted surplus volume, which is generated by different anthropogenic and/or biological activities [1]. They can be divided into several categories according to their source of generation, hazardous property, disposal techniques, and degradation properties (refer to Figure 1).
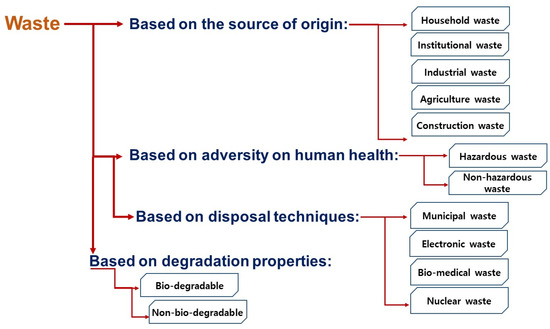
Figure 1.
Types of waste based on different categories.
Due to the structural changes in the societal behavior of an economic system, industrial activities are very important [2]. In fact, it has become an intrinsic part of the modern era to fulfill their day-to-day demands from basic needs to luxurious items by either exploring the primary sources or tapping secondary (end-of-life) materials. In order to run sustainable industrial activities, two points are vital, they are (a) the continuous supply of raw materials and (b) effective disposal of the waste generated by the industrial activities (which is an obvious part of the industrial process). Since the industrial waste generation is a huge volume as per the scale of operations (for the small/medium/large scale industries), their proper handling becomes more important than other waste (relatively in a lower quantity at the individual level, if compared with household wastes or institutional wastes or construction wastes). Though industrial waste varies in its types and characteristics and few have been summarized in Table 1. It is observed that still very less research has been done to manage industrial waste in a sustainable manner [3].

Table 1.
Types of industrial waste.
Kaza et al. [12] have correlated industrial waste generation with a country’s income group like high-income (42.62 kg/capita), upper-middle-income (5.72 kg/capita), and lower-middle-income (0.36 kg/capita) countries. For instance, American industries are quantified to be generated ~7.6 Gt of industrial waste, which is evaluated to be ~3.5 Gt for China in 2019 [10,13]. As per an estimation data from Sweden, annually 66 Mt of waste is produced whereas about 58 Mt belongs to the industrial waste category. Only 16 Mt are reused by the industries themselves, while 26 Mt (including waste from mining activities) are going for dumping as the industrial dumps, and 4.5 Mt waste that consists of non-branch-specific waste (including construction and demolition waste) are sent to landfills. As environmental regulations become stricter worldwide, it is important to find a sustainable solution for industrial waste disposal other than landfilling. Interestingly, as per the disposal techniques, waste can be categorized into four types: municipal solid waste, electronic waste, bio-medical waste, and nuclear waste. It indicates that a significant quantity of industrial waste is merged with other disposal categories, which is inappropriate to be mixed. For example, the waste generated by small and medium enterprises (SMEs) are always dumped as municipal solid waste, sometimes including electronic and mine waste. The waste generated by these SMEs are very different in nature, for instance particle size, moisture content, density, permeability, and heavy metal composition than that of other types of wastes; however, they are vital for the economic and social development of any country and necessitate managing industrial waste to avoid environmental pollution and minimize limited resource consumption. Owing to this, the concept of circular economy is introduced in the industrial sectors that work on the concept of recycling and reuse for efficient utilization of resources and issues associated with disposal.
Additionally, the United Nations Sustainable Development Goals (SDGs), SDG11 and SDG12, also highlight the sustainable management of industrial waste and recycling for the re-use of end-of-life (waste) materials as the potential secondary resource. Henceforth, in-depth understanding is needed to improve waste processes to shift towards sustainability and create an environmentally friendly society, free from the risk of resource exhaustion for their well-being. The opportunities and challenges for reuse and recycling should be preserved. Nevertheless, cumulative discernment, rapid elaboration, and diverse societal, political, and economic challenges result in very different technical and non-technical barriers that present a complex and uncertain issue [14]. Indeed, it also needs to understand the opportunities and challenges for their reuse under the industrial ecology and recycling for solving the associated social and environmental issues. Highlighting these opportunities and challenges is a significant step towards improving waste management outcomes, developing technologies, and implementing alternative solutions.
2. Research Methodology
To extract the articles on the topic presented herein, the initial keyword “industrial waste management” was searched, yielding more than 27 million results. Then after the searched keywords “industrial waste management articles” could reduce the number to a-half. To narrow down the search, we used the same keywords in sciencedirect.com, which limited the search to 192,027 (17,537 reviews; 124,327 research articles; 3432 encyclopedias; and 24,005 book chapters), which could be limited to 129,030 for the years between 2011–2023, and we focused on research articles only (more than 89,000 articles). Later on, red mud management (5938 articles) and coal fly ash management (6384 articles) were chosen to go through. To ensure relevance to the topic of this review article and needful information to share with the readers, about 220 items were manually screened out and included herein.
3. Why Industrial Waste Is a Problem and Sustainable Management Is Required?
The discharge of industrial activities is crucial to producing different forms of waste that widely range from manufacturing to electronics and auto repair. This includes scrap metal, chemicals, plastic waste, and a range of other potentially toxic compounds [15,16]. Industrial waste poses a serious threat to both the environment and human health. It can cause severe contamination of soil, water, and air if it is not disposed of properly. Subsequently, this can have negative impacts on humans, including the health of the workers in the industrial facility and its surroundings. Interestingly, open dumping is yet to be regulated as it is still a common disposal practice in various industries. The legacy of mining activities in the form of debris mountains is usually seen, while the fly-ash mountain is a common view near a coal-burning power plant or smelter using metallurgical industries [17].
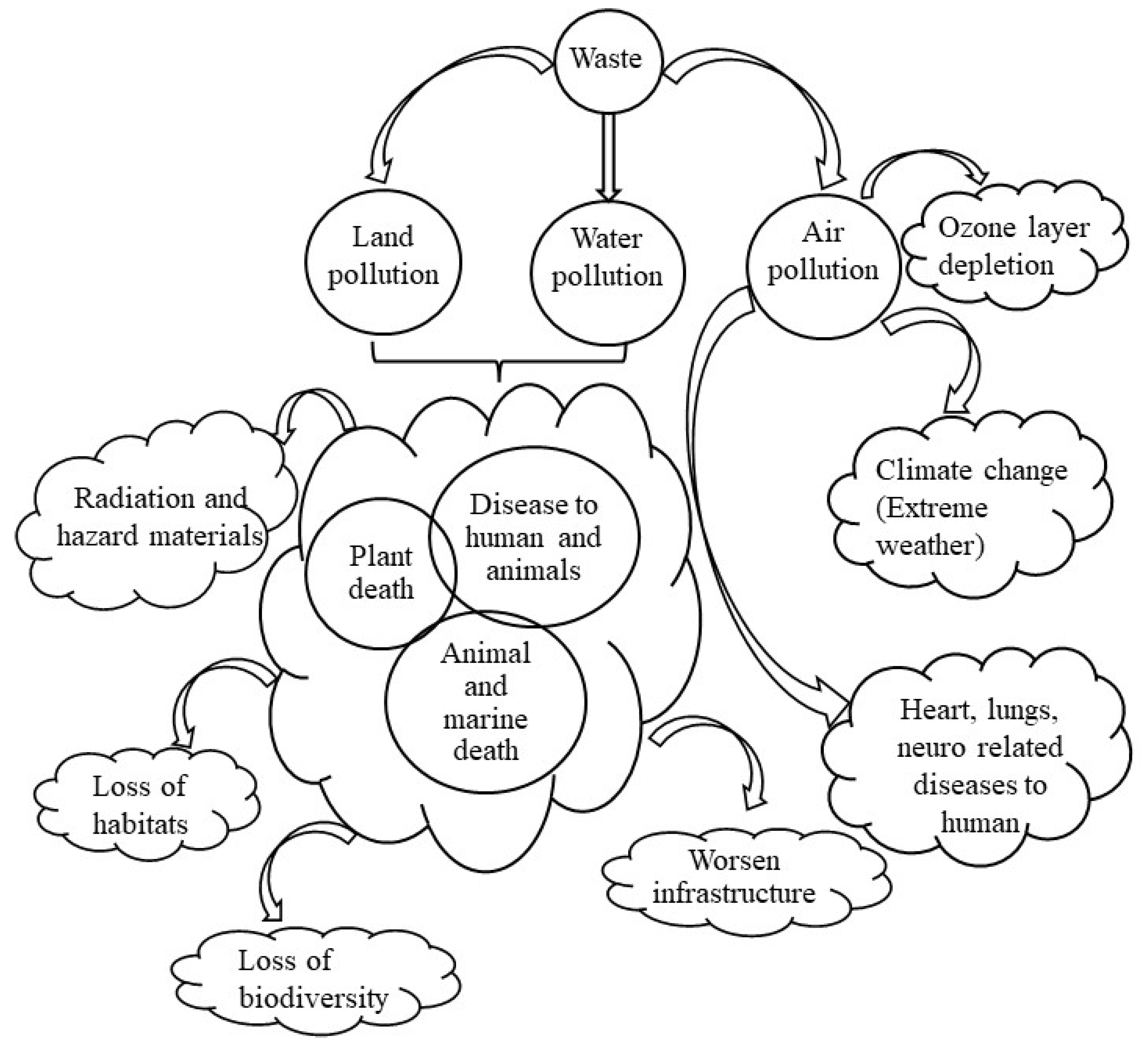
A large part of industrial waste contains heavy metals which can be hazardous and toxic in nature (refer to Table 2), however, infiltration of which can pose significant adversity to the flora and fauna. In and around the dumping yards, leachate generation due to the degradation of organics and/or weathering effects is supposed to happen when water is infiltrating through the wastes, which directly changes the soil nature of the surroundings and contaminates the groundwater. Dust and litter from the waste can be scattered by the wind, which leads to air quality deterioration in the vicinity of disposal sites. Sanitary methods of waste disposal also produce odor and affect the aesthetics of the area. The decomposition of wastes also releases noxious gases like dioxins and other toxins which lead to skin irritation, respiratory problems, and other illness related to the lungs, neuro, and heart [18,19,20]. Consequently, the greenhouse gas (GHG) emission generated by the waste is creating hazards, causing global warming, strong storms, typhoons, and unbearable heat that leads to climate change. The emission of carbon dioxide gas (CO2), a prominent GHGs can increase the rates of carbonation in reinforced concrete structures, reducing the life of infrastructure [21]. It changes the size of the habitat animals need to survive in the environment [22,23,24]. Waste and garbage can also affect animals and marine life as soil and water can be poisoned due to the waste-induced toxic chemicals that arise from industrial waste [25,26,27,28]. Climate change along with soil and water contamination leads to the extinction of species and a threat to biodiversity [29,30,31]. Additionally, waste from nuclear reactors and spent fuel are growing concerns as they pose a serious problem to the biological systems [32,33,34,35]. In nutshell, the issues related to industrial waste that leads to environmental pollution can be seen in Figure 2.

Table 2.
The adversities related to different metal contents in industrial wastes [36].
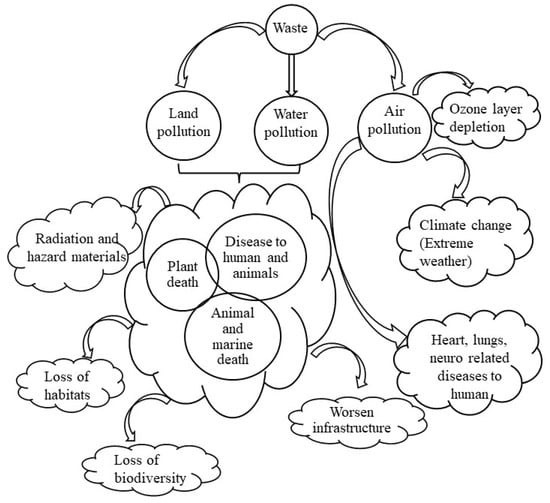
Figure 2.
Adverse effect of waste on the environment and human health.
Air pollution, damage to watersheds, and contamination of soil are issues due to improper management of industrial waste. Since the 1970s, laws have existed to prevent the disposal of chemicals and industrial or radioactive wastes into the ocean. However, several environmental incidents have happened that led to new regulations and care for sustainable management. According to the Environmental Protection Authority Act (EPA)-1968, the ocean-dumping practices have been quantified to 4.5 million tons of industrial waste, 38 million tons of dredged material (34% polluted), and 4.5 million tons of sewage sludge (contaminated with heavy metals). Waste management infrastructure becomes overburdened when materials are eligible for recycling but finds their way to landfills instead of being reused or repurposed. Therefore, it is of utmost importance to manage the waste coming out from different industrial sources.
On the other hand, incessant requirements for industrial products have also created unsustainability in material supply. The raw materials (primary resource materials) used in the industries are limited and created a huge burden in material logistics that is converted as industrial waste. Therefore, proper handling of waste volume along with recycling, reuse, and smart disposal techniques can be a sustainable way to industrial waste management. The decision-making process toward efficient waste management is greatly impeded by environmental pressures, economic growth, and societal sustainability [37]. Nevertheless, sustainable waste management needs in-depth studies on the reduction, treatment, recycling, and final disposal (waste combustion and landfilling) of industrial wastes and it helps in reducing the rapid elaboration, cumulative discernment, and diverse social, political, environmental, and economic challenges [14,38]. It should also contain resource efficiency, carbon neutrality, and endorse cleaner production activities to conserve “waste as a resource” [14].
4. Industrial Waste Regulations
International regulations and laws, such as the Kyoto Protocol (1997), Paris Agreement (2015), COP27 [39] have addressed the issues of carbon emission, climate change, and resource conservation. Recently, COP27 has highlighted the contribution of the waste sector towards global GHG emissions is 10%. It was also estimated that open dumping accounts for 31% of waste management whereas some lower-income countries rely on it for up to 93% of their waste disposal. Mismanaged waste affects health and the environment, and contributes to GHG emissions, as the black carbon aerosols may have 5000 times more global warming potential than CO2 [39]. Therefore, a regulation on waste management not only saves the environment and protects human health, but also mitigates the risk of massive fines and penalties for non-compliance. The Environmental Protection Authority (EPA) of Taiwan sets the laws on industrial waste management namely, “Environmental Protection (Industrial Waste Resource) Regulations 2009” under the hierarchy of eleven principles of environmental protection contained in the EPA-1970. It provides information on (i) characterizing constituents of industrial waste, (ii) waste minimization, (iii) fact sheets, and (iv) assessing risks posed by certain wastes. The waste management hierarchy shows the order of preference as follows:
- Avoidance
- Reuse and recycling
- Recovery of energy
- Treatment and disposal.
Accordingly, the best practice can be to minimize waste generation instead of its treatment and disposal, which is the least preferred approach. Additionally, the EPA can fine over $72,000 per day, per violation for non-compliant hazardous waste disposal or processes. Moreover, the US EPA also provides resources for state-by-state policies and guidance for all waste streams, whereas, the Federal Resource Conservation and Recovery Act (RCRA) provides oversight of hazardous wastes. As such, every state can also apply for EPA state authorization, which turns over the responsibility of hazardous waste management to the state level.
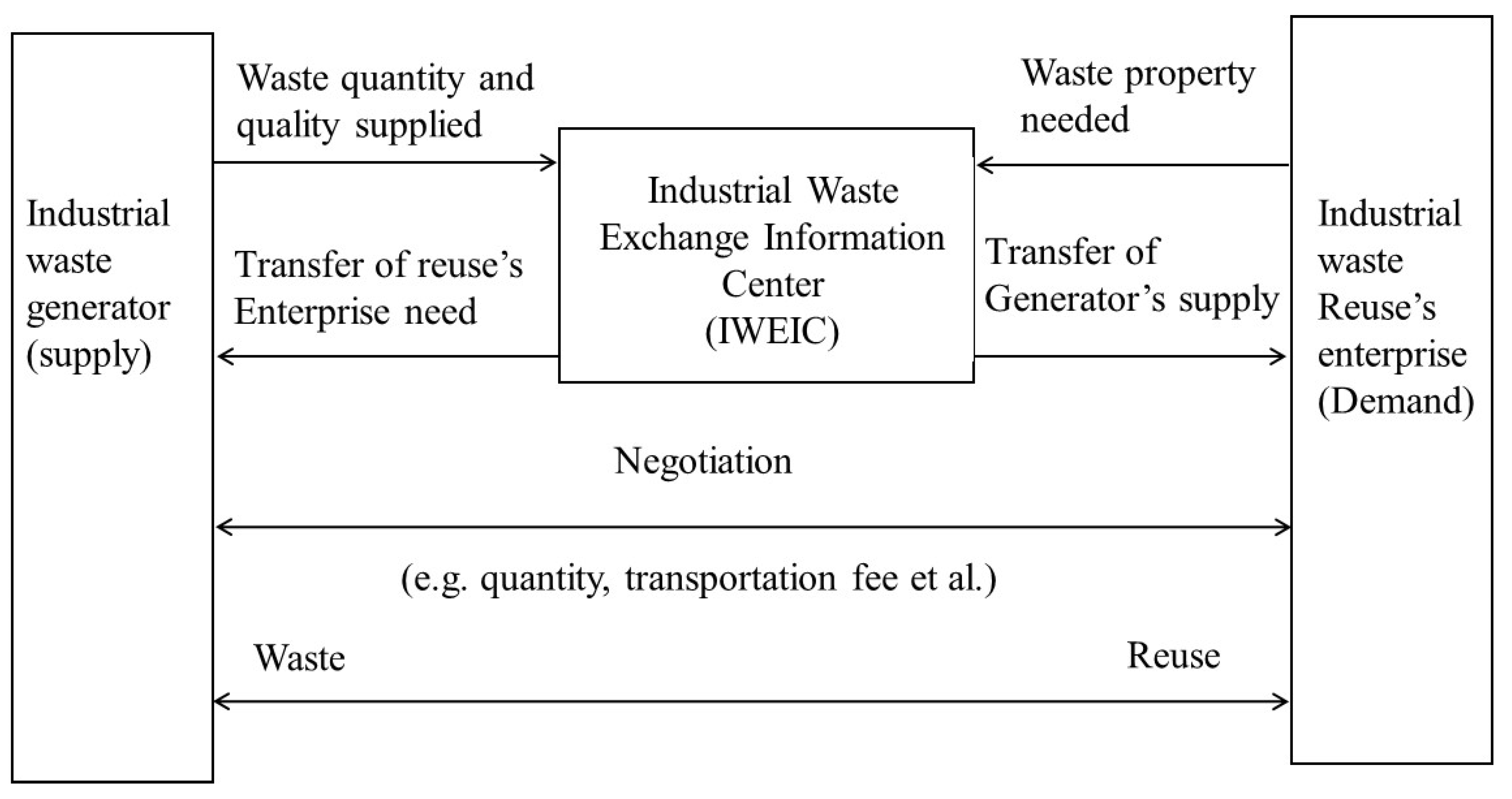
In this context, the Taiwanese government promotes industrial waste recycling under the Waste Disposal Act (WDA), Resource Recycling and Reuse Act (RRRA), and Environmental Basis Law (EBL) by imposing regulations on industrial waste management [40]. The resultant concept has been jointly developed by Taiwan’s EPA and Industrial Development Bureau (under the Ministry of Economic Affairs) after commissioning the Industrial Technology Research Institute (ITRI) that established the Industrial Waste Exchange Information Center (IWEIC) for the promotion of industrial waste exchange, as depicted in Figure 3 [41,42]. Besides, Korea has also endorsed the Framework Act on Resource Circulation (FARC) like Europe in 2016, to build a sustainable material circulation society by minimizing primary resource consumption and waste generation. It has enabled recycling and suitable disposal of the generated waste [43,44]. The FARC has emphasized giving priority to building a circular society through waste management that can reduce the waste at source, reusing the generated wastes, recycling wastes that cannot be reused, and thermally recycling the wastes that are difficult to recycle will be landfilled. India and Thailand governments have implemented hazardous and industrial waste management that includes the Factory Act and Hazardous Substance Act [45,46].
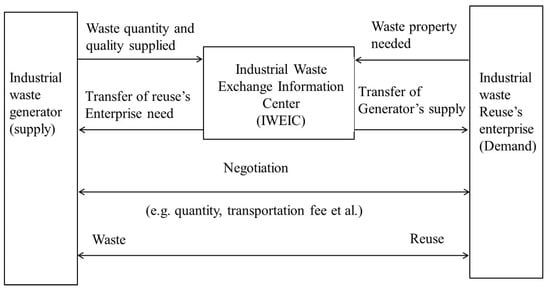
Figure 3.
Exchange schematic of industrial waste developed by IWEIC, Taiwan [41,42].
5. Sustainable Management of Industrial Waste
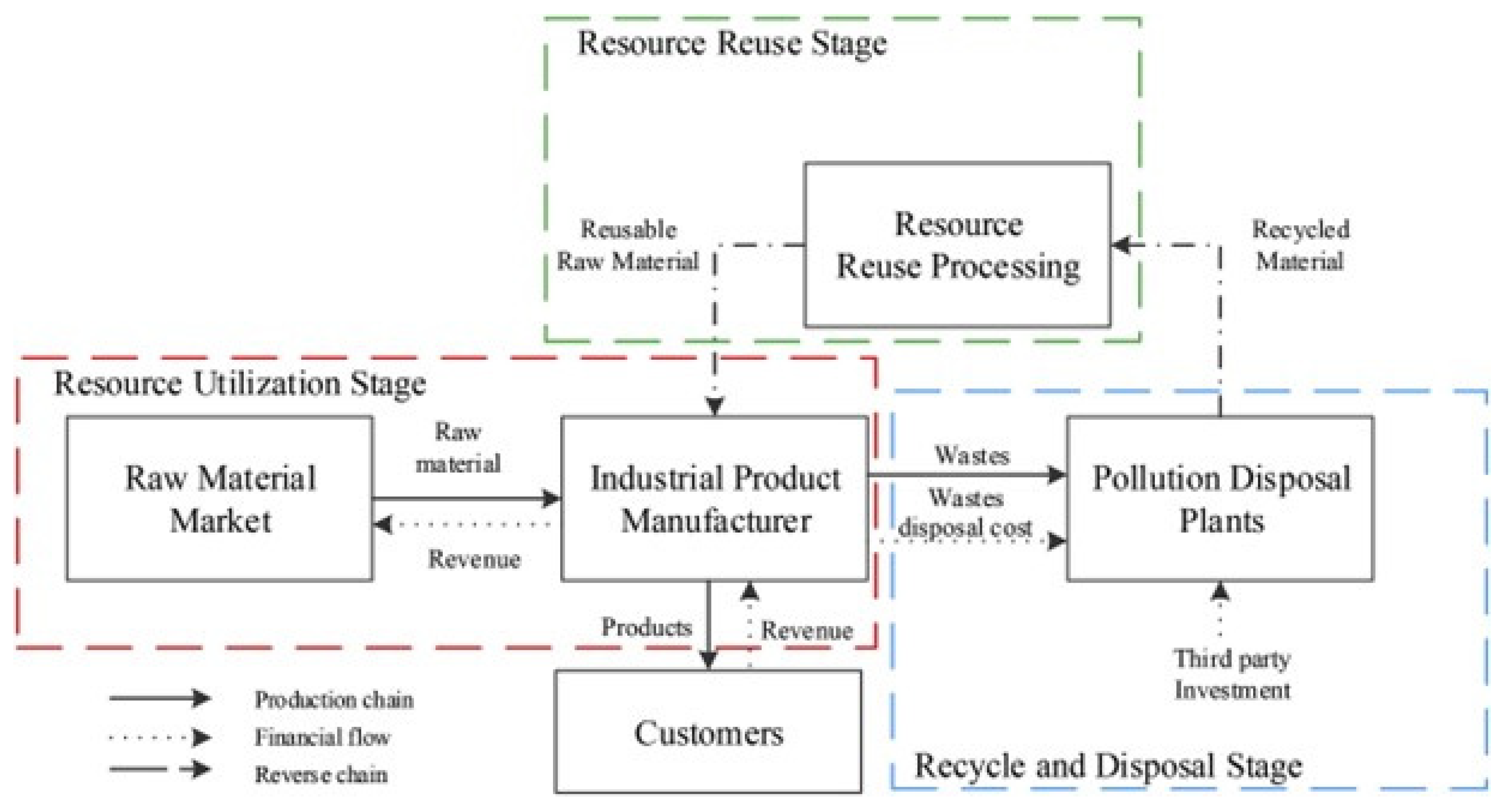
The waste generated from industries is crucial to treat and has been classified into three categories: (i) chemical waste, (ii) solid waste, and (iii) toxic and hazardous waste based on their treatment. The linear economy model has been used in the industry i.e., take-make-dispose, which is unsustainable and creates huge amounts of waste materials that can be either toxic or have the potential to recycle as resources [47]. Environmental problems and resource conservation have been critical issues worldwide therefore, a closed-looped circular economy has been introduced to industrial design and process where the recycling and reuse concept is introduced to convert waste into a resource [44,48]. The industrial circular model proposed by Li et al. [49] showed that the linear economy where raw materials go to manufacturing and disposal can be reversed flow in a closed loop system. Wherein disposed materials are reprocessed and recycled to further use in manufacturing and from that product can be made (as depicted in Figure 4). Thus, the circular model can minimize waste generation, provide secondary raw materials, and generate revenue.
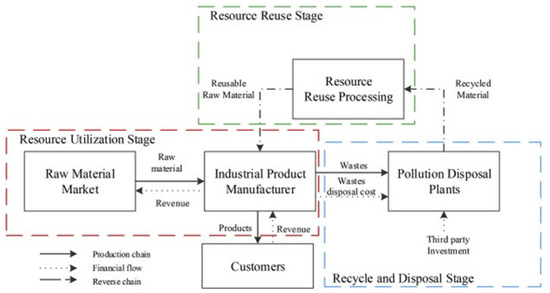
Figure 4.
Processing of industrial waste using the circular economy model [49].
Recently, Kanwal et al. [10] assessed the recyclability of industrial waste for anthropogenic circularity for 18 different classes of industrial waste (refer to Table 3). A lower recyclability of industrial waste of grade (D) indicates a less efficient metal recovery; whereas, a higher degree of material mixing (H) is more accessible and economical to recycle than others. On contrary, a lower degree of mixing with a higher R-value is better in terms of the process economy. For example, red mud, gold tailings, gypsum, and Cu-slag have higher recycling rates than that Fe-vanadium-slag, dried oily sludge, and coal fly ash has lower recyclability. In order to understand industrial waste management, therefore, we have chosen one case study from each type of industrial by-product i.e., (i) red mud from Bayer processing of bauxite ore, and (ii) fly ash from coal-burning; leading towards the circular economy approach that minimizes environmental pollution and to build industrial ecology.

Table 3.
Entropy, grade, and recyclability of industrial waste [10].
5.1. Industrial Waste Management of Red Mud
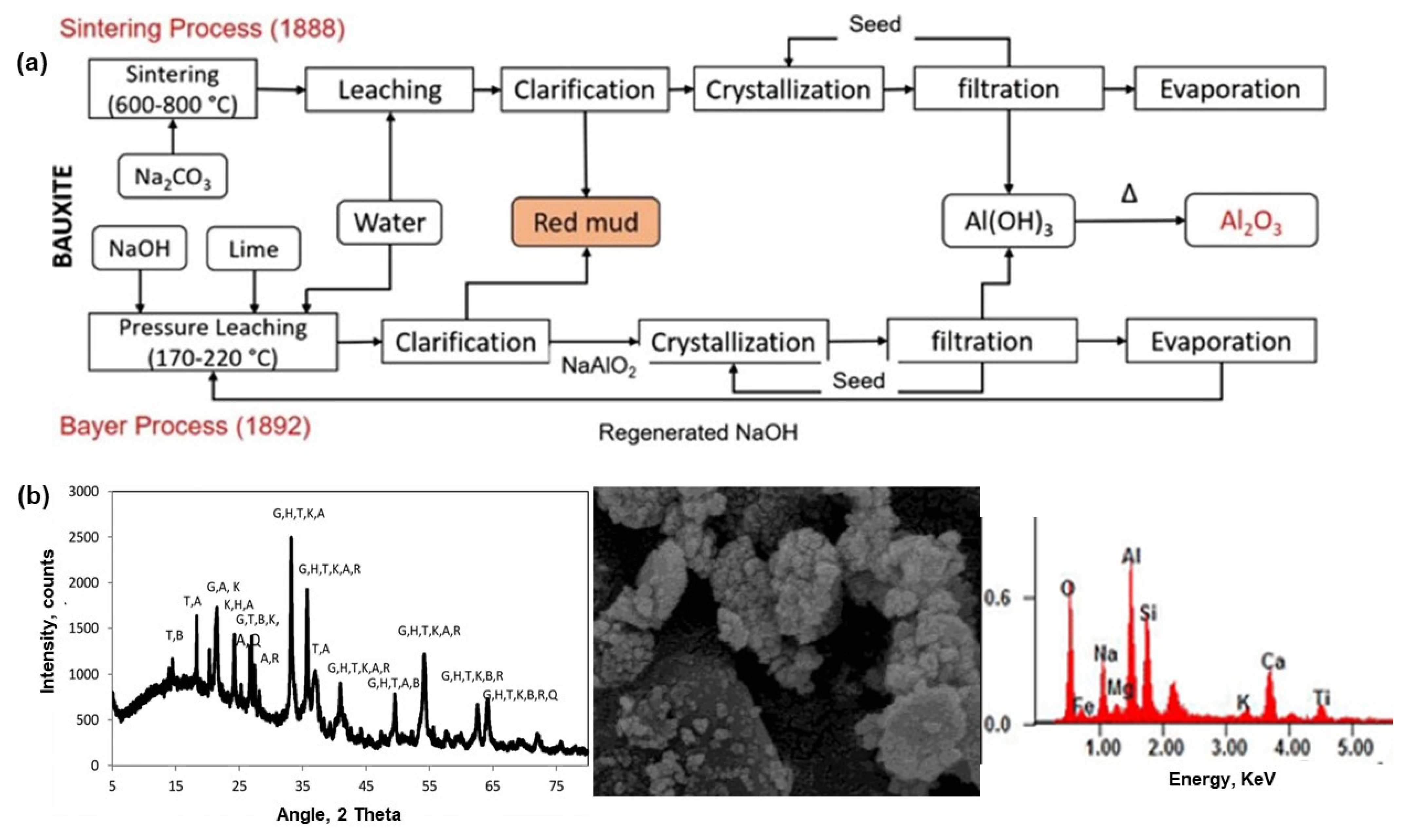
Red mud belongs to one of the major stockpiles of industrial wastes, which is generated by the Bayer process in alumina production from the primary mineral, bauxite (refer Figure 5a) [50]. As an estimate, the global stockpile of red mud is over 4 billion tons and growing at a rate of 175.5 Mt/year [51]. On average, 1.0–1.5 tons of red mud are generated during each ton of primary alumina production [52]. Along with its generation in large quantity, the high alkalinity (i.e., pH between 10 to 13) of red mud is a major hurdle to dispose of this industrial hazard [53], resulting in costlier disposal [54]. Currently, they are majorly stored in artificial ponds/dams and then allowed to be dried in open areas that pose environmental risks. Several incidents of dam failures significantly increase social and environmental threats [55]. Therefore, finding a cost-efficient and environmentally sustainable technique for the safer disposal of red mud is highly desirable. Depending upon their physio-chemical properties, the individual and integrative approach to utilizing red mud as construction materials, contaminants’ removal, neutralizing agent to acidic waste, valuable metals recovery, etc. can be sustainable [56,57,58]. Red mud has been classified according to the production process of alumina, as follows [59]: (i) Bayer process red mud, (ii) sintering process red mud, and (iii) combined process red mud, summarizing their properties in Table 4. The XRD analysis in Figure 5b shows the typical mineral phase therein the sample and SEM-EDX depicts the surface morphology of the red mud.
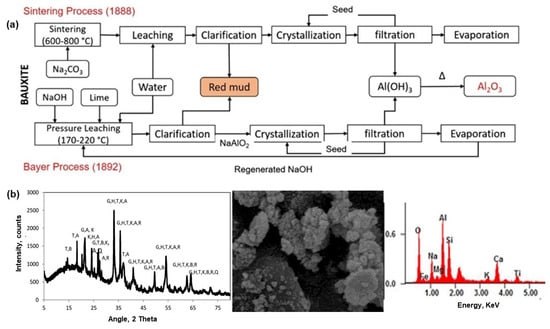
Figure 5.
(a) Schematic for the extraction of alumina from bauxite using Bayer’s and sintering process, while generating red mud as the industrial waste [50]. (b) A typical XRD and SEM-EDX analysis of the red mud sample [60].

Table 4.
The chemical and mineral composition of different types of red mud [59].
As can be seen from Table 4, the composition of all types of red mud is different and their characteristics vary with the origin of the bauxite, which can change over time when stocked in the open. Different types of red mud mainly have Fe2O3, SiO2, Al2O3, TiO2, Na2O, K2O, CaO, and MgO. β-2CaO·SiO2 is the main mineral phase existing in the sintering process of red mud, which differs from the Bayer process red mud, with mineral phases consisting of sodium aluminosilicate, calcite, aragonite, boehmite, and perovskite, along with Fe2O3, suggesting that calcium silicate is the primary phase [50,59]. Among the physical characteristics of red mud, it is a very fine material (average particle size <10 μm) of surface area 64–187 m2/g, contains large water content (700 to 1000 kg/m3) that accounts for 79–93% of the total weight, is porous in structure (void ratio 2.5–3.0), of high compressibility (Eg = 28–40 MPa), and has low shear strength (C = 9.6–74.3 kPa and φ = 13.5–21.0°). Due to the aforementioned characteristics of red mud, it exerts tremendous stress on the environment.
Table 5 reviews the advantages and disadvantages of the existing predominant red mud disposal/management processes [61,62,63,64]. As presented in the table, the dry stacking has advantages over lagooning and marine disposal albeit they are not effective in terms of the environment and resource management. Therefore, right from the stockpiling to the metal recycling from red mud needs to be evaluated to design a sustainable management of this residual waste of global concern.

Table 5.
Pros and cons of the red mud disposal/management process [61,62,63,64].
5.1.1. Stockpiling Design of Yard
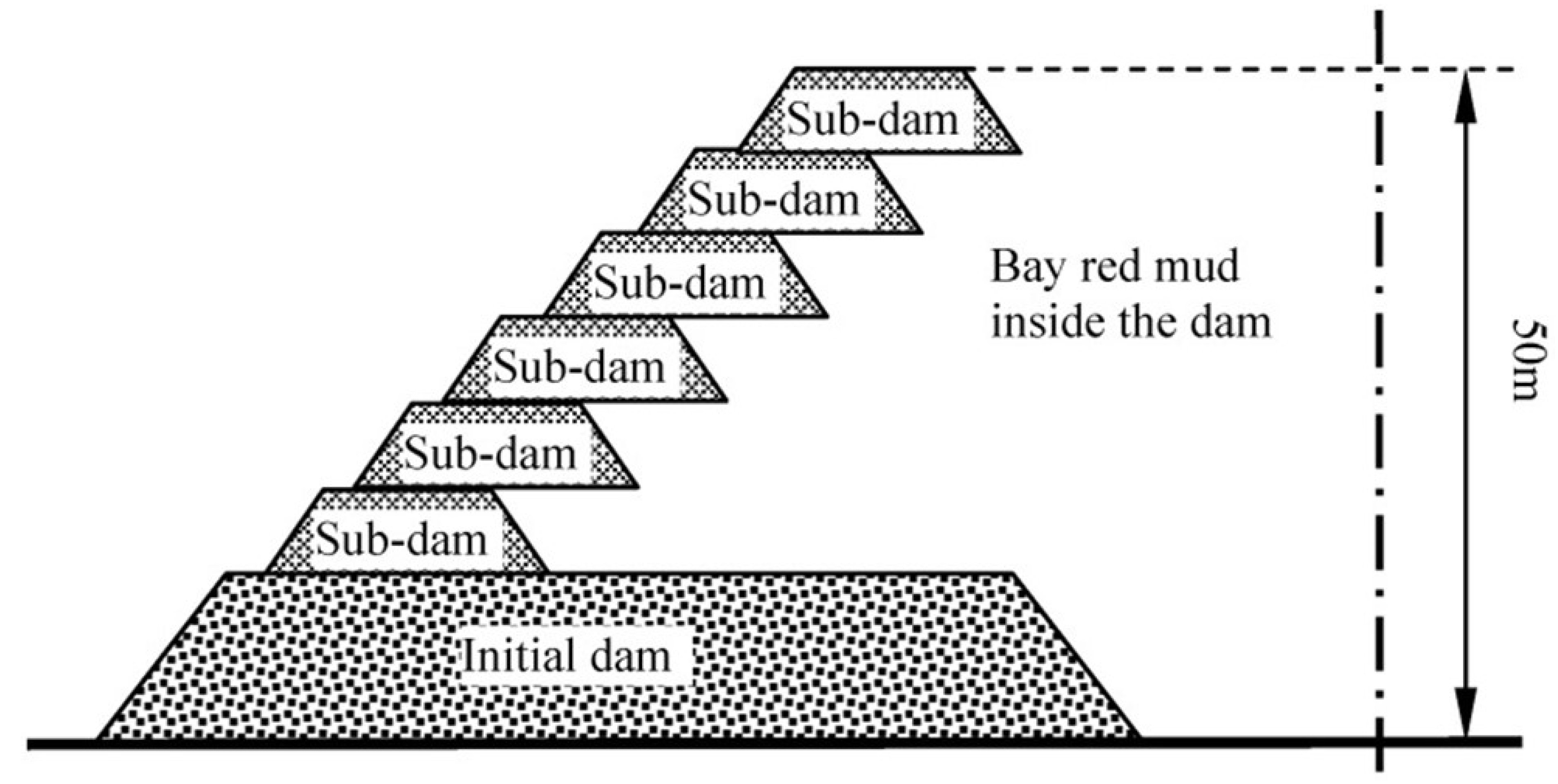
Red mud stocking can be categorized into two parts: (i) wet (involves slurry transportation and stocked after precipitation) and (ii) dry stocking (wherein desiccative red mud is transported and accumulated by air and sun drying). Dry stockpiling with an increased capacity is suitable for the sintering process, however, the construction and maintenance are costlier. To stock slurry, the stockpile dam should be firmer and impermeable contrary to the wet stockpiling, it is suitable for Bayer process red mud. Alternatively, Qiao [65] and Sun [66] designed a mixed stock method using sintering red mud and Bayer red mud in the initial dam, and Bayer red mud in the sub-dams that give advantages of small investment of the initial dam and sub-dam with uncomplicated operation management. The design schematic of a “mixed stocking” is depicted in Figure 6 [59]. Prevention of dam failure is an important safety factor by discharging the accumulated volume of liquid. For this, Zhou [67] recommended increasing the quantity and quality of overflow wells for an improved discharge rate of the liquid, keeping the dam stable, whereas Wang [68] compared several drainage reinforcement methods, such as horizontal, radiation, light, horizontal + vertical jointed well, point drainage, etc. From the finite element analysis of seepage, the study revealed that the combined vertical horizontal seepage drainage yielded better efficiency. Li et al. [69] suggested using finite element analysis in the red mud disposal field, while the development of cracks appearing in dry red mud is studied by Rao [70] who pointed out that the factors like the rate of settlement, dehydration, salt dissolution, and pressure differences leading towards the cracks and dam failure.
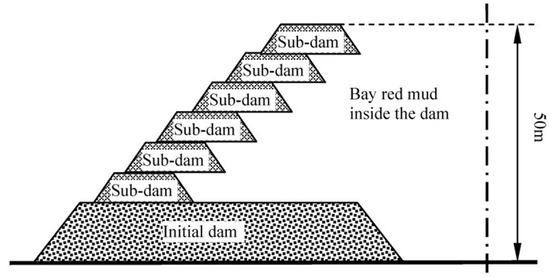
Figure 6.
Schematic of the “mixed stocking” method [59].
5.1.2. Red Mud Diversion to Be Used in Various Applications
Due to a huge stockpiling of red mud in an open environment in continuous mode of generation, there are several efforts have been taken to divert the residue material to different applications. As per the attention given in this direction, it can be divided into three categories: (i) to recover valuable metals from red mud, (ii) use as blending materials (in particular, to cement), and (iii) as a filling material (in road and building construction sites, and abandoned mines, not discussed due to less scientific importance). Besides, it has also been shown as a soil supplement (fertilizer) [71,72,73] but still, a lot has to be done to fix this application as a prominent waste management of red mud, and hence, this is excluded from the scope of this review article.
Metals Recovery from Red Mud
A significant quantity of iron in red mud is attractive to use as a potential source; however, the presence of other impurities like P, S, Na, and Si makes it difficult by creating problems for the fluidity of slag and alkali accumulation [74]. A direct magnetic separation has been employed to physically separate iron with reduced energy costs as compared to the smelting technique [75]. The thus separated magnetic part can undergo iron production (refer to Figure 7) while the non-magnetic portion can be sent to construction materials [74]. Using the magnetic separation, the improvement in iron grade was observed from 41.08% to 45.46% and 20.84% to 35.47% but with a lower recovery rate of up to 35% [76].
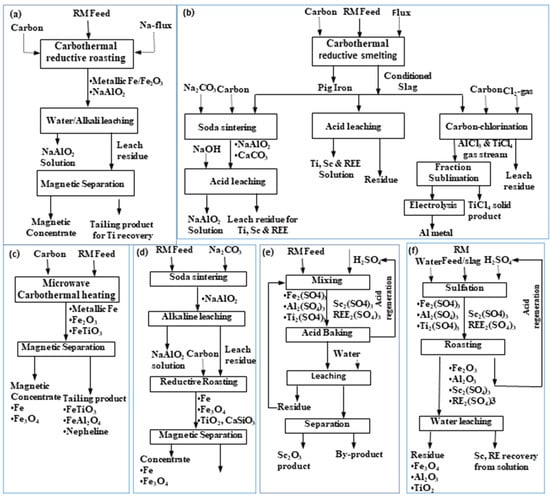
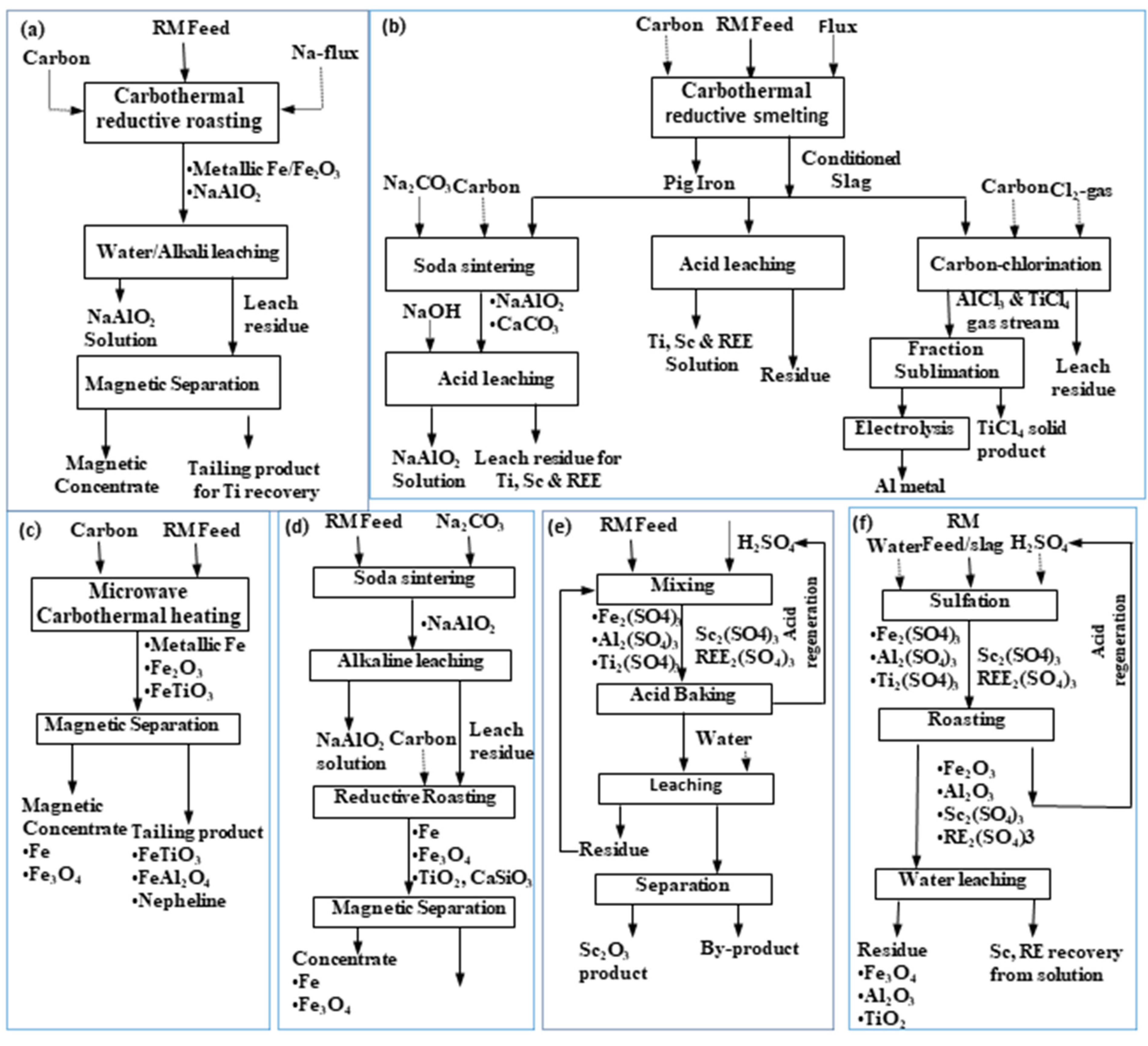
Figure 7.
General flow sheets for roast-reduction: water leaching–magnetic separation process (a), smelting–soda sintering, acid leaching, carbo-chlorination (b), microwave reduction–magnetic separation (c), and soda sintering–alkali leaching–reduction–magnetic separation (d), acid baking–water leaching, and (e) sulfation–roasting–leaching process (f) [50].
Hence, researchers tried to reduce red mud in a smelter furnace to produce pig iron [77,78] though the recovered iron has been found of a complex structure due to mixing with other metals, making it necessary to recover all major elements, like Ti and Al as well (refer to Figure 7). In this context, various reductants like coal [79], coal char [80], coke [81], carbon [82], carbon powder [83], carbon-pellets [84], etc., are employed while low-energy consuming plasma technique has been also studied with iron separation efficacy of 71% and above [85,86]. Alternatively, red mud is directly roast-reduced at different parameters of carbon dosage, temperature, and red mud ratio, getting a total iron content of about 89% with a recovery efficiency >81% [83], while the residual mass undergoing as a construction material. The reactions involved in the roast-reduction process can be as follows [81]:
Further, to enhance the iron recovery efficiency, several researchers explored changing iron grain growth using different additives in the reduction roasting process. The addition of Na2SO4 + CaO leads to a formation of compounds 2CaO⋅Al2O3⋅SiO2, NaO⋅Al2O3⋅2SiO2, 2CaO⋅SiO2, CaO⋅Al2O3, and 12CaO⋅7Al2O3 that ameliorate the magnetic separation between alumina and iron [87]. Moreover, Na2SO4 also improved iron grain growth which increased the iron grade and yielded >92% recovery. Alternatively, Zinoveev et al. [88] added 22.01% K2CO3 with Na2CO3 for reduction roasting performed at 1250 °C and 3 h of duration. However, the high amount of potassium salt was an issue that was significantly lower while using Na2SO4 additive with Na2CO3 (both were 6% of the red mud) with a higher yield of iron about 95% [89,90]. However, as the reduction roasting requires a prolonged energy-intensive process, several researchers applied microwave technique for red mud roasting [91,92], thereby achieving a magnetic concentrate of iron with lower metallization (69.3%) but at a 40% shorter time. Despite showing recovery potential, the pyrometallurgical techniques need good stability of the refractory depending upon the composition and temperature of the molten mass; henceforth, an effective technique is yet to be discovered in terms of environmental beingness [93].
To overcome the pyrometallurgical challenges, the aqueous processes depending upon the solution chemistry of metals have gained tractions in the recent past (refer to Figure 7). Debadatta and Pramanik [94] used 8.0 (N) H2SO4 at a pulp density of 20% and temperature of 100 °C maintained for 24 h yielded a 47% iron leaching, which was much lower than its calcination (at 600 °C) followed by H2SO4 leaching process that yielded 97% of iron extraction from red mud due to the hydrated mineral phases conversion to their anhydrous phases after the calcination of red mud [95]. Using organic acid, Yu et al. [96] applied oxalic acid with UV light that precipitated iron-oxalate with 90% of yield. In other studies, Çengeloğlu et al. [97,98] applied Donnan dialysis for the recovery of Fe, Ti, and Al by dissolving red mud HCl solution (0.05 to 1.0 M concentrations) prior to recovering the metals through the charged heterogeneous and Neosepta CMB and CMX cation exchange membranes. Pepper et al. [99] studied the dissolution of amorphous anatase with only 24% efficacy in diluted H2SO4 which could be enhanced up to 64.5% using 6.0 N H2SO4 at 60 °C and 5% pulp density [100]. Zhang et al. [101] applied HCl leaching for dissolving the metals into the acid solution, which was followed by a two-step solvent extraction process employing Aliquat 336 for iron extraction and then P204 for scandium extraction from the leach liquor. Salman et al. [102] studies acid digestion to enhance the rare earth’s dissolution and subsequently employed ion exchange followed before the solvent extraction for better separation and recovery of the concerned metals. In a recent study, selective leaching of scandium on a pH basis (at pH values ≥2.0 using HNO3 as the lixiviant medium) was employed, exhibiting the interfacial diffusion mechanism with the apparent activation energy value of 19.5 kJ/mol [103].
A combinational pyro+hydroapproach was employed by Erçag and Apak [104] to extract valuable minerals from red mud with coal and dolomite sintered pellet was smelted at 1550 °C for 30 min to produce pig iron while the residual slag was leached in 30% H2SO4 solution at 90 °C to dissolve the anatase phase. Further, the solvent extraction of Ti was performed using 5 vol.% D2EHPA in kerosene to extract ~85% Ti. In another study, Kasliwal and Sai [105] employed soda roasting at 1150 °C, which converted sodium aluminate as water-soluble species to enrich TiO2 as high as 76% in the leached residue. In order to extract rare earth, red mud is roasted with H2SO4 forming rare-earth sulfates and converting the other metal sulfates into corresponding oxides to further leach metals in the water-soluble form [106,107]. The water-soluble rare-earth sulfates decompose at high temperatures (700–850 °C) in comparison to titanium, aluminum, and iron sulfates (340–540 °C). The water leaching step was conducted at high liquid ratios due to the solubility limitations of the rare-earth sulfates, while the leaching was conducted at high agitations or in the presence of ultrasonic waves to ensure proper contact between roasted red mud particles and water [106,107]. The sulfation-roasting-leaching process was followed by scandium precipitation with NaOH at a pH of 7.0–8.0 and oxalic acid at a pH of 1.0–1.25, respectively [108]. Recently, Ding et al. [103] reported 92% Sc leaching using the same sulfation roasting-water leaching process at the optimized condition of roasting at 1023 K for 1 h duration using H2SO4 to red mud ratio 0.9 mL/g followed by leaching at 323 K, pulp density 5 mL/g, stirring speed 200 rpm, and time 2 h. Whereas, Archambo and Kwatra [109] reported rare earth’s extraction from red mud iron nugget slag which could raise their concentration by 100% and subsequently sent to HCl leaching to dissolve the REOs. Finally, the REE-oxalates were recovered by the oxalic acid precipitation route.
A generalized flow sheet for metals recovery is shown in Figure 7. Figure 7a illustrates the iron recovery by dissolving aluminum in the alkali solution via solubilizing the water-soluble sodium aluminate mineral phase of red mud. In contrast, Figure 7b depicts iron recovery through the smelting, while the slag generated in the process was subjected to sintering, acid leaching, and carbochlorination to recover other metal values like Al and Ti along with enrichment of rare earth in the residual mass. Figure 7c shows the carbothermal reduction for iron recovery through the magnetic separation of roast-reduced product, remaining Ti, and Al in the tailing mass. Figure 7d illustrates the soda roasting, NaOH leaching to dissolve sodium aluminate while the residue was roast-reduced with carbon to perform the magnetic separation of iron. Other than the alkaline medium, Figure 7e,f show sulphation roasting with H2SO4, which was followed by a water leaching step to dissolve Sc and rare earths in the solution and leaving other metals in the residue.
Red Mud as a Blending Material
The bulk utilization of red mud to produce building materials like glass ceramics, cement, and geopolymer bricks has made progress in the recent past to minimize waste disposal. Dicalcium silicate (as β-2CaO·SiO2) is a major phase in red mud (refer to Table 4) that can be vital in the crystallization of cement clinker. In comparison to coal fly ash (mainly composed of SiO2 and Al2O3), the use of red mud in cement could reduce energy consumption along with increasing the early strength and resistance to sulfate attack [58,110]. Due to the high Na2O in red mud, the solidification of Na+ helps to avoid the alkali-aggregate reaction [93]. Researchers showed the production of standard Portland cement with a large utilization of red mud (~50 wt.%) through thermal activation and adding metallurgical slag as a modifier [98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113].
The structure of red mud is quite similar to glass-ceramics (CaO-SiO2-Al2O3); hence, numerous studies have been also reported on red mud utilization in that direction [114,115]. It has been observed that the ~85% of red mud + fly ash mixture can be pushed in the glass-ceramic to lower the raw material consumption with environmental benefits [116], albeit the impurity of iron and manganese often alters the color of the final product [117] and yielding a dense product which can be solved by foam ceramics production [118]. To replace the Portland cement, the synthesis of aluminosilicate material with red mud is also tested [119,120,121]. It has been found that red mud can produce an inorganic polymer of compressive strength ~21 MPa and a 3% less water adsorption therein [121].
5.2. Industrial Waste Management of Coal Fly Ash
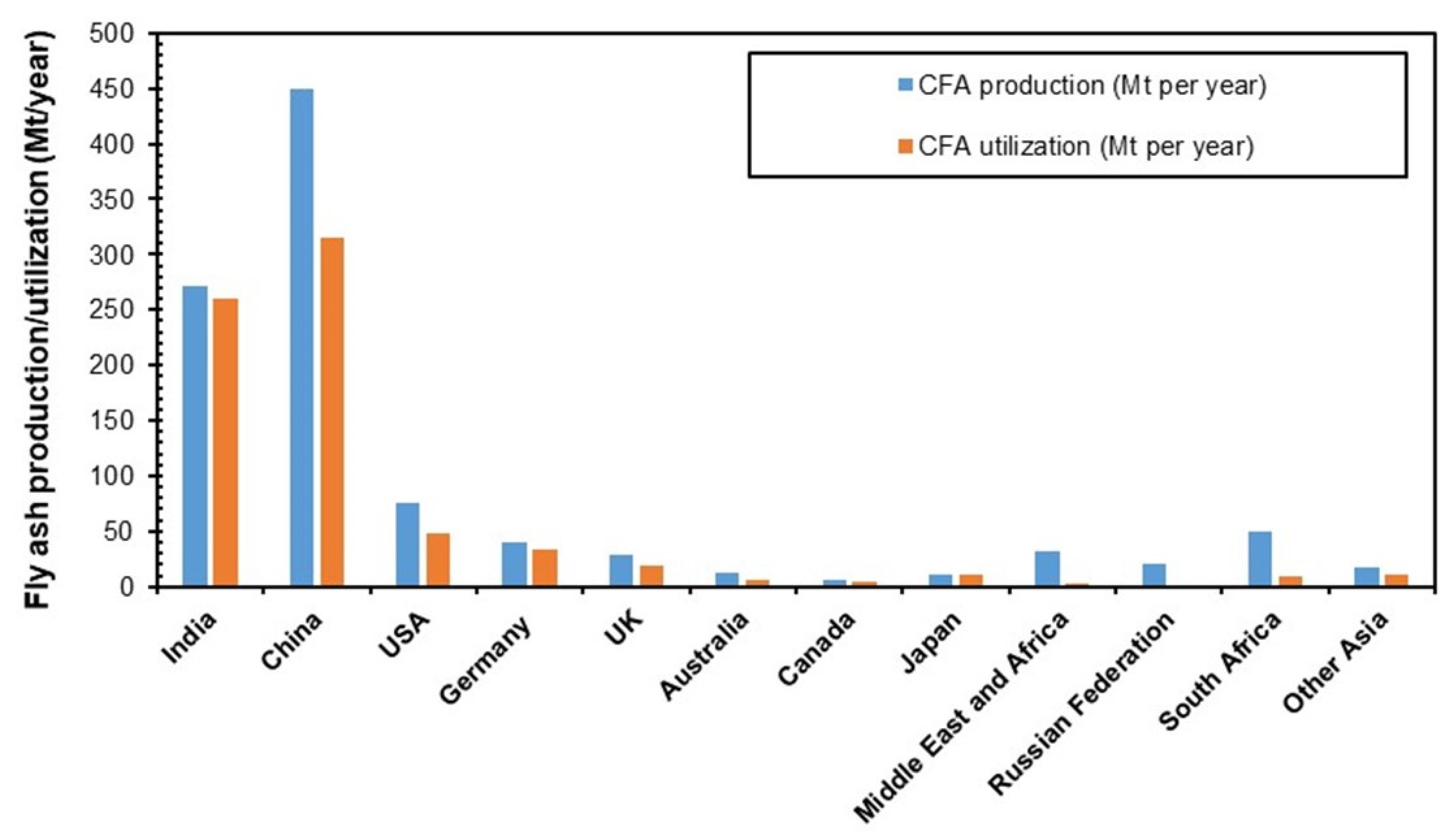
Fly ash is a major residue produced from the combustion of pulverized coal in thermal power plants and steel plants. USA, China, and India produce 75–120 million tons of coal fly ash while Europe, Africa, the Middle East, Australia, Japan, and the Russian Federation collectively produce around 150 million tons of coal fly ash every year. Fly ash utilization is only 1/4 of the total production (refer to Figure 8) [122,123,124,125,126] India and China have less than 50% utilization rate, while the Russian Federation, Middle East, and Africa have the lowest utilization rate [127].
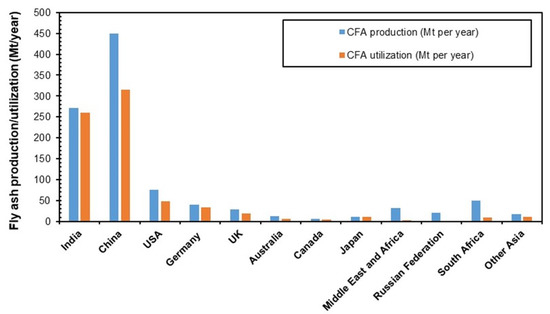
Figure 8.
Production and utilization of coal fly ash [122,123,124,125,126].
Similar to red mud, fly ash also consists of a variety of heavy metals therein (refer to Table 6), therefore, large-scale storage of it also results in severe environmental degradation soon. The potential adverse effects of fly ash are: (i) leaching of potentially toxic substances like Ca, Na, K, Mn, Fe, S, and Pb from ash into soils and groundwater, (ii) changes in plant elemental composition, (iii) increased cycling of these toxic elements through the food chain and (iv) respiratory problems to human beings [128]. Fly ash is generally spherical having 10 to 85% of total coal residue [129], while the main mineral phases are SiO2, Al2O3, Fe2O3, and CaO [130] along with some unburnt carbon (refer to Figure 9) [131,132]. The ASTM Standard C-618 divided fly ash into two broad categories, according to their chemical compositions. If SiO2 + Fe2O3 + Al2O3 is higher than 70%, fly ash is said to be Class F fly ash, whereas if SiO2 + Fe2O3 + Al2O3 is between 50% and 70%, then it is said to be Class C fly ash. Class F fly ash (low-lime fly ash) generally contains less than 10% CaO [133].

Table 6.
Concentrations of trace elements in fly ash.
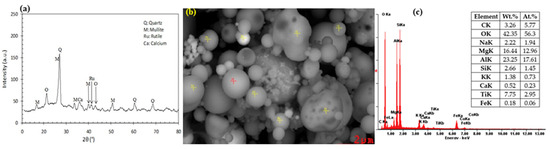
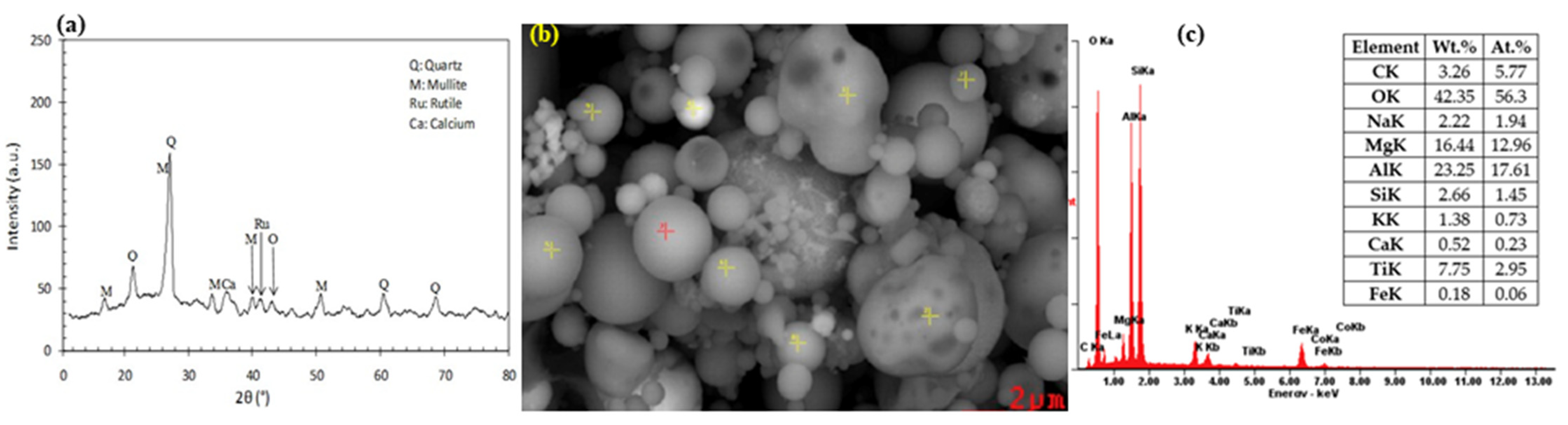
Figure 9.
Typical XRD pattern (a), SEM picture (b), and EDX analysis (c) of the coal fly ash samples [131,132].
The coal-fired thermal power plants (TPP) cannot generate electricity without creating environmental pollution in one form or another, be it air, water, or soil [134,135]. The major fly ash factors that affect the release of pollutants from fly ash to the groundwater include the quality of coal, source of water, pH, soil attenuation capacity, release mechanisms, long-term weathering, and solubility-controlling mechanisms [136]. The major disadvantage of fly ash application to agricultural land is the metal(loid) enrichment and toxicity, and plant nutrient imbalance such as phosphorous deficiency in soil [137,138,139]. Inhaling of fly ash particles imposes health risks by leaching genotoxic compounds that cause diseases like asthma, bronchitis, and even lung cancer [136,140].
To cope with the associated issues with coal fly ash, an average utilization rate of it is 60% [140], which has been separately found to be 68–70% for China (comprehensive utilization of 408 Mt), 54% for the United States (comprehensive utilization of 23.76 Mt), nearly 100% for Japan (12 Mt), 90% for the European Union (comprehensive utilization of 36 Mt), and 63% for India (comprehensive utilization of 106 Mt) [141,142]. The main aim of any environmental policy is to minimize the environmental impact of waste and to motivate recycling on a large scale [142,143,144,145]. According to the European Standard EN 450 “Fly Ash for Concrete” means high lime fly ash obtained from the combustion of pulverized lignite coal cannot be utilized as concrete addition and must be used for filling open cast mines [146]. In the United States, laws and regulations associated with coal fly ash management are different between distinct states. Fly ash management includes the current disposal practices, the cost of alternative disposal methods, as well as the current and potential future utilization of coal fly ash [147]. According to the Resource Conservation and Recovery Act, coal fly ash has been classified as non-hazardous solid waste and suitable for surface impoundments, landfills, and as fill-in surface or underground mines. The US energy protection agency also limits toxic discharges like cadmium, and chromium (less than 90%) from coal-fired power plants’ ash wastewater [148]. The utilization of fly ash in China is a slow-developed process and only building material purposes, construction projects, and Al-extraction are the common practices [149]. According to the Law of the People’s Republic of China on the Prevention and Control of Environmental Pollution by Solid Waste, industrial solid wastes can be termed as (a) general industrial solid wastes and (b) industrial hazardous wastes. General industrial solid wastes are further divided into two categories, i.e., class I and class II, depending on the environmental impact. Coal fly ash falls into class II general industrial wastes and it is not hazardous according to the Chinese environmental protection agency [150]. Japan Coal Energy Center (JCOAL) leads the development of the utilization of coal fly ash. Coal fly ash utilization in Japan is 66% in the cement area, 14% in the civil engineering area, 4% in building, 1% in agriculture, and 15% in other uses [147]. Australian Environmental Protection Act and Management regulations follow the same law and regulations as those of the United States. However, the Department of Environment and Climate Change of New South Wales strictly limits lead (>100 mg/kg), Cadmium (>10 mg/kg), and mercury (>5 mg/kg) content in the coal fly ash and hot water-soluble concentration not to exceed 60 mg/kg [151]. The Ministry of Environment and Forests (MoEF) of India issued a regulation on 14 September 1999 in which existing (old) and new coal-based thermal power plants must utilize 100% of the produced fly ash [152].
5.2.1. Fly Ash Diversion to Be Used in Various Applications
Coal fly ash is rich in metal oxides (like SiO2, Al2O3, Fe2O3, CaO, etc.), suitable to be used as a cement supplement to replace gypsum, zeolite preparation, and geopolymerization while the potential recovery of a significant quantity of rare earth has also been explored. A brief summary of coal fly ash treatment and the action mechanism is shown in Figure 10. Due to coal remains a major energy source worldwide and therefore its continuous generation needs to be taken care of, mainly via (i) the blending with cement and geopolymer materials, and (ii) recovery of metal values particularly the rare earth elements, besides sending it as a filling material.
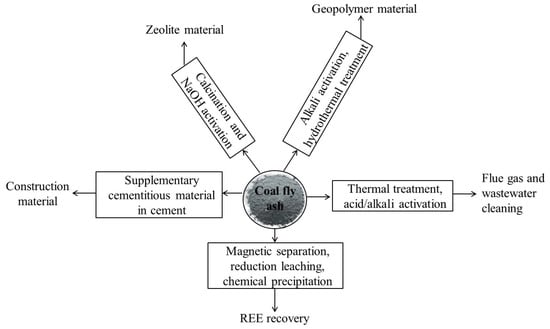
Figure 10.
Various treatment routes to recirculate the fly ash from waste stream to different end applications.
Use in Cement and Geopolymer Production
Due to a huge stockpiling of red mud in an open environment in continuous mode of generation, there are several efforts have been taken to divert the residue material to different applications. The efficient utilization of fly ash is a need of our modern society so that disposal costs can be minimized by decreasing disposal permitting requirements and it will be a source of finance or can replace some scarce or expensive resources. The concept of a circular economy has been implemented to manage fly ash in TPP and has been categorized as the fifth largest raw material resource [153] and used as an alternative to conventional materials in the cement industry [154]. Velandia et al. [155] reported the addition of Na2SO4 as a chemical activator to produce the blended cement that contains about 50% of fly ash. They claimed that the chemical activation by Na2SO4 did not have the same effect on ettringite formation while using fly ash of higher (9–11%) Fe2O3 content. Wilinska et al. [156] employed Ca(OH)2 together with Na2SO4 for the hydration and activation of fly ash-to-cement mixture at a ratio of (80:20). They found that the chemical activation promotes ettringite precipitation after 28 days of hydration. The result was in-line with the development of the pozzolanic activity, ettringite formation, and increased rate of hydrolyzed precipitation [157]. Recently, Toit et al. [158] mixed 70% siliceous coal fly ash with 30% Portland cement and added 5% Na2SO4 as the chemical activator to investigate the effect of mechanical activation by milling the mixture for up to 365 days. An increased rate of the pozzolanic reaction was observed in the following order: unclassified coal fly ash < unclassified coal fly ash + 5% Na2SO4 < mechanically activated coal fly ash < mechanically activated coal fly ash + 5% Na2SO4.
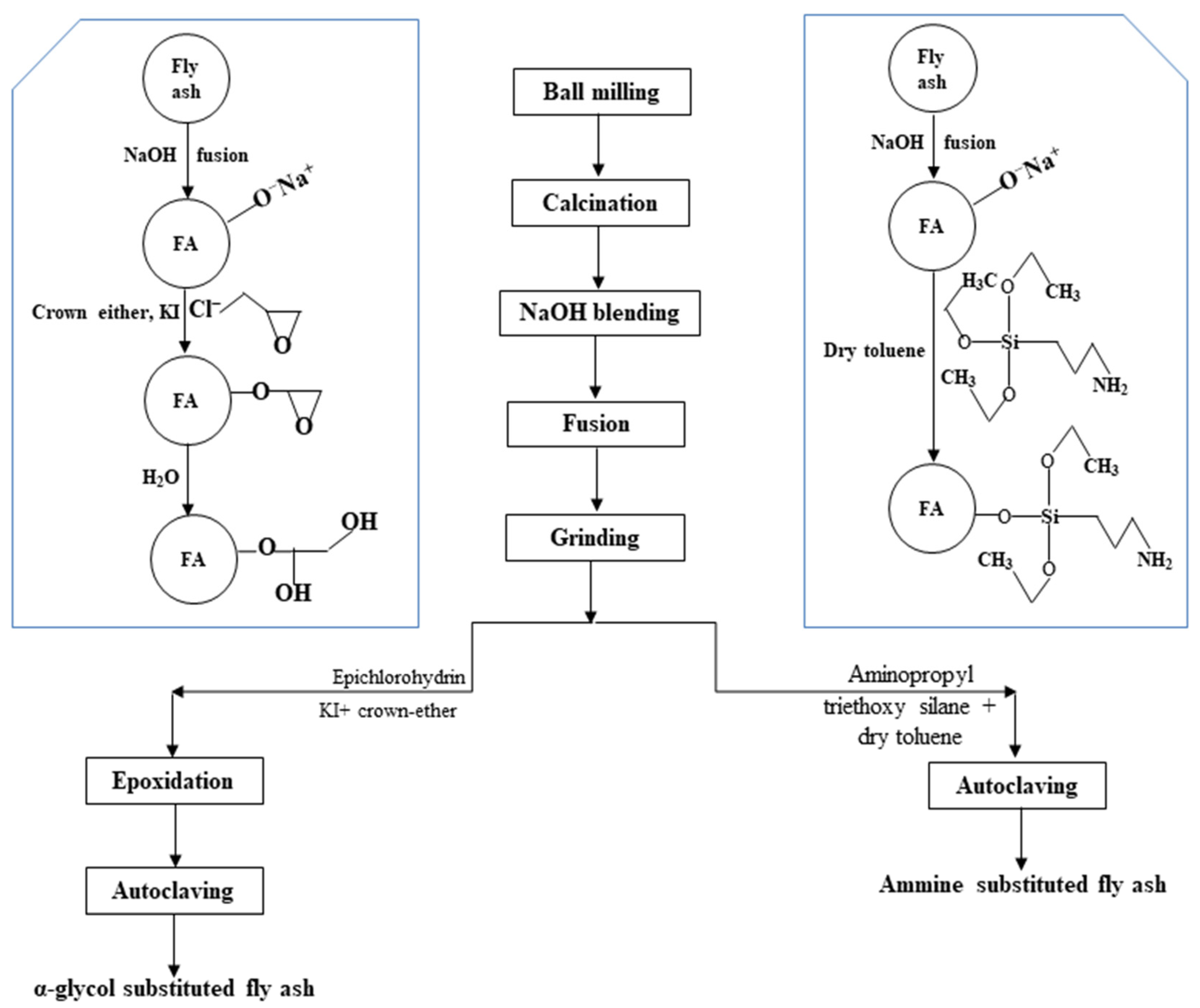
Singh et al. [159] reported that the maximum utilization of fly ash has remained at 44.26% in the cement sector, followed by the reclamation of low-lying areas (10.77%), mine filling (13.0%), fly ash dyke raising, roads and embankments (12.88%), bricks and tiles (11.72%), and agriculture (1.93%) in recent years. Even after application in these sectors, only 55.69% of the total fly ash is utilized. In a scanty work by Gautam et al. [160], the mechanochemical activation technique via ball milling of fly ash was followed by calcination at 600 °C, and then, NaOH fusion at 600 °C for 2 h was performed before the functionalization of fly ash. This study was based on the earlier determined breakage distribution function (between 0.2088 and 0.5253) and specific rate of breakage (between 0.0409 and 0.1896 per min) for a particle size ranging between 20–125 μm [161] along with diffusivity rate of dissolved silica through porous media (i.e., 2.28×10−11 to 2.96×10−10 cm2/s) [131]. To achieve the functionalization of fly ash, the alkali-fused mass was separately treated with epichlorohydrin and aminopropyl triethoxysilane to obtain the α-glycol substituted and amine-substituted fly ash (as illustrated in Figure 11). Both functionalized fly ash exhibited the desired rheological and filtration loss behavior, meeting the standard specifications of API-grade bentonite. The thus obtained functionalized fly ash was found suitable to replace the API-grade bentonite used as the drilling fluid additive in an oil well.
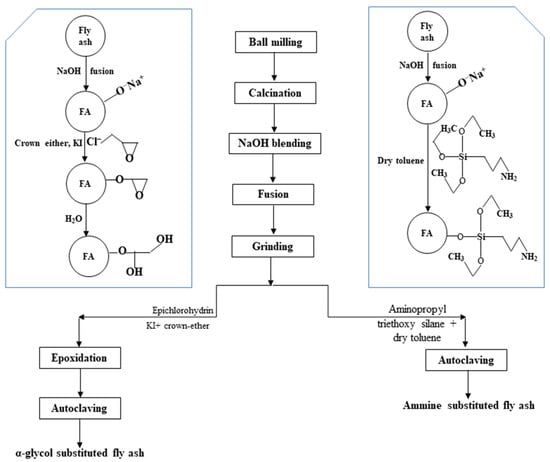
Figure 11.
Novel functionalization of coal fly ash to replace the API-grade bentonite as drilling fluid additive [160].
Fly ash was also converted into a geopolymer precursor, which is used as an additive binder for the production of cement. Fly ash exhibits hydraulic or pozzolanic behavior and reacts with water or aqueous calcium hydroxide to form pozzolanic cement (a hydration product). The direct replacement of fly ash with high bulk volume with cement is not common as it retards hydration, delays setting time, and reduces the development of early-age strength in concrete. However, the utilization of fly ash may be improved by adjusting the fineness, particle size distribution, shape morphology, surface smoothness, bulk density, compressive strength, permeability, porosity, the mineral composition of the phases, and the pozzolanic activity of fly ash [162,163]. Alkali-assisted dissolution of fly ash has become an important area of research in the last two decades to synthesize inexpensive and ecologically sound cement-like construction materials. Fly ash is also used in oil well drilling applications, where it acts as a stabilizing agent for the drilling fluid wastes to avoid groundwater contamination, as the extraction toxicity procedure does not exceed the limits when flying ash and drilling fluid mixture is subjected to land disposal [164,165]. It was also used as a foamable drilling fluid for deep water offshore well operations [166]. Fly ash was used as a solidifier to solidify reserve pit fluids immediately following the well completion, which prevents mobilization of potential contaminants into the soil and/or groundwater [167] and also reduces free water and toxic contaminants by solidification (which is also referred to as encapsulation, briquetting, fixation, and stabilization). Usually, the hydration of the fly ash forms a crystalline structure, consisting of calcium-alumino-silicate which results in a rock-like, monolithic and hardened mass [168]. High-calcium (Class-C) fly ash has the property of cementation and is very useful in stabilizing the drilling fluid.
Valuable and Rare Metals Recovery from Fly Ash
Burnet et al. [169] employed the magnetic separation of fly ash and took the non-magnetic fraction to be mixed with carbon and chlorinated in a fixed bed. Iron was primarily removed at a lower temperature of 400–600 °C followed by Al-recovery at ~900 °C, which was in variance with the results of Mehrotra et al. [170,171] wherein only 25% of Al and Ti were recovered. Leaching of non-magnetic separation in nitric acid followed by Al-crystallization has been also reported. Using the hydrometallurgical techniques, Al and Ti were recovered by acid/alkali leaching-precipitation-solvent extraction, or re-crystallization route [172,173,174].
Focusing on Al-dissolution in alkali leaching at different temperatures, Shoppert et al. [175,176] showed interesting results by controlling the formation of Na6(Al6Si6O24)·Na2X at a higher pulp density of >5% and Na2O concentration 400 g/L. Moreover, the kinetics analysis indicated that the leaching process was limited by the surface chemical reaction at a temperature lower than 100 °C, which was shifted to the diffusion-controlled reaction at a higher temperature of >100 °C. A group of researchers focused on (NH4)HSO4 leaching of coal fly ash to dissolve aluminum in ammonia solutions and found that the surface tension of ammonium aluminum sulfate in water decreased with raising the temperature albeit the as-obtained crystals possessed a rhombic shape with a smooth surface [177]. However, the neutralization of ammonium aluminum sulfate with ammonium hydroxide leads to form the pseudobohemite [178] whose particle size could be enhanced from a median diameter of 20.1 μm to 31.3 μm by adding 0.5 g/L sodium dodecylbenzene sulfonate [179]. A raise in temperature up to ~180 °C resulted in NH4Al3(SO4)2(OH)6 precipitation that hinders the alumina leaching process [180,181]. Furthermore, it has been observed that the particle size distribution was a little affected by varying the calcination temperature [182]. To achieve a higher selectivity on Al-leaching over iron, the use of (NH4)SO4 instead of (NH4)HSO4 has been also suggested [183]. The thermochemical treatment performed at a temperature = 600 °C and (NH4)SO4-to-fly ash ratio = 1:3 for 1 h was subjected to water leaching that resulted in 95% of aluminum [184].
In a study, Xu et al. [185] employed the acid mixture of (NH4)HSO4 in H2SO4 that resulted in the leaching of more than 97% aluminum from the coal fly ash. Whereas, Aphane et al. [186] employed a combined acid and alkali leaching using H2SO4 solution in the first step and NaOH solution in the second step of leaching. The thus leached Na2SiO3 was used for a sol-gel synthesis in the presence of polyethylene glycol (as a surfactant) and H2SO4 (as a catalyst), yielding ultra-pure SiO2 nanoparticles. Several researchers applied pressure acid leaching mainly to target aluminum and iron leaching. Such process includes three stages of magnetic separation for iron separation, floatation for carbon separation, and autoclave pressure leaching in acid solution [187]. Pressure leaching carried out in an HCl solution of 354 g/L concentration at 210 °C and a pulp density of 20% yielded 95% Al leaching after 3 h, which was below 45% at 160 °C [188]. Applying the pressure acid leaching in nitric acid, 80% of Al and 78% of Ga could be leached at the condition of temperature = 220 °C, pulp density = 25%, HNO3 concentration = 340 g/L, and time = 2 h [189]. Alternative to the pressure acid leaching process, Gao et al. [190] have recently applied sequential leaching with HCl following the H2SO4 solution that yielded an overall efficiency of 96.75% Al and 97.64% Fe. Further, the HCl leached solution was taken for precipitation by adding Al(OH)3 to adjust the solution pH at 3.5. Then after heating the solution at 65 °C for 4 h, the sulfuric acid leached solution was added, mixed, and kept for 24 h to synthesize the polymeric aluminum ferric chloride sulfate coagulant that can be used for wastewater treatment. Employing the multi-step dissolution process, Valeev et al. [191] applied HCl leaching to dissolve aluminum and thus obtained AlCl3.6H2O after salting out from the leach liquor was subjected to alkaline treatment and recrystallization of Al(OH)3 by seeding effect with coarse Al(OH)3 addition. The thus obtained Al(OH)3 was calcined at 1000 °C for 1 h to recover the sandy grade alumina as a final product.
To recover the high-pure alumina as a final product, a few researchers also adopted the high-temperature pre-treatment of coal fly ash. Yan et al. [192] used calcination with CaCO3 at 1390 °C for 1 h, which was further dissolved in a Na2CO3 solution at 70 °C for 30 min to dissolve more than 87% aluminum in the alkali solution. The evolved CO2 was recycled to the leached solution to allow Al(OH)3 precipitation, which again sent for calcination between 400–550 °C to finally yield a high-grade γ-Al2O3 (65/550) with a surface area of 230 m2/g. Recently, Yang et al. [193] applied the NaOH molten-salt calcination that transformed the Al-rich mullite phase into nepheline (NaAlSiO4) at a mass ratio of NaOH:Fly ash = 0.8:1 and temperature = 400 °C. The calcined mass leached with 40% NaOH solution at 260 °C for 1 h could dissolve 94% of alumina, leaving silicon in the residue as NaCaHSiO4 mineral phase.
To design a clean process, the electrolytic treatment of coal fly ash has been explored as a potential alternative to chemical treatment. Using the anode made of TaOx/IrOx-coated Ti and Ti-cathode, Shi et al. [194] performed a controlled reduction of iron, thereby allowing different iron products like Fe3O4, FeOOH, and Fe0 with a purity of above 98% at a current density of 2500 Amp/m2. In their subsequent study [195], the alteration of the charge sequence of Al3+, Fe3+, and H2O was achieved to first separate Fe0 and leave Al2(SO4)3 in the solution at a higher current density of 5000 Amp/m2. As a continuous improvement on the process, they further utilized the heat produced during the electrolytic process for the in-situ hydrolysis of titanium as Ti(OH)4, and overall recovery of 97.6% iron, 93.1% titanium, and 88.5% alumina was achieved [196].
Coal is a good source of gallium and germanium that gets enriched in fly ash after burning coal. Meawad et al. [197] investigated water/acid/alkali leaching followed by precipitation, distillation, ion flotation, adsorption (with activated carbon), and solvent extraction. In a study conducted by Arroyo [198], leaching with oxalic acid yielded 90% Ge and sulfuric acid yielded 82% Ga, which was differing from the leaching with corrosive (6.0 M) HCl followed by Fe-precipitation and solvent extraction of Ga with LIX54 [199]. Font et al. [200] used water leaching at 90 °C for yielding 86% Ge, while Hernandez-Exposito et al. [201] employed ion-flotation with different reagents (hydroquinone, pyrogallol, catechol, and resorcin) and observed that they yielded 100% Ge extraction from the fly ash at pH ranges between 4.0 to 7.0. In contrast, in the leaching conducted at alkaline pH (~12.0), Wang et al. [202] observed 55–69% of Se, which was also supported by the study of Iwashita et al. [203].
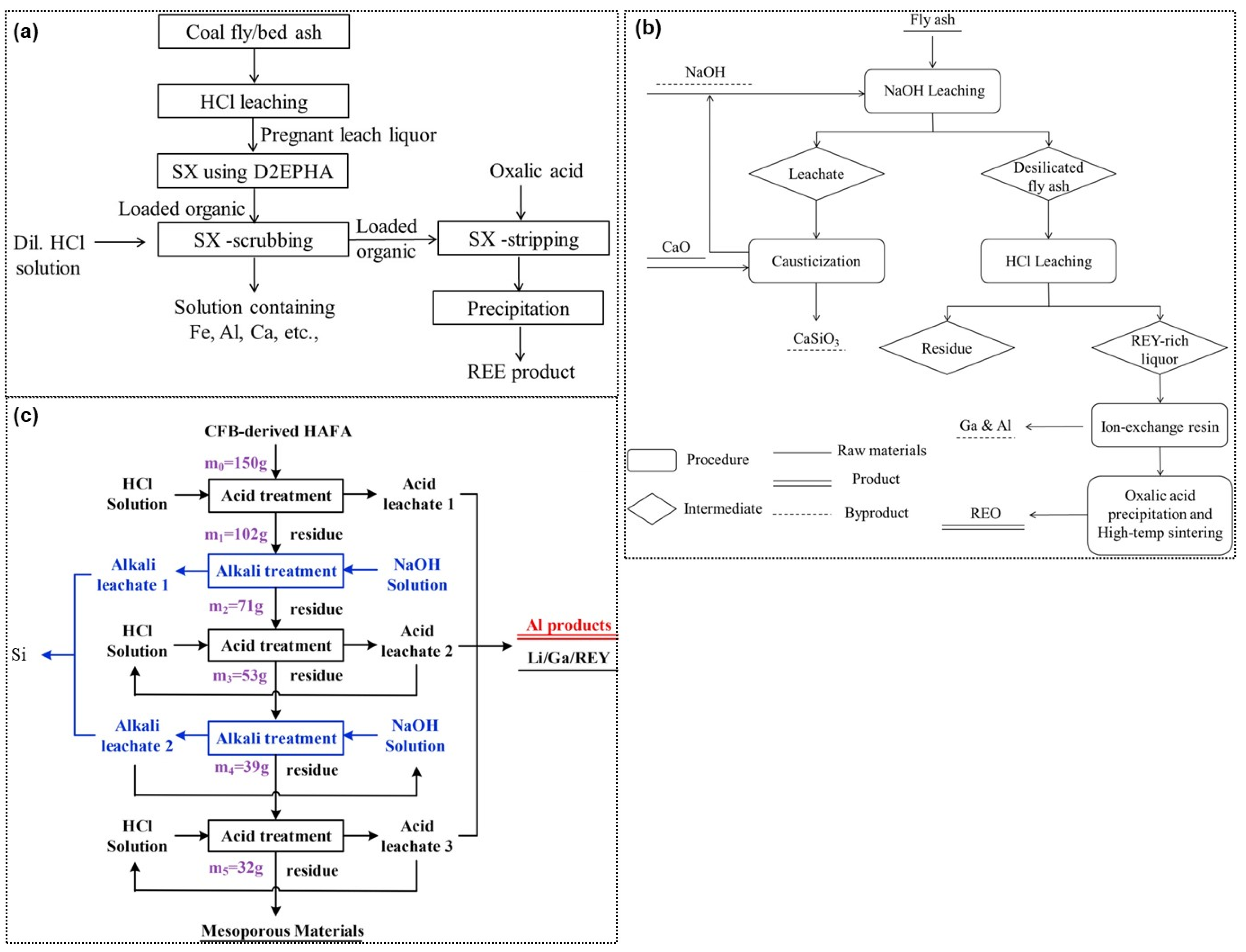
In the recent past, the contents of rare earth in coal fly ash have become an attraction and plenty of lab studies have been conducted and reviewed [204]. Pan et al. [205] tested three different samples of origins from the lab muffle, circulating fluidized bed, and pulverized coal furnace to investigate the liberation of rare earths from the fly ash samples. The results showed a 50% higher leaching efficiency in the HCl solution while using the samples from the muffle and circulating fluidized bed than that was obtained with the pulverized coal furnace. The major route searched for the rare earth’s extraction from coal fly ash is shown in Figure 11. As with the suitability of rare earth forming chloride species, Honaker et al. [206] applied HCl leaching of coal fly ash (refer to Figure 12a). The leach liquor containing rare earth metal chloride species were separated by solvent extraction with D2EHPA and subsequently the loaded organic was stripped with oxalic acid to recover metal precipitates in their oxalate form. Using P204 extractant in n-heptane, the extraction order was obtained to be La < Ce < Pr < Nd [207], which consisted of the rule of lanthanide shrinkage. At the optimized condition of 6 vol.% P204, feed solution pH 2.1, and the organic-to-aqueous ratio of 1, the extraction efficiency of La, Ce, Pr, Nd, and Y was above 89%, 94%, 95%, 96%, and 99%, respectively.
Alternatively, a direct sulfuric acid leaching of fly ash performed at 1.5 M H2SO4 at a pulp density of 20% for 5 h has been reported to yield 50% light and 90% heavy rare earth dissolution efficiency [208]. Looking at the difficulties in acid leaching, Wang et al. [209] applied caustic leaching to first remove silica as CaSiO3. The leached residue was subjected to an HCl leaching to dissolve rare earth in an acid solution, which was passed through an ion exchange resin to separate Ga and Al while the rare earth remaining in the raffinate were precipitated using oxalic acid (refer to Figure 12b). Alternately, Tang et al. [210] introduced an alkaline fusion of fly ash and observed that the added fluxes (Na2CO3, Na2O2, NaOH, and KOH) were able to break the matrix of fly ash and making rare earth amenable to leach out in acid solution (with the efficiency between 57% to 65%). Furthermore, to improve the recovery efficiency, a sequential acid, and alkali treatment were applied by Ma et al. [211] thereby, 78% Al2O3, 80% Li, 72% Ga, and 55% rare earth were extracted after 2-step HCl treatment(refer to Figure 12c). Moreover, a second NaOH treatment was able to extract 63% Si and then re-leaching of residue in HCl solution presented abundant mesopores of a high specific surface area, i.e., 273 m2/g [212]. In another work, Wen et al. [213] applied mechanically enhanced alkaline pre-treatment of fly ash at a ratio of 1:8 followed by leaching in 3.0 M HCl solution at 90 °C for 90 min, yielding >79% extraction efficiency of La, Nd, and Ce.
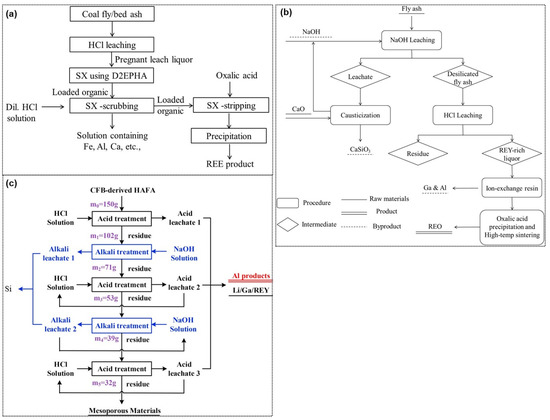
Figure 12.
General flow sheets of different treatment routes to recover rare earths from the coal fly ash, consisting the HCl leaching-solvent extraction technique (a), two-step alkali and acid leaching (b), and multi-stage alkali and acid leaching process (c) [206,209,211].
Figure 12.
General flow sheets of different treatment routes to recover rare earths from the coal fly ash, consisting the HCl leaching-solvent extraction technique (a), two-step alkali and acid leaching (b), and multi-stage alkali and acid leaching process (c) [206,209,211].
6. Perspectives
In nutshell, this review identifies the challenges associated with industrial waste which is disposed of directly into the environment. The open disposal of industrial waste (herein, red mud, and coal fly ash) created environmental havoc. However, they can be managed in a sustainable manner by proper characterization, scientific treatments, resource recovery, and implementing a circular economy in the industrial process. Fly ash and red mud have enough potential to be used as a secondary source, as they are widely used in the construction sectors but due to the presence of metals, their applications in construction are limited. Therefore, this study emphasizes the removal of metals from these byproducts using the hydrometallurgical method and further to be used for the construction purpose. The reclamation of several critical elements such as rare earths, titanium, gallium, and germanium have been discussed comprehensively to achieve the aim of sustainable waste management. It needs to be emphasized that the techniques related to critical metals’ recovery from these waste materials are still on their infantilism and testing at the pilot scale is highly desirable to make it applicable at the commercial scale. The real challenge lies in their low contents and in further raising their concentration to make them suitable feed for economic exploitation.
7. Conclusions
This study assessed the challenges and regulations associated with industrial solid waste. Two case studies on industrial waste i.e., red mud and fly ash were evaluated to determine the potential of industrial waste. It is noted that the circular economy concept used in the industrial process not only minimizes waste generation but also generates secondary resource materials. However, it is recommended to characterize the materials to identify their potential and improved the utilization of secondary materials. The circular economy in the red mud process highlights that valuable metals present in red mud can be recovered and the remaining materials can be used as blending materials in cement and filling material. Moreover, fly ash has tremendous applications in the cement industry, and metal can be recovered through hydrometallurgy. Thus, this study elaborates the industrial waste management in a sustainable manner where the industrial waste will be a source of secondary materials and minimize the pollution load. The circular economy has created a sustainable pathway between industry and the environment and helps in achieving SDGs.
Author Contributions
Conceptualization, R.R.S., S.I. and P.P.; Methodology, D.K.R. and H.K.; Literature Analysis, R.R.S., S.I., D.K.R. and P.P.; Resources, R.R.S., D.K.R., S.I., H.K. and P.P.; Data Curation, R.R.S., D.K.R., S.I. and P.P.; Writing-Original Draft Preparation, R.R.S., D.K.R., S.I. and P.P.; Writing-Review & Editing, R.R.S., D.K.R., S.I., H.K. and P.P.; Visualization, R.R.S. and D.K.R.; Supervision, R.R.S. and P.P.; Project Administration, S.I. and H.K.; Funding Acquisition, S.I. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Brain Pool Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (Grant No. 2021H1D3A2A01100016) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Project no. 2020R1I1A1A01074249).
Data Availability Statement
Not applicable.
Acknowledgments
The authors are very thankful to the Referees whose valuable and insightful inputs were very crucial to revise the manuscript in its present form.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sonjica, B.P. Waste Act: National Policy on Thermal Treatment of General and Hazardous Waste. National Environmental Management: Waste Act, 2008 (Act No. 59 of 2008). 2009. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/32439777.pdf (accessed on 5 November 2022).
- Zhangqi, Z.; Zhuli, C.; Lingyun, H. Technological innovation, industrial structural change and carbon emission transferring via trade: An agent-based modeling approach. Technovation 2022, 110, 102350. [Google Scholar] [CrossRef]
- El Haggar, S. Sustainable Industrial Design and Waste Management: Cradle-to-Cradle for Sustainable Development; Academic Press, Elsevier: San Diego, CA, USA, 2010. [Google Scholar]
- Millati, R.; Cahyono, R.B.; Ariyanto, T.; Azzahrani, I.N.; Putri, R.U.; Taherzadeh, M.J. Agricultural, industrial, municipal, and forest wastes: An overview. In Sustainable Resource Recovery and Zero Waste Approaches, 1st ed.; Taherzadeh, M., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Saint Louis, MO, USA, 2019; pp. 1–22. [Google Scholar]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Awasthi, M.K.; Dussap, C.G.; Pandey, A. Assessing the impact of industrial waste on environment and mitigation strategies: A comprehensive review. J. Hazard. Mater. 2020, 398, 123019. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Rhee, Y.H. Current research trends of microbiological leaching for metal recovery from industrial wastes. Curr. Res. Technol. Educ. Topics. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1289–1292. [Google Scholar]
- Matinde, E.; Simate, G.S.; Ndlovu, S. Mining and metallurgical wastes: A review of recycling and re-use practices. J. South. Afr. Inst. Min. Metall. 2018, 118, 825–844. [Google Scholar] [CrossRef]
- Achawa, O.W.; Danso-Boatengb, E. Environmental management in the oil, gas and related energy industries in Ghana. Int. J. Chem. Eng. 2013, 4, 117–122. [Google Scholar]
- Asim, N.; Badiei, M.; Torkashvand, M.; Mohammad, M.; Alghoul, M.A.; Gasaymeh, S.S.; Sopian, K. Wastes from the petroleum industries as sustainable resource materials in construction sectors: Opportunities, limitations, and directions. J. Clean. Prod. 2021, 284, 125459. [Google Scholar] [CrossRef]
- Kanwal, Q.; Li, J.; Zeng, X. Mapping recyclability of industrial waste for anthropogenic circularity: A Circular economy approach. ACS Sustain. Chem. Eng. 2021, 9, 11927–11936. [Google Scholar] [CrossRef]
- Pudasainee, D.; Kurian, V.; Gupta, R. Coal: Past, present, and future sustainable use. In Future Energy: Improved, Sustainable and Clean Options for Our Planet, 3rd ed.; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–48. [Google Scholar]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- Analysis of the Development Status of China’s Industrial Solid Waste Treatment Industry in 2020. Available online: https://www.citymine.com.cn/a/1396.html (accessed on 11 November 2022).
- Bui, T.D.; Tsai, F.M.; Tseng, M.L.; Ali, M.H. Identifying sustainable solid waste management barriers in practice using the fuzzy Delphi method. Resour. Conserv. Recycl. 2020, 154, 104625. [Google Scholar] [CrossRef]
- Babu, B.R.; Parande, A.K.; Basha, C.A. Electrical and electronic waste: A global environmental problem. Waste Manag. Res. 2007, 25, 307–318. [Google Scholar]
- Mir, D.F. Environmental behaviour in Chicago automotive repair micro-enterprises (MEPs). Bus. Strategy Environ. 2008, 17, 194–207. [Google Scholar] [CrossRef]
- Misra, V.; Pandey, S.D. Hazardous waste, impact on health and environment for development of better waste management strategies in future in India. Environ. Int. 2005, 31, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, O.M.; Breum, N.O.; Ebbehøj, N.; Hansen, Å.M.; Ivens, U.I.; van Lelieveld, D.; Malmros, P.; Matthiasen, L.; Nielsen, B.H.; Nielsen, E.M.; et al. Collection of domestic waste. Review of occupational health problems and their possible causes. Sci. Total Environ. 1995, 170, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H. Textbook of Geotechnical Engineering, 4th ed.; Khan, I.H., Ed.; PHI Learning Private Limited: Delhi, India, 2019; pp. 382–383. [Google Scholar]
- Aluko, O.O.; Obafemi, T.H.; Obiajunwa, P.O.; Obiajunwa, C.J.; Obisanya, O.A.; Odanye, O.H.; Odeleye, A.O. Solid waste management and health hazards associated with residence around open dumpsites in heterogeneous urban settlements in Southwest Nigeria. Int. J. Environ. Health Res. 2022, 32, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Banthia, N.; Grace, J.R. Carbonation in concrete infrastructure in the context of global climate change–Part 1: Experimental results and model development. Cem. Concr. Compos. 2012, 34, 924–930. [Google Scholar] [CrossRef]
- Ash, H.J.; Gemmell, R.P.; Bradshaw, A.D. The introduction of native plant species on industrial waste heaps: A test of immigration and other factors affecting primary succession. J. Appl. Ecol. 1994, 31, 74–84. [Google Scholar] [CrossRef]
- Urry, J. Consuming the planet to excess. Theory Cult. Soc. 2010, 27, 191–212. [Google Scholar] [CrossRef]
- Hasnat, G.T.; Kabir, M.A.; Hossain, M.A. Major environmental issues and problems of South Asia, particularly Bangladesh. In Handbook of Environmental Materials Management; Chaudhary, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–40. [Google Scholar]
- Bouma, J. Implications of the nexus approach when assessing water and soil quality as a function of solid and liquid waste management. In Environmental Resource Management and the Nexus Approach: Managing Water, Soil, and Waste in the Context of Global Change; Hettiarachchi, H., Ardakanian, R., Eds.; Springer International Publishing: Dresden, Germany, 2016; pp. 179–209. [Google Scholar]
- Pandia, S.; Tanata, S.; Rachel, M.; Octiva, C.; Sialagan, N. February. Effect of fermentation time of mixture of solid and liquid wastes from tapioca industry to percentage reduction of TSS (Total Suspended Solids). IOP Conf. Ser.: Mater. Sci. Eng. 2018, 309, 012086. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Poll. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste mismanagement in developing countries: A review of global issues. Int. J. Environ. Res. Public Health. 2019, 16, 1060. [Google Scholar] [CrossRef]
- Ahmed, M. Effect of industrial waste on seed bank and growth of wild plants in Dhabeji area, Karachi, Pakistan. Pak. J. Bot. 2009, 41, 1659–1665. [Google Scholar]
- Martinez, N.; Mariana, C. Impact of pharmaceutical waste on biodiversity. In Ecopharmacovigilance, The Handbook of Environmental Chemistry; Gómez-Oliván, L.M., Ed.; Springer: Cham, Switzerland, 2017; pp. 235–253. [Google Scholar]
- Ji, S.; Ma, S. The effects of industrial pollution on ecosystem service value: A case study in a heavy industrial area, China. Environ. Dev. Sustain. 2022, 24, 6804–6833. [Google Scholar] [CrossRef]
- Little, R.D.; Maul, P.R.; Smith, G.M.; Towler, P.A. A comparison of hazardous and solid radioactive waste treatment and disposal. Energy Environ. 1994, 5, 255–275. [Google Scholar] [CrossRef]
- Saleh, H.E. Introductory chapter: Introduction to hazardous waste management. In Management of Hazardous Wastes; El-Din, H., Saleh, M., Rahman, O.A., Eds.; IntechOpen: Rijeka, Croatia, October 2016; pp. 1–19. [Google Scholar]
- Paul, S.N.; Frazzoli, C.; Sikoki, F.D.; Babatunde, B.B.; Orisakwe, O.E. Natural occurring radioactive materials (NORMs) from mining sites in Nigeria: A systematic review of geographical distribution and public health concern. J. Environ. Radioact. 2022, 249, 106889. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R. Emerging Domains of Material Science, 1st ed.; Thanuj International Publishers: Tamilnadu, India, 2022. [Google Scholar]
- Pathak, P.; Srivastava, R.R.; Ojasvi. Assessment of legislation and practices for the sustainable management of waste electrical and electronic equipment in India. Renew. Sustain. Energy Rev. 2017, 78, 220–232. [Google Scholar] [CrossRef]
- Araee, E.; Manavizadeh, N.; Bosjin, S.A. Designing a multi-objective model for a hazardous waste routing problem considering flexibility of routes and social effects. J. Ind. Prod. Eng. 2020, 37, 33–45. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Theisen, H.; Vigil, S.A. Integrated Solid Waste Management: Engineering Principle and Management Issue; McGraw Hill Inc.: New York, NY, USA, 1993. [Google Scholar]
- Side Event: Unmanaged Waste—A Hidden Cause of Climate Change. COP27, 2022. Available online: https://www.unodc.org/unodc/en/environment-climate/cop27-unmanaged-waste.html (accessed on 15 November 2022).
- Environmental Protection Administration to Amend the “Greenhouse Gas Reduction and Management Act” to “Climate Change Response Act”. Available online: http://cemnt.epa.gov.tw/eng/ (accessed on 8 November 2022).
- Cheng, C.H.; Huang, K.; Yeh, L.W. Industrial waste exchange and recycling status in Taiwan. Chem. Eng. Inf. 2002, 23, 48–55. (In Chinese) [Google Scholar]
- Tsai, W.T.; Chou, Y.H. A review of environmental and economic regulations for promoting industrial waste recycling in Taiwan. Waste Manag. 2004, 24, 1061–1069. [Google Scholar] [CrossRef]
- K-Assembly. The Framework Act on Resource Circulation. Republic of Korea National Assembly. 2016. Available online: https://www.law.go.kr/lsSc.do?section=&menuId=1&subMenuId=15&tabMenuId=81&eventGubun=060101&query=%EC%9E%90%EC%9B%90%EC%88%9C%ED%99%98%EA%B8%B0%EB%B3%B8%EB%B2%95#undefined (accessed on 6 November 2022).
- Kim, I.; Jang, Y. Material efficiency and greenhouse gas reduction effect of industrial waste by material circulation in Korea. J. Clean. Prod. 2022, 376, 134053. [Google Scholar] [CrossRef]
- Hazardous Waste Rules—Central Pollution Control Board. Available online: https://cpcb.nic.in/rules/ (accessed on 6 November 2022).
- Otwong, A.; Jongmeewasin, S.; Phenrat, T. Legal obstacles for the circular economy in Thailand: Illegal dumping of recyclable hazardous industrial waste. J. Clean. Prod. 2021, 302, 126969. [Google Scholar] [CrossRef]
- Pathak, P.; Rout, P.R. Urban Mining for Waste Management and Resource Recovery: Sustainable Approaches; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2021. [Google Scholar]
- Tejaswini, M.S.S.R.; Pathak, P.; Gupta, D.K. Sustainable Approach for Valorization of Solid Wastes as a Secondary Resource through Urban Mining. J. Environ. Manag. 2022, 319, 115727. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.-Q.; Lee, C. The waste treatment and recycling efficiency of industrial waste processing based on two-stage data envelopment analysis with undesirable inputs. J. Clean. Prod. 2020, 242, 118279. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Evaluation of red mud as a polymetallic source—A review. Miner. Eng. 2021, 171, 107084. [Google Scholar] [CrossRef]
- Archambo, M.; Kawatra, S.K. Red mud: Fundamentals and new avenues for utilization. Miner. Process. Extr. Metall. Rev. 2021, 42, 425–450. [Google Scholar]
- Zhang, R.; Zheng, S.; Ma, S.; Zhang, Y. Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process. J. Hazard. Mater. 2011, 189, 827–835. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, C.; Wu, Y. Characterization of red mud derived from a combined Bayer process and bauxite calcination method. J. Hazard. Mater. 2007, 146, 255–261. [Google Scholar] [CrossRef]
- Mayes, W.M.; Jarvis, A.P.; Burke, I.T.; Walton, M.; Feigl, V.; Klebercz, O.; Gruiz, K. Dispersal and attenuation of trace contaminants downstream of the ajka bauxite residue (red mud) depository failure. hungary. Environ. Sci. Technol. 2011, 45, 5147–5155. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. Options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Rai, S.; Wasewar, K.; Lataye, D.; Mukhopadhyay, J.; Yoo, C. Feasibility of red mud neutralization with seawater using Taguchi’s methodology. Int. J. Environ. Sci. Technol. 2013, 10, 305–314. [Google Scholar] [CrossRef]
- Snars, K.; Gilkes, R. Evaluation of bauxite residues (red muds) of different origins for environmental applications. Appl. Clay Sci. 2009, 46, 13–20. [Google Scholar] [CrossRef]
- Liu, D.Y.; Wu, C.S. Stockpiling and Comprehensive Utilization of Red Mud Research Progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. Treatment from abandoned mine landfill leachates. Adsorption technology. J. Mater. Res. Technol. 2019, 8, 2732–2740. [Google Scholar] [CrossRef]
- Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Review of Current Bauxite Residue Management, Disposal and Storage: Practices, Engineering and Science, Asia-Pacific Partnership Project ATF-06-3, CSIRO Document DMR-3608, The discovery, commercialization, and Development of the Aluminum Industry in France, May 2009. Available online: https://www.researchgate.net/publication/290676558_The_discovery_commercialization_and_development_of_the_aluminum_industry_in_France (accessed on 7 November 2022).
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Swain, B.; Akcil, A.; Lee, J. Red mud valorization an industrial waste circular economy challenge; review over processes and their chemistry. Crit. Rev. Environ. Sci. Technol. 2022, 52, 520–570. [Google Scholar] [CrossRef]
- Qiao, Y.H. Technical study of mixed pond for red mud from Bayer process and red mud from sintering process. Light Met. 2004, 10, 18–20. (In Chinese) [Google Scholar]
- Sun, Y.D. Research and implementation on storage process of “half-drying mixed red mud”. Energy Sav. Non-Ferr. Metall. 2009, 25, 20–25. (In Chinese) [Google Scholar]
- Zhou, Y.L. Some design problems of the red mud stockpiling yard. Light Met. 1992, 5, 15–17. (In Chinese) [Google Scholar]
- Wang, J.W. Red mud dam reinforcement measures analysis. Nonferrous Met. Eng. Res. 2009, 30, 39–41. [Google Scholar]
- Li, Q.M.; Wang, Y.H.; Fu, S.G. Application of finite element method in safety assessment of red mud disposal site. Light Met. 2007, 6, 12–16. (In Chinese) [Google Scholar]
- Rao, P.P. The characteristics and genesis discussion of fracture in dry red mud disposal yard. Ind. Const. 2010, 40, 73–77. [Google Scholar]
- Chao, X.; Zhang, T.A.; Lyu, G.; Liang, Z.; Chen, Y. Sustainable application of sodium removal from red mud: Cleaner production of silicon-potassium compound fertilizer. J. Clean. Prod. 2022, 352, 131601. [Google Scholar] [CrossRef]
- Gautam, M.; Agrawal, M. Effects of red mud addition in soil fertilized with cowdung manure on growth performance and metal accumulations in Brassica juncea cultivars Kranti and Pusa Bold. Commun. Soil Sci. Plant Anal. 2019, 50, 1214–1231. [Google Scholar] [CrossRef]
- Berta, K.M.; Kurdi, R.; Lukács, P.; Penk, M.; Somogyi, V. Red mud with other waste materials as artificial soil substitute and its effect on Sinapis alba. J. Environ. Manag. 2021, 287, 112311. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.; Mishra, B.; Apelian, D.; Blanpain, B. CR3 Communication: Red Mud—A Resource or a Waste? JOM 2013, 65, 340. [Google Scholar]
- Xiang, Q.; Liang, X.; Schlesinger, M.E.; Watson, J.L. Low-temperature reduction of ferric iron in red mud. Light Metals. In Proceedings of the Sessions, TMS Annual Meeting, Warrendale, PA, USA, 11–15 February 2001; pp. 157–162. [Google Scholar]
- Peng, X.; Huang, G. Method for Recovering Iron Concentrates from Alumina Red Mud. Google Patents CN101648159B, China, 8 October 2011. [Google Scholar]
- Kumar, R.; Srivastava, J.; Premchand, P. Utilization of iron values of red mud for metallurgical applications. In Environmental and Waste Management; Bandopadhyay, A., Goswami, N.G., Rao, P.R., Eds.; National Metallurgical Laboratory: Jamshedpur, India, 1998; pp. 108–119. [Google Scholar]
- Valeev, D.; Zinoveev, D.; Kondratiev, A.; Lubyanoi, D.; Pankratov, D. Reductive smelting of neutralized red mud for iron recovery and produced pig iron for heat-resistant castings. Metals 2020, 10, 32. [Google Scholar] [CrossRef]
- Zhu, D.; Chun, T.; Pan, J.; He, Z. Recovery of Iron From high-iron red mud by reduction roasting with adding sodium salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Liu, W.; Sun, S.; Zhang, L.; Jahanshahi, S.; Yang, J. Experimental and simulative study on phase transformation in Bayer red mud soda-lime roasting system and recovery of Al, Na and Fe. Miner. Eng. 2012, 39, 213–218. [Google Scholar] [CrossRef]
- Swagat, S.R.; Archana, P.; Jayasankar, K.; Ajit, K.M.; Kumar, C.S.; Partha, S.M.; Barada, K.M. Statistical modeling studies of Iron recovery from red mud using thermal plasma. Plasma Sci. Technol. 2013, 15, 459. [Google Scholar]
- Raspopov, N.A.; Korneev, V.P.; Averin, V.V.; Lainer, Y.A.; Zinoveev, D.V.; Dyubanov, V.G. Reduction of iron oxides during the pyrometallurgical processing of red mud. Russ. Metall. 2013, 1, 33–37. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Application of Bayer red mud for iron recovery and building material production from alumosilicate residues. J. Hazard. Mater. 2009, 161, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, J.; Xu, H.; Zhao, K.; Shi, X. Nuggets production by direct reduction of high Iron red mud. J. Iron Steel Res. Int. 2013, 20, 24–27. [Google Scholar] [CrossRef]
- Jayasankar, K.; Ray, P.K.; Chaubey, A.K.; Padhi, A.; Satapathy, B.K.; Mukherjee, P.S. Production of pig iron from red mud waste fines using thermal plasma technology. Int. J. Miner. Metall. Mater. 2012, 19, 679–684. [Google Scholar] [CrossRef]
- Changming, D.; Chao, S.; Gong, X.; Ting, W.; Xiange, W. Plasma methods for metals recovery from metal–containing waste. Waste Manag. 2018, 77, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.J.; Zhu, D.Q.; Pan, J.; He, Z. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Q. 2014, 53, 183–189. [Google Scholar] [CrossRef]
- Zinoveev, D.; Grudinsky, P.; Zakunov, A.; Semenov, A.; Panova, M.; Valeev, D.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Influence of Na2CO3 and K2CO3 addition on iron grain growth during carbothermic reduction of red mud. Metals 2019, 9, 1313. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Rao, M.; Jiang, T.; Zhuang, J.; Zhang, Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J. Hazard. Mater. 2014, 280, 774–780. [Google Scholar] [CrossRef]
- Rao, M.; Zhuang, J.; Li, G.; Zeng, J.; Jiang, T. Iron Recovery from Red Mud by Reduction Roasting-Magnetic Separation. In Light Metals 2013, The Minerals, Metals & Materials Series; Sadler, B.A., Ed.; Springer: Cham, Switzerland, 2016; pp. 125–130. [Google Scholar]
- Samouhos, M.; Taxiarchou, M.; Tsakiridis, P.E.; Potiriadis, K. Greek “red mud” residue: A study of microwave reductive roasting followed by magnetic separation for a metallic iron recovery process. J. Hazard. Mater. 2013, 254, 193–205. [Google Scholar] [CrossRef]
- Cardenia, C.; Balomenos, E.; Wai Yin Tam, P.; Panias, D. A combined soda sintering and microwave reductive roasting process of bauxite residue for iron recovery. Minerals 2021, 11, 222. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Debadatta, D.; Pramanik, K. A study on chemical leaching of iron from red mud using sulphuric acid. Res. J. Chem. Environ. 2013, 17, 50–56. [Google Scholar]
- Uzun, D.; Gülfen, M. Dissolution kinetics of iron and aluminum from red mud in sulphuric acid solution. Indian J. Chem. Technol. 2007, 14, 263–268. [Google Scholar]
- Yu, Z.L.; Shi, Z.X.; Chen, Y.M.; Niu, Y.J.; Wang, Y.X.; Wan, P.Y. Red-mud treatment using oxalic acid by UV irradiation assistance. Trans. Nonferrous Met. Soc. China 2012, 22, 456–460. [Google Scholar] [CrossRef]
- Çengeloğlu, Y.; Kir, E.; Ersöz, M. Recovery and Concentration of Al(III), Fe(III), Ti(IV), and Na(I) from Red Mud. J. Colloid Interface Sci. 2001, 244, 342–346. [Google Scholar] [CrossRef]
- Çengeloğlu, Y.; Kir, E.; Ersoz, M.; Buyukerkek, T.; Gezgin, S. Recovery and concentration of metals from red mud by Donnan dialysis. Colloids Surf. A Physicochem. Eng. Asp. 2003, 223, 95–101. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.E.; Markopoulos, C.H. Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Erçag, E.; Apak, R. Furnace smelting and extractive metallurgy of red mud: Recovery of TiO2, Al2O3 and pig iron. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1997, 70, 241–246. [Google Scholar] [CrossRef]
- Zhang, X.K.; Zhou, K.G.; Chen, W.; Lei, Q.Y.; Huang, Y.; Peng, C.H. Recovery of iron and rare earth elements from red mud through an acid leaching-stepwise extraction approach. J. Cent. South Univ. 2019, 26, 458–466. [Google Scholar] [CrossRef]
- Salman, A.D.; Juzsakova, T.; Rédey, Á.; Le, P.C.; Nguyen, X.C.; Domokos, E.; Abdullah, T.A.; Vagvolgyi, V.; Chang, S.W.; Nguyen, D.D. Enhancing the recovery of rare earth elements from red mud. Chem. Eng. Technol. 2021, 44, 1768–1774. [Google Scholar] [CrossRef]
- Shoppert, A.; Loginova, I.; Napol’skikh, J.; Kyrchikov, A.; Chaikin, L.; Rogozhnikov, D.; Valeev, D. Selective scandium (Sc) extraction from bauxite residue (red mud) obtained by alkali fusion-leaching method. Materials 2022, 15, 433. [Google Scholar] [CrossRef]
- Kasliwal, P.; Sai, P.S.T. Enrichment of titanium dioxide in red mud: A kinetic study. Hydrometallurgy 1999, 53, 73–87. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Kazantzis, N.K.; Emmert, M.H. Selective process steps for the recovery of scandium from Jamaican bauxite residue (red mud). ACS Sustain. Chem. Eng. 2018, 6, 1478–1488. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Emmert, M.H.; Narayanan, R.P. Bauxite Residue Recycling. U.S. Patent US 11,028,461, 8 June 2021. [Google Scholar]
- Qiu, X.R.; Qi, Y.Y. Reasonable utilization of red mud in the cement industry. Cem. Technol. 2011, 6, 103–105. (In Chinese) [Google Scholar]
- Archambo, M.S.; Kawatra, S.K. Extraction of rare earths from red mud iron nugget slags with oxalic acid precipitation. Miner. Process. Extr. Metall. 2022, 43, 656–663. [Google Scholar] [CrossRef]
- Feng, X.-p.; Liu, X.-m.; Sun, H.-h.; Bai, X.; Niu, X.-l. Study on the High Use Ratio of Red Mud in Cementitious Material. Multipurp. Util. Miner. Resour. 2007, 4, 35–37. [Google Scholar]
- Nikbin, I.M.; Aliaghazadeh, M.; Sh, C.; Fathollahpour, A. Environmental impacts and mechanical properties of lightweight concrete containing bauxite residue (red mud). J. Clean. Prod. 2018, 172, 2683–2694. [Google Scholar] [CrossRef]
- Liu, R.-X.; Poon, C.S. Utilization of red mud derived from bauxite in self-compacting concrete. J. Clean. Prod. 2016, 112, 384–391. [Google Scholar] [CrossRef]
- Peng, F.; Liang, K.-M.; Shao, H.; Hu, A.-M. Nano-crystal glass-ceramics obtained by crystallization of vitrified red mud. Chemosphere 2005, 59, 899–903. [Google Scholar] [CrossRef]
- Zhang, P.-X.; Yan, J.Q. Infrared spectra of crystallization of glasses using red mud as raw materials. J. Inorg. Mater. 2000, 15, 751–755. [Google Scholar]
- Yang, J.; Zhang, D.; Hou, J.; He, B.; Xiao, B. Preparation of glass-ceramics from red mud in the aluminium industries. Ceram. Int. 2008, 34, 125–130. [Google Scholar] [CrossRef]
- Dry, C.; Meier, J.; Bukowski, J. Sintered coal ash/flux materials for building materials. Mater. Struct. 2004, 37, 114–121. [Google Scholar] [CrossRef]
- Chen, X.; Lu, A.; Qu, G. Preparation and characterization of foam ceramics from red mud and fly ash using sodium silicate as foaming agent. Ceram. Int. 2013, 39, 1923–1929. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization. Constr. Build. Mater. 2013, 38, 865–871. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Thang, N.H.; Kien, P.T.; Hinode, H.; Bacani, F.T.; Gallardo, S.M. Optimizing Ternary-blended Geopolymers with Multi-response Surface Analysis. Waste Biomass Valorization 2016, 7, 929–939. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.C.; Sahoo, S.K.; Maharana, H. Progress of red mud utilization: An overview. Am. Chem. Sci. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Dimas, D.; Maragkos, I.; Panias, D. Utilization of metallurgical solid by-products for the development of inorganic polymeric construction materials. Glob. Nest J. 2009, 11, 127–136. [Google Scholar]
- Yu, X.; Cui, Y.; Chen, Y.; Chang, I.; Wu, J. The drivers of collaborative innovation of the comprehensive utilization technologies of coal fly ash in China: A network analysis. Env. Sci. Pollut. Res. 2022, 29, 56291–56308. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gacem, A.; Choudhary, N.; Rai, A.; Kumar, P.; Yadav, K.K.; Abbas, M.; Khedher, N.B.; Awwad, N.S.; Barik, D.; et al. Status of Coal-Based Thermal Power Plants, Coal Fly Ash Production, Utilization in India and Their Emerging Applications. Minerals 2022, 12, 1503. [Google Scholar] [CrossRef]
- Report on Fly Ash Generation at Coal/Lignite Based Thermal Power Stations and Its Utilization in the Country for the Year 2020–21. Available online: https://cea.nic.in/wp-content/uploads/tcd/2021/09/Report_Ash_Yearly_2020_21.pdf (accessed on 10 December 2022).
- Valeev, D.; Bobylev, P.; Osokin, N.; Zolotova, I.; Rodionov, I.; Salazar-Concha, C.; Verichev, K. A review of the alumina production from coal fly ash, with a focus in Russia. J. Clean. Prod. 2022, 363, 132360. [Google Scholar] [CrossRef]
- 2021 Coal Combustion Product (CCP) Production & Use Survey Report. Available online: https://acaa-usa.org/wp-content/uploads/2022/12/2021-Production-and-Use-Survey-Results-FINAL.pdf (accessed on 10 December 2022).
- Bhatt, A.; Priyadarshini, S.; Mohanakrishnan, A.A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00263. [Google Scholar] [CrossRef]
- Ugurlu, A. Leaching characteristics of fly ash. Environ. Geol. 2004, 46, 890–895. [Google Scholar] [CrossRef]
- Stockel, R.F.; Bridgewater, N.J. Coal Ash Fertilizer Compositions. U.S. Patent US4,469,503, 9 April 1984. [Google Scholar]
- Ahmaruzzaman, A. A review on the utilization of fly ash. Prog. Energ. Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Rajak, D.K.; Guria, C.; Ghosh, R.; Agarwal, S.; Pathak, A.K. Alkali assisted dissolution of fly ash: A shrinking core model under finite solution volume condition. Int. J. Miner. Process. 2016, 155, 106–117. [Google Scholar] [CrossRef]
- Längauer, D.; Čablík, V.; Hredzák, S.; Zubrik, A.; Matik, M.; Danková, Z. Preparation ofSynthetic Zeolites from Coal Fly Ash by Hydrothermal Synthesis. Materials 2021, 14, 1267. [Google Scholar] [CrossRef]
- Sear, L.K.A. Properties and Use of Coal Fly Ash: A Valuable Industrial By-Product; Thomas Telford: London, UK, 2001. [Google Scholar]
- Page, A.L.; Elseewi, A.A.; Straughan, I.R. Physical and chemical properties of fly ash from coal-fired power plants with reference to environmental impacts. In Residue Reviews: Residues of Pesticides and Other Contaminants in the Total Environment; Gunther, F.A., Gunther, J.D., Eds.; Springer: New York, NY, USA, 1979; Volume 71, pp. 83–120. [Google Scholar]
- Singh, R.K.; Gupta, N.C.; Guha, B.K. Fly ash disposal in ash ponds: A threat to ground water contamination. J. Inst. Eng. India Ser. A 2016, 97, 255–260. [Google Scholar] [CrossRef]
- Dutta, M. Fly Ash—An Environment and Health Perspective. Available online: http://toxicslink.org/docs/06007_Fly_Ash_Not_So_Inert_After_All.pdf (accessed on 10 November 2022).
- Adriano, D.C.; Webber, J.; Bolan, N.S.; Paramasivam, S.; Koo, B.J.; Sajwan, K.S. Effects of high rates of coal fly ash on soil, turf grass and groundwater quality. Water Air Soil Pollut. 2002, 139, 365–385. [Google Scholar] [CrossRef]
- Gupta, D.K.; Rai, U.N.; Tripathi, R.D.; Inouhe, M. Impacts of fly-ash on soil and plant responses. J. Plant Res. 2002, 115, 401–409. [Google Scholar] [CrossRef]
- Kukier, U.; Sumner, M.E. Boron availability to plants from coal combustion by-products. Water Air Soil Pollut. 1996, 87, 93–110. [Google Scholar] [CrossRef]
- Borm, J.A.P. Toxicity and occupational health hazards of coal fly ash (CFA). A review of data and comparison to coal mine dust. Ann. Occup. Hyg. 1997, 41, 659–676. [Google Scholar] [CrossRef]
- Jin, S.; Zhao, Z.; Jiang, S.; Sun, J.; Pan, H.; Jiang, L. Comparison and summary of relevant standards for comprehensive utilization of fly ash at home and abroad. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Shenzhen, China, 23–25 October 2020; Volume 621, p. 012006. [Google Scholar]
- Kar, K.K. Handbook of Fly Ash; Elsevier: Kidlington, Oxford, UK, 2021; pp. 477–479. [Google Scholar]
- Council directive 2008/98/EC on waste and repealing certain Directives. Off. J. Eur. Union L. 312. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 15 November 2022).
- Sear, L.K.A. Coal fired power station ash products and EU regulation. Coal Combust. Gasif. Prod. 2009, 1, 63–66. [Google Scholar] [CrossRef]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Feuerborn, H.J. Calcareous ash in Europe-a reflection on technical and legal issues. In Proceedings of the 2nd Hellenic Conference on Utilisation of Industrial By-Products in Construction, AianiKozani, Greece, 1–3 June 2009; Volume 1. [Google Scholar]
- Moon, S.T. Regulatory and Legal Applications: Fly ash use in cement and cementitious products. In Proceedings of the World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 22–25 April 2013. [Google Scholar]
- U.S. Environmental Protection Agency. Laws & Regulations-Summary of the Resource Conservation and Recovery Act: U.S. Environmental Protection Agency; 2015. Available online: https://pubs.usgs.gov/fs/2015/3037/pdf/fs2015-3037.pdf (accessed on 1 November 2022).
- Tang, Z.; Ma, S.; Ding, J.; Wang, Y.; Zheng, S.; Zhai, G. Current Status and prospect of fly ash utilization in China. In Proceedings of the World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 22–25 April 2013; pp. 1–7. [Google Scholar]
- He, Y.; Luo, Q.; Hu, H. Situation analysis and countermeasures of China’s fly ash pollution prevention and control. Procedia Environ. Sci. 2012, 16, 690–696. [Google Scholar] [CrossRef]
- Yunusa, I.A.; Loganathan, P.; Nissanka, S.P.; Manoharan, V.; Burchett, M.D.; Skilbeck, C.G.; Eamus, D. Application of coal fly ash in agriculture: A strategic perspective. Crit. Rev. Environ. Sci. Technol. 2012, 42, 559–600. [Google Scholar] [CrossRef]
- Dwivedi, A.; Jain, M.K. Fly ash–waste management and overview: A review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Fan, C.; Wang, B.; Ai, H.; Qi, Y.; Liu, Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. J. Clean. Prod. 2021, 319, 128790. [Google Scholar] [CrossRef]
- Bilodeau, A.; Malhotra, V. High-volume fly ash system: Concrete solution for sustainable development. ACI Mater. J. 2000, 97, 41–48. [Google Scholar]
- Velandia, D.F.; Lynsdale, C.J.; Provis, J.L.; Ramirez, F.; Gomez, A.C. Evaluation of activated high volume fly ash systems using Na2SO4, lime and quicklime in mortars with high loss on ignition fly ashes. Constr. Build. Mater. 2016, 128, 248–255. [Google Scholar] [CrossRef]
- Wilińska, I.; Pacewska, B.; Ostrowski, A. Investigation of different ways of activation of fly ash–cement mixtures. J. Therm. Anal. Calorim. 2019, 138, 4203–4213. [Google Scholar] [CrossRef]
- Joseph, S.; Snellings, R.; Cizer, Ö. Activation of Portland cement blended with high volume of fly ash using Na2SO4. Cem. Concr. Compos. 2019, 104, 103417. [Google Scholar] [CrossRef]
- du Toit, G.; van der Merwe, E.M.; Kruger, R.A.; McDonald, J.M.; Kearsley, E.P. Characterisation of the Hydration Products of a Chemically and Mechanically Activated High Coal Fly Ash Hybrid Cement. Minerals 2022, 12, 157. [Google Scholar] [CrossRef]
- Singh, H.; Brar, G.S.; Mudadhar, G.S. Evaluation of characteristics of fly ash-reinforced clay bricks as building material. J. Build. Phys. 2017, 40, 530–543. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C.; Rajak, D.K.; Pathak, A.K. Functionalization of fly ash for the substitution of bentonite in drilling fluid. J. Pet. Sci. Eng. 2018, 166, 63–72. [Google Scholar] [CrossRef]
- Rajak, D.K.; Raj, A.; Guria, C.; Pathak, A.K. Grinding of class-F fly ash using planetary ball mill: A simulation study to determine the breakage kinetics by direct-and back-calculation method. S. Afr. J. Chem. Eng. 2017, 24, 135–147. [Google Scholar] [CrossRef]
- Bentz, D.P. Powder additions to mitigate retardation in High-Volume fly ash mixtures. ACI Mater. J. 2010, 107, 508–514. [Google Scholar]
- Bentz, D.P.; Ferraris, C.F. Rheology and setting of high volume fly ash mixtures. Cem. Concr. Compos. 2010, 32, 265–270. [Google Scholar] [CrossRef]
- Deeley, G.M.; Canter, L.W.; Laguros, J.G. Stabilization of drilling fluid waste with fly ash. MRS Proc. 1986, 86, 77–85. [Google Scholar] [CrossRef]
- Thompson, L.F. Drilling fluid waste minimization and stabilization using polymer technology. Soc. Petro. Eng. 1994, 361–370. [Google Scholar]
- Totten, P.L.; King, B.L.; Griffith, J.E. Foamable Drilling Fluid and Methods of Use in Well Drilling Operations. U.S. Patent US 5,716,910, 10 February 1998. [Google Scholar]
- Ramirez, P., Jr. Reserve Pit Management: Risks to Migratory Birds. U.S. Fish & Wildlife Service Region 6 Environmental Contaminants Program. 2009. Available online: https://www.researchgate.net/profile/Pedro-Ramirez14/publication/280946626_Reserve_Pit_Management_Risks_to_Migratory_Birds/links/55ce0a9b08ae502646a70adc/Reserve-Pit-Management-Risks-to-Migratory-Birds.pdf (accessed on 5 November 2022).
- Conner, J.R.; Hoeffner, S.L. A critical review of stabilization/solidification technology. Crit. Rev. Environ. Sci. Technol. 1998, 28, 397–462. [Google Scholar] [CrossRef]
- Burnet, O.; Murtha, M.J.; Wijatno, H. Recovery of alumina from fly ash by high temperature chlorination. In Proceedings of the 3rd Kentucky Coal Refuse Disposal and Utilization Seminar, Lexington, KY, USA, 11–12 May 1977; Rose, J.G., Ed.; University of Kentucky: Lexington, KY, USA, 1977; pp. 83–88. [Google Scholar]
- Mehrotra, A.K.; Behie, L.A.; Bishnoi, P.R.; Svrcek, W.Y. High temperature chlorination of coal ash in a fluidized bed. 1. Recovery of aluminium. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 37–44. [Google Scholar] [CrossRef]
- Mehrotra, A.K.; Behie, L.A.; Bishnoi, P.R.; Svrcek, W.Y. High temperature chlorination of coal ash in a fluidized bed recovery of iron, silicon, and titanium. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 44–50. [Google Scholar] [CrossRef]
- Matjie, R.H.; Bunt, J.R.; Heerden, J.H.P. Extraction of alumina from coal fly ash generated from a selected low rank bituminous. S. Afr. Coal. Miner. Eng. 2005, 18, 299–310. [Google Scholar] [CrossRef]
- Halina, M.; Ramesha, S.; Yarmob, M.A.; Kamarudin, R.A. Nonhydrothermal synthesis of mesoporous materials using sodium silicate from coal fly ash. Mater. Chem. Phys. 2007, 101, 344–351. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Liu, Y.; Zhai, Y. Extraction of alumina from coal fly ash with sulfuric acid leaching method. Chin. J. Process Eng. 2011, 11, 254–258. [Google Scholar]
- Shoppert, A.; Loginova, I.; Valeev, D. Kinetics Study of Al Extraction from Desilicated Coal Fly Ash by NaOH at Atmospheric Pressure. Materials 2021, 14, 7700. [Google Scholar] [CrossRef]
- Shoppert, A.; Valeev, D.; Loginova, I.; Chaikin, L. Complete extraction of amorphous aluminosilicate from coal fly ash by alkali leaching under atmospheric pressure. Metals 2020, 10, 1684. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Li, L.; Wang, Y.; Li, M. Crystallization mechanism of ammonium aluminum sulfate during cooling process. J. Cryst. Growth 2021, 560, 126064. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, Y.; Li, L. Synthesis and characterization of pseudoboehmite by neutralization method. Ceram. Int. 2021, 47, 15923–15930. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Li, L.; Wang, Y. Effect of SDBS on Crystallization Behavior of Pseudoboehmite. J. Phys. Chem. C 2021, 125, 26039–26048. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Li, L.; Wang, Y.; Li, M. Thermodynamics of ammonioalunite precipitation in ammonium aluminum sulfate solution. Hydrometallurgy 2020, 195, 105393. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Li, L.; Wang, Y.; Li, M. Kinetics of extracting alumina by leaching coal fly ash with ammonium hydrogen sulfate solution. Chem. Pap. 2019, 73, 2289–2295. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, P.; Li, L. Synthesis of alumina with coarse particle by precipitating aluminum ammonium sulfate solution with ammonia. Adv. Powder Technol. 2016, 27, 124–129. [Google Scholar] [CrossRef]
- Van der Merwe, E.M.; Gray, C.L.; Castleman, B.A.; Mohamed, S.; Kruger, R.A.; Doucet, F.J. Ammonium sulphate and/or ammonium bisulphate as extracting agents for the recovery of aluminium from ultrafine coal fly ash. Hydrometallurgy 2017, 171, 185–190. [Google Scholar] [CrossRef]
- Doucet, F.J.; Mohamed, S.; Neyt, N.; Castleman, B.A.; van der Merwe, E.M. Thermochemical processing of a South African ultrafine coal fly ash using ammonium sulphate as extracting agent for aluminium extraction. Hydrometallurgy 2016, 166, 174–184. [Google Scholar] [CrossRef]
- Xu, D.; Li, H.; Bao, W.; Wang, C. A new process of extracting alumina from high-alumina coal fly ash in NH4HSO4+ H2SO4 mixed solution. Hydrometallurgy 2016, 165, 336–344. [Google Scholar] [CrossRef]
- Aphane, M.E.; Doucet, F.J.; Kruger, R.A.; Petrik, L.; van der Merwe, E.M. Preparation of sodium silicate solutions and silica nanoparticles from South African coal fly ash. Waste Biomass Valorization 2020, 11, 4403–4417. [Google Scholar] [CrossRef]
- Valeev, D.; Kunilova, I.; Alpatov, A.; Mikhailova, A.; Goldberg, M.; Kondratiev, A. Complex utilisation of ekibastuz brown coal fly ash: Iron & carbon separation and aluminum extraction. J. Clean. Prod. 2019, 218, 192–201. [Google Scholar]
- Valeev, D.; Kunilova, I.; Shoppert, A.; Salazar-Concha, C.; Kondratiev, A. High-pressure HCl leaching of coal ash to extract Al into a chloride solution with further use as a coagulant for water treatment. J. Clean. Prod. 2020, 276, 123206. [Google Scholar] [CrossRef]
- Hou, H.; Shao, S.; Ma, B.; Li, X.; Shi, S.; Chen, Y.; Wang, C. Sustainable process for valuable-metal recovery from circulating fluidized bed fly ash through nitric acid pressure leaching. J. Clean. Prod. 2022, 360, 132212. [Google Scholar] [CrossRef]
- Gao, J.M.; Wang, B.; Li, W.; Cui, L.; Guo, Y.; Cheng, F. High-efficiency leaching of Al and Fe from fly ash for preparation of polymeric aluminum ferric chloride sulfate coagulant for wastewater treatment. Sep. Purif. Technol. 2023, 306, 122545. [Google Scholar] [CrossRef]
- Valeev, D.; Shoppert, A.; Mikhailova, A.; Kondratiev, A. Acid and acid-alkali treatment methods of al-chloride solution obtained by the leaching of coal fly ash to produce sandy grade alumina. Metals 2020, 10, 585. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Liu, N.; Gao, Y.; Meng, Y.; Li, K.; Chen, X. Green synthesis of mesoporous γ-Al2O3 from coal fly ash with simultaneous on-site utilization of CO2. J. Hazard. Mater. 2018, 359, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, H.; Peng, T.; Zeng, L.; Chao, L. Separation of alumina from aluminum-rich coal fly ash using NaOH molten salt calcination and hydrochemical process. Clean Technol. Environ. Policy 2022, 24, 1507–1519. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, K.X.; Zhang, T.A.; Zhu, X.F. Electrolysis designed for clean production of selective iron products from coal fly ash leachate. Hydrometallurgy 2021, 203, 105617. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, K.X.; Zhang, T.A.; Zhu, X.F. Simultaneous separation of Fe & Al and extraction of Fe from waste coal fly ash: Altering the charge sequence of ions by electrolysis. Waste Manag. 2022, 137, 50–60. [Google Scholar] [PubMed]
- Shi, Y.; Jiang, K.X.; Zhang, T.A.; Zhu, X.F. Simultaneous and clean separation of titanium, iron, and alumina from coal fly ash in one spot: Electrolysis-hydrolysis method. Sep. Purif. Technol. 2022, 294, 121247. [Google Scholar] [CrossRef]
- Meawad, A.S.; Bojinova, D.Y.; Pelovski, Y.G. An overview of metals recovery from thermal power plant solid wastes. Waste Manag. 2010, 30, 2548–2559. [Google Scholar] [CrossRef]
- Arroyo, F.; Font, O.; Chimenos, J.M.; Pereira, C.F.; Querol, X.; Coca, P. IGCC fly ash valorisation. Optimisation of Ge and Ga recovery for an industrial application. Fuel Process. Technol. 2014, 124, 222–227. [Google Scholar] [CrossRef]
- Gutie’rrez, B.; Pazos, C.; Coca, J. Recovery of gallium from coal fly ash by a dual reactive extraction process. Waste Manag. Res. 1997, 15, 371–382. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Lopez-Soler, A.; Chimenos, J.M.; Fernandez, A.I.; Burgos, S.; Garcia, P.F. Germanium extraction from gasification fly ash. Fuel 2005, 84, 1384–1392. [Google Scholar] [CrossRef]
- Hernandez-Exposito, A.; Chimenos, J.M.; Fernandez, A.I.; Font, O.; Querol, X.; Coca, P.; Garcia, P.F. Ion flotation of germanium from fly ash aqueous leachates. Chem. Eng. J. 2006, 118, 69–75. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Burken, J.G.; Ban, H.; Ladwig, K. The leaching characteristics of selenium from coal fly ashes. J. Environ. Qual. 2007, 36, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, A.; Sakaguchi, Y.; Nakajima, T.; Takanashi, H.; Ohki, A.; Kambara, S. Leaching characteristics of boron and selenium for various coal fly ashes. Fuel 2005, 84, 479–485. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Powell, M.A.; Equeenuddin, S.M. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. Int. J. Coal Sci. Technol. 2016, 3, 267–283. [Google Scholar] [CrossRef]
- Pan, J.; Long, X.; Zhang, L.; Shoppert, A.; Valeev, D.; Zhou, C.; Liu, X. The Discrepancy between Coal Ash from Muffle, Circulating Fluidized Bed (CFB), and Pulverized Coal (PC) Furnaces, with a Focus on the Recovery of Iron and Rare Earth Elements. Materials 2022, 15, 8494. [Google Scholar] [CrossRef]
- Honaker, R.Q.; Zhang, W.; Werner, J. Acid leaching of rare earth elements from coal and coal ash: Implications for using fluidized bed combustion to assist in the recovery of critical materials. Energy Fuels 2019, 33, 5971–5980. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, X.; Zhou, C.; Yang, F.; Ji, W. Study on Solvent Extraction of Rare Earth Elements from Leaching Solution of Coal Fly Ash by P204. Minerals 2022, 12, 1547. [Google Scholar] [CrossRef]
- Rao, K.A.; Md, S.; Ramadevi, G.; Thakurta, S.G.; Sreenivas, T. On the characterization and leaching of rare earths from a coal fly ash of Indian origin. Sep. Sci. Technol. 2021, 56, 541–557. [Google Scholar]
- Wang, Z.; Dai, S.; Zou, J.; French, D.; Graham, I.T. Rare earth elements and yttrium in coal ash from the Luzhou power plant in Sichuan, Southwest China: Concentration, characterization and optimized extraction. Int. J. Coal Geol. 2019, 203, 1–14. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, C.; Zhang, N.; Pan, J.; Cao, S.; Hu, T.; Ji, W.; Wen, Z.; Nie, T. Extraction of rare earth elements from coal fly ash by alkali fusion–acid leaching: Mechanism analysis. Int. J. Coal. Prep. Uti. 2022, 42, 536–555. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, S.; Zhang, H.; Cheng, F. Novel extraction of valuable metals from circulating fluidized bed-derived high-alumina fly ash by acid–alkali–based alternate method. J. Clean. Prod. 2019, 230, 302–313. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, X.; Guo, Y.; Cheng, F. Extraction of valuable metals and preparation of mesoporous materials from circulating fluidized bed-derived fly ash via an acid–alkali-based alternate method. ACS Omega 2020, 5, 31295–31305. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhou, C.; Pan, J.; Cao, S.; Hu, T.; Ji, W.; Nie, T. Recovery of rare-earth elements from coal fly ash via enhanced leaching. Int. J. Coal Prep. Uti. 2022, 42, 2041–2055. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).